 Open Access
Open Access
CASE REPORT
Carotid Artery Pseudoaneurysm in a Pediatric Patient Following ECMO: Management with Carotid Artery Ligation and Pseudoaneurysm Resection under Balloon Occlusion-Guided DSA
1 Department of Cardiothoracic Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, 210008, China
2 Clinical Teaching Hospital of Medical School, Nanjing Children’s Hospital, Nanjing University, Nanjing, 210093, China
* Corresponding Author: Wei Peng. Email:
# These authors contributed equally to the present study and should be regarded as joint first authors
Congenital Heart Disease 2025, 20(1), 55-60. https://doi.org/10.32604/chd.2025.063072
Received 04 January 2025; Accepted 06 March 2025; Issue published 18 March 2025
Abstract
Background: Carotid artery pseudoaneurysm in children is rare; typically caused by trauma; surgical interventions and infection. These aneurysms can lead to significant neurological and vascular risks; and their management remains challenging. While endovascular therapy has become the standard for giant pseudoaneurysms in adults; its use in children is limited. No established guidelines or long-term safety data exist for pediatric endovascular treatment. We present a child who developed a carotid artery pseudoaneurysm after venoarterial extracorporeal membrane oxygenation (VA-ECMO) support and heart transplantation; highlighting the management strategies and outcomes. Case Description: A 4-year-old boy with dilated cardiomyopathy was admitted for congestive heart failure and subsequently required VA-ECMO support due to worsening hemodynamics. After heart transplantation; the patient developed a persistent hoarseness and a rapidly enlarging neck mass. Imaging confirmed the presence of a giant carotid artery pseudoaneurysm. Balloon occlusion-guided digital subtraction angiography (DSA) revealed adequate collateral circulation; allowing successful carotid artery ligation and pseudoaneurysm resection. Postoperative recovery was uneventful; with no neurological deficits or complications; and regular follow-up confirmed no further adverse sequelae. Conclusions: Management of carotid artery pseudoaneurysms in pediatric patients remains challenging. Under the guidance of DSA and with the assistance of balloon occlusion; precise aneurysm resection and vascular reconstruction can be achieved. In the event that vascular conditions limit the success of the repair; the balloon’s ability to occlude the parent artery and supplying vessels can safely facilitate the ligation of the parent artery of the aneurysm.Keywords
Supplementary Material
Supplementary Material FileA 4-year-old boy (19 kg, 108 cm) was admitted with congestive heart failure and diagnosed with dilated cardiomyopathy. Despite optimal treatment, his hemodynamic status worsened on day 10, necessitating venoarterial extracorporeal membrane oxygenation (VA-ECMO) support. A right-sided neck incision was made for cannula insertion into the common carotid artery and jugular vein, with proximal ligation of the artery. He was simultaneously listed for emergency heart transplantation. On day 16, sputum cultures tested positive for Acinetobacter baumannii, prompting imipenem therapy, which resolved the infection four days later. On day 25, he underwent successful heart transplantation, followed by repair of arteries and veins after ECMO weaning. On postoperative day 6, blood cultures were positive for Klebsiella pneumoniae, and linezolid was added to treatment, resolving the infection nine days later. However, on day 49, the patient developed persistent hoarseness with a rapidly enlarging neck lump (45 mm × 40 mm) (Fig. 1). Ultrasound and CT angiography (CTA) confirmed a pseudoaneurysm (Fig. 2 and Fig. 3A; Videos S1 and S2).
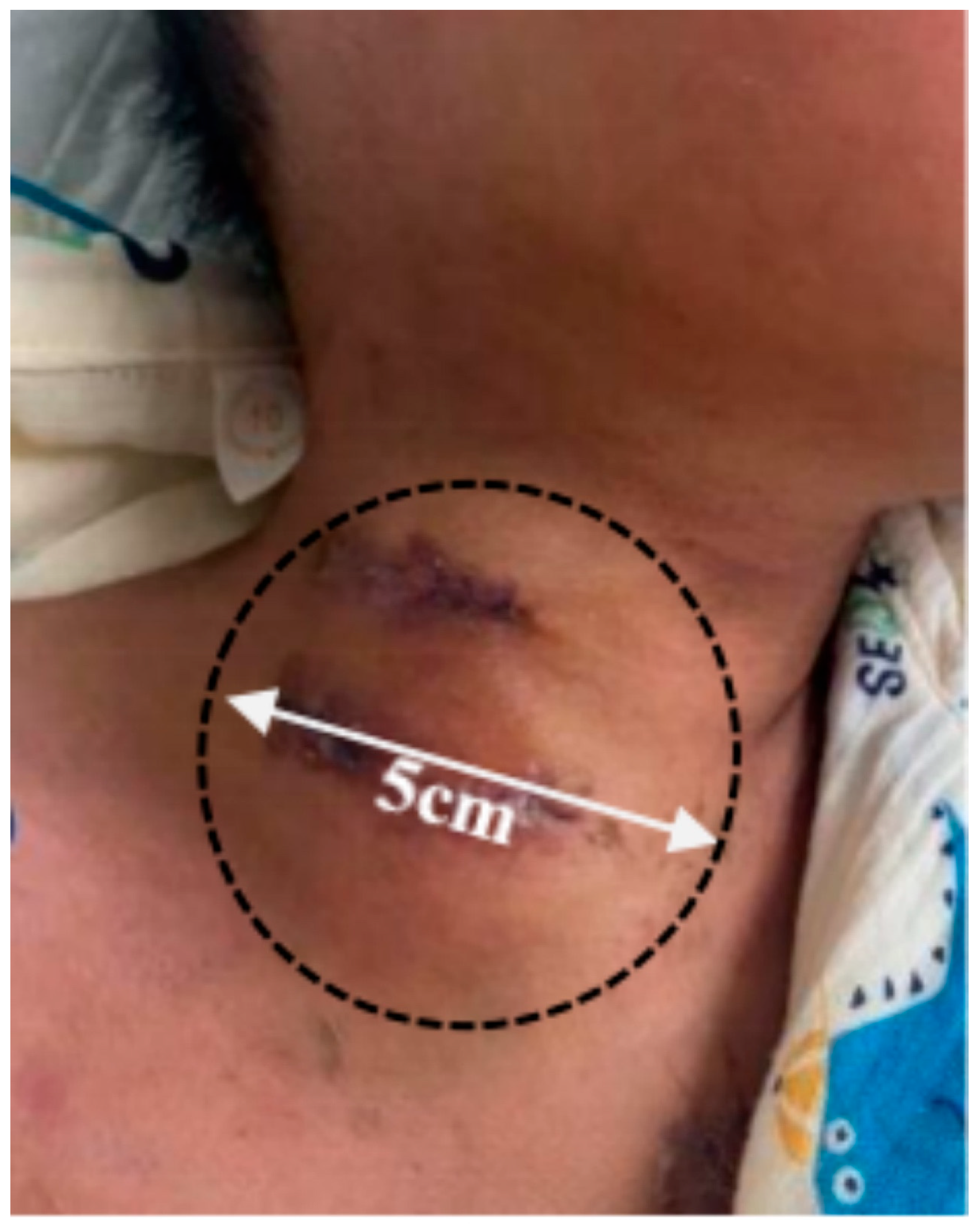
Figure 1: Preoperative surface lump measurement of patient.
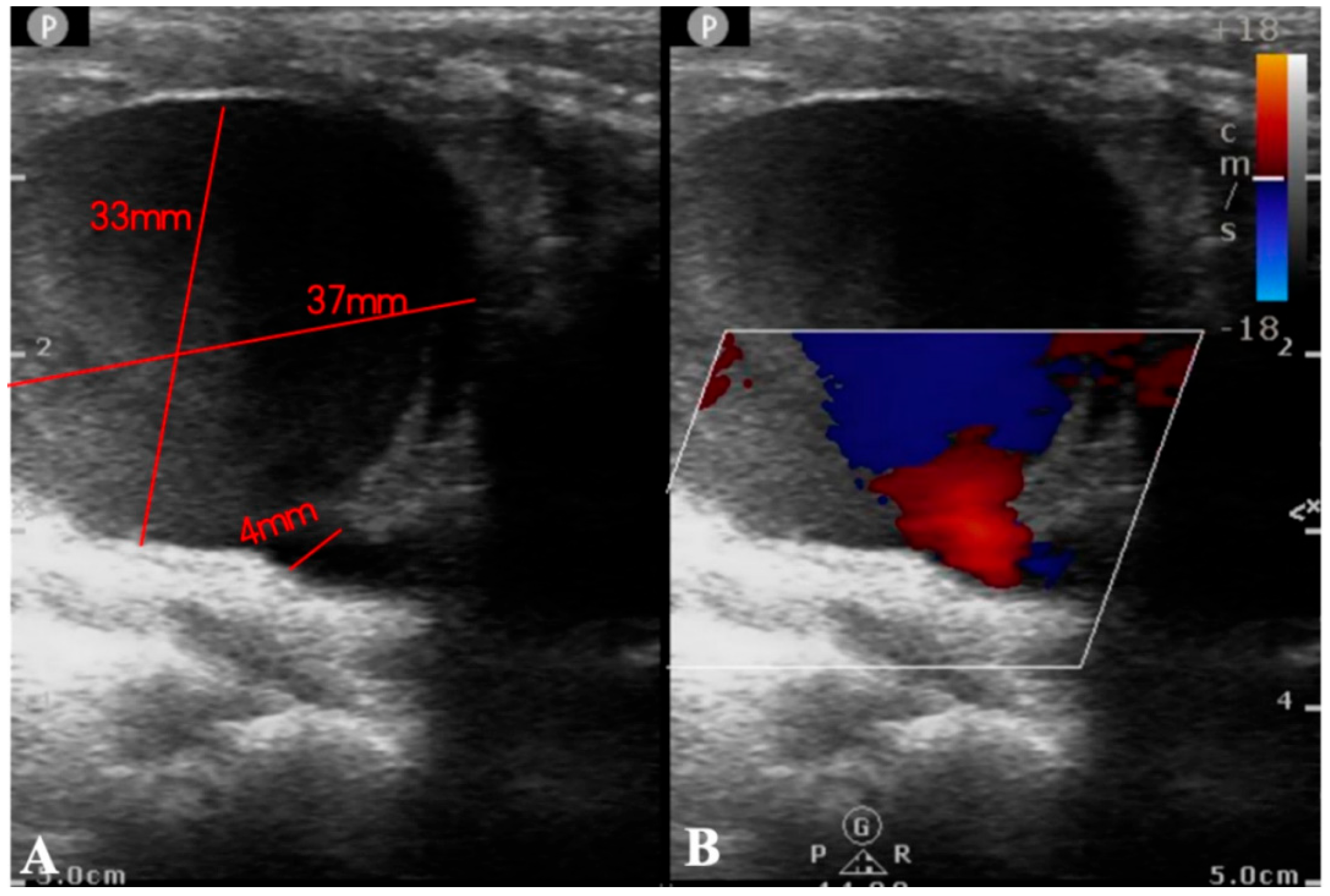
Figure 2: Ultrasound examination reports. (A): Ultrasound examination revealed a low echogenicity area of approximately 37 × 33 mm2, contiguous with the internal carotid artery, featuring an orificium fistulae with a diameter of 4 mm. (B): Color Doppler Flow Imaging (CDFI) demonstrated a color flow jet entering from the internal carotid artery, with visible red and blue turbulent flow signals within.
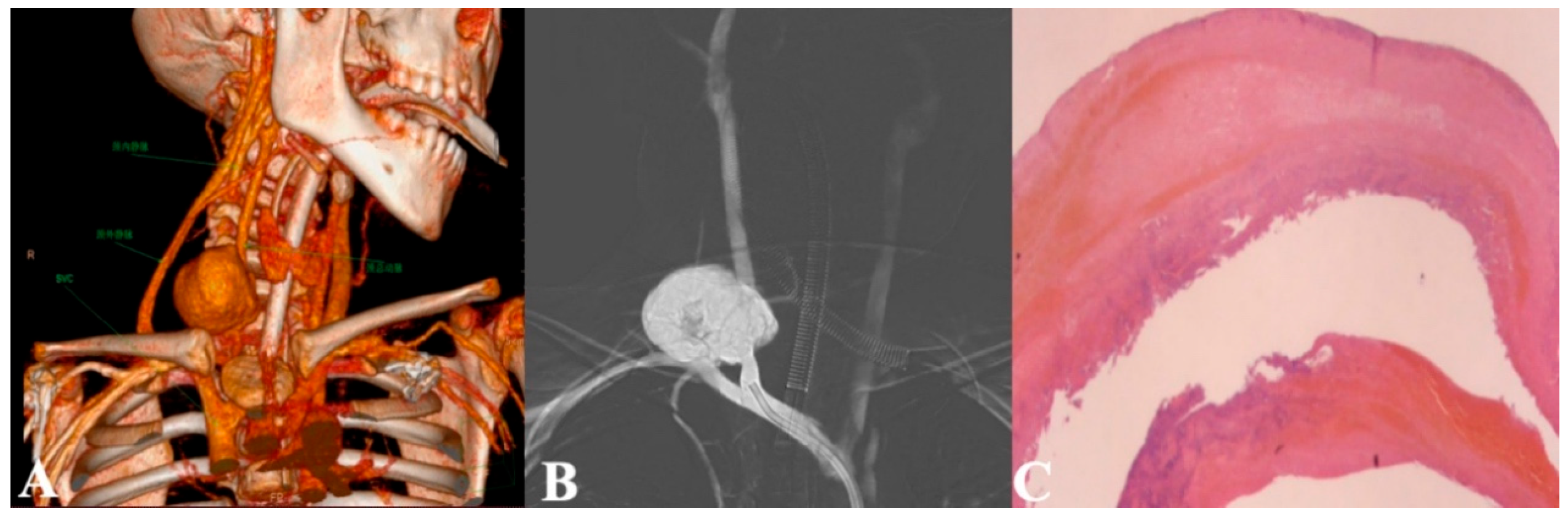
Figure 3: (A): Presurgical CT contrast scan suggesting a common carotid artery pseudoaneurysm: 3-D reconstruction coronal plane; (B): Arteriogram demonstrating a pseudoaneurysm of the right common carotid artery; (C): Pathologic examination of the carotid pseudoaneurysm, mainly composed of thrombus and fibrous components, accompanied by infiltration of inflammatory cells.
Emergency surgery was attempted to repair the artery. Initially, a guidewire was inserted via the right femoral artery, followed by right common carotid artery angiography, which revealed a large pseudoaneurysm of the right common carotid artery. The cerebral circle of Willis was found to be intact, and no abnormalities were observed. Subsequently, a 6 F balloon was deployed to completely occlude the aneurysm orifice, and angiographic findings indicated no blood flow through the aneurysm or the right common carotid artery. A 2.5 cm horizontal incision was then made along the original ECMO cannulation site in the neck, which revealed a fragile vessel wall with significant bleeding. The balloon was withdrawn after occluding the superior and inferior ends of the orifice, and the aneurysm wall was resected. Direct suturing and ligation were performed at the superior and inferior ends. Subsequently, the vessel is completely isolated and repaired. Digital subtraction angiography (DSA) assessment showed adequate collateral blood supply, allowing successful direct suturing and closure of the artery. Postoperative pathology confirmed the pseudoaneurysm diagnosis (Fig. 3C). The patient recovered well, with resolution of hoarseness and no neurological complications. Monthly ultrasound surveillance over an 8-month follow-up period revealed no detectable thrombi or adverse sequelae.
Carotid artery pseudoaneurysms are exceedingly rare, with an incidence rate ranging from 0.026‰ to 0.030‰ [1]. In the early stages, inward expansion of the pseudoaneurysm may compress the laryngeal nerve, hypoglossal nerve, or sympathetic trunk, thereby inducing cranial nerve and peripheral nerve dysfunction [2,3], such as the hoarseness observed in the present case [4]. As the mass enlarges further in the later stages, it may become palpable in the neck and is often misdiagnosed as a cervical infection [5]. Premature incision or lack of intervention can easily lead to rupture, posing a life-threatening risk. Therefore, due to its rarity and unpredictability, the natural course of the disease is generally poor, with a stroke rate as high as 24% [6]. The most common cause of pseudoaneurysm in children is infection, with other causes including anticoagulant therapy and, less frequently, iatrogenic injuries [3,7,8]. In our case, the patient experienced a systemic infection (Klebsiella pneumoniae positive in the blood) after surgery and surgical trauma to the neck vessels. In addition, heparin was administered for anticoagulation during ECMO therapy, and immunosuppressants (tacrolimus, mycophenolate mofetil) were used after transplantation. These factors collectively contributed to the formation of a pseudoaneurysm.
Asymptomatic carotid pseudoaneurysms can be managed conservatively with regular follow-up. However, surgery is generally accepted as the treatment of choice for symptomatic carotid pseudoaneurysms. There are some case reports in the literature describing the pathogenesis and treatment of carotid pseudoaneurysm, including a review by Welleweerd et al. [9]. Although similar reports have been published, carotid pseudoaneurysms after ECMO remain a rare phenomenon in children. There has been controversy over the preferred surgical treatment of pseudoaneurysms [7].
Several case reports of endovascular treatments have indeed demonstrated promising outcomes [2]. However, in pediatric patients, particularly those of younger ages, the long-term efficacy and safety of these treatments have not been fully validated, rendering them potentially inappropriate [8]. In contrast, open surgery exhibits greater advantages in managing large pseudoaneurysms caused by infections [10]. We have observed that poor vascular conditions resulting from infections and secondary surgeries often elevate the risk of failure in repairing the vascular wall [11,12]. In such instances, where repair surgery cannot be successfully performed, carotid artery ligation is considered as an alternative approach. Furthermore, some medical centers have attempted to use the great saphenous vein as a substitute for damaged arterial blood vessels, although this method has not been extensively reported in pediatric patients [13]. Given the complexity of the aneurysm and the intricate anatomy that rendered dissection challenging, we opted for DSA-guided techniques primarily to mitigate the risk of aneurysm rupture and to avoid cranial nerve paralysis [14]. The balloon occlusion facilitated by DSA effectively sealed the aneurysm sac, enabling meticulous dissection. However, due to significant vascular wall damage from puncture and infection, the aneurysm repair attempt failed. Consequently, leveraging the balloon’s capacity to occlude the parent artery and supplying vessels, we safely performed ligation of the parent artery of the aneurysm. The DSA balloon not only provides a clear surgical field during the procedure but also ensures the smooth progression of surgery even in the event of rupture. Even with simultaneous ligation and gradual balloon deflation, arterial bleeding was effectively minimized. In the future, the availability of pediatric-appropriate balloons that can achieve complete occlusion during ligation would further enhance the safety of such procedures. Additionally, when the condition is relatively non-urgent, the DSA balloon facilitates the most effective assessment of collateral circulation, thereby reducing surgical risks and enhancing success rates. More importantly, the application of the DSA balloon offers surgeons more surgical options and decision-making bases. If collateral circulation assessment reveals insufficient flow, the use of artificial blood vessels to replace damaged arteries can be considered to effectively restore cerebral blood supply. By doing so, we can not only ensure the safety and effectiveness of the surgery but also improve treatment outcomes and prognosis for patients. Observing good collateral blood supply on DSA, we ultimately decided to complete the pseudoaneurysm resection and carotid artery ligation under balloon occlusion on DSA. The patient did not experience neurological dysfunction postoperatively and recovered well. Typically, during ECMO, surgeons routinely ligate vessels above the carotid artery catheterization site and perform vessel release and repair when ECMO is withdrawn. However, there is ongoing debate about the necessity of repair [15].
Our case suggests that repair may not always be effective due to the fragility of the vessels, which can still lead to aneurysm formation and potentially require further surgery. Ligation or repair of the carotid artery in children with ECMO-associated infections requires further investigation through multicenter, large-cohort studies to identify the most suitable and safe approach for pediatric patients.
Acknowledgement:
Funding Statement: Supported by the Scientific Research Project of Jiangsu Maternity and Child Health Care Association (FYX202201).
Author Contributions: The authors confirm contribution to the paper as follows: Yaqi Zhang and Liang Hu contributed equally to this manuscript. Yaqi Zhang, Liang Hu and Wei Peng conceived the project. Yaqi Zhang wrote the main manuscript text and prepared figures and tables. Liang Hu, Bo Qian and Yuxi Zhang critically revised the work for intellectual content. Wei Peng and Jirong Qi gave approval of the final manuscript and agree to be accountable for all aspects of the work. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors will supply the relevant data in response to reasonable requests.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and National Research Committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This research was approved by the Institutional Review Board in Children’s Hospital of Nanjing Medical University and the approval number is 202411014-1 and informed consent was acquired.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material and the CARE checklist are available online at https://doi.org/10.32604/chd.2025.063072.
References
1. Norat P, Sokolowski JD, Gorick CM, Soldozy S, Kumar JS, Chae Y, et al. Intraarterial transplantation of mitochondria after ischemic stroke reduces cerebral infarction. Stroke Vasc Interv Neurol. 2023;3(3):e000644. doi:10.1161/svin.122.000644. [Google Scholar] [CrossRef]
2. Welleweerd JC, de Borst GJ, de Groot D, van Herwaarden JA, Lo RT, Moll FL. Bare metal stents for treatment of extracranial internal carotid artery aneurysms: long-term results. J Endovasc Ther. 2015;22(1):130–4. doi:10.1177/1526602814566405. [Google Scholar] [CrossRef]
3. Welleweerd JC, de Borst GJ, Carotid Aneurysm Registry Project Group. Extracranial carotid artery aneurysm: optimal treatment approach. Eur J Vasc Endovasc Surg. 2015;49(3):235–6. doi:10.1016/j.ejvs.2014.11.007. [Google Scholar] [CrossRef]
4. El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31(4):702–12. doi:10.1067/mva.2000.104101. [Google Scholar] [CrossRef]
5. Sundarrajan C, Isa SA, Caruso JP, Ban VS, Shah GB, Whittemore BA, et al. Treatment of large infectious extracranial carotid artery pseudoaneurysms in children: a systematic review of the literature. Childs Nerv Syst. 2021;37(5):1461–70. doi:10.1007/s00381-021-05084-0. [Google Scholar] [CrossRef]
6. Atalay YB, Piran P, Chatterjee A, Murthy S, Navi BB, Liberman AL, et al. Prevalence of cervical artery dissection among hospitalized patients with stroke by age in a nationally representative sample from the United States. Neurology. 2021;96(7):e1005–11. doi:10.1212/WNL.0000000000011420. [Google Scholar] [CrossRef]
7. Esposito A, Menna D, Giordano AN, Cappiello AP. Adjunctive techniques in endovascular repair of postcarotid endarterectomy pseudoaneurysm: Case report and literature review. Catheter Cardiovasc Interv. 2023;101(5):900–6. doi:10.1002/ccd.30619. [Google Scholar] [CrossRef]
8. Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ, et al. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48(3):470–2. doi:10.1097/00005373-200003000-00016. [Google Scholar] [CrossRef]
9. Welleweerd JC, den Ruijter HM, Nelissen BG, Bots ML, Kappelle LJ, Rinkel GJ, et al. Management of extracranial carotid artery aneurysm. Eur J Vasc Endovasc Surg. 2015;50(2):141–7. doi:10.1016/j.ejvs.2015.05.002. [Google Scholar] [CrossRef]
10. Welleweerd JC, Moll FL, de Borst GJ. Technical options for the treatment of extracranial carotid aneurysms. Expert Rev Cardiovasc Ther. 2012;10(7):925–31. doi:10.1586/erc.12.61. [Google Scholar] [CrossRef]
11. Haq TU, Yaqoob J, Munir K, Usman MU. Endovascular-covered stent treatment of posttraumatic cervical carotid artery pseudoaneurysms. Australas Radiol. 2004;48(2):220–3. doi:10.1111/j.1440-673.2004.01302.x. [Google Scholar] [CrossRef]
12. Pulli R, Dorigo W, Pratesi G, Fargion A, Pratesi C. Single-center experience on endovascular repair of noninfected extracranial internal carotid artery pseudoaneurysms. Ann Vasc Surg. 2013;27(5):672.e13–7. doi:10.1016/j.avsg.2012.07.028. [Google Scholar] [CrossRef]
13. Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–7. doi:10.3760/cma.j.issn.0253-2727.2019.01.010. [Google Scholar] [CrossRef]
14. Srivastava SD, Eagleton MJ, O’Hara P, Kashyap VS, Sarac T, Clair D. Surgical repair of carotid artery aneurysms: a 10-year, single-center experience. Ann Vasc Surg. 2010;24(1):100–5. doi:10.1016/j.avsg.2009.09.006. [Google Scholar] [CrossRef]
15. Pourhassan S, Grotemeyer D, Fokou M, Heinen W, Balzer K, Ramp U, et al. Extracranial carotid arteries aneurysms in children: single-center experiences in 4 patients and review of the literature. J Pediatr Surg. 2007;42(11):1961–8. doi:10.1016/j.jpedsurg.2007.07.052. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools