 Open Access
Open Access
REVIEW
Reverse Potts Shunt in Children with Suprasystemic Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis
1 Department of Cardiothoracic Surgery, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, China
2 National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing, 400014, China
* Corresponding Author: Yuhao Wu. Email:
Congenital Heart Disease 2025, 20(1), 1-12. https://doi.org/10.32604/chd.2025.063152
Received 06 January 2025; Accepted 21 February 2025; Issue published 18 March 2025
Abstract
Background Pulmonary arterial hypertension (PAH) is a progressive condition with a poor prognosis in children. Lung transplantation (Ltx) remains the ultimate option when patients are refractory to PAH-specific therapy. Reverse Potts shunt (RPS) has been introduced to treat suprasystemic PAH. This study aims to investigate the clinical outcomes of suprasystemic PAH in children. Methods Embase, Pubmed, and the Cochrane Library databases were searched for related studies that reported the clinical outcomes of suprasystemic PAH following RPS in children. To investigate the clinical outcomes of RPS, meta-analyses of the early and overall mortalities were performed. Results Nine studies were included in this study. The estimated early mortality was 14.4% (95% CI, 7.1% to 23.1%), and the overall mortality/Ltx was 23.2% (95% CI, 14.4% to 32.9%). The estimated 1-year survival was 86.3% (95% CI, 75.9% to 88.7%). A qualitative review showed that the median value of 5-year survival free from Ltx of patients undergoing RPS was 68.6% (range: 65% to 92.3%). Compared to Ltx, RPS did not significantly increase the early mortality (OR, 2.48, 95% CI 0.75 to 8.24, p = 0.14). RPS also significantly improved the New York Heart Association/World Health Organization functional class, reduced the BNP/NT-pro BNP levels, decreased the PAH-specific therapy, and increased the six-minute-walking distance. Conclusions RPS may serve as an alternative treatment for suprasystemic drug-refractory PAH. Further large-scale and prospective cohort studies are needed to validate these findings.Keywords
Supplementary Material
Supplementary Material FilePulmonary arterial hypertension (PAH) is a progressive and life-threatening condition with a poor prognosis, especially in children [1]. The causes of PAH in children may be idiopathic, heritable, or associated with congenital heart diseases (CHD) [2]. Despite profound advances in the PAH-specific therapy, including prostanoids, endothelin receptor antagonists, and 5-phosphodiesterase inhibitors, lung transplantation (Ltx) remains the ultimate alternative when patients are refractory to maximal medications [3].
In 2004, Blanc et al. [4] introduced a novel reverse Potts shunt (RPS) for children with suprasystemic PAH. An anastomosis between the left pulmonary artery (LPA) and the descending aorta (DAO) is created. RPS may delay the need for Ltx and provide an option for ineligible recipients of Ltx. RPS unloads the right ventricle (RV) and reduces the pulmonary arterial pressure (PAP). In addition, RPS also improves the RV function and the RV-PA coupling [5]. This intervention may convert severe PAH into Eisenmenger physiology, which has a better life expectancy [4]. A recent study based on the global TOPP registry also showed a higher survival rate in PAH with open shunts [6]. Compared to the atrial septostomy, RPS may direct the highest oxygenated blood to the cerebral and coronary arteries [7]. In addition, cyanosis or the risks of thromboembolism are theoretically limited to the lower part of the body [8]. In 2013, the transcatheter Potts shunt (TPS) creation [9] and patent ductus arteriosus (PDA) stenting [8] were reported as alternatives to surgical anastomosis. However, TPS is technically challenging with some severe complications such as hemothorax and stent dislodgement [10]. PDA stenting is only available when a tiny PDA or its remnant exists [8].
In 2022, Mendel et al. [11] published a meta-analysis regarding RPS in adults and children with severe PAH. Their quantitative results showed reduced oxygen saturation (SaO2) in both upper and lower limbs following RPS. However, the early and overall mortalities have not been determined yet. In this study, we aim to perform an updated meta-analysis that investigates the outcomes of RPS in children with suprasystemic PAH and provides the latest evidence for clinical practice.
This study was performed according to the 2020 PRISMA guideline [12]. The study protocol was registered on the PROSPERO website (CRD42024591496). The Institutional Review Board (IRB) of the Children’s Hospital of Chongqing Medical University waived the ethical approval. The specific framework in terms of population, intervention, comparator, outcome, and study design (PICOS) was as follows: (P) Children with suprasystemic PAH; (I) RPS; (O) Early mortality, overall mortality/Ltx; and (S) Comparative studies or single-arm case series.
2.1 Literature Search and Study Screen
The Embase, PubMed, and Cochrane CENTRAL websites were searched in September 2024 for studies that reported the clinical outcomes of pediatric PAH following RPS. The search strategies are presented in Supplemental Material S1. The references of retrieved studies were searched manually for eligible studies. References were imported into Endnote software to remove duplicate records.
We included studies that met the following criteria: (i) Comparative studies or single-arm case series; (ii) Studies that reported the clinical outcomes of suprasystemic PAH following RPS in children; and (iii) RPS was created through either surgical or transcatheter procedure.
We excluded studies that met one of the following criteria: (i) Several studies were based on similar population or study period; (ii) Studies that reported atrial septostomy; or (iii) Adult patients who were older than 20 years of age. When multiple studies originated from a similar population or study period, we only included a study with the most complete data set. Editorial letters, single-case reports, reviews, or conference abstracts were also excluded. Literature search and screening were performed by Yong An and Gang Wang independently, and Jiangtao Dai checked the accuracy.
2.2 Data Extraction and Variable Definition
We extracted the following items: (1) Baseline data: authors, study area, study periods, study design, RPS approaches, study populations, causes of PAH, operative age, New York Heart Association/World Health Organization (NYHA/WHO) functional class before RPS, mean PAP, and pulmonary vascular resistance (PVR); (2) Primary outcomes: Early mortality, overall mortality/Ltx, and survival free from Ltx; (3) Secondary outcomes: upper and lower limb SaO2, NYHA/WHO functional class, BNP/NT-pro BNP level, and six minute-walking distance (6MWD) after RPS. The approaches of RPS mainly included surgical and transcatheter approaches. The surgical approach created a shunt between the LPA and the DAO either by direct anastomosis or by inserting a conduit. A valved conduit might be considered in infrasystemic or borderline suprasystemic PAH with worsening conditions. The transcatheter approach was performed by stenting a tiny PDA or by puncturing between LPA and DAO with the placement of a covered stent. Children were defined as patients younger than 20 years of age. Early mortality was defined as death before discharge or within 30 days after surgery. Overall mortality/Ltx was defined as postoperative death or Ltx during the hospitalization or during the follow-up after discharge. Data were extracted by Yong An and Gang Wang independently. The consultation with Jiangtao Dai resolved any disagreements.
2.3 Quality Assessment and Risk of Bias (RoB)
The quality assessment and RoB rating were performed by Yong An and Gang Wang independently. The consultation with Zhengxia Pan resolved any disagreements. RoB was evaluated using the Methodological Index for Non-Randomized Studies (MINORS) tool [13]. RoB was determined based on eight items. An overall score above 12 indicated low RoB, 8 to 12 indicated moderate RoB, and less than 8 indicated high RoB [14].
All analyses were performed using Stata 12.0 and OpenMeta Analyst softwares. Engauge Digitizer software was used to extract the survival rate from Kaplan-Meier curves as necessary. Statistical heterogeneity was assessed via the χ2−Q and I2 statistics, with I2 > 50% showing significant heterogeneity. To investigate the clinical outcomes of RPS, we performed meta-analyses of the prevalence, which included early and overall mortalities. A random-effects model was used for all analyses regardless of heterogeneity. Mortality and survival free from Ltx were pooled using the inverse variance method of DerSimonian-Laird. The Freeman-Tukey transformation was used for adjustment. Subgroup analyses of mortality regarding study design and surgical approach were performed. We also compared the clinical outcomes of RPS versus Ltx. Odds ratios (OR) were adopted for dichotomous data. A p-value of 0.05 or less was considered statistically significant.
The initial search from electronic databases yielded 381 studies. A manual search of the references of the retained studies did not find any eligible studies. After the removal of duplicates, 138 studies were excluded. After screening for relevance in titles and abstracts, only 49 studies were qualified for the full-text assessment. Eventually, our report was based on 9 studies [15,16,17,18,19,20,21,22,23]. Exclusion rationales following full-text evaluation are recorded in Supplemental Material S2. The PRISMA flowchart is depicted in Fig. 1.
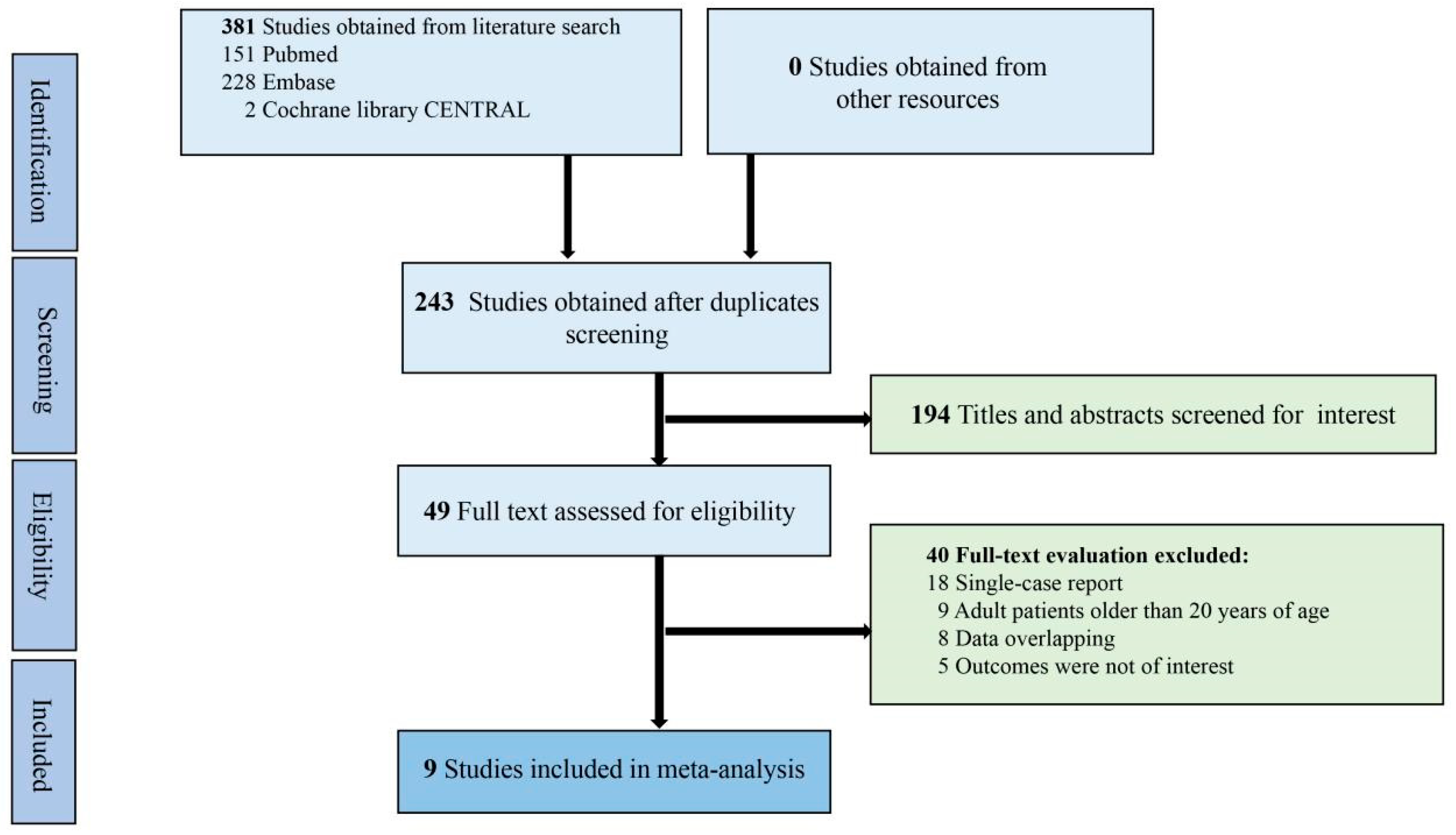
Figure 1: The PRISMA flow diagram of literature retrieval
Seven studies [15,16,17,18,20,21,22] were single-arm case series reporting the outcomes of RPS, and two studies [19,23] compared RPS to Ltx in pediatric PAH (Table 1). We included 166 participants, involving 107 patients receiving RPS and 59 patients receiving Ltx. The causes of PAH mainly included idiopathic, heritable, or associated with CHD. The indication of RPS was generally suprasystemic drug-refractory PAH with NYHA/WHO functional class III to IV [15,16,17,18,19,20,21,22,23]. To evaluate PAH and anatomical features before operations, echocardiogram and right heart catheterization were generally performed. Severe RV dysfunction, decompensated conditions, and uncontrolled hemoptysis might contraindicate RPS. Prostacyclin was not available in one study [17]. Surgical approach [15,17,18,19,22,23], PDA stenting [16,17,18,19,21], and transcatheter shunt creation [20,21] were reported for establishing Potts shunt (Table 1). The duration of follow-up was reported in 8 studies [15,16,17,18,19,21,22,23] ranging from 14 to 103 months, and the 5-year survival free from Ltx was only reported in 2 studies [21,23]. We used the MINORS tool for RoB evaluation. One study [19] was rated low RoB, and the other 8 were considered moderate RoB (Supplemental Material S3).
Table 1: Baseline characteristics of included studies
| Authors, Study Area, and Study Year | Study Design | Approaches | Cases (n) | Causes of PAH | Diagnosis of PAH before RPS | Median or Mean Age | Preoperative NYHA/WHO Function Class | Preoperative mPAP (mmHg) | Preoperative PVR (Wood Units) | Shunt Size | Mean or Median Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Labombarda, France, 2009 [15] | Retrospective case series | Surgical RPS | 2 | 2 IPAH | Echocardiogram/Catheterization | 7 y | IV | 93 | 22.5 | NA | 24 m |
| Latus, Germany, 2011–2013 [16] | Retrospective case series | Transcatheter RPS(PDA stenting) | 4 | 2 CHD 2 IPAH | Echocardiogram/Catheterization | 3.1 m | NA | 54 | NA | 4 to 5 mm | 14 m |
| Baruteau, France, 2003–2014 [17] | Retrospective case series | Surgical/Transcatheter RPS (PDA stenting) | 24 | NA | Echocardiogram/Catheterization | 7.8 y | Mostly IV | NA | NA | 8 to 10 mm | 25 m |
| Gorbachevsky, Russia, 2013–2016 [18] | Retrospective case series | Surgical/Transcatheter RPS(PDA stenting) | 8 | 3 CHD 5 IPAH | Echocardiogram/Catheterization | 13.5 m | III to IV | 86.6 | NA | 65% to 70% of the diameter of DAO | 17 m |
| Lancaster, USA, 2013–2020 [19] | Prospective comparative study | Surgical/Transcatheter RPS(PDA stenting) | 23 | 43% with CHD | Echocardiogram/Catheterization | 10.3 y | III to IV | 73 | 21 | 80% to 90% of the diameter of DAO | 22 m |
| Lung transplantation | 31 | 39% with CHD | - | 7.9 y | NA | NA | NA | - | 40 m | ||
| Mirabile, France, 2016–2017 [20] | Prospective Case series | Transcatheter RPS (Shunt creation) | 10 | 3 CHD 2 IPAH 5 HPAH | Echocardiogram/Catheterization | 10.7 y | III to IV | NA | 21.2 | 7 to 10 mm | NA |
| Haddad, France, 2009–2018 [21] | Retrospective case series | Transcatheter RPS (PDA stenting + Shunt creation) | 13 | 2 CHD 4 IPAH 7 HPAH | Echocardiogram/Catheterization | 8.7 y | III to IV | 83 | 21 | 7 to 9 mm | 77 m |
| de Castro, Brazil, 2023 [22] | Retrospective case series | Surgical RPS | 3 | 3 IPAH | Echocardiogram/Catheterization | 3.3 y | NA | NA | NA | 6 to 7 mm | 23 m |
| Valdeolmillos, France, 2004–2020 [23] | Retrospective comparative study | Surgical RPS | 20 | 7 CHD 9 IPAH 4 HPAH | Echocardiogram/Catheterization | 8 y | Mostly III to IV | 74 | 19.8 | NA | 103 m |
| Lung transplantation | 28 | 11 CHD 23 IPAH 5 HPAH 2 Veino-occlusive diseases | - | 14.9 y | NA | 82 | 20.7 | - | 71 m |
Early mortality
We collected early mortality following RPS from all 9 included studies [15,16,17,18,19,20,21,22,23]. The meta-analysis indicated that the estimated early mortality was 14.4% (95% CI, 7.1% to 23.1%; I2 = 0%; Fig. 2). Two studies [19,23] compared RPS to Ltx, and we found that compared to Ltx, RPS did not significantly increase the early mortality (OR, 2.48, 95% CI 0.75 to 8.24, p = 0.14; I2 = 0%; Fig. 3). The causes of early mortality in our included studies involved pulmonary hypertensive crisis [18,22], RV failure [17,19,23], respiratory failure [19,20,23], and cerebral damage [20]. Extracorporeal membrane oxygenation (ECMO) was reported in two studies [19,20]. One study [19] reported that all three patients who were on preoperative ECMO were dead after RPS. The other study [20] also reported 100% in-hospital mortality in patients on ECMO during RPS.
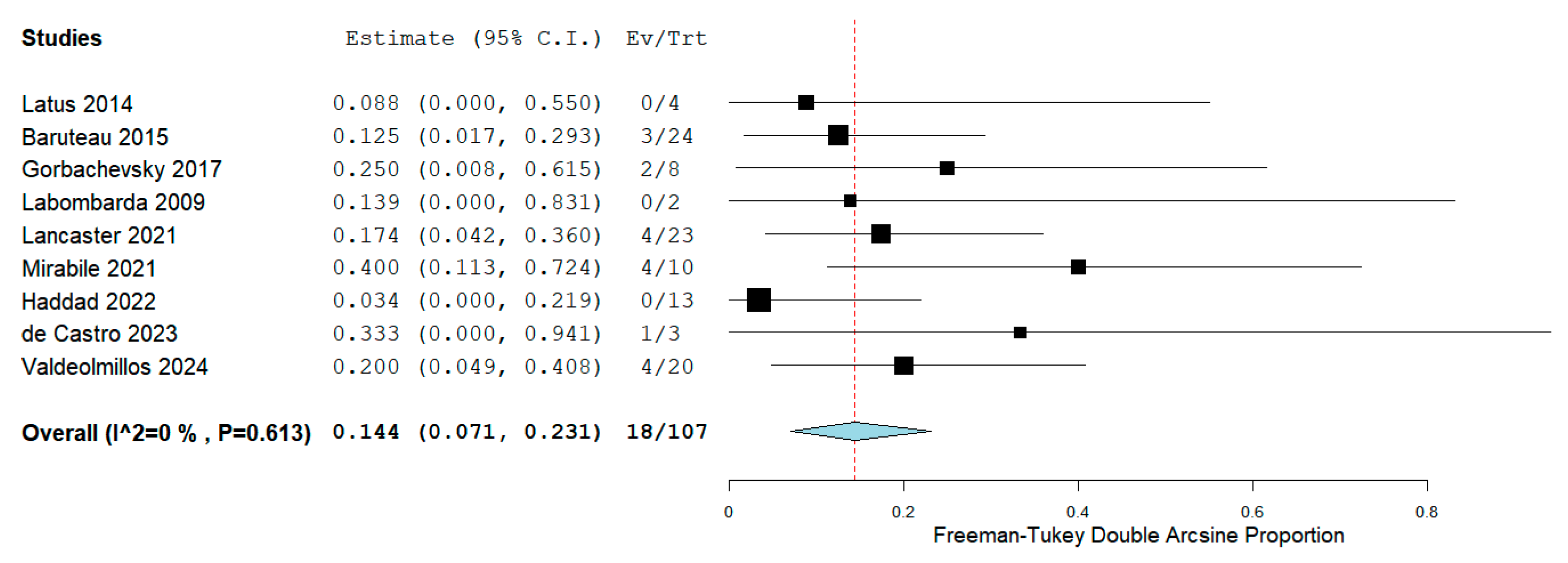
Figure 2: Forest plot of the early mortality
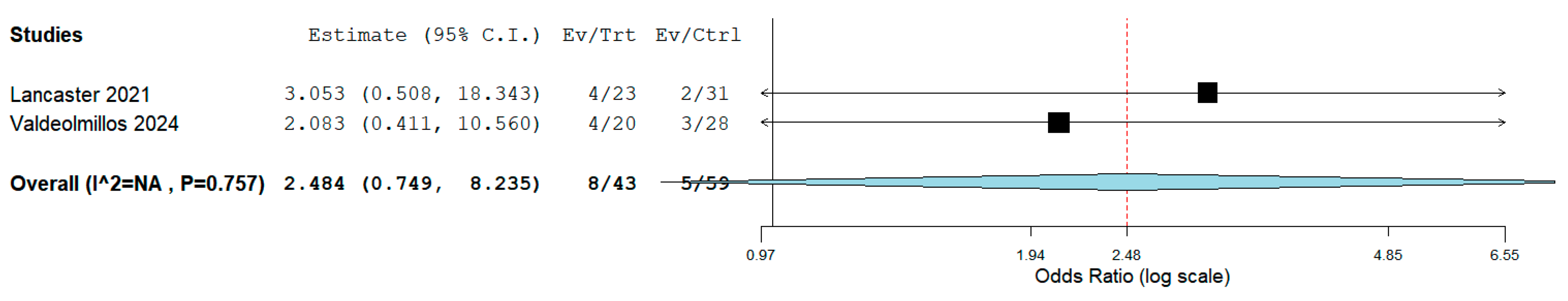
Figure 3: Comparison of the early mortality between reverse Potts shunt versus lung transplantation
Overall mortality/Ltx
We collected overall mortality/Ltx following RPS from all 9 studies [15,16,17,18,19,20,21,22,23]. The meta-analysis indicated that the estimated overall mortality/Ltx was 23.2% (95% CI, 14.4% to 32.9%; I2 = 0%; Fig. 4). Two studies [19,23] compared RPS to Ltx regarding mortality/Ltx. However, Lancaster et al. [19] did not provide the exact number of deaths or Ltx after discharge despite efforts to request data from authors via Email; therefore, we could not perform a meta-analysis comparing RPS to Ltx regarding overall mortality/Ltx. The causes of mortality after discharge involved lung infections [17,19,21] and RV failure [23]. Furthermore, Ltx was reported in 4 cases [17,19,23] after RPS due to uncontrolled hemoptysis or RV failure.
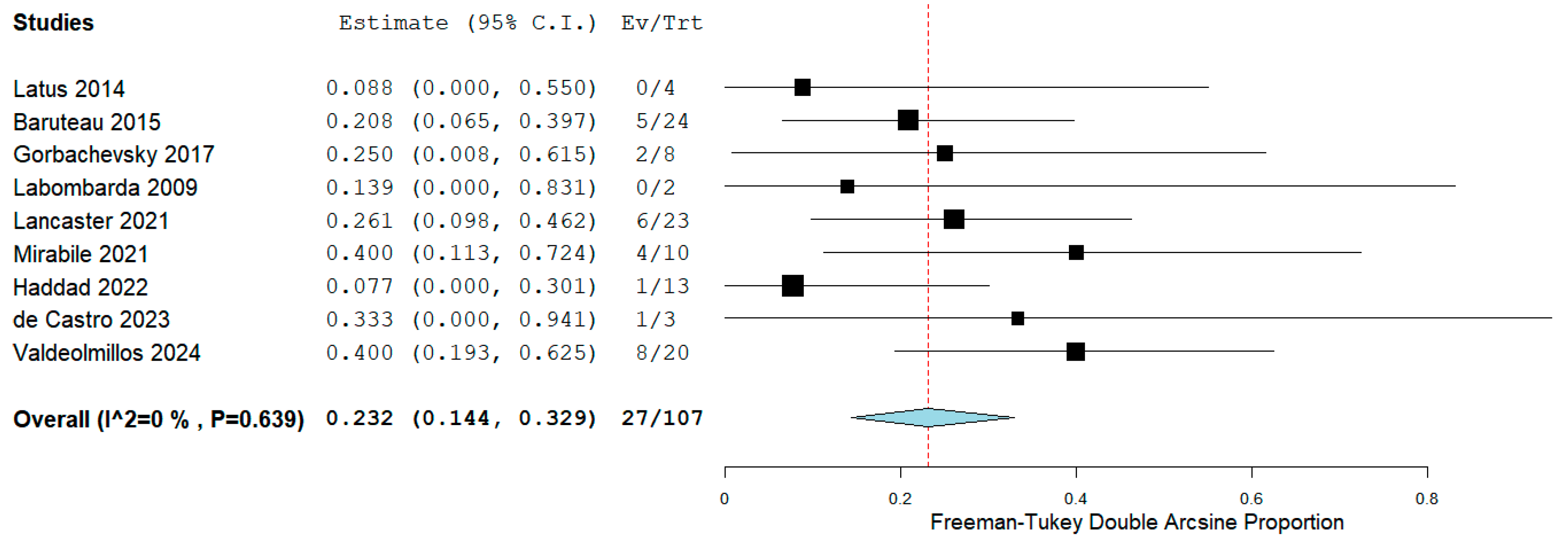
Figure 4: Forest plot of the overall mortality or lung transplantation
One-year survival and 5-year survival free from Ltx
A total of 7 [15,16,17,18,21,22,23] studies reported the 1-year survival without Ltx after RPS. The estimated 1-year survival without Ltx was 86.3% (95% CI, 75.9% to 88.7%; I2 = 0%; Fig. 5). Two studies [21,23] reported the 5-year survival free from Ltx, but the exact numbers of patients with 5-year survival were not reported. Therefore, a quantitative analysis could not be conducted. Haddad et al. [21] reported a 5-year survival free from Ltx of 92.3% after the transcatheter RPS. Valdeolmillos et al. [23] reported a 5-year survival without Ltx of 68.6% after RPS. In addition, we extract the 5-year survival without Ltx from one study [19] using the Engauge Digitizer software. They reported a 5-year survival without Ltx of 65% after RPS [19]. As a result, the median value of 5-year survival free from Ltx of patients undergoing RPS was 68.6% (range: 65% to 92.3%). Lancaster et al. [19] and Valdeolmillos et al. [23] showed that the long-term survival of patients undergoing RPS was not different from that of Ltx recipients.
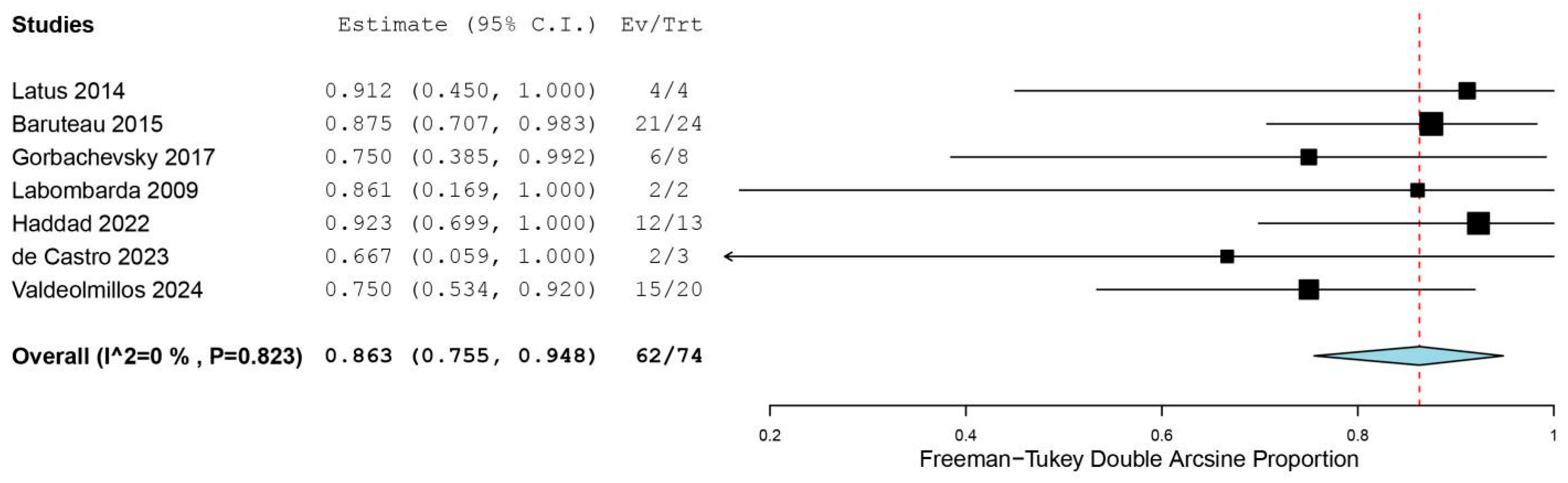
Figure 5: Forest plot of the one-year survival free from lung transplantation
Subgroup analyses of mortality
Subgroup analyses of mortality regarding study design and surgical approach were performed (Supplemental Material S4). Two studies [19,20] were prospective in design, and seven [15,16,17,18,21,22,23] were retrospective studies. We found that studies in prospective design (26.9%) entailed a higher early mortality. Three studies [15,22,23] reported surgical RPS in 25 cases, three [15,22,23] reported transcatheter RPS in 27 cases [16,20,21], and three reported a hybrid approach in 55 case [17,18,19]. The surgical group entailed the highest early mortality (17.0%), as compared to the transcatheter and hybrid groups. Regarding overall mortality/Ltx, we found that studies in prospective design (33.0%) entailed a higher overall mortality/Ltx. The surgical group entailed the highest overall mortality/Ltx (34.7%) as compared to the transcatheter and hybrid groups.
Our included studies reported diverse secondary outcomes [15,16,17,18,19,20,21,22,23], so quantitative analyses could not be performed. We only performed a qualitative review of the upper and lower limb SaO2, the NYHA/WHO functional class, the BNP/NT-pro BNP level, the use of PAH-specific therapy, and the 6MWD after RPS.
Baruteau et al. [17] reported that RPS did not significantly influence the upper limb SaO2. They reported a significant change in the lower limb SaO2 from 95.9% to 81.6% after RPS. Gorbachevsky et al. [18] had similar findings regarding the SaO2 (Upper limb: 95.7% vs. 94.8%; Lower limb: 95.0% vs. 84.3%).
Baruteau et al. [17], Gorbachevsky et al. [18], Lancaster et al. [19], Haddad et al. [21], and Valdeolmillos et al. [23] reported that RPS significantly downgraded the NYHA/WHO functional class.
Baruteau et al. [17] reported that RPS significantly reduced the serum NT-proBNP level. Lancaster et al. [19] reported that RPS significantly reduced the serum BNP level (799 vs. 170 ng/L) in the last follow-up. However, Haddad et al. [21] and Valdeolmillos et al. [23] did not find a significant reduction in the NT-proBNP levels.
Baruteau et al. [17], Lancaster et al. [19], and Valdeolmillos et al. [23] reported that RPS significantly decreased the PAH-specific therapy. However, Haddad et al. [21] did not find a significant reduction in the PAH-specific therapy.
Baruteau et al. [17] reported that RPS significantly increased the 6MWD (260 vs. 522 m). Haddad et al. [21] and Valdeolmillos et al. [23] did not find any significant improvement in the 6MWD after RPS.
Before introduction of the PAH-specific therapy, the 5-year survival of PAH in children is 29% [24]. With the PAH-specific therapy, the 5-year survival free from Ltx ranges from 51% to 57% [24,25]. However, the ultimate option for drug-refractory PAH is restricted to Ltx, which is very limited with insufficient donors, transplant rejection, and poor long-term survival in children. Additionally, morbidity following pediatric Ltx is also significant. Hypertension and diabetes are the most common at the early stage. Chronic renal dysfunction (30%) and bronchiolitis obliterans syndrome (37%) are frequently observed five years following pediatric Ltx [26]. In recent years, RPS has been proposed as a novel alternative in children with suprasystemic drug-refractory PAH. To our knowledge, this is the first and latest meta-analysis that reports the early and late outcomes of RPS in children with severe PAH. Even though a quantitative comparison of death or Ltx after discharge could not be performed, two studies [19,23] reported a similar long-term survival between patients undergoing RPS and Ltx. RPS did not significantly increase the early mortality compared to Ltx (p = 0.14). Subgroup analyses showed that studies with prospective design and surgical approach entailed higher early/overall mortalities. However, these results required cautious explanation due to the small sample sizes [20,22]. More recently, a unidirectional valved conduit was reported to enable a broader application to patients with infrasystemic or borderline suprasystemic PAH [17,27]. Such a modification maintains the right-to-left shunt, prevents blood flow inversion, and maximizes RV protection [27,28].
In patients with suprasystemic PAH, cardiac function is often compromised. Therefore, it is possible to utilize ECMO during RPS. However, preoperative ECMO initiation seems to be a significant risk factor (HR, 10.7) for early mortality [29] in the literature. Grady et al. [29] reported a mortality of 64% in patients who were placed on ECMO preoperatively. Two of our included studies reported the use of ECMO during RPS. However, the in-hospital mortality was significant. Lancaster et al. [19] reported a 100% in-hospital mortality (3 out of 3) in children who were on preoperative ECMO, and they no longer provided RPS for acutely decompensated children. Mirabile et al. [20] also reported a 100% in-hospital mortality in patients on ECMO during RPS. They concluded that elective ECMO might be an option at the early stage of the learning curve, but ECMO did not improve the prognosis of PAH patients with critical conditions. Preoperative ICU admission, use of intravenous inotropes, mechanical ventilation, and World Symposium on Pulmonary Hypertension (WSPH) group 3 are also strong indicators for early and late mortality following RPS [29]. Therefore, decompensated children with PAH may not be good candidates for RPS. RV dysfunction is associated with early and late deaths after RPS [17,19,23]. RPS may be considered in PAH patients at an early stage with preserved RV function, with Ltx being performed with dysfunctional RV.
Three different approaches have been reported for RPS in the literature [29]. Due to the increased risks associated with thoracotomy or sternotomy in children with end-stage PAH, a transcatheter approach has been proposed [10,30]. The transcatheter approach may help avoid adhesions to the left pulmonary hilum resulting from previous open operations [19]. In a small group of patients, RPS can be performed by stenting a tiny arterial duct or its remnant [8,16]. Later, TPS creation is reported in the absence of PDA [9,10]. This procedure creates a connection between the LPA and the DAO. The proximity of LPA-to-DAO facilitates the creation of such a connection, which potentially reduces peri-operative complications [9,10,31]. Haddad et al. [21] reported a satisfied long-term survival of 92.3% following the transcatheter RPS. However, frequent reinterventions, protrusion to the aorta, and uncontrolled bleeding still raise concerns about this procedure [19,21].
In 2021, Grady et al. [29] published a study based on an international registry including 13 institutions. They reported 17 early deaths (15%) after RPS. Eighteen patients (20%) died or underwent Ltx after discharge. Unfortunately, we cannot include this study in our research due to significant data overlapping (Supplemental Material S2). Compared to this study, we included more participants with longer follow-up period, and we found that the early mortality was not significantly different between RPS and Ltx. We also found that RPS significantly improved the NYHA/WHO functional class, reduced the BNP/NT-pro BNP levels, decreased the PAH-specific therapy, and increased the 6MWD. In 2022, Mendel et al. [11] published a meta-analysis regarding RPS in adults and children with severe PAH. Compared to this meta-analysis, we explored the early and late outcomes in patients receiving RPS, and we also compared the clinical outcomes of RPS versus Ltx.
Limitations of this research are acknowledged here. First, most of our included studies are retrospective in design, with a small number of participants and a short follow-up period. The number of included participants is relatively small. We excluded single-case reports in this study; therefore, the mortality in our study seemed to be higher than that in the literature. Second, one study [17] originates from a multicenter study (Marie Lannelongue Hospital, Necker-Enfants Malades Hospital, and Bambino Gesu Children’s Hospital). As a result, their data may overlap with those of other included studies. Third, shunt size is crucial for postoperative outcomes after RPS, but the standard cannot be determined based on our research. Fourth, our study cannot clarify whether a surgical approach should be prioritized over a TPS creation or a PDA stenting. Last, RV dysfunction contraindicates Potts shunt, and preoperative evaluation of the RV function is critical. However, data from echocardiogram, catheterization, and magnetic resonance imaging are too incomplete to provide specific guidance.
In conclusion, RPS may be an alternative in the treatment of suprasystemic drug-refractory PAH and may delay the ultimate Ltx. The early and long-term survival of patients undergoing RPS is similar to that of lung transplant recipients. An ideal role of RPS is to delay RV dysfunction maximally and serve as an actual bridge to Ltx. Further large-scale and prospective cohort studies are warranted to clarify the safety and effectiveness of RPS in the long term.
Acknowledgement:
Funding Statement: Chongqing Medical University Program for Youth Innovation in Future Medicine (W0204); Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission (CSTB2023NSCQ-BHX0010); Chongqing Postdoctoral Research Project Special Support (2023CQBSHTB3074); Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202400421).
Author Contributions: Yuhao Wu and Yong An conceived the study; Yong An designed the protocol; Gang Wang and Jiangtao Dai collected the data; Zhengxia Pan and Yuhao Wu drafted the manuscript which was revised following critical review by all authors. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data of this study were available from the corresponding author upon reasonable request.
Ethics Approval: The Institutional Review Board of the Children’s Hospital of Chongqing Medical University waived ethical approval of this study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2025.063152.
Abbreviations
| Pulmonary arterial hypertension | |
| Lung transplantation | |
| Congenital heart diseases | |
| Reverse Potts shunt | |
| Left pulmonary artery | |
| Descending aorta | |
| Right ventricle | |
| Transcatheter Potts shunt | |
| Patent ductus arteriosus | |
| Oxygen saturations | |
| New York Heart Association/World Health Organization | |
| Pulmonary artery pressure | |
| Pulmonary vascular resistance | |
| Six-minute-walking distance | |
| Methodological Index for Non-Randomized Studies | |
| Extracorporeal membrane oxygenation | |
| Confidence intervals |
References
1. Dong W, Hong Z, Wang A, Jiang K, Zhu H, zhang F, et al. Risk stratification and prognosis of pulmonary arterial hypertension associated with congenital heart disease. Congenit Heart Dis. 2024;19(3):325–39. 10.32604/chd.2024.052267. [Google Scholar] [CrossRef]
2. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1):1801916. 10.1183/13993003.01916-2018. [Google Scholar] [CrossRef]
3. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879. 10.1183/13993003.00879-2022. [Google Scholar] [CrossRef]
4. Blanc J, Vouhé P, Bonnet D. Potts shunt in patients with pulmonary hypertension. N Engl J Med. 2004;350(6):623. 10.1056/nejm200402053500623. [Google Scholar] [CrossRef]
5. Aggarwal M, Mark Grady R, Choudhry S, Anwar S, Eghtesady P, Singh GK. Potts shunt improves right ventricular function and coupling with pulmonary circulation in children with suprasystemic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2018;11(12):e007964. 10.1161/CIRCIMAGING.118.007964. [Google Scholar] [CrossRef]
6. Ploegstra MJ, Ivy DD, Beghetti M, Bonnet D, Alehan D, Ablonczy L, et al. Long-term outcome of children with newly diagnosed pulmonary arterial hypertension: results from the global TOPP registry. Eur Heart J Qual Care Clin Outcomes. 2024;10(1):66–76. 10.1093/ehjqcco/qcad020. [Google Scholar] [CrossRef]
7. Grady RM. Beyond transplant: roles of atrial septostomy and Potts shunt in pediatric pulmonary hypertension. Pediatr Pulmonol. 2021;56(3):656–60. 10.1002/ppul.25049. [Google Scholar] [CrossRef]
8. Boudjemline Y, Patel M, Malekzadeh-Milani S, Szezepanski I, Lévy M, Bonnet D. Patent ductus arteriosus stenting (transcatheter Potts shunt) for palliation of suprasystemic pulmonary arterial hypertension: a case series. Circ Cardiovasc Interv. 2013;6(2):e18–20. 10.1161/CIRCINTERVENTIONS.112.000091. [Google Scholar] [CrossRef]
9. Esch JJ, Shah PB, Cockrill BA, Farber HW, Landzberg MJ, Mehra MR, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32(4):381–7. 10.1016/j.healun.2013.01.1049. [Google Scholar] [CrossRef]
10. Boudjemline Y, Sizarov A, Malekzadeh-Milani S, Mirabile C, Lenoir M, Khraiche D, et al. Safety and feasibility of the transcatheter approach to create a reverse potts shunt in children with idiopathic pulmonary arterial hypertension. Can J Cardiol. 2017;33(9):1188–96. 10.1016/j.cjca.2017.06.004. [Google Scholar] [CrossRef]
11. Mendel B, Christianto C, Angellia P, Holiyono I, Prakoso R, Siagian SN. Reversed potts shunt outcome in suprasystemic pulmonary arterial hypertension: a systematic review and meta-analysis. Curr Cardiol Rev. 2022;18(6):e090522204486. 10.2174/1573403X18666220509203335. [Google Scholar] [CrossRef]
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [Google Scholar] [CrossRef]
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6. 10.1046/j.1445-2197.2003.02748.x. [Google Scholar] [CrossRef]
14. Wu Y, Wang G, Dai J, Li H, Li Y, Wu C, et al. Slide tracheoplasty for congenital tracheal stenosis repair: a systematic review and meta-analysis. Laryngoscope. 2022;132(8):1532–41. 10.1002/lary.29771. [Google Scholar] [CrossRef]
15. Labombarda F, Maragnes P, Dupont-Chauvet P, Serraf A. Potts anastomosis for children with idiopathic pulmonary hypertension. Pediatr Cardiol. 2009;30(8):1143–5. 10.1007/s00246-009-9485-3. [Google Scholar] [CrossRef]
16. Latus H, Apitz C, Moysich A, Kerst G, Jux C, Bauer J, et al. Creation of a functional Potts shunt by stenting the persistent arterial duct in newborns and infants with suprasystemic pulmonary hypertension of various etiologies. J Heart Lung Transplant. 2014;33(5):542–6. 10.1016/j.healun.2014.01.860. [Google Scholar] [CrossRef]
17. Baruteau AE, Belli E, Boudjemline Y, Laux D, Lévy M, Simonneau G, et al. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47(3):e105–10. 10.1093/ejcts/ezu445. [Google Scholar] [CrossRef]
18. Gorbachevsky SV, Shmalts AA, Barishnikova IY, Zaets SB. Potts shunt in children with pulmonary arterial hypertension: institutional experience. Interact Cardiovasc Thorac Surg. 2017;25(4):595–9. 10.1093/icvts/ivx209. [Google Scholar] [CrossRef]
19. Lancaster TS, Shahanavaz S, Balzer DT, Sweet SC, Mark Grady R, Eghtesady P. Midterm outcomes of the Potts shunt for pediatric pulmonary hypertension, with comparison to lung transplant. J Thorac Cardiovasc Surg. 2021;161(3):1139–48. 10.1016/j.jtcvs.2020.10.163. [Google Scholar] [CrossRef]
20. Mirabile C, Malekzadeh-Milani S, Bojan M, Raisky O, Gaudin R, Bonnet D, et al. A case series of transcatheter Potts Shunt creation in a pediatric population affected with refractory pulmonary artery hypertension: focus on the role of ECMO. Perfusion. 2021;36(4):415–20. 10.1177/0267659120954169. [Google Scholar] [CrossRef]
21. Haddad RN, Levy M, Szezepanski I, Malekzadeh-Milani S, Bonnet D. Long-term outcomes of transcatheter Potts shunt in children with suprasystemic pulmonary arterial hypertension. Front Cardiovasc Med. 2022;9:1028304. 10.3389/fcvm.2022.1028304. [Google Scholar] [CrossRef]
22. de Castro MF, Oliveira EC, Nunes MCP, Oliveira C, Almeida MGC, Barbosa JAA. Reverse potts for the treatment of severe idiopathic pulmonary hypertension in children. Braz J Cardiovasc Surg. 2023;38(4):e20220320. 10.21470/1678-9741-2022-0320. [Google Scholar] [CrossRef]
23. Valdeolmillos E, Le Pavec J, Audié M, Savale L, Jais X, Montani D, et al. Thirty years of surgical management of pediatric pulmonary hypertension: mid-term outcomes following reverse Potts shunt and transplantation. J Thorac Cardiovasc Surg. 2024;168(3):943–54. 10.1016/j.jtcvs.2023.11.045. [Google Scholar] [CrossRef]
24. van Loon RLE, Roofthooft MTR, Delhaas T, van Osch-Gevers M, ten Harkel ADJ, Strengers JLM, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol. 2010;106(1):117–24. 10.1016/j.amjcard.2010.02.023. [Google Scholar] [CrossRef]
25. Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart. 2010;96(17):1401–6. 10.1136/hrt.2009.182378. [Google Scholar] [CrossRef]
26. Bryant R 3rd, Morales D, Schecter M. Pediatric lung transplantation. Semin Pediatr Surg. 2017;26(4):213–6. 10.1053/j.sempedsurg.2017.07.005. [Google Scholar] [CrossRef]
27. Rosenzweig EB, Ankola A, Krishnan U, Middlesworth W, Bacha E, Bacchetta M. A novel unidirectional-valved shunt approach for end-stage pulmonary arterial hypertension: early experience in adolescents and adults. J Thorac Cardiovasc Surg. 2021;161(4):1438–46.e2. 10.1016/j.jtcvs.2019.10.149. [Google Scholar] [CrossRef]
28. Ozturk M, Tongut A, Desai M, d’Udekem Y, Yerebakan C. Unidirectional reversed Potts shunt with reinforced valved femoral vein homograft. JTCVS Tech. 2022;17:164–5. 10.1016/j.xjtc.2022.10.014. [Google Scholar] [CrossRef]
29. Mark Grady R, Canter MW, Wan F, Shmalts AA, Coleman RD, Beghetti M, et al. Pulmonary-to-systemic arterial shunt to treat children with severe pulmonary hypertension. J Am Coll Cardiol. 2021;78(5):468–77. 10.1016/j.jacc.2021.05.039. [Google Scholar] [CrossRef]
30. Hansmann G, Apitz C. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii67–85. 10.1136/heartjnl-2015-309103. [Google Scholar] [CrossRef]
31. Sizarov A, Raimondi F, Bonnet D, Boudjemline Y. Vascular anatomy in children with pulmonary hypertension regarding the transcatheter Potts shunt. Heart. 2016;102(21):1735–41. 10.1136/heartjnl-2016-309352. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools