 Open Access
Open Access
CASE REPORT
Aorta–Right Atrial Tunnel in an Infant
Department of Cardiothoracic Surgery, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
* Corresponding Author: Hao Zhang. Email:
Congenital Heart Disease 2025, 20(1), 103-113. https://doi.org/10.32604/chd.2025.063108
Received 05 January 2025; Accepted 21 February 2025; Issue published 18 March 2025
Abstract
Aorta–right atrial tunnel (ARAT) is an extremely rare congenital heart malformation with an average age of diagnosis of approximately 20 years. Its clinical symptoms are varied and often atypical. ARAT usually originates from the left or right sinus of Valsalva. In this case report, we aim to share a rare case of ARAT originating from the noncoronary sinus in an infant. The patient presented with a cardiac murmur, a dilated right heart, and a tortuous tunnel originating from the dilated noncoronary sinus and terminating at the right atrium in echocardiogram and computed tomography angiography. The patient underwent surgical closure of the tunnel to prevent possible heart failure. Postoperative echocardiography revealed complete closure of the tunnel with no residual flow. No evidence of aortic valve regurgitation or aortic root dilation was detected during 6-month follow-up.Keywords
Supplementary Material
Supplementary Material FileA 50-day-old boy was referred to our department for a continuous heart murmur. He had a fever and cough 3 weeks prior and was diagnosed with pneumonia when a cardiac murmur was first found. The patient’s neonatal pneumonia was successfully resolved following clinical intervention. It is postulated that the onset of this condition may be associated with congenital structural cardiac abnormalities and a persistent left-to-right shunt. The patient was asymptomatic at admission with no sign of growth retardation. Cardiac auscultation revealed a grade 2/6 continuous murmur on the parasternal border. Electrocardiography findings and cardiothoracic ratio were in the normal range.
An echocardiogram revealed a dilated right atrium and ventricle with a normal left ventricular size and function. The aortic valve was tricuspid with mild aortic regurgitation. The noncoronary sinus of Valsalva (NCS) was dilated with a continuous turbulent flow between the NCS and the right atrium, which was approximately 8 mm in diameter. An atrial septal defect (ASD) (10 mm) was detected. To confirm the diagnosis of aorta–right atrial tunnel, a multidetector computed tomography angiography (CTA) was performed. CTA revealed that the left and right coronary arteries and branches were normal. The NCS was enlarged, from which a tortuous tunnel originated and terminated at the right atrial–superior vena cava junction (Fig. 1 and Fig. 2).
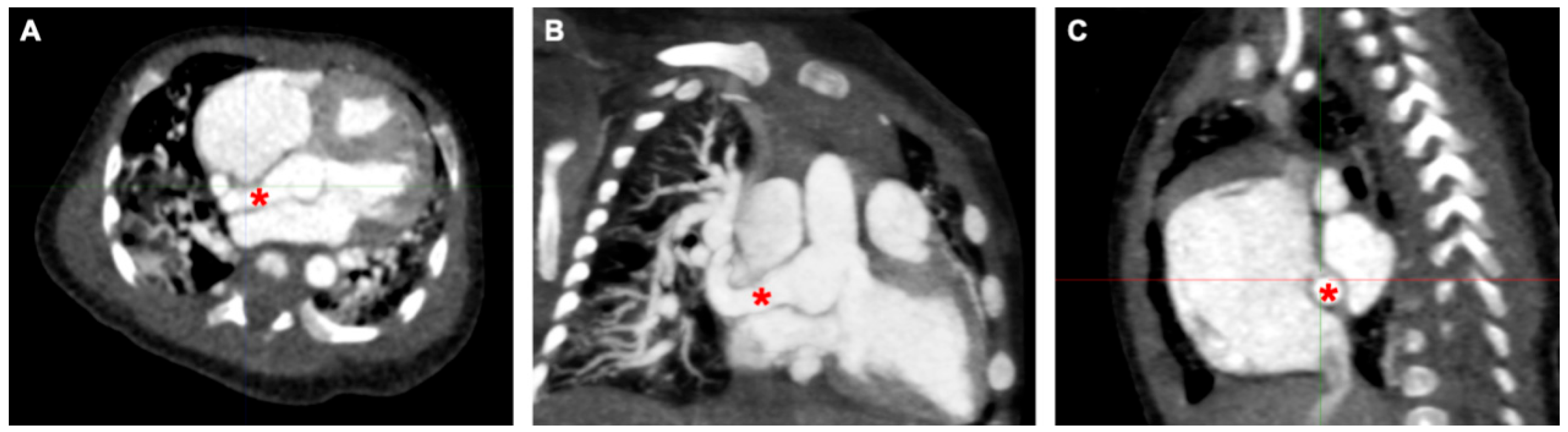
Figure 1: The course of ARAT in computed tomography across different imaging planes. (A) Axial view: An anomalous channel arising from the noncoronary sinus of Valsalva, coursing posterior to the right atrium. (B) Coronal reconstruction: The channel terminating at the right atrial–superior vena cava junction. (C) Sagittal reconstruction. *Aorta–right atrial tunnel.
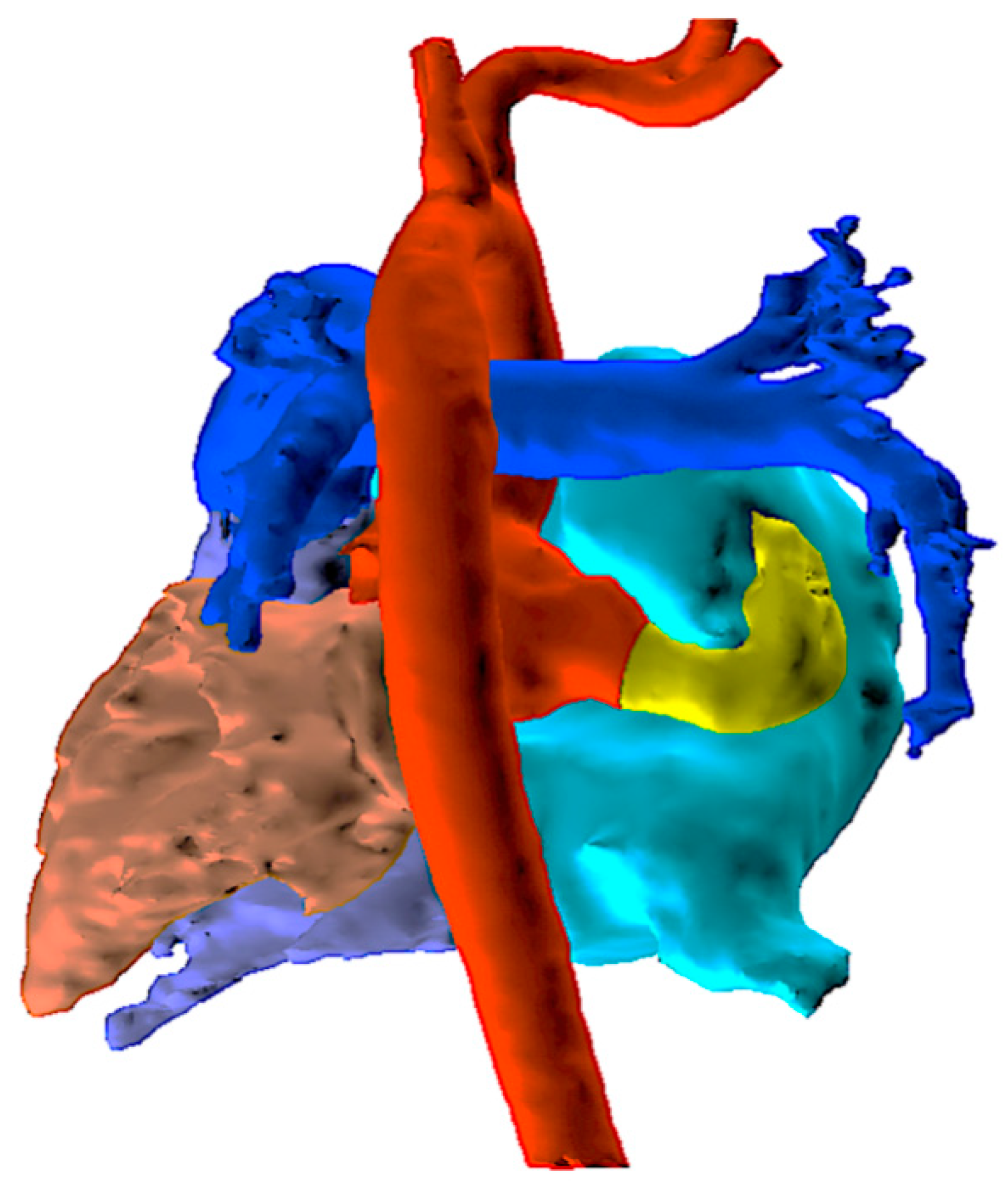
Figure 2: Three-dimensional reconstruction of the cardiac structure based on CT images using the Mimics software, viewed from the posterior aspect. The yellow channel represents ARAT, arising from the dilated noncoronary sinus, coursing posterior to the right atrium. CT, computed tomography; ARAT, aorta–right atrial tunnel.
The patient was diagnosed with aorta–right atrial tunnel (ARAT), which was an extremely rare condition. Considering the obviously dilated right heart detected by both echocardiogram and CTA, the infant may have already been suffering from abnormal left to right shunt, and was at risk of developing congestive heart failure in near future if the tunnel remained untreated. Therefore, we decided to perform surgical closure of the tunnel. A median sternotomy was performed, and cardiopulmonary bypass with aorto-bicaval cannulation was conducted. The abnormal tunnel was discernible, originating from the aortic root and traveling along the posterior aspect of the right atrium. The tunnel was successfully dissected and looped before cross-clamp without any electrocardiographic change. The aorta was cross-clamped, and cold anterograde cardioplegia was used to arrest the heart. In this way, we were able to open the root of the aorta and directly explore the relationship between the coronary arteries and the aortic opening of the tunnel, as well as the structure of the aortic valve. A transverse incision was made in the aorta to expose the aortic valve and coronary ostium, both of which appeared normal in morphology. Therefore, we decided to close the aortic end of the tunnel directly. Pericardial pledget-reinforced closure was performed at the aortic end to prevent long-term expansion or aneurysm. Right atriotomy was performed, and the atrial end of the tunnel was composed of several small openings, which was consistent with cardiac ultrasound results. Therefore, the tunnel was transected and closed with a polypropylene running suture outside the right atrium. The ASD was closed using a pericardial patch (Fig. 3).
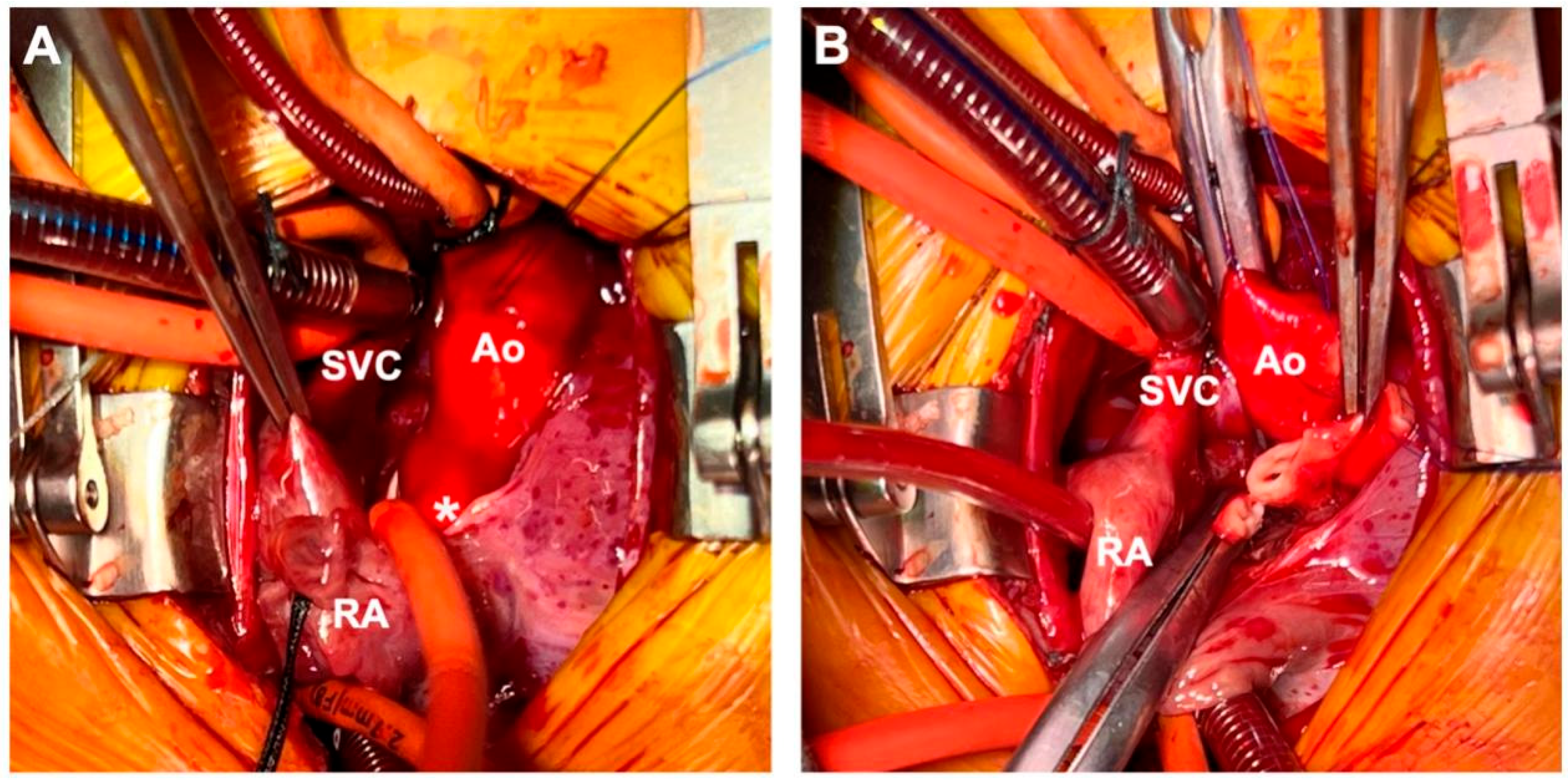
Figure 3: Surgery images. (A) ARAT was looped, and the patient’s electrocardiogram was monitored for any significant changes before cardioplegic perfusion and aortic cross-clamp. (B) The tunnel was transected in the middle part. *ARAT, aorta–right atrial tunnel.
The patient was weaned off ventilation on the second postoperative day and was discharged after 1 week. Postoperative echocardiogram revealed complete closure of the tunnel with no residual flow. During the 6-month postoperative follow-up, both electrocardiographic monitoring and echocardiography showed no evidence of aortic valve regurgitation or aortic root dilation. The infant exhibited age-appropriate growth and developmental milestones, with no documented comorbidities or subsequent hospital admissions.
ARAT is first described by Coto et al. in 1980 [1]. The embryogenesis of this condition is attributed to a congenital weakness of the elastic lamina in the aortic media [2]. We reviewed all relevant articles and identified a total of 64 cases in 49 publications, most of which were case reports (Table 1). Although the first reported case of ARAT originated from the NCS, this type accounts for the smallest proportion. This case is the fourth reported and the only one in which a cardiac structural abnormality was detected during the neonatal period [1,3,4].
Two-dimensional echocardiography can detect a channel-like structure extending from the aortic root to the right atrium with continuous systolic and diastolic flow on Doppler. Mild to severe enlargement of right heart chambers can manifest. In patients with heart failure, dilated left cardiac chambers and atrioventricular valve regurgitation can be detected. CTA and cardiac catheterization are generally used to confirm the anatomical structure of coronary arteries. Most ARAT cases originate from the left sinus of Valsalva (38/64) and the right sinus of Valsalva (23/64). Clinically, a differential diagnosis should be made with rupture of a congenital sinus of Valsalva aneurysm and coronary cameral fistulas. Rupture of a sinus of Valsalva aneurysm originates mostly from the right sinus of Valsalva and NCS and rarely from the left sinus of Valsalva. Congenital sinus of Valsalva aneurysm has a typical windsock appearance, whereas ARAT usually has an apparent tubular-shaped extra-cardiac course. In our case, the patient was diagnosed during the neonatal period, which suggests that this malformation was a congenital structure instead of a rupture of an aneurysm. Coronary cameral fistula is characterized by the dilation of the coronary arteries and intra-myocardial coronary artery branches. In patients with ARAT, coronary arteries can be completely normal (46/64), especially when ARAT originates from the NCS. Even if the coronary artery arises from the tunnel, usually adjacent to its origin, the remote part of the ipsilateral coronary artery is always of normal caliber.
The average age of diagnosis was 22.8 ± 18.2 years. A total of 24 patients (37.5%) were asymptomatic at the time of diagnosis. Clinical symptoms ranged from nonspecific ones (such as palpitations, exertional dyspnea, tachypnea, chest pain, and fatigability) to severe ones related to heart failure (such as pedal edema, abdominal distension, and facial puffiness). Although patients can be asymptomatic, surgery is recommended once a diagnosis is made because of the potential long-term risks, including sinus or atrial tachyarrhythmia (eight cases), congestive heart failure (four cases) [5,6,7,8], and endocarditis (three cases). The diameter of the channel ranged from 5 to 35 mm, but the degree of shunting depended mainly on the size of the right atrial orifices and if there was a narrowing of the atrial opening. The pulmonary to systemic flow ratio, based on cardiac magnetic resonance imaging (MRI) or cardiac catheterization, varied from 1.1:1 to 6.1:1, with a median of 1.67:1. There were nine patients diagnosed at or under 1 year of age, and four patients underwent surgery during the neonatal period; three of them were prenatally diagnosed, with the earliest diagnosis at 24 weeks of gestation [9,10,11,12]. All neonates exhibited severe right atrial and right ventricular enlargement and hemodynamic instability immediately after birth. In this case, the patient had a history of pneumonia during the neonatal period, potentially associated with abnormal shunting, and exhibited significant right heart enlargement upon admission, which warranted early intervention.
Among all patients, 46 patients underwent surgical treatment, 12 underwent percutaneous closure, and 6 were followed up. Surgical methods mainly included direct running sutures, pledgeted closure, and patch closure at the aortic and right atrial ends. The tunnel can also be looped and ligated off-pump. For patients requiring aortic occlusion during surgery, it is crucial to control the channel first to ensure proper and effective perfusion of the myocardial protective solution. For those with coronary arteries originating directly from the channel, multiple techniques were used to reconstruct coronary arteries and the sinus of Valsalva, including reimplantation of the involved coronary artery with or without a patch, closure of the channel distal to the takeoff of the coronary artery, and coronary artery bypass grafting [8,13,14,15,16,17,18]. Interventional closure devices included vascular plugs, ventricular septal defect and ASD closure devices, duct occluders, and coils. Caution must be exercised to avoid perforation during the procedure, as it may necessitate conversion to mid-sternal surgery [19]. Overall, the surgical outcomes were favorable, with only one early patient death reported [17].
In conclusion, ARAT is an extremely rare congenital heart malformation with an average age of diagnosis of approximately 20 years. Its clinical symptoms are varied and often atypical. ARAT usually originates from the left or right sinus of Valsalva and needs to be differentiated from the rupture of a congenital sinus of Valsalva aneurysm and coronary cameral fistulas. Patients diagnosed during the neonatal period or presenting with clinical manifestations typically exhibit high-volume shunt flow, requiring early surgical intervention. Timely operative management effectively reduces the risk of impending cardiac failure, while potentially preventing long-term complications, such as infective endocarditis and development of atrial fibrillation. The outcomes are generally favorable.
Table 1: General characteristics of patients in different articles.
| Author | Year | Gender | Age | ECG | Associated Lesions | Origin | Coronary Origin | Qp/Qs | Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chigullapalli et al. [13] | 2024 | F | 24 years | RCS | Proximal part of tunnel | 2:1 | Surgery | ||
| 2 | Dlm et al. [20] | 2024 | M | 14 years | RCS | Proximal part of tunnel | Surgery | |||
| 3 | Ulular et al. [14] | 2022 | F | 27 years | Atrial fibrillation | PFO | RCS | Proximal part of tunnel | Surgery | |
| 4 | Onorato et al. [21] | 2020 | M | 27 years | Infective endocarditis | RCS | Normal | 1.1:1 | Occlutech® PDA Occluder | |
| 5 | Fontes et al. [22] | 2019 | M | 52 years | LCS | Proximal part of tunnel | 1.8:1 | Follow-up | ||
| 6 | Jain et al. [5] | 2018 | M | 26 years | AI, TR | RCS | Normal | Surgery | ||
| 7 | Narin et al. [9] | 2017 | M | Neo | PDA | LCS | Normal | Amplatzer Duct Occluder II | ||
| 8 | Dellis et al. [23] | 2017 | F | 38 years | PDA | LCS | Normal | Surgery | ||
| 9 | Khan et al. [15] | 2017 | M | 55 years | RCS | Proximal part of tunnel | Surgery | |||
| 10 | Dingli et al. [24] | 2017 | F | 25 years | LCS | Normal | 1.2:1 | Follow-up | ||
| 11 | Looi et al. [19] | 2016 | F | 38 years | Atrial fibrillation | LCS | Normal | 1.7:1 | Surgery | |
| 12 | Lee et al. [25] | 2016 | M | 31 years | LCS | Normal | 1.54:1 | Follow-up | ||
| 13 | Kim et al. [26] | 2016 | M | 42 years | Atrial fibrillation | Infective endocarditis, MR, AI | LCS | Normal | Surgery | |
| 14 | Altiparmak et al. [27] | 2015 | F | 57 years | RCS | Normal | 2.2:1 | Amplatzer Vascular Plug II | ||
| 15 | Katayama et al. [6] | 2015 | F | 50 years | TR | LCS | Normal | Surgery | ||
| 16 | Venkataraman et al. [7] | 2015 | F | 29 years | RCS | Normal | Surgery | |||
| 17 | Tossios et al. [28] | 2014 | F | 47 years | LCS | Normal | 1.5:1 | Surgery | ||
| 18 | Iyisoy et al. [29] | 2014 | F | 18 years | LCS | Proximal part of tunnel | Surgery | |||
| 19 | Omeroglu et al. [30] | 2013 | F | 31 years | LCS | Normal | 2.5:1 | Surgery | ||
| 20 | Kim et al. [31] | 2013 | F | 36 years | RCS | Normal | 3.3:1 | Surgery | ||
| 21 | Thanopoulos et al. [32] | 2013 | M | 4 years | RCS | Normal | Amplatzer Duct Occluder II | |||
| 22 | Salehi et al. [8] | 2013 | M | 71 years | MR, TR, AI | RCS | Proximal part of tunnel | Surgery | ||
| 23 | Kim et al. [33] | 2013 | F | 42 years | LCS | Normal | Follow-up | |||
| 24 | Baykan et al. [34] | 2012 | F | 3 years | VSD | LCS | Normal | 1.88:1 | Amplatzer Vascular Plug | |
| 25 | Winter et al. [35] | 2012 | F | 23 years | LCS | Normal | Amplatzer Septal Occluder | |||
| 26 | M | 3 years | LCS | Normal | 1.2:1 | Follow-up | ||||
| 27 | Hu et al. [36] | 2012 | M | 38 years | LCS | Normal | Surgery | |||
| 28 | Matter et al. [37] | 2011 | F | 2 months | Sinus tachycardia | LCS | Normal | Surgery | ||
| 29 | OMaoldomhnaigh et al. [4] | 2011 | M | 8 years | NCS | Normal | Surgery | |||
| 30 | Sung et al. [38] | 2011 | M | 73 years | RCS | Normal | 1.4:1 | Surgery | ||
| 31 | Myers et al. [39] | 2011 | M | 33 years | Infective endocarditis | LCS | Normal | Surgery | ||
| 32 | Chandra et al. [40] | 2011 | F | 12 years | ASD | RCS | Normal | 3:1 | Amplatzer Duct Occluder | |
| 33 | Altekin et al. [41] | 2011 | M | 39 years | Sinus tachycardia | RCS | Normal | 1.5:1 | Follow-up | |
| 34 | Krishna et al. [16] | 2010 | F | 11 years | LCS | Normal | 1.8:1 | Surgery | ||
| 35 | M | 24 years | RCS | Normal | 1.6:1 | Surgery | ||||
| 36 | Deshpande et al. [10] | 2010 | 2 days | RCS | Normal | Surgery | ||||
| 37 | Subban et al. [42] | 2009 | F | 21 years | LCS | Normal | 1.67:1 | Amplatzer Duct Occluder | ||
| 38 | Mahesh et al. [11] | 2008 | 4 days | RCS | Proximal part of tunnel | Coils | ||||
| 39 | Aggarwal et al. [43] | 2007 | F | 12 years | Sinus tachycardia | LCS | Normal | 1.2:1 | Surgery | |
| 40 | M | 33 years | Atrial fibrillation | RCS | Proximal part of tunnel | 2.7:1 | Surgery | |||
| 41 | Al-Hay et al. [44] | 2005 | M | 6 months | LCS | Normal | Surgery | |||
| 42 | Sreedharan et al. [45] | 2005 | M | 11 years | LCS | Normal | 1.4:1 | Coils | ||
| 43 | Sivakumar et al. [46] | 2006 | M | 19 years | LCS | Proximal part of tunnel | 3.2:1 | Amplatzer Vascular Plug | ||
| 44 | Gajjar et al. [17] | 2005 | F | 9 years | PDA | LCS | Normal | 1.3:1–6.3:1 | Surgery | |
| 45 | F | 45 years | ASD | LCS | Proximal part of tunnel | Surgery | ||||
| 46 | F | 23 years | LCS | Proximal part of tunnel | Surgery | |||||
| 47 | M | 15 years | ASD | LCS | Normal | Surgery | ||||
| 48 | M | 18 years | RCS | Proximal part of tunnel | Surgery | |||||
| 49 | M | 42 years | LCS | Proximal part of tunnel | Surgery | |||||
| 50 | M | 3 years | RCS | Proximal part of tunnel | Surgery | |||||
| 51 | M | 10 years | RCS | Normal | Coils | |||||
| 52 | F | 17 years | LCS | Normal | Surgery | |||||
| 53 | Kursaklioglu et al. [47] | 2004 | M | 27 years | LCS | Normal | 1.6:1 | Surgery | ||
| 54 | Moraes et al. [3] | 2004 | M | 1 year | NCS | Normal | Surgery | |||
| 55 | Türkay et al. [18] | 2003 | M | 29 years | RCS | Proximal part of tunnel | 2.1:1 | Surgery | ||
| 56 | Tsai et al. [48] | 2002 | F | 2 years | LCS | Normal | 1.8:1 | Surgery | ||
| 57 | Kalangos et al. [49] | 2000 | M | 18 years | LCS | Normal | 1.5:1 | Surgery | ||
| 58 | M | 7 years | LCS | Normal | 1.3:1 | Surgery | ||||
| 59 | Danilowicz et al. [12] | 1989 | F | 3 days | ASD, PDA | RCS | Normal | Surgery | ||
| 60 | Rosenberg et al. [2] | 1986 | F | 7 years | LCS | Normal | Surgery | |||
| 61 | F | 6 months | LCS | Proximal part of tunnel | 1.7:1 | Surgery | ||||
| 62 | M | 15 years | LCS | Normal | 1.3:1 | Surgery | ||||
| 63 | M | 8 months | LCS | Proximal part of tunnel | Surgery | |||||
| 64 | Coto et al. [1] | 1980 | M | 25 years | Sinus tachycardia | NCS | Normal | Surgery |
Acknowledgement:
Funding Statement: The study was supported by the National Clinical Key Specialty Construction Project (Grant number: 10000015Z155080000004), Shanghai Research Center for Pediatric Cardiovascular Diseases (Grant number: 2023ZZ02024).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Xu Liu, Yanjun Sun, Hao Zhang; data collection: Xu Liu, Xiafeng Yu; analysis and interpretation of results: Xu Liu, Yiwei Liu; draft manuscript preparation: Xu Liu, Yiwei Liu, Hao Zhang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, Hao Zhang, upon reasonable request.
Ethics Approval: The study was approved by the Ethics Committee of SCMC (SCMCIRB-K2024198-1), and parental written informed consent was obtained prior to the initiation of the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2025.063108.
Abbreviations
| Aorta–right atrial tunnel | |
| Noncoronary sinus of Valsalva |
References
1. Coto EO, Caffarena JM, Such M, Marques JL. Aorta–right atrial communication. Report of an unusual case. J Thorac Cardiovasc Surg. 1980;80(6):941–4. [Google Scholar]
2. Rosenberg H, Williams WG, Trusler GA, Smallhorn J, Rowe RD, Moes CAF, et al. Congenital aortico-right atrial communications: The dilemma of differentiation from coronary-cameral fistula. J Thorac Cardiovasc Surg. 1986;91(6):841–7. [Google Scholar]
3. Moraes F, Santos CL, Moraes CR. Aortic-right atrial tunnel. Cardiol Young. 2004;14(1):86–8. doi:10.1017/s1047951104001167. [Google Scholar] [CrossRef]
4. OMaoldomhnaigh C, Ramsay JM, Finley JP, Andrews D, Murray C. Bilateral aortico-atrial tunnels. Pediatr Cardiol. 2011;32(8):1199–201. doi:10.1007/s00246-011-9979-7. [Google Scholar] [CrossRef]
5. Jain J, Wani A, Kulkarni A, Yelne P. Aorta-right Atrial Tunnel Presenting with Heart Failure in an Adult. Heart Views. 2018;19(4):152–5. doi:10.4103/HEARTVIEWS.HEARTVIEWS_74_18. [Google Scholar] [CrossRef]
6. Katayama Y, Ozawa T, Iga A, Hisatake S, Watanabe Y. Surgical repair of aorta-right atrial tunnel in an adult. Circ J. 2015;79(4):892–3. doi:10.1253/circj.CJ-14-202. [Google Scholar] [CrossRef]
7. Venkataraman R, Vaidyanathan K, Nainar M. Aorto-right atrial tunnel: imaging and correlation. Eur J Cardiothorac Surg. 2015;47(2):390. doi:10.1093/ejcts/ezu199. [Google Scholar] [CrossRef]
8. Salehi A, Cui WW. Aorta-right atrial tunnel in an elderly patient. Anesth Analg. 2013;117(6):1282–5. doi:10.1213/ANE.0b013e3182a74791. [Google Scholar] [CrossRef]
9. Narin N, Pamukcu O. Percutaneous closure of aorta-right atrial tunnel in a newborn. Cardiol Young. 2017;28(1):142–3. doi:10.1017/S1047951117001780. [Google Scholar] [CrossRef]
10. Deshpande SR, Fyfe DA. Aorto–Right Atrial Tunnel: Fetal Heart Failure, Diagnosis, and Treatment. Pediatr Cardiol. 2010;31(2):299–300. doi:10.1007/s00246-009-9567-2. [Google Scholar] [CrossRef]
11. Mahesh K, Francis E, Kumar RK. Aorta to Right Atrial Tunnel. JACC Cardiovasc Interv. 2009;1(6):716–7. doi:10.1016/j.jcin.2008.05.011. [Google Scholar] [CrossRef]
12. Danilowicz D, Presti S, Colvin S, Rutkowski M. Congenital fistulous tract between aorta and right atrium presenting as heart failure in a newborn. Pediatr Cardiol. 1989;10(2):93–7. doi:10.1007/BF02309921. [Google Scholar] [CrossRef]
13. Chigullapalli S, Malani SK, Nalawade D, Musuku MR. Aorto-atrial tunnel arising from sinus of Valsalva aneurysm in a young female. BMJ Case Rep. 2024;17(3):1–5. doi:10.1136/bcr-2023-59254. [Google Scholar] [CrossRef]
14. Ulular O, Bolat B, Gulcan O. A new surgical approach for aorta-right atrial tunnel with right coronary artery orifice. Turk J Thorac Cardiovasc Surg. 2022;30(1):121–4. doi:10.5606/tgkdc.dergisi.2022.21200. [Google Scholar] [CrossRef]
15. Khan SAM, Scholtz L, Synders FA, Villiers J. An unusual case of aorta-right atrial tunnel with windsock aneurysm: imaging, diagnosis and treatment. Cardiovasc J Afr. 2017;28(4):e1–5. [Google Scholar]
16. Krishna CS, Baruah DK, Reddy GV, Panigrahi NK, Suman K, Kumar PVN. Aorta-right atrial tunnel. Tex Heart Inst J. 2010;37(4):480–2. doi:10.5830/CVJA-2016-073. [Google Scholar] [CrossRef]
17. Gajjar T, Voleti C, Matta R, Iyer R, Dash PK, Desai N. Aorta-right atrial tunnel: clinical presentation, diagnostic criteria, and surgical options. J Thorac Cardiovasc Surg. 2005;130(5):1287–92. doi:10.1016/j.jtcvs.2005.07.021. [Google Scholar] [CrossRef]
18. Türkay C, Golbasi I, Belgi A, Tepe S, Bayezid O. Aorta-right atrial tunnel. J Thorac Cardiovasc Surg. 2003;125(5):1058–60. doi:10.1067/mtc.2003.87. [Google Scholar] [CrossRef]
19. Looi JL, Gabriel RS. Multi-modality imaging in congenital aorto-right atrial tunnel. Eur Heart J Cardiovasc Imaging. 2016;17(5):586. doi:10.1093/ehjci/jev360. [Google Scholar] [CrossRef]
20. Dlm WY, Deng MB, Xie XJ. Aorta-right atrial tunnel with anomalous circumflex artery origin. Acta Cardiol. 2024;79:846–7. doi:10.1080/00015385.2024.2375050. [Google Scholar] [CrossRef]
21. Onorato EM, Costante AM, Andreini D, Bartorelli AL. Infective endocarditis of an asymptomatic congenital aorta-right atrial tunnel: a case report. Eur Heart J Case Rep. 2020;4(2):1–5. doi:10.1093/ehjcr/ytaa039. [Google Scholar] [CrossRef]
22. Fontes A, Dias-Ferreira N, Ladeiras-Lopes R, Oliveira M, Braga P. Aorta-right atrium tunnel: an unexpected diagnosis. Eur Heart J Cardiovasc Imaging. 2019;20(12):1352. doi:10.1093/ehjci/jez154. [Google Scholar] [CrossRef]
23. Dellis SL, Pennel T, Said-Hartley Q, Zilla P. Sinus of Valsalva-right atrial tunnel causing heart failure in a 38-year-old. J Thorac Cardiovasc Surg. 2018;155(1):e51–3. doi:10.1016/j.jtcvs.2017.06.005. [Google Scholar] [CrossRef]
24. Dingli P, Reichmuth L, Yamagata K, Felice H. Aorto-right atrial tunnel draining into a pedunculated mass. J Echocardiogr. 2017;15(3):1–2. doi:10.1007/s12574-017-0328-6. [Google Scholar] [CrossRef]
25. Lee S, Kim SW, Im SI, Yong HS, Choi CU, Lim HE, et al. Aorta-Right Atrial Tunnel: Is Surgical Correction Mandatory? Circulation. 2016;133(13):e454–7. doi:10.1161/CIRCULATIONAHA.115.020161. [Google Scholar] [CrossRef]
26. Kim GS, Kim DW, Jeong IS, Ahn BH, Oh SG. Aorta-right atrial tunnel in a patient with multivalvular endocarditis. J Card Surg. 2016;31(12):738–9. doi:10.1111/jocs.12863. [Google Scholar] [CrossRef]
27. Altiparmak IH, Erkuş ME, Bozkurt S, Aksoy M, Sezen Y. Case images: successful treatment of an aneurysmatic aorta-right atrial tunnel by vascular plug: a very rare case. Arch Turk Soc Cardiol. 2015;43(3):316. doi:10.5543/tkda.2015.74943. [Google Scholar] [CrossRef]
28. Tossios P, Sarlis G, Aidonidis G, Karatzopoulos A, Grosomanidis V, Kouskouras K. Aorta-right atrial tunnel: imaging and surgical repair in an adult patient. J Cardiothorac Vasc Anesth. 2014;28(5):1314–8. doi:10.1053/j.jvca.2013.03.025. [Google Scholar] [CrossRef]
29. Iyisoy A, Celik T, Celik M, Sag C. Aorta-right atrial tunnel: an interesting type of a congenital coronary artery anomaly. Korean Circ J. 2014;44(3):193–5. doi:10.4070/kcj.2014.44.3.193. [Google Scholar] [CrossRef]
30. Omeroglu SN, Goksedef D, Balkanay OO, Ipek G. Aorta-right atrial tunnel in an adult. Eur J Cardiothorac Surg. 2014;45(3):580–1. doi:10.1093/ejcts/ezt348. [Google Scholar] [CrossRef]
31. Kim KW, Kim JH, Choe WJ. Aorta-right atrial tunnel. Eur Heart J Cardiovasc Imaging. 2014;15(1):112. doi:10.1093/ehjci/jet104. [Google Scholar] [CrossRef]
32. Thanopoulos BV, Ninios V, Germanakis J. Catheter closure of a right aortico-atrial tunnel in a patient 4 years of age. JACC Cardiovasc Interv. 2013;6(1):e1–2. doi:10.1016/j.jcin.2012.07.020. [Google Scholar] [CrossRef]
33. Kim KN, Cho KI, Kim JJ, Kang JH, Goo JJ, Lee JY, et al. A case of aorta-right atrial tunnel presented with an asymptomatic murmur. Korean Circ J. 2013;43(9):640–3. doi:10.4070/kcj.2013.43.9.640. [Google Scholar] [CrossRef]
34. Baykan A, Narin N, Ozyurt A, Uzum K. Aorta-right atrial tunnel closure using the transcatheter technique: a case of a 3-year-old child. Cardiol Young. 2013;23(3):457–9. doi:10.1017/S1047951112001151. [Google Scholar] [CrossRef]
35. Winter RJD, Blom NA, Straver B, Bouma BJ, Backx APCM, Clur SA, et al. Two cases of aorto-right atrial tunnel: clinical presentation, imaging and percutaneous closure. Neth Heart J. 2012;20(12):509–12. doi:10.1007/s12471-012-0330-6. [Google Scholar] [CrossRef]
36. Hu B, Zhou Q, Guo RQ. Misdiagnosis of aorta-right atrial tunnel. Echocardiography. 2012;29(2):e43–4. doi:10.1111/j.1540-8175.2011.01547.x. [Google Scholar] [CrossRef]
37. Matter M, Elgamal MA, Rahman AA, Almarsafawy H. Aortico-right atrial tunnel in an infant. Pediatr Cardiol. 2011;32(6):849–50. doi:10.1007/s00246-011-9971-2. [Google Scholar] [CrossRef]
38. Sung YM, Merchant N. Imaging of congenital aorta-right atrial tunnel with electrocardiogram gated 64-multi-slice computed tomography. Ann Thorac Surg. 2011;92(2):743. doi:10.1016/j.athoracsur.2011.01.002. [Google Scholar] [CrossRef]
39. Myers PO, Milas F, Panos A. Multimodality imaging in the evaluation of aorta-right atrial tunnel. Eur J Cardiothorac Surg. 2011;40(4):e153. doi:10.1016/j.ejcts.2011.05.044. [Google Scholar] [CrossRef]
40. Chandra S, Vijay S, Kaur D, Dwivedi S. Congenital Aorta Right Atrial Fistula: Successful Transcatheter Closure with the Amplatzer Occluder. Pediatr Cardiol. 2011;32(7):1057–9. doi:10.1007/s00246-011-0026-5. [Google Scholar] [CrossRef]
41. Altekin RE, Basarici I, Koc S, Kucuk M, Yanikoglu A, Demir I. Aorta-right atrial tunnel leading to heart failure. J Cardiol Cases. 2011;4(2):e87–9. doi:10.1016/j.jccase.2011.06.003. [Google Scholar] [CrossRef]
42. Subban V, Sankardas MA, Janakiraman E. Left aortic sinus to right atrial tunnel. Eur Heart J. 2010;31(2):164. doi:10.1093/eurheartj/ehp331. [Google Scholar] [CrossRef]
43. Aggarwal SK, Sai V, Ramnathiyer V. Imaging Features of Aorto-Right Atrial Tunnel: A Report of Two Cases. Congenit Heart Dis. 2007;2(6):429–32. doi:10.1111/j.1747-0803.2007.00137.x. [Google Scholar] [CrossRef]
44. Al-Hay AA, Sethia B, Shinebourne EA. A tunnel from the left sinus of Valsalva to the right atrium. Cardiol Young. 2005;15(1):79–81. doi:10.1017/S1047951105000168. [Google Scholar] [CrossRef]
45. Sreedharan M, Baruah B, Dash PK. Aorta-right atrial tunnel—a novel therapeutic option. Int J Cardiol. 2006;107(3):410–2. doi:10.1016/j.ijcard.2005.01.056. [Google Scholar] [CrossRef]
46. Sivakumar K, Shahani JM, Francis E. Transcatheter Closure of Aortico Right Atrial Tunnel—a Rare Cardiac Anomaly. Congenit Heart Dis. 2006;1(6):324–6. doi:10.1111/j.1747-0803.2006.00056.x. [Google Scholar] [CrossRef]
47. Kursaklioglu H, Iyisoy A, Celik T, Kose S, Amasyali B, Isik E. Aortico-right atrial tunnel in an adult patient. Int J Cardiovas Imaging. 2005;21(4):383–5. doi:10.1007/s10554-004-7252-3. [Google Scholar] [CrossRef]
48. Tsai YC, Wang JN, Yang JY, Wu JM. Aortico-cameral communication from left sinus Valsalva aneurysm to right atrium via a tortuous tunnel with aneurysmal dilatation. Pediatr Cardiol. 2002;23(1):108–9. doi:10.1007/s00246-001-0029-8. [Google Scholar] [CrossRef]
49. Kalangos A, Beghetti M, Vala D, Chraibi S, Faidutti B. Aorticoright atrial tunnel. Ann Thorac Surg. 2000;69(2):635–7. doi:10.1016/s0003-4975(99)01346-6. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools