 Open Access
Open Access
REVIEW
Maternal Diabetes Mellitus and Congenital Heart Diseases: Systematic Review
1 Division of Tocogynecology, University Hospital Polydoro Ernani of São Thiago, Federal University of Santa Catarina (UFSC), Florianópolis, 88036-800, Brazil
2 Brazilian Institute for Digital Convergence, Federal University of Santa Catarina (UFSC), Florianópolis, 88040-900, Brazil
3 Department of Fetal Medicine, Biodesign Laboratory DASA/PUC, Rio de Janeiro, 22451-900, Brazil
4 Department of Pediatrics, Pediatric Cardiology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, 21941-617, Brazil
5 Department of Obstetrics, Paulista School of Medicine-Federal University of São Paulo (EPM-UNIFESP), São Paulo, 04039-001, Brazil
6 Discipline of Woman Health, Municipal University of São Caetano do Sul (USCS), São Caetano do Sul, 09521-160, Brazil
7 Department of Clinical Analyses, Federal University of Santa Catarina (UFSC), Florianópolis, 88040-900, Brazil
* Corresponding Author: Edward Araujo Júnior. Email:
(This article belongs to the Special Issue: Prenatal Diagnosis of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(1), 89-101. https://doi.org/10.32604/chd.2025.063014
Received 01 January 2025; Accepted 25 February 2025; Issue published 18 March 2025
Abstract
Introduction: Diabetes mellitus (DM), a metabolic disorder, leads to organ damage due to chronic hyperglycemia with multiple pathogenic processes. Gestational diabetes mellitus (GDM) poses risks to mothers and offspring, increasing the incidence of structural congenital heart disease (CHD) and myocardial hypertrophy in newborns. Objective: This review aimed to examine the association between maternal diabetes mellitus and CHD. Methods: This systematic review used the STROBE and TRIPOD checklists registered in PROSPERO (CRD42024513858). It focused on diagnostic test accuracy using the Munn et al. protocol for systematic assessment, emphasizing the “PIRD”: Population, Index Test, Reference Test, Diagnosis of Interest. This review aimed the following PIRD model question: ‘Does diabetic pregnant woman influence in fetal cardiac malformation?’ using PRISMA 2020 statement. A systematic review was conducted on 19 October 2023 in the following databases: PubMed/MEDLINE, Embase (Elsevier), CINAHL (EBSCO), Scopus (Elsevier), Web of Science (Clarivate Analytics), LILACS, and SciELO. Only articles in English, Spanish, and Portuguese languages were selected. Results: Seven studies between 2018 and 2023 were selected. The studies differed in terms of the cardiac ultrasound parameters used to assess CHD and diagnose diabetes mellitus in pregnancy. They highlight the importance of fetal echocardiography in detecting CHD prenatally and assessing the impact of diabetes mellitus on fetal cardiac health, recommending proactive care planning and early intervention for better outcomes. Conclusions: The studies highlight the impact of maternal diabetes mellitus, particularly GDM, on fetal cardiac development and support early detection by fetal echocardiography. Standardization and collaboration are essential to refine management and outcomes in high-risk pregnancies.Keywords
Supplementary Material
Supplementary Material FileDiabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia due to defects in insulin secretion, action, or both. Chronic hyperglycemia leads to organ damage, affecting the eyes, kidneys, nerves, heart, and blood vessels [1]. Pathogenic processes vary from autoimmune destruction leading to insulin deficiency to resistance to insulin action. Abnormalities in carbohydrate, fat, and protein metabolism result from inadequate insulin action on target tissues. Inadequate insulin secretion and reduced tissue responses to insulin contribute to impaired insulin action. Often, both secretion and action problems coexist in patients, making it difficult to determine the primary cause of hyperglycemia [2].
Gestational diabetes mellitus (GDM), defined as carbohydrate intolerance first diagnosed during pregnancy, poses significant risks to both mothers and their offspring [3]. Maternal consequences include increased rates of operative and cesarean section deliveries, hypertensive disorders, and increased future risk of type 2 diabetes mellitus and metabolic syndrome, including obesity and cardiovascular morbidities. In addition, delivery of macrosomic or large for gestational age fetuses is associated with an increased risk of cesarean section, postpartum hemorrhage, birth trauma, and shoulder dystocia. Today, GDM is characterized by “carbohydrate intolerance of variable severity with onset or first recognition during pregnancy,” regardless of post-pregnancy insulin treatment or persisting conditions [4]. It recognizes the possibility of pre-existing glucose intolerance prior to pregnancy. GDM affects up to 14% of pregnancies annually in the United States and contributes significantly to perinatal morbidity and mortality, as well as ongoing maternal health problems. Among all types of diabetes in pregnancy, GDM accounts for 90–95% of cases. Historically, references to diabetes in pregnancy date back to Bennewitz in 1823, who considered it a transient symptom, and Priscilla White’s pioneering study in the early 20th century challenged the notion of diabetes as a contraindication to pregnancy [5]. Jorgan Pedersen’s 1952 hyperglycemia-hyperinsulinism hypothesis explaining fetal pancreatic islet hypertrophy remains influential. The St. Vincent Declaration of 1989 emphasized global cooperation in the prevention, treatment, and cure of diabetes, underscoring its local, regional, and national importance [6]. Currently, GDM is a major concern for health care providers and patients, with national audits challenging the St. Vincent Declaration and revealing poor pregnancy outcomes. The historical evolution and contemporary understanding of GDM highlight its multifaceted impact, urging continued research and comprehensive health care approaches [7].
GDM increases the incidence of cardiac malformations in newborns. Malformations such as ventricular septa defect, transposition of the great arteries, aortic stenosis, pulmonary atresia, and dextrocardia are common, occurring in 8.5% of cases even with adequate glycemic control. Hypertrophic cardiomyopathy, present in 30% of cases, may result in sudden intrauterine fetal death [8].
Congenital heart disease (CHD), which results from abnormal development of the fetal heart structure, often during the formation of the four chambers and valves, is assessed by fetal echocardiography [9]. The prevalence of CHD is significantly higher than that of chromosomal anomalies and neural tube defects. Genetic or environmental risk factors contribute, and fetal echocardiography is the standard of care for early detection, offering high sensitivity and specificity. Early interventions, such as transplacental pharmacological therapy, are effective in treating fetal arrhythmias and associated heart failure [10].
GDM affects fetal cardiac development, causing myocardial hypertrophy due to fetal hyperinsulinemia resulting from inadequate glycemic control. Fetal Doppler echocardiography is essential for the diagnosis of morphologic and functional changes in the fetal and pediatric heart [11]. Preconception glycemic control reduces the risk of CHD [12]. Pregestational diabetes mellitus increases the risk of CHD, with teratogenicity attributed to oxidative stress induced by hyperglycemia [13]. In this systematic review, we assessed the association between maternal diabetes mellitus and CHD.
This study was conducted using the critical appraisal and data extraction for systematic reviews, strengthening the reporting of observational studies in epidemiology (STROBE) [14] and the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) [15] checklists. The study protocol was registered in the Prospero International Prospective Register of Systematic Reviews (CRD42024513858).
Diagnostic test accuracy is a critical aspect of medical and health sciences and provides valuable insight into the performance of diagnostic tests. Munn et al. [16] have provided protocols that outline a systematic approach to assessing diagnostic test accuracy to ensure reliability and validity in the evaluation of diagnostic tests. In this case, the suggested question format is “PIRD”: Population, Index Test, Reference Test, Diagnosis of Interest (Fig. 1). This review aimed the following PIRD model question: ‘Does diabetic pregnant woman influence in fetal cardiac malformation?’, using PRISMA 2020.
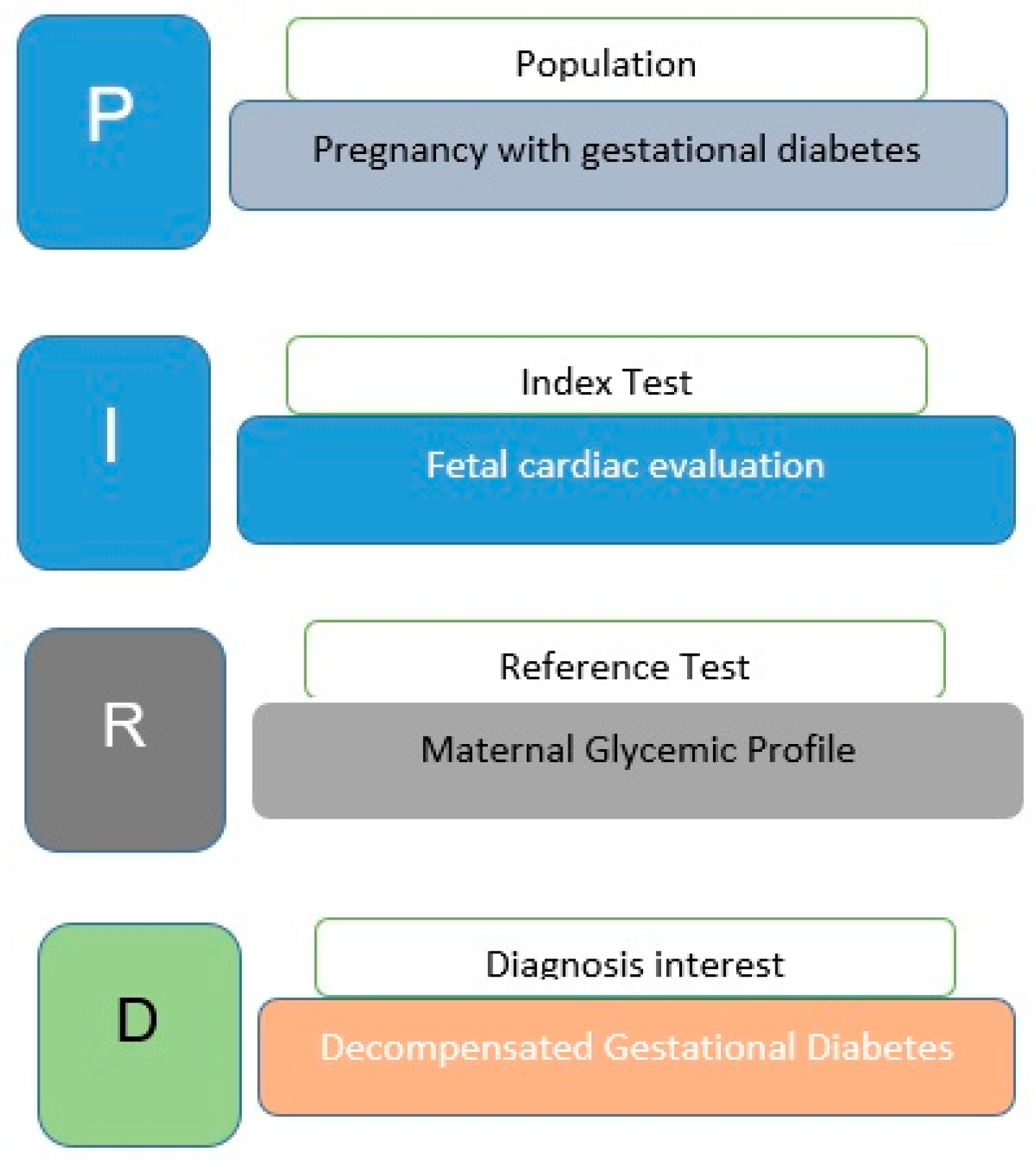
Figure 1: Review definition.
Details of search locations (databases), terms, and inclusion and exclusion criteria are shown in Table 1. The full search definitions for each database are shown in Table 2.
Table 1: Search definition.
| Search Location | Search Terms | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|
| PubMed/MEDLINE; Embase (Elsevier); CINAHL (EBSCO); Scopus (Elsevier); Web of Science (Clarivate Analytics); LILACS; SciELO | “Diabetes Gestacional” “Diabetes Induzida pela Gravidez” “Diabetes Induzida por Gravidez” “Diabetes Mellitus Gestacional” “Diabetes Inducida por el Embarazo” “Diabetes, Gestational”[Mesh] “Pregnancy-Induced Diabetes” “Gestational Diabetes” “Gestational Diabetes Mellitus” “Diabetes Mellitus” “C Peptide” “Feto” “Estruturas Fetais” “Fetos”“estructuras fetales” “Fetus”[Mesh] “Fetus” “Fetuses” “Fetal Structures” “Fetal Structure” “Coração” “Heart” “Cardiac “Livers” “Ultrasound” “Ultrasonography” | Articles that use fetal cardiac malformation to evaluate fetuses of pregnant women with gestational diabetes studies performed in human Any diabetes types Studies published between 2018–2023 Diagnostic method: ultrasound or fetal echocardiography | Articles that do not evaluate cardiac malformation studies not performed in human Review articles Articles not written in English, Portuguese or Spanish Articles that were not completely available on the database |
Table 2: Search definitions by database.
| Database | Date | Filters | Papers | Search Strings |
|---|---|---|---|---|
| PubMed/MEDLINE | 2018–2023 | Any formats | 75 | (“Diabetes, Gestational”[Mesh] OR” Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus” OR “C Peptide”) AND (“Fetus”[Mesh] OR “Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure”) AND (“Heart”[Mesh] OR “Heart” OR “Livers”) AND (“Ultrasound” OR “Ultrasonography”) |
| Embase (Elsevier) | 2018–2023 | Medicine | 33 | (“Gestational Diabetes” OR “Diabetes Mellitus”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure”) AND (“Heart”) AND (“Ultrasound” OR “Ultrasonography”) |
| CINAHL (EBSCO) | 2018–2023 | Any formats | 5 | (“Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus” OR “C Peptide”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure”) AND (“Heart” OR “Hearts”) AND (“Malformation”) |
| Scopus (Elsevier) | 2018–2023 | medicine | 160 | (“Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus” OR “C Peptide”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure”) AND (“Heart” OR “Hearts”) AND (“Malformation”) |
| Web of Science (Clarivate Analytics) | 2018–2023 | Any formats | 6 | (“Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure”) AND (“Heart” OR “Hearts”) AND (“Malformation”) |
| LILACS | 2018–2023 | Any formats | 10 | (“Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus” OR “Diabetes Gestacional” OR “Diabetes Induzida pela Gravidez” OR “Diabetes Induzida por Gravidez” OR “Diabetes Mellitus Gestacional” OR “Diabetes Onducida por el Embarazo”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure” OR “Feto” OR “Estruturas Fetais” OR “Fetos” OR “Estructuras Fetales”) AND (“Heart” OR “Corazon” OR “Malformation”) |
| SciELO | 2028–2023 | Any formats | 3 | (“Pregnancy-Induced Diabetes” OR “Gestational Diabetes” OR “Gestational Diabetes Mellitus” OR “Diabetes Mellitus” OR “Diabetes Gestacional” OR “Diabetes Induzida pela Gravidez” OR “Diabetes Induzida por Gravidez” OR “Diabetes Mellitus Gestacional” OR “Diabetes Inducida por el Embarazo”) AND (“Fetus” OR “Fetuses” OR “Fetal Structures” OR “Fetal Structure” OR “Feto” OR “Estruturas Fetais” OR “Fetos” OR “Estructuras Fetales”) AND (“Heart” OR “Corazon” OR “Malformation”) |
The systematic literature review proposal was submitted to the PROSPERO platform—International prospective register of systematic reviews, 2023 (available at https://www.crd.york.ac.uk (accessed on 24 February 2025)) under the registration number: CRD42024468567, prepared according to the recommendations of the Cochrane Collaboration and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17] (Fig. 2).
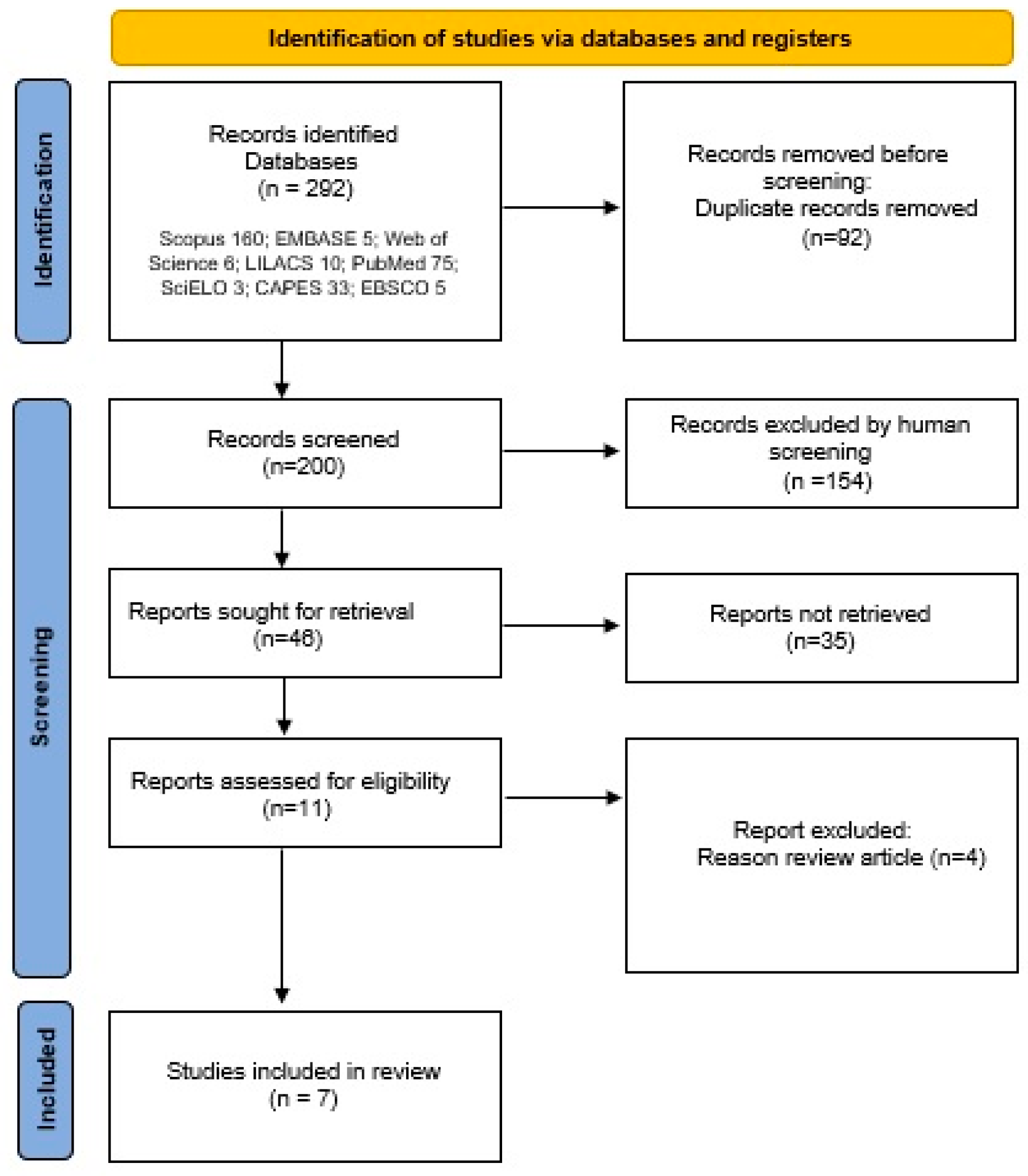
Figure 2: PRISMA flow diagram.
On 19 October 2023, an initial search yielded 292 articles, which were then exported to Rayyan QCRI1 (available at https://rayyan.ai (accessed on 24 February 2025)). Subsequently, 92 duplicates were removed, and the remaining articles were analyzed by two expert reviewers. They assessed the titles and abstracts and applied inclusion and exclusion criteria to filter the papers. The reviewers then thoroughly reviewed the full texts and together selected a total of 7 articles between 2018 and 2023. These steps are shown in Fig. 2.
It appears that the research was narrowed down to a select few papers after applying inclusion/exclusion criteria and identified some challenges in the literature. Specifically, a limited number of small studies were found on fetal CHD changes identified by ultrasound in pregnant women with diabetes mellitus. In addition, the research papers identified vary in their methodologies for assessing fetal CHD and diagnosing diabetes mellitus in gestation.
Given these challenges, it’s important to carefully evaluate the methods and results of each paper in order to draw meaningful conclusions. Consider factors such as sample size, study design, ultrasound techniques used, and criteria for diagnosing diabetes mellitus. By critically analyzing these aspects, you can better understand the current state of knowledge regarding fetal CHD changes in diabetes mellitus and identify potential areas for further research or refinement of diagnostic approaches.
Raafat et al. [11] compared cardiac structure and function in fetuses of diabetic and non-diabetic mothers using fetal echocardiography. Measurements included cardiac thickness and myocardial performance index. Postnatal follow-up by specialists ensured ongoing assessment of heart health. The study recommended comprehensive prenatal cardiac screening of diabetic mothers because of the increased risk of cardiac dysfunction (higher myocardial performance index) and hypertrophic cardiomyopathy. Early detection allows for proactive care planning after birth, potentially improving health outcomes for these newborns.
Joshi et al. [12], a hospital-based retrospective review, analyzed the medical records of 324 fetuses to examine the reasons for fetal echocardiography and its rate of detection of cardiac problems prenatally. Several echocardiographic techniques were used to assess cardiac structure and function. The most common indication was maternal GDM. Fetal echocardiography, which was becoming increasingly common, was a reliable prenatal diagnostic tool for detecting heart problems that were critical to improving neonatal health outcomes. It is widely used in pediatric cardiology and awareness of its benefits is essential. Despite its limitations, it’s recognized as vital in resource-limited settings like Nepal, improving early detection and treatment of CHD.
Wang et al. [18] compared fetal cardiac growth in pregnant women with and without GDM using fetal echocardiography at various stages of late pregnancy. A mixed model analysis adjusted for covariates identified differences between the GDM and control groups. GDM did not affect left heart growth in late pregnancy, but it did affect the right heart, with significant differences observed. This suggests that GDM may affect structural and functional growth of the right heart in late pregnancy.
Bogo et al. [19] performed echocardiography examinations of newborns of mothers with GDM to ensure consistency with the same observer. Three measurements per examination were averaged for analysis. Newborns of diabetic mothers had thicker heart muscles before delivery, but this decreased after birth. Heart function improved after birth. Careful management of GDM during pregnancy may reduce its effects on the heart health of newborns.
Aguilera et al. [20] recruited 73 women with GDM and 73 with uncomplicated pregnancies and performed fetal heart scans at 35–36 weeks’ gestation followed by postnatal echocardiograms. Advanced imaging techniques were used to assess myocardial deformation rates. The effects of maternal diabetes treatment on offspring cardiac health were analyzed. Newborns of mothers with GDM had rounder hearts with weaker squeezing action during late pregnancy and infancy, along with a reduced ability to relax between beats and pump blood effectively. The type of treatment for gestational diabetes did not affect these differences, suggesting potential long-term negative effects on the heart health of offspring.
In a cross-sectional study conducted by Reza Alipour et al. [10], 114 pregnant women were assessed by fetal echocardiography. Participants were referred based on international guidelines, ensuring that those at risk were screened. Most pregnant women referred for fetal echocardiography had diabetes mellitus; while most newborns had normal hearts, some had heart problems or abnormal rhythms. The study highlighted the effectiveness of fetal echocardiography in the prenatal detection of heart problems.
Sharma et al. [8] in a descriptive cross-sectional study aimed to determine the prevalence of abnormal fetal echocardiography in diabetic pregnant women. Fetal echocardiography was performed primarily at 22–32 weeks for GDM and 24–26 weeks for pre-existing diabetes mellitus. Of 104 GDM mothers, 15.38% had abnormal fetal echocardiographic findings. Significant differences were noted between GDM and pre-existing diabetes mellitus, with specific heart defects observed, including tetralogy of Fallot, single ventricle and tricuspid atresia. Ventricular septal defects were observed in GDM and pre-existing diabetes mellitus with higher prevalence in pre-existing diabetes mellitus. Table 3 summarizes the main findings of the included studies.
Table 3: Evaluation of congenital heart disease parameters.
| Reference, Year, Country | Study Design | Standard Diagnostic/Reference Guideline | n | Common Abnormalities | Contributions | Study Limitations |
|---|---|---|---|---|---|---|
| Sharma and Tiwari, 2020, Nepal | Prospective cross-sectional study | Fetal echocardiography in diabetic pregnancies | 104 diabetic pregnant women | Fetuses of diabetic mothers may have echogenic foci, septal defects, great vessel changes, and hypertrophic cardiomyopathy, which may result in intrauterine death | Study highlights early detection of diabetes for better maternal-fetal health and addresses cardiac issues such as ventricular septal defect, and aortic stenosis management | Limited sample size may affect generalizability of findings. Long-term postnatal follow-up data needed for comprehensive findings |
| Reza Alipour et al., 2022, Iran | Prospective cross-sectional study | Fetal echocardiography | 114 pregnant women (36.8% diabetic women) | Premature atrial contraction, ventricular septal defect, congestive heart failure, complete heart block, atrioventricular septal defect, hypoplastic left heart syndrome, tetralogy of Fallot, aortic stenosis | Article highlight’s role of fetal echocardiography in early diagnosis of CHD, impact of maternal disease, validation of prenatal accuracy, and importance of early detection | The study’s single hospital focus, cross-sectional design, selective echocardiography criteria, and limitations in ventricular septal defect detection sensitivity limitations affect generalizability and comprehensiveness |
| Aguilera et al., 2020, England | Prospective longitudinal study | Fetal cardiac scans | 73 diabetic and 73 non-pregnant women | More globular right ventricles, reduced right global longitudinal systolic strain, left global longitudinal systolic strain | Gestational diabetes mellitus has been associated withalterations in fetal cardiac function and structure compared tocontrols and persistent cardiac changes in infancy | Study limitations: potential bias from early heart checks, lack of effect of fasting blood glucose data, reliance on single heart movement assessment |
| Bogo et al., 2020, Brazil | Retrospective cohort study | Echocardiographic data | 48 diabetic pregnant women | Common abnormality is hypertrophic cardiomyopathy, myocardial performance index, change in the ratio of mitral and tricuspid E/A waves | Study highlights potential cardiac complications in newborns of diabetic mothers and emphasizes postnatal echocardiographic screening for early detection despite well-controlled maternal diabetes. Long-term implications require continued surveillance | Study limitations: small sample size limits generalizability, focus on insulin-treated pregnant women, retrospective design affects reliability of data |
| Wang et al., 2022, China | Prospective longitudinal study | Fetal echocardiography or 1hPPG ≥180 mg/dL or 2hPPG ≥153 mg/dL) | 63 gestational diabetes mellitus, 67 healthy pregnant | Right ventricle and TAPSE show significant differences and interaction in gestational diabetes mellitus versus control groups, suggesting structural and functional changes in gestational diabetes mellitus-affected hearts | Gestational diabetes mellitus affects fetal heart growth, particularly the right side, in late pregnancy. Echocardiographic assessment shows significant differences, suggesting an effect of gestational diabetes mellitus on fetal right heart development | Study lacks analysis of effects of gestational diabetes mellitus treatment on fetal heart development and only examines late pregnancy, leaving uncertainties about earlier effects and long-term effects |
| Joshi et al., 2019, Nepal | Retrospective cross-sectional study | Fetal echocardiography | 324 fetuses, 30.1% from gestational diabetic mothers | Echogenic intra-cardiac foci, isolated ventricle septal defect | Study highlights fetal echocardiography for prenatal diagnosis of congenital heart disease, especially in cases of gestational diabetes, advocating early evaluation and global comparative analysis to improve management | The study’s single hospital setting, limited cases, potential biases, and retrospective design limit generalizability, comprehensive understanding, and long-term follow-up of fetal echocardiography and congenital heart disease |
| Raafat et al., 2020, Egypt | Prospective cross-sectional study | Fetal echocardiography | Study compared 40 controlled diabetic, 60 non-diabetic mothers | Higher myocardial performance index and hypertrophic cardiomyopathy (thicker interventricular septum, and myocardial walls) in diabetic mellitus group | Study finds higher risk of heart problems in babies of diabetic mothers, suggesting prenatal heart screenings for early detection and intervention | Limitations: small sample size, exclusion criteria bias, lack of prediction of postpartum symptoms, requires postpartum follow-up for clinical assessment |
The studies presented provide valuable insights into the impact of maternal diabetes, particularly GDM, on fetal cardiac development. They underscore the importance of fetal echocardiography as a vital prenatal diagnostic tool for detecting cardiac problems, which is critical for improving neonatal health outcomes. However, they also highlight several challenges and variations in methodology, sample size, and diagnostic criteria that require careful evaluation to draw meaningful conclusions.
Each study used different echocardiographic/cardiac ultrasound examination parameters to assess fetal cardiac development in the context of maternal diabetes mellitus. In a prospective cross-sectional case-control study, Raafat et al. [11] used fetal echocardiography to compare cardiac structure and function in newborns of diabetic and non-diabetic mothers, highlighting the importance of comprehensive prenatal cardiac screening for newborns of diabetic mothers. The authors observed a higher myocardial performance index and hypertrophic cardiomyopathy in the diabetes group compared to controls. Joshi et al. [12] conducted a hospital-based retrospective review analyzing fetal medical records, highlighting the reliability of fetal echocardiography in detecting cardiac problems prenatally. In this study, the most common indication was GDM (30.31%). Limitations of the study include its single-center, retrospective design and lack of long-term postnatal follow-up. Ventricular septal defects, anomalies of the great arteries and hypertrophic cardiomyopathy are the most common heart defects found in the studies described previously. Wang et al. [18] compared fetal heart growth in pregnant women with and without GDM and found differences in left and right heart growth, suggesting potential effects of GDM on structural and functional development. This study showed an effect of gestational diabetes mellitus on fetal right heart development from echocardiographic assessment. However, it only assessed late pregnancy, leaving uncertainties about early and long-term effects. Bogo et al. [19] and Aguilera et al. [20] examined cardiac examinations and fetal heart scans in newborns of mothers with GDM and found differences in heart structure and function, suggesting potential long-term adverse effects on offspring heart health. Bogo et al. [19] focused-on insulin-treated pregnant women, whereas Aguillera et al. did not have data on fasting blood glucose. The two studies found changes in the heart function of the fetuses of diabetic mothers that persisted into the postnatal period.
In addition, Sharma and Tiwari [8] and Reza Alipour et al. [10] conducted cross-sectional studies evaluating pregnant women using fetal echocardiography, highlighting the efficacy of prenatal detection of cardiac problems in diabetic pregnant women. Sharma and Tiwari [8] included 104 diabetic pregnant women, however, in Reza Alipour et al.’s [8] study, 36.8% of 114 pregnant women were diabetic. Despite these differences, the two studies taken together contribute to our understanding of the complex relationship between maternal diabetes mellitus and coronary heart disease in the fetus. Limitations of these studies include the limited sample in single-center and lack of long-term postnatal follow-up.
The following meta-analyses are worth mentioning Papazoglou et al. [21] and Zhang at al. [22] although being published before the search period of this review, are noteworthy in relation to this topic. Papazoglou et al. [21] described, similarly to the studies included in this review, that maternal DM increases the risk of CHD compared to the general population. In addition, these authors observed a higher association of pregestational DM with CHD compared with gestational DM, emphasizing that preconception care is crucial to reduce the adverse effects of hyperglycemia on cardiogenesis. Zhang et al. [22] observed a heightened rate of congenital cardiac and extracardiac anomalies in the conceptuses of women with diabetes. Similarly to Papazoglou et al. [21], these researchers noted that the incidence of these malformations was higher in pregestational DM than in gestational DM. It is therefore important to emphasize that screening for diabetes in pregnant women can lead to enhanced glycemic control and facilitate the identification of fetuses at risk of congenital anomalies.
However, challenges such as small sample sizes, variations in diagnostic criteria, and the retrospective nature of some studies limit the generalizability and reliability of the findings. For example, small sample sizes such as Sharma and Tiwari [8] and Bogo et al. [19] may limit the statistical power and generalizability of the findings. In addition, variations in diagnostic criteria for diabetes diagnosis and assessment of fetal CHD may result in inconsistencies in findings between studies [11,19,20]. Finally, the retrospective nature of some studies may introduce biases and limitations in data collection and analysis [11,19]. It’s important to consider these challenges and variations when interpreting results and drawing conclusions.
4.3 Implications and Future Directions
Despite these challenges, the studies provided valuable insights into the impact of maternal diabetes on fetal cardiac development and highlight the importance of early detection and management. It is recommended that future research endeavors concentrate on standardizing the echocardiographic parameters to be evaluated during the fetal heart examination from diabetic mothers. Furthermore, it is advised that the study samples be increased and the long-term results analyzed to achieve a more complete comprehension of the impact of maternal diabetes on fetal heart health. Collaboration between researchers, clinicians and policymakers is imperative in addressing these challenges and improving outcomes for at-risk newborns. The analysis of the results of each study will facilitate a more comprehensive understanding of the current state of knowledge regarding changes in fetal heart disease in maternal diabetes and the identification of potential areas for research or for improving diagnostic approaches.
These studies highlight the impact of maternal diabetes, particularly GDM, on fetal cardiac development and emphasize the role of fetal echocardiography in early detection. Despite differences in the parameter assessed by the cardiac examination and sample size, they collectively advance our understanding of this complex relationship. Addressing challenges such as small sample sizes and standardization of diagnostic criteria is critical for future research. Collaboration among stakeholders is essential to improve management and outcomes for at-risk infants. Further studies should prioritize standardization and long-term outcome assessment to refine our understanding of the impact of maternal diabetes on fetal cardiac health.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Roberto Noya Galluzzo, Edward Araujo Júnior. Data collection: Roberto Noya Galluzzo, Karine Souza Da Correggio. Analysis and interpretation of results: Heron Werner, Nathalie Jeanne Bravo-Valenzuela, Aldo von Wangenheim. Draft manuscript preparation: Roberto Noya Galluzzo, Karine Souza Da Correggio, Heron Werner, Nathalie Jeanne Bravo-Valenzuela, Edward Araujo Júnior, Alexandre Sherlley Casimiro Onofre. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material (PRISMA 2020 Checklist) is available online at https://doi.org/10.32604/chd.2025.063014.
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. [Google Scholar]
2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–8. [Google Scholar]
3. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8(11):639–49. [Google Scholar]
4. Capobianco G, Gulotta A, Tupponi G, Dessole F, Pola M, Virdis G, et al. Materno-fetal and neonatal complications of diabetes in pregnancy: A retrospective study. J Clin Med. 2020;9(9):2707. [Google Scholar]
5. Schaefer-Graf U, Napoli A, Nolan CJ, Diabetic Pregnancy Study Group. Diabetes in pregnancy: A new decade of challenges ahead. Diabetologia. 2018;61(5):1012–21. [Google Scholar]
6. Leese B. Diabetes Mellitus and the St Vincent Declaration: The economic implications. PharmacoEconomics. 1995;7(4):292–307. [Google Scholar]
7. Ashwal E, Hod M. Gestational diabetes mellitus: where are we now? Clin Chim Acta. 2015;451:14–20. [Google Scholar]
8. Sharma J, Tiwari S. Abnormal fetal echocardiography in diabetic pregnant women at a tertiary care hospital: a descriptive cross-sectional study. J Nepal Med Assoc. 2020;58(227):456–8. [Google Scholar]
9. Sun HY. Prenatal diagnosis of congenital heart defects: echocardiography. Transl Pediatr. 2021;10(8):2210–24. [Google Scholar]
10. Reza Alipour M, Moradi H, Mahdieh Namayandeh S, Majidpoure F, Pezeshkpour Z, Sarebanhasanabadi M. Abnormal findings in fetal echocardiography and maternal disease: a cross-sectional study. Int J Reprod Biomed. 2022;20(5):405412. [Google Scholar]
11. Raafat M, Aborizk S, Saraya M, Soliman HH. Role of fetal echocardiography in morphologic and functional assessment of fetal heart in diabetic mothers. Egypt J Radiol Nucl Med. 2020;51:84. [Google Scholar]
12. Joshi A, Shrestha RPB, Shrestha PS. Indications of fetal echocardiography and detection of congenital heart disease prenatally in tertiary care hospital. Kathmandu Univ Med J. 2019;17(67):195–200. [Google Scholar]
13. Aloqab FW, Almajed MR, Binsanad NA, Al Amer SR, Kalis NN. Maternal diabetes as a teratogenic factor for congenital heart defects in infants of diabetic mothers. Birth Defects Res. 2023;115(7):764–9. [Google Scholar]
14. Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [Google Scholar]
15. Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. [Google Scholar]
16. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5. [Google Scholar]
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [Google Scholar]
18. Wang Y, Liu H, Hu X, Hu X, Zhang J, Zhang H, et al. The effect of gestational diabetes mellitus on fetal right heart growth in late-term pregnancy: a prospective study. Echocardiography. 2022;39(8):1101–12. [Google Scholar]
19. Bogo MA, Pabis JS, Bonchoski AB, Santos DCD, Pinto TJF, Simões MA, et al. Cardiomyopathy and cardiac function in fetuses and newborns of diabetic mothers. J Pediatr. 2021;97(5):520–4. [Google Scholar]
20. Aguilera J, Semmler J, Anzoategui S, Zhang H, Nicolaides K, Charakida M. Cardiac function in gestational diabetes mellitus: A longitudinal study from fetal life to infancy. BJOG. 2021;128(2):272–9. [Google Scholar]
21. Papazoglou AS, Moysidis DV, Panagopoulos P, Kaklamanos EG, Tsagkaris C, Vouloagkas I, et al. Maternal diabetes mellitus and its impact on the risk of delivering a child with congenital heart disease: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35(25):7685–94. [Google Scholar]
22. Zhang TN, Huang XM, Zhao XY, Wang W, Wen R, Gao SY. Risks of specific congenital anomalies in offspring of women with diabetes: a systematic review and meta-analysis of population-based studies including over 80 million births. PLoS Med. 2022;19(2):e1003900. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools