DOI:10.32604/cmc.2021.012095

| Computers, Materials & Continua DOI:10.32604/cmc.2021.012095 |  |
| Article |
Modelling and Analysis of Bacteria Dependent Infectious Diseases with Variable Contact Rates
1Innovative Internet University for Research, Kanpur, 208017, India
2P.P.N.(P.G.) College, CSJM University, Kanpur, 208001, India
3College of Computer and Information Sciences, Majmaah University, Majmaah, 11952, Saudi Arabia
*Corresponding Author: Sunil Kumar Sharma. Email: s.sharma@mu.edu.sa
Received: 20 June 2020; Accepted: 30 August 2020
Abstract: In this research, we proposed a non-linear SIS model to study the effect of variable interaction rates and non-emigrating population of the human habitat on the spread of bacteria-infected diseases. It assumed that the growth of bacteria is logistic with an intrinsic growth rate is a linear function of infectives. In this model, we assume that contact rates between susceptibles and infectives as well as between susceptibles and bacteria depend on the density of the non-emigrating population and the total population of the habitat. The stability theory has been analyzed to analyzed to study the crucial role played by bacteria in the increased spread of an infectious disease. It is shown that as the density of non-emigrating population increases, the spread of an infectious disease increases. It is shown further that as the emigration increases, the spread of the disease decreases in both the cases of contact mentioned above rates, but this spread increases as these contact rates increase. It suggested that the control of bacteria in the human habitat is very useful to decrease the spread of an infectious disease. These results are confirmed by numerical simulation.
Keywords: Mathematical modelling; density dependent contact rates; stability analysis
Most of the deaths worldwide are caused due to infectious diseases. A severe threat to the wellbeing and public health is caused not only due to the new infectious diseases but also due to the increasing prevalence of drug-related diseases as well as the resurgence of chronic infectious diseases. Recently, considerable evidence found to suggest that common strategies are adopted by different pathogens to cause disease and infection. The infectious organism causes Infectious diseases; these are bacteria, viruses, fungi, etc. Under normal circumstances, disease symptoms may not develop when the immune system of the host is fully functional, but an infectious disease ensues if the immune system of the host is compromised. Bacteria, protozoa, viruses, etc. cause most of the infections in living organisms. Bacteria is a unicellular prokaryotic micro-organism. In the human habitat, due to household emission, various kinds of carriers and vectors grow and survive. They become agents to carry bacteria to food and water of Susceptibles, causing them to be infected by various diseases. Notably, in habitats, which are not clean enough, various types of bacteria such as mycobacterium tuberculosis, vibrio cholera etc. are present, the growth rate of which depends upon the following factors:
• The Bacteria discharges by infective in the environment.
• The characteristics of household discharges such as nutrients, salts, amino acids, and vitamins etc.
• The characteristics of bacteria related to particular diseases such as TB, Typhoid etc.
• The natural conditions such as the climate of the habitat.
Therefore, we assume that the bacteria population density is proportional to the density of carriers, such as house flies, and hence its growth rate is assumed to follow the logistic model [1]. These bacteria get transported by carriers to susceptible and their food as well as water, making them infectives indirectly [2]. Mathematical models have been used in the study of the spread and control of infectious diseases for a long time. In classical models, the contact rates between susceptible and infectives have been assumed to be constant [3–6], but recently the effect of density-dependent contact rate, death rate etc. on the spread of infectious diseases have been studied. However, the effect of the non-emigrating population of the habitat on the spread of the infectious disease has never been considered in various studies. However, in a realistic situation, the contact rates between susceptible and infectives as well as between susceptible and bacteria depends upon the density of non-emigrating population [7–9]. The massive consumption of alcohol affects almost all parts of the body, which in turn responsible for gonorrhea, which is a bacterial disease. This disease infects the parts of the body like urine, eyes, throat, vagina anus, and the female’s fallopian tubes, uterus etc. [10]. Recently researchers work in the field of application of mathematical modeling multiscale fast correlation filtering tracking algorithm [11], grammar model [12], grammar mapping and the integer modulo Arithmetic [13] and decision model of knowledge [14] transfer The spread of an infectious disease can be controlled by using awareness programs. However, the disease remains endemic due to immigration [11,12,15,16].
Therefore, we proposed a non-linear mathematical model to study the effects of the following aspects of the spread of bacteria-infected diseases:
• Effect of the non-emigrating population of the habitat.
• Effect of emigration dependent contact rate between susceptible and infective population as well as between susceptible and bacteria population.
• Effects of discharge of bacteria by infectives in the habitat.
• Effect of control of bacteria by using chemicals and pesticides.
2 Mathematical Model with Emigration Dependent Contact Rates
Let the total human population of the habitat at time t be N(t), which consists of the susceptible population density X(t) and infective population density Y(t). We assume that the susceptible population of the habitat may be affected by the bacterial populations with density B(t) (Fig. 1). The bacteria population grows logistically with its intrinsic growth rate coefficient as a linear function of infective population Y(t). The contact rate
where, N0 is the non-emigrating population density of the habitat which is a fraction of N,

Figure 1: Interaction phenomena between susceptibles and infectives
Similarly, the emigration dependent contact between susceptibles and bacteria population is assumed to be a non-negative linear function of the total population as,
where,
In view of the above situation, the diseases dynamics model given below:
where, X(0) = X0 > 0,
The model system Eq. (3) has the following parameters:
• A: Immigration rate of the human population from outside.
•
•
•
•
• d: Natural death rate coefficient.
•
•
•
•
•
•
The model system Eq. (3) is reduced as follows by using X = N − Y and
With the initial conditions:
Region of attraction: The following set gives the domain region of the model system Eqs. (4)–(6) as
where,
3 Basic Reproduction Number and Equilibrium Analysis of the Model
The basic reproduction number R0 is used to measure the transmission potential of a disease. It is the average number of secondary infections produced by an infection in a human habitat where the living population is susceptible [17,18].
The non-linear mathematical model Eqs. (4)–(6) has three non-negative equilibria as:
1.
2.
3.
It is noted that when
Proof. The equilibrium point E0 exists obviously. For the model system Eqs. (4)–(6), we prove the existence of
By using Eqs. (10) and (11) we define a polynomial function in Y as,
We note from Eq. (12),
•
•
Thus, the equation F(Y) = 0 has at least one root
then, by using Eq. (12) in Eq. (13), we get
Hence F′(Y) < 0 for R0 > 1. Thus a unique root of F(Y) = 0,
The other equilibrium point
By using the equations Eqs. (16) and (17) in Eq. (15) and setting
We define the following function
It is noted from Eq. (18)
•
•
Hence, the equation H(Y) = 0 has at least one root in
By using Eq. (18) in Eq. (19), we have
Provided R1 > 1, where R1 is the reproduction number. Hence the equation Eq. (18) have unique root in the interval
The stability of the equilibrium points
Proof. See Appendix A.
The equilibrium point
where, Bm is defined in Section 2 Eq. (7).
Proof. See Appendix B: It is noted from Eq. (4), the inequalities Eqs. (23) and (24) are stronger than inequalities Eqs. (21) and (22), as expected. Further, the inequalities Eqs. (21) and (23) are satisfied automatically when
5 Numerical Simulation of the Model
By using MAPLE, we show the existence and stability of the equilibrium point
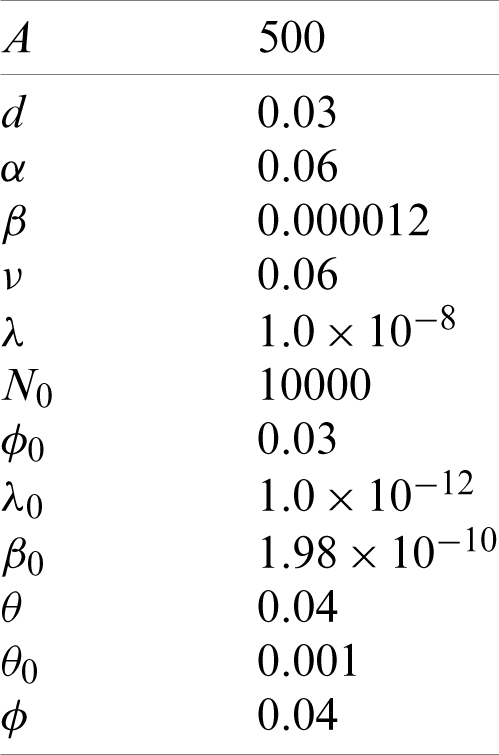
For these values of parameters, the value of the non-trivial equilibrium point
The Jacobian matrix at
The eigenvalues of the above matrix at
Since the sign of one eigenvalue is negative and two of them having negative real part, therefore the non-trivial equilibrium point
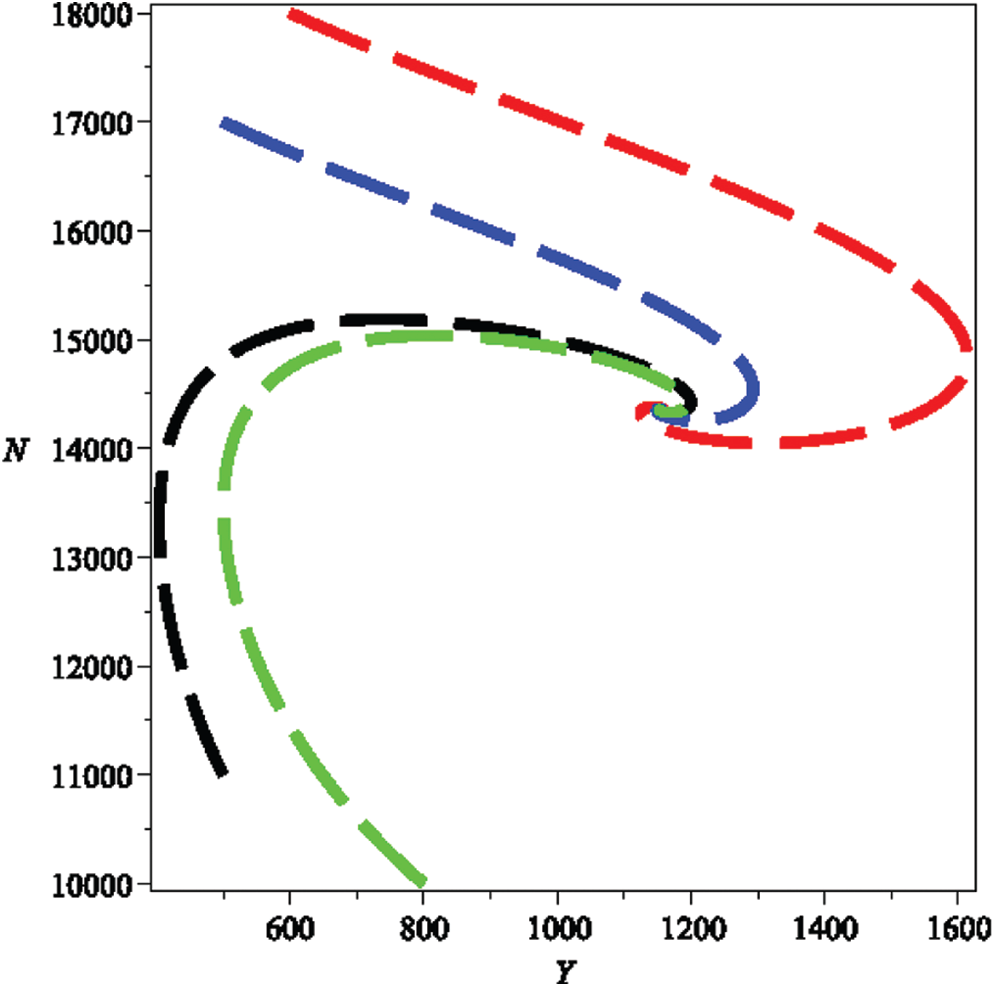
Figure 2: Phase plots between infected human population density Y(t) and human population density N(t)
It depicted in Fig. 3, as

Figure 3: Effect of transmission coefficient

Figure 4: Effect of emigration coefficient

Figure 5: Effect of transmission coefficient

Figure 6: Effect of emigration coefficient

Figure 7: Effect of growth rate coefficient

Figure 8: Effect of the depletion rate coefficient

Figure 9: Effect of the natural growth rate coefficient of bacteria population

Figure 10: Effect of constant immigration rate A on infective population density Y

Figure 11: Effect of non-emigrating population N0 on infective population density Y
In this study, an SIS non-linear model with emigration has been proposed and analyzed to study the effects of the following factors on the spread of bacteria-infected diseases.
• Effect of non-emigrating population.
• Effect of the emigration dependent contact rate between susceptibles and infectives, which is dependent on non-emigrating population and the total human population in the habitat.
• Effect of the emigration dependent contact rate between susceptibles and bacteria population, which depends on non-emigrating population and the total population in the habitat.
• Effect of the discharge rate of bacteria by the infectives in the habitat.
• Effect of the growth rate of bacteria, which is assumed to follow the logistic model, the growth rate of which is a linear function of the infective population.
Using the stability theory, the analysis of the model has shown the following results.
• As the discharge rate of bacteria by infectives increases, the spread of bacteria-infected disease increases.
• As the non-emigrating population density increases, the spread of bacteria-infected diseases increases.
• As the direct contact rate between susceptibles and infectives increases, the spread of a bacteria infected disease increases.
• As the contact rate between susceptibles and bacteria population increases, the spread of bacteria infected disease increases.
• As the emigration rate increases, the spread of bacteria-infected disease decreases.
• As the natural growth rate of bacteria increases, the spread of an infectious disease increases.
• As the immigration rate increases, the spread of bacteria-infected disease increases.
• As the control rate of bacteria in the habitat increases, the spread of the disease decreases.
The simulation study of the non-linear model confirms the above outcomes. It has been concluded that if the bacteria population in the habitat controlled by using pesticides, the spread of bacteria-infected disease can be reduced considerably.
Acknowledgement: The authors would like to express their heartfelt thanks to the editors and anonymous referees for their most valuable comments and constructive suggestions which leads to the significant improvement of the earlier version of the manuscript. The authors are thankful to the Deanship of Scientific Research at Majmaah University.
Funding Statement: Dr. Sunil Kumar Sharma would like to thank Deanship of Scientific Research at Majmaah University for supporting this work under the Project No. R-2021-8.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. E. I. Mondragon, L. Esteva and E. M. B. Rosevo, “Mathematical model for the growth of Mycobacterium tuberculosis in the granuloma,” Mathematical Biosciences, vol. 15, no. 2, pp. 407–428, 2018. [Google Scholar]
2. R. I. Joh, H. Wang, H. Weiss and J. S. Weitz, “Dynamics of indirectly transmitted infectious diseases with immunological threshold,” Bulletin of Mathematical Biology, vol. 71, no. 4, pp. 845–862, 2009. [Google Scholar]
3. S. R. Verma, R. Naresh, M. Agarwal and S. Sundar, “Role of environmental factors on the spread of bacterial diseases: A modeling study,” Computational Ecology and Software, vol. 10, no. 2, pp. 59–73, 2020. [Google Scholar]
4. C. I. Siettos and L. Russo, “Mathematical modeling for disease dynamics,” Virulence, vol. 4, no. 4, pp. 295–306, 2013. [Google Scholar]
5. A. Huppert and G. Catriel, “Mathematical modelling and prediction in infectious diseases epidemiology,” Clinical Microbiology and Infection, vol. 19, no. 11, pp. 999–1005, 2013. [Google Scholar]
6. J. Wang and S. Liao, “A generalized cholera model and epidemic/endemic analysis,” Journal of Biological Dynamics, vol. 6, no. 2, pp. 568–589, 2012. [Google Scholar]
7. S. Singh, J. Singh and J. B. Shukla, “Modelling and analysis of the effects of density dependent contact rates on the spread of carrier dependent infectious diseases with environmental discharges,” Modelling Earth System Environment, vol. 5, no. 1, pp. 21–32, 2019. [Google Scholar]
8. S. Singh, J. Singh, S. K. Sharma and J. B. Shukla, “The effect of density dependent emigration on the spread of infectious diseases: A modeling study,” Revista Latinoamericana de Hipertension, vol. 14, no. 1, pp. 83–88, 2019. [Google Scholar]
9. S. K. Sharma, J. B. Shukla, J. Singh and S. Singh, “The effect of density dependent migration on the spread of infectious diseases: A mathematical model,” Revista de la Universidad Del Zulia, vol. 10, no. 27, pp. 184–211, 2019. [Google Scholar]
10. A. Raza, M. S. Arif, M. Bibi and M. Mohsin, “Stochastic numerical analysis for impact of heavy alcohol consumption on transmission dynamics of gonorrhoea epidemic,” Computers, Materials & Continua, vol. 62, no. 3, pp. 1125–1142, 2020. [Google Scholar]
11. Y. T. Chen, J. Wang, S. J. Liu, X. Chen, J. Xiong et al., “Multiscale fast correlation filtering tracking algorithm based on a feature fusion model,” Concurrency and Computation: Practice and Experience, vol. 47, no. 5, pp. e5533, 2019. [Google Scholar]
12. P. He, Z. L. Deng, C. Z. Gao, X. N. Wang and J. Li, “Model approach to grammatical evolution: deep-structured analyzing of model and representation,” Soft Computing, vol. 21, no. 18, pp. 5413–5423, 2017. [Google Scholar]
13. P. He, Z. L. Deng, H. F. Wang and Z. S. Liu, “Model approach to grammatical evolution: Theory and case study,” Soft Computing, vol. 20, no. 9, pp. 3537–3548, 2016. [Google Scholar]
14. C. R. Wu, Y. W. Chen and F. Li, “Decision model of knowledge transfer in big data environment,” China Communications, vol. 13, no. 7, pp. 100–107, 2016. [Google Scholar]
15. I. Z. Kiss, J. Cassell, M. Recker and P. L. Simon, “The impact of information transmission on 642 epidemic outbreaks,” Mathematical Biosciences, vol. 225, no. 1, pp. 1–10, 2010. [Google Scholar]
16. A. K. Misra, A. Sharma and J. B. Shukla, “Modeling and analysis of effects of awareness programs by media on the spread of infectious diseases,” Mathematical and Computer Modelling, vol. 53, no. 5–6, pp. 1221–1228, 2011. [Google Scholar]
17. W. Wang and X. Q. Zhao, “Basic reproduction numbers for reaction-diffusion epidemic models,” SIAM Journal on Applied Dynamical Systems, vol. 11, no. 4, pp. 1652–1673, 2012. [Google Scholar]
18. P. V. D. Driessche, “Reproduction number of infectious disease models,” Infectious Disease Modeling, vol. 2, no. 3, pp. 288–303, 2017. [Google Scholar]
19. B. Dasbasi and I. Ozturk, “On the stability analysis of the general mathematical modeling of bacterial infection,” International Journal of Engineering & Applied Sciences, vol. 10, no. 2, pp. 93–117, 2018. [Google Scholar]
Appendix A. Proof of Theorem 4.1
Proof. The model system Eqs. (4)–(6) as:
For the above model system Eqs. (4)–(6), the Jacobian matrix is defined for the system as
The Jacobian matrix for the first equilibrium point
From the above Jacobian matrix, it is clear that one of the eigenvalues is
It is clear from the above Jacobian matrix, one eigenvalue
Differentiating Eq. (27) with respect to t, we get
The linearization of the functions gives,
Then by Eq. (28), we have
Here we choose the constant k1 such that
The derivative
By combining conditions Eqs. (35), (36) and on substituting the value of k1 in Eq. (34), we get the conditions as stated in Theorem 4.
Appendix B. Proof of Theorem 4.2
Proof. We consider the Lyapunov function,
On differentiating Eq. (39) with respect to ‘t’, we get
By choosing
For
and,
By combining the inequalities Eqs. (45)–(46)
Hence the non-trivial equilibrium point is non-linearly stable in
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |