 Open Access
Open Access
ARTICLE
Predictors of Early Right Ventricular Dysfunction after Cone Reconstruction for Ebstein’s Anomaly: A Retrospective Cohort Study
1 Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, China
2 National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital, Guangzhou Medical University, Guangzhou, 510080, China
3 Department of Cardiovascular Surgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
4 Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, 510080, China
* Corresponding Authors: Haiyun Yuan. Email: ; Shusheng Wen. Email:
Congenital Heart Disease 2025, 20(1), 13-25. https://doi.org/10.32604/chd.2025.063437
Received 14 January 2025; Accepted 24 February 2025; Issue published 18 March 2025
Abstract
Background: Although Cone reconstruction has been shown to improve biventricular function over time, postoperative right ventricular dysfunction (RVD) is frequently observed, signiffcantly affecting reoperation and long-term prognosis. This study aims to identify the predictors for postoperative RVD. Methods: This retrospective cohort study included 51 patients with Ebstein’s anomaly who underwent the Cone reconstruction. RVD was deffned as right ventricular fractional area change (RV-FAC) less than 35% and tricuspid annular plane systolic excursion (TAPSE) less than 17 mm through pre-discharge echocardiography. Univariate and multivariate analyses were used to analyze the pre-operative predictors. Results: The median age at surgery was 37.7 (±15.3) years, RVD was documented in 25 patients (49%) of the 51 patients. Patients with RVD had signiffcantly higher right ventricular end-systolic volume index (RVESVi) (p = 0.001), right ventricular end-diastolic volume index (RVEDVi) (p = 0.03), and septal leaffet displacement (p = 0.003). Multivariate analysis conffrmed that septal leaffet displacement was independently associated with postoperative RVD (p = 0.02). Additionally, RVD was not related to the cardiopulmonary bypass time, ICU stay and total hospital time. Conclusions: This study suggests that preoperative right ventricular ejection fraction (RVEF) reduction, severe septal leaffet displacement and signiffcant right ventricular dilatation are key predictors of early postoperative RVD. RVD may exacerbate tricuspid regurgitation, and this ffnding indicates that predicting RVD may aid in identifying high-risk patients prone to recurrence of tricuspid regurgitation after Cone reconstructionKeywords
Ebstein’s anomaly is a rare congenital heart disease, occurring in 1–5 per 200,000 live births and accounting for 1% of congenital heart disease [1]. EA is characterized by the downward displacement of the tricuspid valve leaflets, primarily affecting the septal leaflet, followed by the posterior and anterior leaflets. It is also associated with dilation and atrialization of the right ventricle (RV), alongside various degrees of hypertrophy and thinning of the wall [2,3,4]. The anterior leaflet may present with redundancy, rupture, and limited mobility. Additionally, dilation of the tricuspid annulus often leads to tricuspid regurgitation, which can reduce the effective volume of the RV [5].
The Cone procedure, a surgical technique aimed at reconstructing the right ventricular inflow tract, has shown promising results in reducing tricuspid regurgitation and improving right ventricular function, consequently improving patient quality of life and prognosis [6]. Related studies have reported favorable clinical outcomes post-Cone reconstruction, with diminished reoperation rates and mortality compared to other surgeries, alongside better long-term outcomes [7,8]. However, postoperative right ventricular dysfunction (RVD) remains a significant concern, often leading to complications such as tricuspid regurgitation, arrhythmias, and heart failure. International literature has reported predictors for RVD after the Cone procedure, such as marked right ventricular dilatation and decreased right ventricular ejection fraction (RVEF), however, the pathophysiological underlying early postoperative RVD remains inconclusive [9,10]. The pathophysiological mechanisms underlying RVD are multifactorial. During Cone reconstruction, the RV may experience acute volume or pressure overload. This can be due to increased venous return, or changes in ventricular interdependence. Factors such as hypoxia, myocardial ischemia, microemboli, air emboli, arrhythmias, and prolonged cardiopulmonary bypass (CPB) time also can lead to RVD [9].
Cardiac magnetic resonance (CMR) is widely acknowledged as the gold standard for assessing right ventricular size and function. It provides precise evaluations of right ventricular volume and ejection fraction, characterized by excellent reproducibility and minimal variability. It is crucial for physicians to assess surgical risks, refine surgical strategies, and forecast postoperative outcomes. While CMR can provide detailed quantitative measures, we chose to use echocardiography as the primary imaging modality due to its widespread availability and routine use in clinical practice. The recent guidelines propose several easier two-dimensional measurement methods, such as the right ventricular fractional area change (RV-FAC) and tricuspid annular plane systolic excursion (TAPSE). These parameters are limited in the individual evaluation of right ventricular function, thus they are often combined in the examination to assess RVD. This comprehensive approach provides a more accurate evaluation of tricuspid valve function and its impact on right ventricular performance [11,12]. The aim of this study was to identify preoperative and operative predictors of early postoperative RVD and to determine the relationship between RVD and postoperative tricuspid regurgitation severity.
This study included 51 patients with EA who underwent Cone reconstruction at Guangdong Provincial People’s Hospital from January 2014 to December 2024. Bases for exclusion in this study are as follows: (1) a history of prior cardiac surgery and radiofrequency ablation; (2) the presence of other complex cardiac malformations (such as transposition of the great arteries, valvular disease); (3) poor image quality or failure to complete the relevant examinations for this study; (4) age less than 10 years old, etc. [9,13]. Guangdong Provincial People’s Hospital (GDPPH) Ethics Research Committee approved this retrospective study and waived the requirement for informed consent. The ethics committee reference code is KY2024-693-02. Subjects’ privacy and personal identification information were removed. The data of the patients was stored anonymously in the hospital database. Follow-ups were conducted by contacting the patients or their legal guardians via telephone and WeChat. Transthoracic echocardiography was used to assess the patients before and after the procedure. In this study, vasoactive-inotropic score (Vis) is determined by the dose of vasoactive drugs within the first 24 h postoperatively.
The Cone reconstruction, an advancement of Carpentier’s repair technique, is predominantly utilized for patients who have no significant valvular abnormalities and possess a sufficiently large anterior leaflet [14]. During the procedure, the anterior, posterior, and septal leaflets of the tricuspid valve are carefully detached, and then severing the anomalous papillary muscles and fibrous tissues, preserving only the chordae tendineae directed towards the cardiac apex. With the premise of fully detaching and utilizing all leaflets, especially the ectopic and underdeveloped septal leaflet, the detached leaflets are rotated clockwise and the corresponding edges are sutured to form a 360° conical structure with the apex directed towards the apex of the heart. The dilated atrialized RV is then folded longitudinally, and the tricuspid annulus is appropriately reduced, with the constructed tricuspid valve is then being sutured onto the new annulus [15].
CMR was conducted using a 3.0T scanner. In cases where patients had multiple CMR scans, the most recent one before the Cone reconstruction was chosen for analysis, given its relevance to the current clinical state. Biventricular volumes were assessed at end-diastole and end-systole through manual delineation of the endocardial and epicardial contours. Right ventricular volume and functional results were reported in a 4-chamber stack, see Fig. 1 below. Classification criteria for right heart dysfunction was based on RVEF: Normal: RVEF ≥ 45%; Mild Dysfunction: RVEF 40%–44%; Moderate Dysfunction: RVEF 30%–39%; Severe Dysfunction: RVEF < 30%. Severe right heart dilatation is typically defined using the following criteria: right ventricular end-diastolic volume index (RVEDVi) ≥ 160 mL/m2 or right ventricular end-systolic volume index (RVESVi) ≥ 80 mL/m2. The Celermajer index was calculated as the right atrium plus the atrialized portion of the RV (functional RV þ left atrium þ left ventricle) from the four-chamber view. All CMR examinations were reviewed and contours were confirmed by a pediatric cardiologist with extensive CMR experience. The aforementioned procedures were independently performed by two experienced radiologists, with discrepancies resolved by consensus [16].
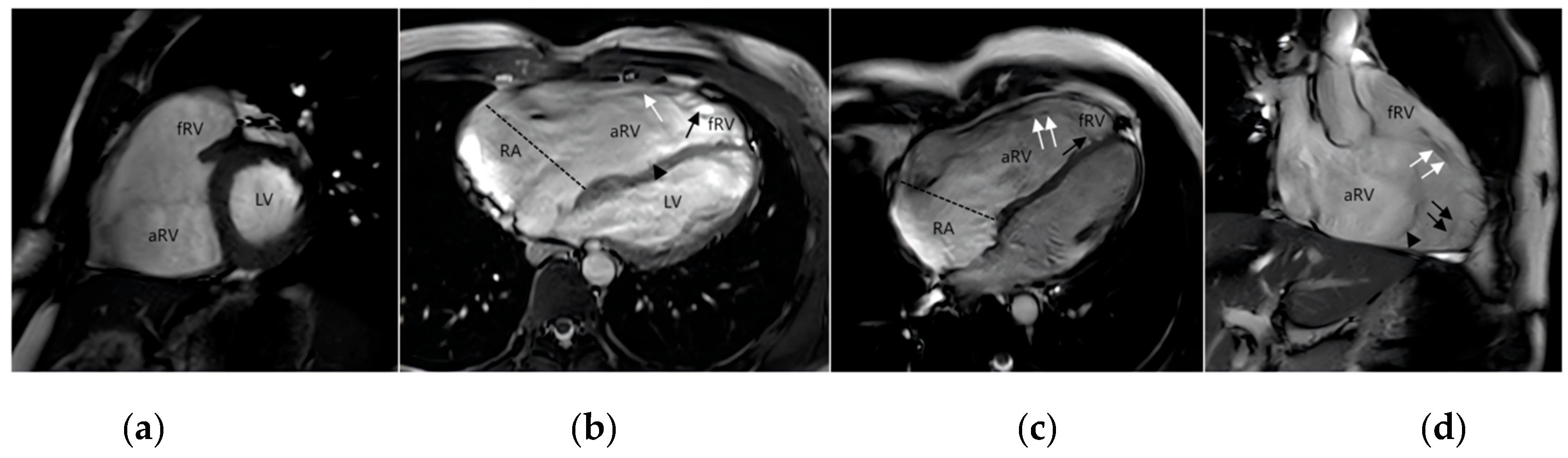
Figure 1: Magnetic resonance imaging of EA. (a) the short-axis image; (b) horizontal axis bitmap; (c) four-chamber heart view; (d) the inflow-outflow tract view of the right ventricle. The atrialized right ventricle (aRV) is located between the atrioventricular junction (dotted line) and the orifice of the functional tricuspid valve. Functional right ventricle (fRV) is the part of the right ventricle that lies between the aRV and the pulmonary valve. Anatomically, the right ventricle is a combination of aRV and fRV. The functional orifice of the tricuspid valve rotates toward the apex and upward toward the right ventricular outflow tract. Anterior valve (white↑), septal valve (black↑) and posterior valve (▲).
Cardiologists performed all preoperative and postoperative two-dimensional echocardiographic measurements. The dataset included examinations conducted three months prior to surgery, immediately before discharge after surgery, and three months postoperatively. The three-month follow-up echocardiograms were specifically utilized to track temporal changes in right ventricular function. The tricuspid valve was typically evaluated in the apical four-chamber view and inflow tract of RV view, see Fig. 2 below. The long diameter of the right atrium is defined as the distance from the midpoint of the tricuspid valve annulus to the roof of the right atrium at end-systole, when the right atrium is maximally dilated. Similarly, the long diameter of the RV is defined as the distance from the midpoint of the tricuspid valve annulus to the apex of the RV at end-diastole, when the RV is maximally filled. These measurements are taken in the apical four-chamber view. Fifteen patients with EA were randomly selected for reproducibility assessment. For intra-observer variability, the same investigator analyzed the samples at least one month later and was blinded to the initial results. For inter-observer variability, another investigator, who was blinded to the clinical data and results, analyzed the same samples. Patients were classified as having RVD if they met both of the following criteria in pre-discharge echocardiography: RV-FAC less than 35% and TAPSE less than 17 mm.
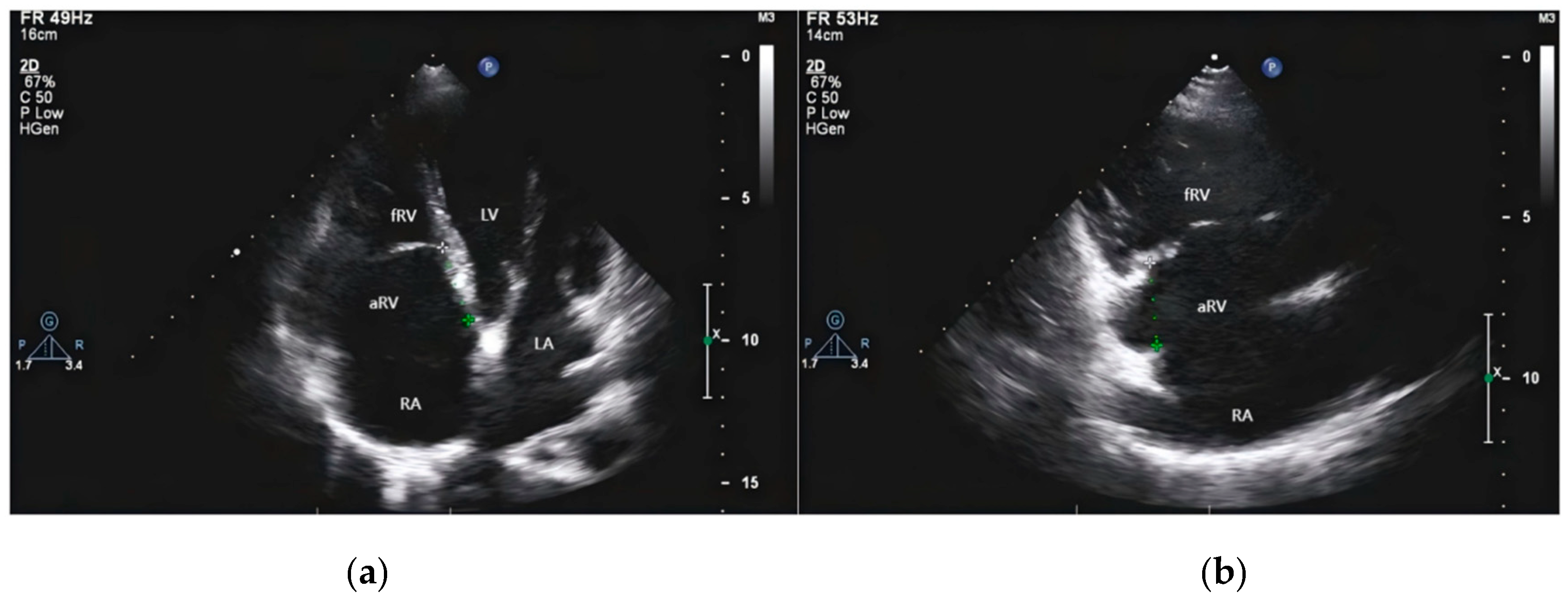
Figure 2: Transthoracic echocardiography of EA. (a) Four-chamber section shows two-dimensional images of dilated right atrium (RA), atrialized right ventricle (aRV), functional right ventricle (fRV) and the distance between the anterior mitral valve and the tricuspid septal valve. (b) The section of the inflow tract of the two cavities of the right ventricle shows the enlarged RA and aRV, and the posterior tricuspid valve is short and obviously moved downward.
The RVFAC was calculated as [(right ventricular end-diastolic area-right ventricular end-systolic area)/right ventricular end-diastolic area] from the four-chamber view. The TAPSE was obtained from a four-chamber view: Place the M-mode cursor through the tricuspid annulus, parallel to the interventricular septum, ensuring it crosses the lateral aspect of the tricuspid annulus, and then measure the vertical distance from the maximal diastolic position to the systolic position of the tricuspid annulus. Color Doppler can evaluate the severity of tricuspid regurgitation by multiple views. By measuring the vena contracta (VC), the patients were divided into three groups according to the severity: VC ≤ 3 mm was mild, 3 mm < VC < 7 mm was moderate, and VC ≥ 7 mm was severe [17].
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) and compared to an independent t-test or a Mann-Whitney. The categorical data were expressed as frequency (percentage) and analyzed using the chi-squared test or Fisher’s exact Test. Univariate and multivariate logistic regression analyses were employed to identify the predictors of RVD following Cone reconstruction. The receiver operating characteristic (ROC) curve was utilized to ascertain the optimal critical value for RVD prediction post-CR. The area under curve (AUC) was computed to assess the accuracy of predictors for postoperative RVD. A p-value of less than 0.05 is considered statistically significant. Data for this study were analyzed using R software (version 4.0, R Foundation, Vienna, Austria).
3.1 Population Characteristics
After excluding patients who were under 10 years of age (n = 13), had a history of cardiac surgery (n = 2), had a history of radiofrequency ablation (n = 5), had other complex cardiac malformations (n = 5), did not undergo surgery after CMR (n = 22), underwent alternative surgical procedures after CMR (n = 18), had poor image quality (n = 4), incomplete imaging data (n = 3), a total of 51 patients with EA were included in the study, see Fig. 3 below, and their baseline clinical characteristics are summarized in Table 1. The age of the patients was 37.7 ± 15.3 years, with a predominance of females (38/51, 75%). The majority of patients were classified as New York Heart Association (NYHA) Class I and NYHA Class II (43/51, 84%). In preoperative Carpentier’s classification, type A and type B were predominant, with no statistically significant difference between the two groups (p = 0.47), see Table 1.
Table 1: Baseline characteristics of the study population.
| Parameters | All Patients (n = 51) | Non-RVD (n = 26) | RVD (n = 25) | p-Value |
|---|---|---|---|---|
| Age (y) | 37.7 ± 15.3 | 36.5 ± 16.1 | 38.9 ± 14.8 | 0.587 |
| Body mass index (kg/m2) | 22.1 ± 3.2 | 22.0 ± 3.5 | 22.2 ± 3.0 | 0.803 |
| Sex | 0.935 | |||
| Male | 13 (25%) | 6 (23%) | 7 (28%) | |
| Female | 38 (75%) | 20 (77%) | 18 (72%) | |
| Carpentier’s classification | 0.466 | |||
| A | 18 (35%) | 9 (35%) | 9 (36%) | |
| B | 22 (43%) | 13 (50%) | 9 (36%) | |
| C | 11 (22%) | 4 (15%) | 7 (28%) | |
| D | 0 (0%) | 0 (0%) | 0 (0%) | |
| NYHA class | 0.283 | |||
| NYHA I | 24 (47%) | 16 (61%) | 6 (24%) | |
| NYHA II | 19 (37%) | 7 (27%) | 14 (56%) | |
| NYHA III | 8 (16%) | 3 (12%) | 5 (20%) | |
| NYHA IV | 0 (0%) | 0(0%) | 0 (0%) | |
| Symptoms | 0.312 | |||
| chest distress | 10 (20%) | 4 (15%) | 6 (24%) | |
| breathlessness | 8 (16%) | 5 (19%) | 3 (12%) | |
| palpitation | 14 (27%) | 5 (19%) | 9 (36%) | |
| Preoperative ASD | 1.000 | |||
| YES | 27 (53%) | 14 (54%) | 13 (52%) | |
| NO | 24 (47%) | 12 (46%) | 12 (48%) | |
| Cardiothoracic ratio (%) | 0.60 ± 0.09 | 0.57 ± 0.08 | 0.63 ± 0.08 | 0.008** |
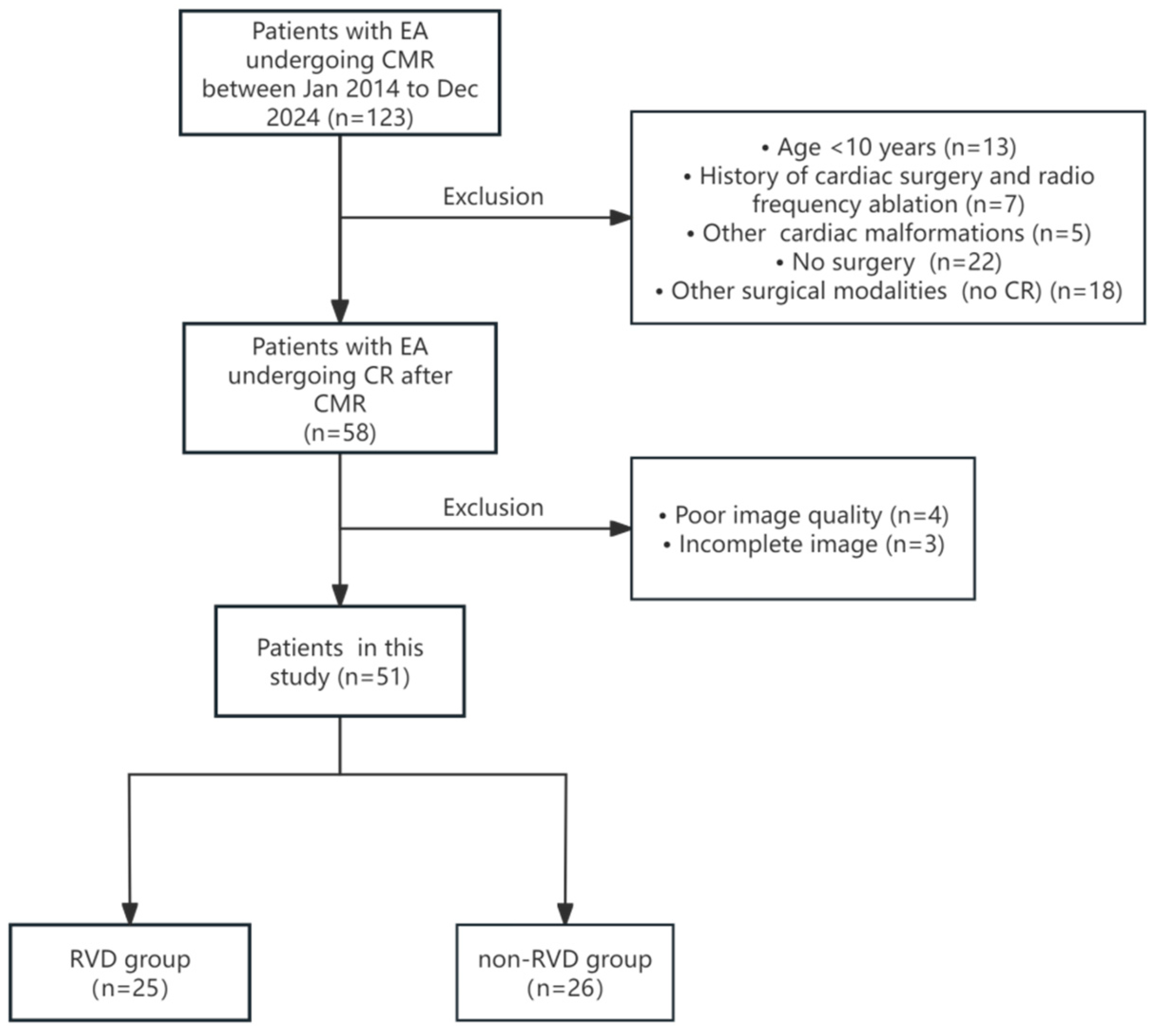
Figure 3: Flowchart depicting patient inclusion criteria. EA, Ebstein anomaly; CMR, cardiovascular magnetic resonance; CR, Cone reconstruction; RVD, right ventricular dysfunction.
Echocardiographic results comparison revealed a significant difference in the degree of septal leaflet displacement between the two groups preoperatively (p = 0.003), but there was no difference in the degree of posterior leaflet displacement. Additionally, there was no significant difference in the diameter of right atrium and RV between the two groups. Postoperative echocardiograms showed that 25 patients (49%) had RVD, see Table 2.
Table 2: Comparison of preoperative CMR and echocardiography parameters in all patients, RVD group and non-RVD group after Cone reconstruction.
| Parameters | All Patients (n = 51) | Non-RVD (n = 26) | RVD (n = 25) | p-Value |
|---|---|---|---|---|
| Preoperative CMR | ||||
| LVEDVi (mL/m2) | 62.7 (52.7, 72.6) | 57.8 (52.6, 67.4) | 64.1 (56.4, 73.9) | 0.214 |
| LVESVi (mL/m2) | 24.5 (19.8, 31.1) | 22.7 (19.2, 30.2) | 24.9 (21.7, 31.7) | 0.178 |
| LVSVi (mL) | 56.8 ± 13.3 | 56.0 ± 12.7 | 57.6 ± 14.0 | 0.668 |
| LVCO (mL) | 4.2 (3.8, 4.9) | 4.4 (3.8;5.0) | 4.1 (3.8;4.7) | 0.678 |
| LVEF (%) | 59.2 (55.1, 62.9) | 59.7 (55.3, 63.9) | 59.0 (53.0, 61.4) | 0.332 |
| RVEDVi (mL/m2) | 183.5 (140.9, 226.0) | 153.0 (113.1, 201.2) | 208.5 (179.3, 246.5) | 0.003** |
| RVESVi (mL/m2) | 103.5 (64.9, 126.4) | 71.7 (52.1, 106.4) | 115.9 (95.6, 152.9) | 0.001** |
| RVEF (%) | 46.9 ± 8.8 | 50.0 ± 8.3 | 43.6 ± 8.1 | 0.007** |
| Celermajer index | 0.72 (0.53, 0.89) | 0.65 (0.52, 0.89) | 0.74 (0.62, 1.12) | 0.263 |
| GOSE | 2.0 (2.0, 2.0) | 2.0 (2.0, 2.0) | 2.0 (2.0, 3.0) | 0.126 |
| Right heart dysfunction: | 0.003** | |||
| Normal | 20 (39%) | 16 (61%) | 4 (16%) | |
| Mild | 18 (35%) | 7 (27%) | 11 (44%) | |
| Moderate | 9 (18%) | 3 (12%) | 6 (24%) | |
| Severe | 4 (8%) | 0 (0%) | 4 (16%) | |
| Preoperative echocardiography | ||||
| Septal leaflet (mm) | 29.6 ± 10.9 | 25.3 ± 11.2 | 34.0 ± 8.8 | 0.003** |
| Posterior leaflet (mm) | 39.6 ± 15.7 | 36.5 ± 16.8 | 42.8 ± 14.1 | 0.151 |
| Right atrium (mm) | 74.7 ± 20.0 | 70.0 ± 19.9 | 79.3 ± 9.4 | 0.108 |
| Right ventricle (mm) | 47.2 ± 13.5 | 45.1 ± 12.6 | 49.5 ± 14.4 | 0.251 |
| LVEF (%) | 65.8 ± 6.8 | 67.1 ± 7.0 | 64.5 ± 6.4 | 0.172 |
The RVD group had a significantly lower RVEF on preoperative CMR (p = 0.007), and poorer right ventricular function (p = 0.003). The degree of cardiac dilation was more severe in RVD patients. Patients with RVD exhibited a higher RVEDVi (p = 0.03) and RVESVi (p = 0.01). The Celermajer index was not different between the two groups preoperatively, see Table 2.
Patients with RVD had a higher Vismax (24 h) (p = 0.02). The ICU time was longer in the RVD group, but the difference was not statistically significant. The difference in hospital time between the two groups was also not statistically significant. There was no significant difference in CPB time and Aortic occlusion (ACC) time. There was no statistically significant difference in the degree of tricuspid regurgitation between the two groups postoperatively, and left ventricular function was normal in all 51 patients postoperatively. In our study, we observed that patients had RVD postoperatively continued to exhibit RVD in subsequent evaluations three months later. Notably, we observed that in some patients with RVD, the severity of valvular regurgitation worsened over time (p = 0.014), see Table 3.
Table 3: Comparison of clinical outcomes after Cone in all patients with EA, RVD group and non-RVD group.
| Parameters | All Patients (n = 51) | Non-RVD (n = 26) | RVD (n = 25) | p-Value |
|---|---|---|---|---|
| Vis Max (24 h) | 10.0(6.3, 13.0) | 7.8 (5.0, 10.0) | 11.0(8.0, 17.0) | 0.002** |
| Vis Mean (24 h) | 6.0 (4.3, 9.1) | 5.4 (4.0, 7.4) | 9.0 (4.5, 11.3) | 0.010* |
| CPB time (min) | 129.0 (112.5, 156.5) | 125.0 (103.0, 166.0) | 132.0 (120.0, 152.0) | 0.528 |
| ACC time (min) | 83.0 (67.5, 97.8) | 83.0 (66.0, 109.0) | 83.0 (70.0, 95.0) | 0.831 |
| ICU time (d) | 2 (2, 4) | 2 (2, 4) | 3 (2, 4) | 0.456 |
| Hospital time (d) | 20 (16, 22) | 19 (16, 22) | 20 (16, 23) | 0.691 |
| Postoperative Degree of tricuspid regurgitation | ||||
| Normal | 15 (29%) | 10 (39%) | 5 (20%) | 0.444 |
| Mild | 23 (45%) | 11 (42%) | 12 (48%) | |
| Moderate | 9 (18%) | 4 (15%) | 5 (20%) | |
| Severe | 4 (8%) | 1 (4%) | 3 (12%) | |
| Three Months after surgery | ||||
| Normal | 11 (21%) | 10 (39%) | 1 (4%) | 0.014* |
| Mild | 25 (49%) | 11 (42%) | 14 (56%) | |
| Moderate | 10 (20%) | 4 (15%) | 6 (24%) | |
| Severe | 5 (10%) | 1 (4%) | 4 (16%) |
3.5 Predictors of RVD after Cone Reconstruction
Univariate and multivariate logistic regression analysis were carried out to determine the predictors of RVD after Cone reconstruction. The results are summarized in Table 4. Univariate logistic regression analysis showed that RVESVi, RVEDVi, RVEF and Valve displacement were significantly correlated with postoperative RVD. In multivariate logistic regression analysis, Valve displacement was still an important predictor of postoperative RVD [Odds ratio (OR) 1.088; Confidence interval (CI): (1.004–1.179); p = 0.020].
Table 4: Univariate and multivariate logistic regression analysis of RVD after Cone reconstruction.
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| LVEDVi | 1.023 (0.989–1.064) | 0.213 | ||
| LVESVi | 1.046 (0.994–1.116) | 0.119 | ||
| LVEF | 0.984(0.920–1.050) | 0.638 | ||
| RVEDVi | 1.014(1.004–1.025) | 0.007* | ||
| RVESVi | 1.023(1.009–1.043) | 0.005* | ||
| RVEF | 0.902(0.823–0.972) | 0.013* | ||
| Valve displacement | 1.092(1.023–1.165) | 0.008* | 1.088(1.004–1.179) | 0.020* |
ROC analysis is used to determine the cut-off value of meaningful parameters. Only the cut-off values of the parameters with significant significance (p < 0.05) in univariate analysis were evaluated. The sensitivity and specificity of RVESVi > 83.04 for predicting early RVD were 65% and 88%, respectively. The sensitivity and specificity of RVESVi Valve displacement > 26.50 for predicting RVD were 62% and 84%, respectively, see Table 5. ROC curve analysis was used to evaluate the predictive effectiveness of different parameters on RVD after Cone reconstruction. The highest predictive efficacy of RVD after Cone was observed in RVESVi, and the AUC was 0.768, see Fig. 4 below.
Table 5: Receiver operating characteristic curve analysis for predicting RVD after Cone reconstruction.
| Parameters | AUC (95% CI) | Cut-Off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| RVESVi | 0.768(0.634–0.901) | 83.04 | 65 | 88 |
| RVEDVi | 0.742(0.605–0.878) | 175.66 | 69 | 76 |
| RVEF | 0.728(0.586–0.870) | 48.97 | 62 | 84 |
| Valve displacement | 0.742(0.602–0.882) | 26.50 | 62 | 84 |
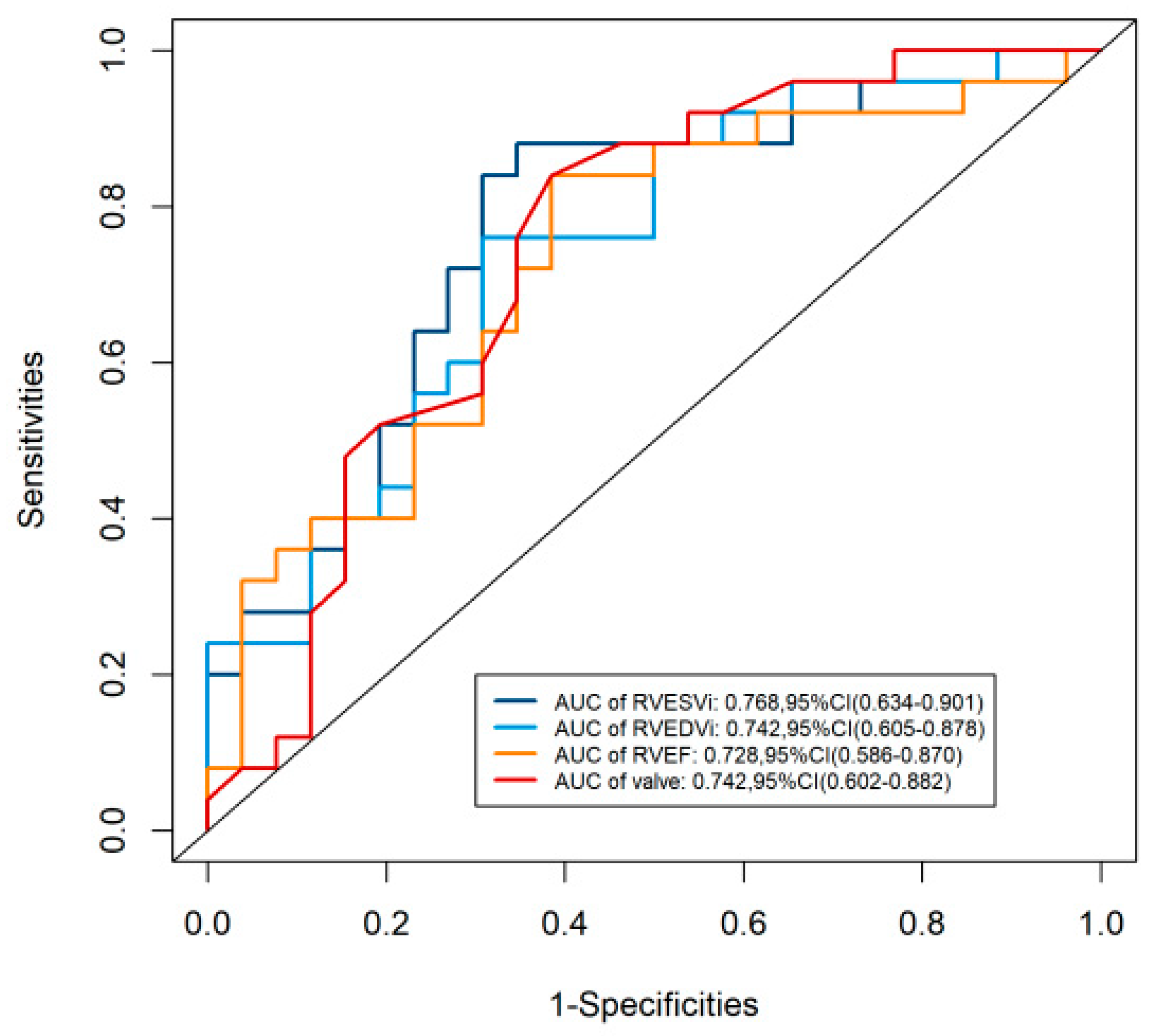
Figure 4: ROC for predicting RVD after Cone reconstruction. The highest predictive efficacy of RVD after Cone reconstruction was observed in RVESVi. Abbreviation: AUC, area under curve; CI, confidence interval; EDVi, end-diastolic volume index; ESVi, end-systolic volume index; EF, ejection fraction; RV, right ventricle.
This study conducted a comparative analysis of preoperative right ventricular structural and functional between patients exhibiting RVD and those without RVD following the Cone reconstruction. Our findings further corroborate that a diminished RVEF, impaired right ventricular function, and severe right heart dilatation are significant predictors of early postoperative RVD, aligning with established literature [9,18]. Additionally, this study identified that the degree of septal leaflet displacement on echocardiography influences the risk of early postoperative RVD, with patients exhibiting displacement exceeding 26.5 mm being particularly vulnerable. The RVD was not associated with the CPB time, total hospital stay, and ICU time, but the maximum dose of vasoactive medication used postoperatively was correlated with RVD.
The results of this study demonstrate that, although the Cone reconstruction yields substantial clinical enhancements and a notable reduction of valvular regurgitation in individuals with EA, RVD persists as a prevalent postoperative occurrence, impacting nearly half of the subjects [19,20]. RVD may lead to exacerbation of tricuspid regurgitation, arrhythmias, and heart failure, all of which are postoperative complications that markedly influence the necessity for reoperation and long-term prognosis [8,21]. Significantly, our study reveals that patients with RVD exhibit an augmentation of tricuspid regurgitation in early postoperative follow-up. Overall, this study shows that the severity of septal leaflet displacement is a key predictor of RVD. Patients with more severe tricuspid valve abnormalities before surgery have a higher risk of developing persistent RVD. Preoperative right ventricular dilatation and dysfunction assessed by CMR are also strong predictors of RVD.
The study revealed a correlation between right ventricular volume and the risk of RVD. An excessively enlarged RV may result in annular dilatation and compromised right ventricular function, thereby necessitating extended surgical time and more complex procedures [22]. Moreover, the remodeling of chordae tendineae and valvular structures, with RV folding, can further impair the functional RV, exacerbating the decline in right ventricular function [23,24]. Relevant literature indicates that a reduced RVEF and significant right ventricular dilatation are risk factors for early postoperative RVD, and progressive right ventricular dilatation and dysfunction are commonly observed in EA [25,26]. Due to the difficulty in early detection of right heart failure and the chronic nature of right ventricular remodeling, which will delay the timing of surgical repair, patients often exhibit severe RVD in the early postoperative period following the Cone procedure [27].
The significance of this study is to underscore the importance of preoperative assessment for the Cone procedure. Identifying patients at high risk for postoperative RVD can facilitate the prediction of tricuspid regurgitation recurrence after the Cone reconstruction and guide the use of postoperative vasoactive medications. It is well established that, given the geometric shape and position of the RV, CMR is the preferred imaging modality for assessing its size and function [28]. However, the latest guidelines indicate that two-dimensional echocardiography plays a significant complementary role in evaluating the tricuspid valve. Our study results demonstrate that the combination of CMR and echocardiography provides crucial additional data on tricuspid valve anatomy, degree of right ventricular expansion, and its function, which is essential for guiding individualized surgical plans for each patient [16,29]. Furthermore, our findings suggest that postoperative RVD does not lead to increased hospital time and intensive care unit (ICU) time, but there is an increase in the dosage of vasoactive medications used. This may be attributed to the lower ejection fraction following the Cone procedure; although improvements in ventricular function and reduction of tricuspid regurgitation can ameliorate this condition, vasoactive medications are still required to maintain adequate cardiac output [29].
As a single-center cohort, our study has several limitations. Primarily, the sample size is relatively small. Secondly, in this study, we used absolute measurements to evaluate the patient’s condition. However, taking into account the large age range of patients (from 10 years old to adulthood), absolute measurements may not fully reflect the differences between different age groups. For example, the same measurements may appear larger in young patients and relatively small in adult patients. Therefore, future studies should consider introducing relative measurements or age standardization methods to assess the disease more accurately. Lastly, this study mainly relied on echocardiography to evaluate the severity of tricuspid regurgitation rather than CMR. CMR provides a more accurate measurement of regurgitation fraction and has a unique advantage in evaluating right ventricular volume and function. Future studies should consider combining CMR and echocardiography to more comprehensively evaluate the severity of tricuspid regurgitation and its effect on right ventricular function.
The study indicates that preoperative decrease of RVEF, significant valvular displacement, and dilatation of the RV are important predictors for the early RVD following the Cone reconstruction. RVD may exacerbate tricuspid regurgitation, and this finding underscores that predicting RVD may aid in identifying high-risk patients for recurrent tricuspid regurgitation after the Cone reconstruction, as well as guiding the use of postoperative vasoactive medications. Therefore, patients with the aforementioned high-risk factors preoperatively, indicating a potential for postoperative RVD, can be considered for early preventive treatment.
Acknowledgement:
Funding Statement: This research was funded by E Fund Congenital Heart Disease Medical Talent Cultivation and Education Fund (grant number [2023QT0009]) and the Science and Technology Planning Project of Guangdong Province (grant number [2023B03J1255]).
Author Contributions: Study conception and design: Haiyun Yuan, Shusheng Wen; Data collection: Jing Ling, Naijimuding Abudurexiti; Analysis and interpretation of results: Yuting Huang; Draft manuscript preparation: Jiaxiong Wu, Zirui Peng, Runzhang Liang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: The study protocol was approved by the GDPPH Ethics Research Committee. The Ethics Committee reference code is KY2024-693-02. The GDPPH Ethics Research Committee approved this retrospective study and waived the requirement for informed consent.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Rydzewska K, Sylwestrzak O, Krekora M, Słodki M, Respondek-Liberska M. Ebstein’s anomaly: epidemiological analysis and presentation of different prenatal management. J Matern Fetal Neonatal Med. 2022;35(17):3297–304. [Google Scholar]
2. Holst KA, Connolly HM, Dearani JA. Ebstein’s anomaly. Methodist Debakey Cardiovasc J. 2019;15(2):138–44. [Google Scholar]
3. Attenhofer Jost CH, Connolly HM, Dearani JA, et al. Ebstein’s anomaly. Circulation. 2007;115(2):277–85. [Google Scholar]
4. Dearani JA, Mora BN, Nelson TJ, Haile DT, O’Leary PW. Ebstein anomaly review: what’s now, what’s next? Expert Rev Cardiovasc Ther. 2015;13(10):1101–9. [Google Scholar]
5. Cherry C, DeBord S, Moustapha-Nadler N. Ebstein’s anomaly: a complex congenital heart defect. AORN J. 2009;89(6):1098–110; quiz 1111–4. [Google Scholar]
6. Li X, Wang SM, Schreiber C, Cheng W, Lin K, Sun JY, et al. More than valve repair: Effect of cone reconstruction on right ventricular geometry and function in patients with Ebstein anomaly. Int J Cardiol. 2016;206:131–7. [Google Scholar]
7. Lange R, Burri M, Eschenbach LK, Badiu CC, da Silva JP, Nagdyman N, et al. Da Silva’s cone repair for Ebstein’s anomaly: effect on right ventricular size and function. Eur J Cardiothorac Surg. 2015;48(2):316–20; discussion 320–1. [Google Scholar]
8. Silva GVRD, Miana LA, Caneo LF, Turquetto ALR, Tanamati C, Penha JG, et al. Early and Long-Term Outcomes of Surgical Treatment of Ebstein’s Anomaly. Braz J Cardiovasc Surg. 2019;34(5):511–6. [Google Scholar]
9. Alsaied T, Castrillon CD, Christopher A, Da Silva J, Morell VO, Lanford L, et al. Cardiac MRI predictors of right ventricular dysfunction after the Da Silva cone operation for Ebstein’s anomaly. Int J Cardiol Congenit Heart Dis. 2022;7:100342. [Google Scholar]
10. Carney M, Gupta A, Christopher A, Olivieri L, Da Silva J, Diaz Castrillon C, et al. Large Right Atrial Size on Cardiac MRI is Associated with Post-operative Right Ventricular Dysfunction after the Cone Operation for Ebstein Anomaly. Pediatr Cardiol. 2024. [Google Scholar]
11. Melillo F, Fabiani D, Santoro A, Oro P, Frecentese F, Salemme L, et al. Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation. J Clin Med. 2024;13(17):5076. [Google Scholar]
12. Mah K, Mertens L. Echocardiographic Assessment of Right Ventricular Function in Paediatric Heart Disease: A Practical Clinical Approach. CJC Pediatr Congenit Heart Dis. 2022;1(3):136–57. [Google Scholar]
13. Tian X, Yang Y, Luo X, Cao L, Zhou X, Xu H, et al. Preoperative right ventricular longitudinal strain as a prognosticator of postoperative residual or recurrent tricuspid regurgitation in Ebstein anomaly: a cardiovascular magnetic resonance study. Cardiovasc Diagn Ther. 2024;14(4):563–75. [Google Scholar]
14. Burri M, Mrad Agua K, Cleuziou J, Beran E, Nagdyman N, Kühn A, et al. Cone versus conventional repair for Ebstein’s anomaly. J Thorac Cardiovasc Surg. 2020;160(6):1545–53. [Google Scholar]
15. Possner M, Gensini FJ, Mauchley DC, Krieger EV, Steinberg ZL. Ebstein’s Anomaly of the Tricuspid Valve: an Overview of Pathology and Management. Curr Cardiol Rep. 2020;22(12):157. [Google Scholar]
16. Qureshi MY, O’Leary PW, Connolly HM. Cardiac imaging in Ebstein anomaly. Trends Cardiovasc Med. 2018;28(6):403–9. [Google Scholar]
17. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Federico G, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35–71. [Google Scholar]
18. Rydman R, Shiina Y, Diller GP, Niwa K, Li W, Uemura H, et al. Major adverse events and atrial tachycardia in Ebstein’s anomaly predicted by cardiovascular magnetic resonance. Heart. 2018;104(1):37–44. [Google Scholar]
19. Lianza AC, Rodrigues ACT, Mercer-Rosa L, Vieira MLC, de Oliveira WAA, Afonso TR,et al. Right Ventricular Systolic Function After the Cone Procedure for Ebstein’s Anomaly: Comparison Between Echocardiography and Cardiac Magnetic Resonance. Pediatr Cardiol. 2020;41(5):985–95. [Google Scholar]
20. Perdreau E, Tsang V, Hughes ML, Ibrahim M, Kataria S, Janagarajan K, et al. Change in biventricular function after cone reconstruction of Ebstein’s anomaly: an echocardiographic study. Eur Heart J Cardiovasc Imaging. 2018;19(7):808–15. [Google Scholar]
21. Steinmetz M, Broder M, Hösch O, Lamata P, Kutty S, Kowallick JT, et al. Atrio-ventricular deformation and heart failure in Ebstein’s anomaly—a cardiovascular magnetic resonance study. Int J Cardiol. 2018;257:54–61. [Google Scholar]
22. Yurekli I, Kestelli M, Cakir H. Which One Predominates in Ebstein Anomaly: Tricuspid Regurgitation or Right Ventricular Dysfunction? Ann Thorac Surg. 2020;109(5):1626. [Google Scholar]
23. Hetzer R, Hacke P, Javier M, Miera O, Schmitt K, Weng Y, et al. The long-term impact of various techniques for tricuspid repair in Ebstein’s anomaly. J Thorac Cardiovasc Surg. 2015;150(5):1212–9. [Google Scholar]
24. Dearani JA. Cone the valve be repaired? J Thorac Cardiovasc Surg. 2015;149(4):1150–1. [Google Scholar]
25. Yu S, Yang K, Chen X, Lu M, Zhao K, Yang S, et al. Cardiac remodeling after tricuspid valve repair in Ebstein’s anomaly: a magnetic resonance study. Eur Radiol. 2023;33(3):2052–61. [Google Scholar]
26. Neijenhuis RML, Tsang VT, Marek J, Issitt R, Bonello B, Von Klemperer K, et al. Cone reconstruction for Ebstein anomaly: Late biventricular function and possible remodeling. J Thorac Cardiovasc Surg. 2021;161(3):1097–108. [Google Scholar]
27. Schultz K, Haeffele CL. Heart failure in the adult Ebstein patient. Heart Fail Rev. 2020;25(4):623–32. [Google Scholar]
28. Geerdink LM, van Everdingen WM, Kuipers IM, Fejzic Z, du Marchie Sarvaas GJ, Frerich S, Ter Heide H,et al.Comprehensive Evaluation of Pediatric Patients with Ebstein Anomaly Requires Both Echocardiography and Cardiac Magnetic Resonance Imaging.Pediatr Cardiol. 2023;44(1):75–85. [Google Scholar]
29. Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122(4):428–36. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools