 Open Access
Open Access
REVIEW
Challenges in the Transition and Transfer of Young Adults with Congenital Heart Disease in Latin America and the Caribbean: The “Timeliness Principle”
1 Department of Pediatric and Adult Congenital Heart Disease, Somer Incare Cardiovascular Center & Somer Clinic, Rionegro-Antioquia, 054040, Colombia
2 Adult Congenital Heart Disease, National Autonomous University of Mexico, National Institute of Cardiology “Ignacio Chavez”, CDMX, 14080, Mexico
* Corresponding Author: John J. Araujo. Email:
Congenital Heart Disease 2025, 20(1), 61-75. https://doi.org/10.32604/chd.2025.062927
Received 31 December 2024; Accepted 21 February 2025; Issue published 18 March 2025
Abstract
Today, more than 90% of children who are born with congenital heart disease survive and reach adulthood, especially in developed countries. Consequently, the population of adults with congenital heart disease has increased significantly over the last few decades. In Latin America and the Caribbean countries, this same scenario is occurring at an accelerated pace. Loss to follow-up is a global problem in adults with congenital heart disease, ranging from 30–60%. In Latin America and Caribbean countries, it is estimated that less than 10% of adults with congenital heart disease are being followed. The small number of specialists and adult congenital heart disease specialized centers, as well as virtually nonexistent transition and transfer programs, are some of the reasons for this. This article is a narrative review of the current status of the transition and transfer of young adults with congenital heart disease, with a special focus on Latin America and Caribbean countries. It describes the general concepts of transition and transfer, analyzes barriers and, finally, presents specialized care alternatives that would reduce losses and improve this population’s care.Keywords
The growing number of adults with congenital heart disease (ACHDs) is a direct result of the successful survival of children undergoing congenital heart disease (CHD) repair. Over the past decades, and up to the beginning of the 90s, there were believed to be more children than adults with CHD, and moderate and complex CHD cases in adults were thought to be exceptional. However, with the development of pediatric cardiovascular surgery and cardiovascular intensive care units, the ratio has reversed. Today, in developed countries in America (United States and Canada), Central and Western Europe, and Oceania, as well as some Asian countries (Japan, South Korea, China, Singapore and Indonesia), there are more adults than children with CHD [1].
The changes in the demographic profile of ACHD that have occurred in developed countries are also occurring in Latin America (LATAM) and Caribbean countries. Today, more than 90% of children with CHD survive and reach adulthood and, as a result, the number of ACHDs living in LATAM and Caribbean countries has noticeably increased over the last decade [2].
Successful repair, recovery and rehabilitation of children born with CHD has been conducted well throughout childhood and adolescence by pediatric cardiologists, who are directly responsible for providing follow-up and treatment. However, in adulthood, this population ends up in a “dark area” where specialized CHD care has not been able to be carried out properly (losses to follow-up).
The new adult CHD population has a different cardiovascular profile than adult cardiologists are used to. Lack of knowledge regarding CHD, its natural course in adults, pathophysiology and changing clinical behavior are some of the things that prevent adult cardiologists from successfully providing appropriate ACHD follow-up.
On the other hand, the diseases acquired in adulthood modify the course of CHDs and are beyond the scope of pediatric cardiologists.
The lack of specialized ACHD centers and almost nonexistent transition and transfer programs complicate the picture even further. As a result of all these problems, young ACHDs are lost to follow-up, with all the repercussions which will occur sooner or later due to CHD decompensation.
2 Profile of Adults with Congenital Heart Disease in Latin America and the Caribbean, “the Timeliness Principle”
“Any child who survives to congenital heart disease surgery will become an adult with postoperative congenital heart disease.”
This phrase is indisputable and, ideally, 100% of children born with a CHD should be diagnosed promptly, and those who require surgical correction should also receive it promptly. So, having understood this point, we now know that the “timeliness principle” is the key element that marks the survival outcomes for CHD in a population and region.
The word “principle” comes from the Latin “principium,” meaning “beginning,” and “opportunity” means an opportune or convenient time or circumstance for something. Extrapolating these ideas, the “principle of opportunity” is defined as the perfect timing for diagnosing and providing specialized care for children and adults with CHD. When, for whatever reason, CHD care and follow-up do not occur at the opportune time, there will be immediate and delayed consequences (mortality and morbidity, respectively).
Congenital heart defects are the most common birth defect in the world. The prevalence in LATAM and the Caribbean is 8–13 children with CHD per 1000 live births [3]. More than 60,000 children with CHD are born each year. Of these, 45% have moderate and complex CHD, 25% of cases are severe and require urgent surgical or hemodynamic procedures. Without intervention, 14% and up to 30% die before one month and one year of age, respectively. In undeveloped countries, it is estimated that less than 10% of children with complex CHD receive timely care during their first year of life [4].
In countries with more resources, better healthcare systems and timely care, the outlook is better, achieving 90% survival. However, when repair is delayed, even with mild CHDs, almost 50% develop serious sequelae (pulmonary hypertension, heart failure, arrhythmias, among others). These, coupled with others comorbidities, as respiratory and gastrointestinal infections, malnutrition, parasitosis, tropical diseases. Increase child mortality. In Mexico, Torres-Cosme and cols, showed that CHDs accounted for 55% of all deaths from congenital anomalies in children under age one in 2013 [5].
Ultimately, all these children with repaired CHDs will become the new population of adults with postoperative CHD. But unlike developed countries with better opportunities, this population’s phenotype has a higher burden of morbidity.
Congenital heart diseases that should have been repaired with no sequelae now present in adults, with more hemodynamic repercussions (serious arrhythmias, stroke, valvular regurgitation, and sudden death, among others). It is also not surprising that there is a high number of unrepaired CHD cases of varying complexity diagnosed in developing countries.
All this is due to delayed availability of specialized care and late access to surgical repair in childhood, the “timeliness principle.”
In a general context, the ACHD population followed in specialized centers is derived from three groups:
a. CHDs that were repaired or underwent some palliative procedure during childhood or adolescence.
b. CHDs that did not require repair and have been followed since childhood.
These two groups account for 90% of the ACHDs followed at specialized centers [6].
c. Natural survival CHDs not diagnosed in childhood or adolescence. These are accepted to account for up to 10% of cases.
Based on regional experiences at the major cardiovascular centers in LATAM and the Caribbean that have specialized ACHD centers, we now know that 40% of the adults seen come from the pediatric setting. That is, childhood survivors of repaired CHDs and unrepaired CHDs followed to adulthood and now seen in specialized ACHD centers. Thirty percent consist of newly diagnosed adults (those who were never diagnosed in childhood or adolescence). While most (more than 60%) are simple CHDs, it is not surprising that the remaining 40% are unrepaired complex defects with serious hemodynamic sequelae.
Finally, the remaining 30% of adults consist of CHDs with previous partial repair or palliative procedures, having complex CHDs that needed to be repaired in two or three surgical stages (forms of univentricular heart, cyanotic CHDs), and which for some reason did not end in repair (lapses in care or follow-up) [7].
Estimated data show that there are more than 1.8 million ACHDs in South America and 657,000 in Central America and the Caribbean, and this population will grow by 5% to 6% annually.
Nowadays, in South America there are 2.3 million of children with CHDs, compared to 1.8 million ACHDs. Due to improved survival of children with CHD and centralization of ACHD, it is estimated that the number of ACHDs will equal that of children by 2030 (if child growth remains constant). Even more interesting is the estimate that between 2030 and 2040 the number of ACHD could exceed that of children with CHD similar to what has occurred in developed countries [8] (Fig. 1).
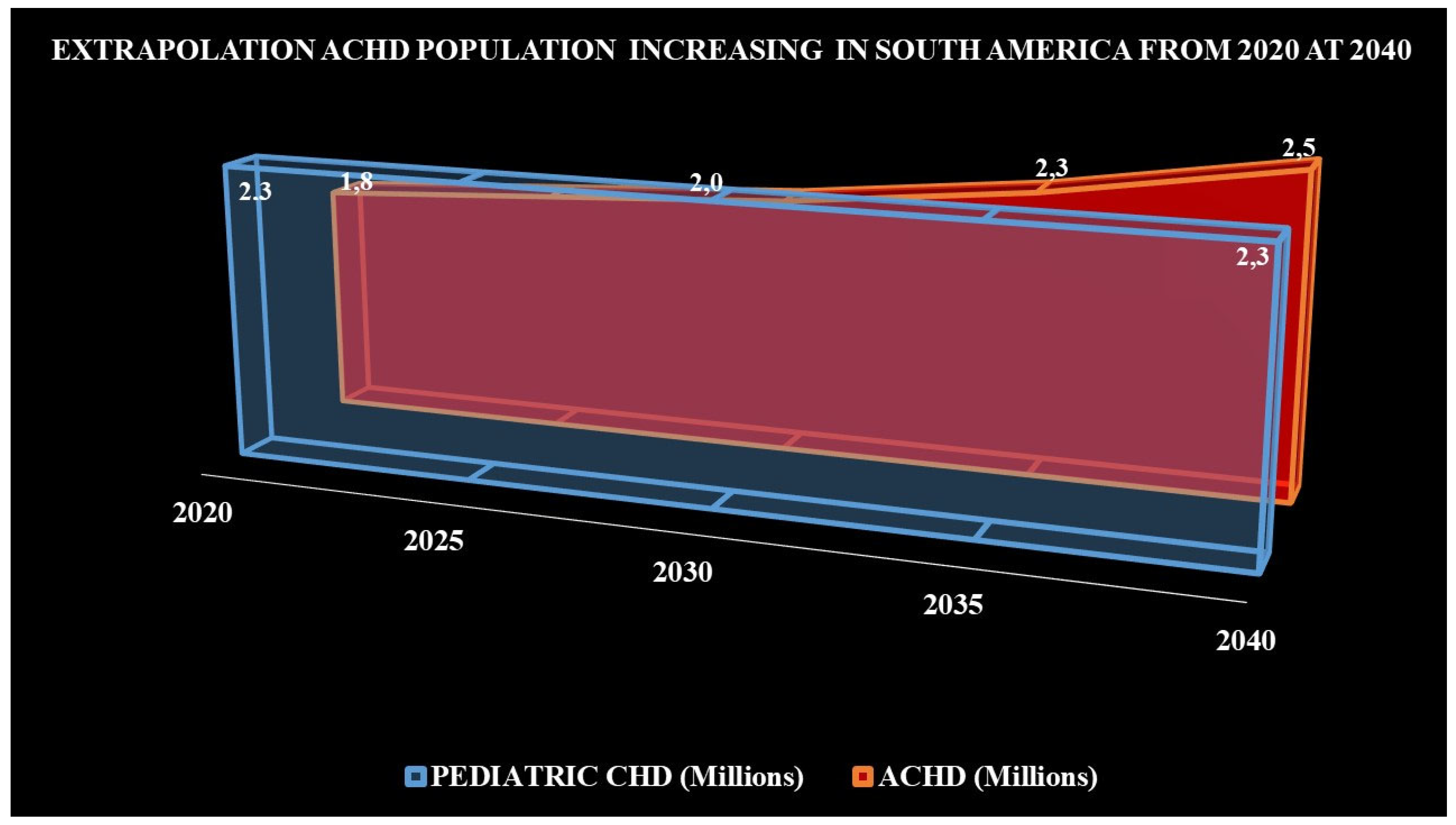
Figure 1: Projected growth of ACHD, from 2020 to 2040 in South America (Taken from Araujo J [8]).
Number (millions) of children with CHD (light blue color), if their growth were remained constant through the time. Number (millions) of ACHD (red color), with an annual growth of 5%–6%, their number could be similar to the pediatric population in 2030, and between 2035 and 2040, the number of ACHD could exceed that of children with CHD.
3 Development Adult Congenital Heart Disease Specialty in Latin America and Caribbean Countries
Due to complex of CHDs, we must understand the pathophysiology and natural evolution throughout life. Analyzing each particular case and making the right decisions. 50 years, only 10% children with complex CHDs survived, and little was known about natural evolution in adulthood [9]. Secondary to the successful repair CHDs in the pediatric population, the demographic profile of the survivors of CHD changed. In fact, ACHDs with simple defects continues to predominate throughout the world, but the number of ACHDs with complex defects grows every year. Nowadays we know that in Europe, 40% ACHDs with complex defects achieved a 60-year life expectancy [10].
Thanks to the success of care in ACHDs in developed countries, the population grew rapidly and the situation was reversed. There are now more adults than children with CHDs are, and a significant number with complex defects. Thus, in 2012, following a joint petition by the American Board of Pediatrics and the American Board of Internal Medicine, the specialty of ACHD was created, due to the need to provide specialized care to this new population [11].
In LATAM and the Caribbean countries, adults cardiologists have handled the ACHDs, traditionally simple CHDs, which survived until adulthood naturally. As explained before, we thought that moderate and complex congenital heart disease were a problem only in the pediatric population, so adult cardiology had no interest in learning about that. However, just as happened in developed countries, the demographic profile of ACHDs has changed at an accelerated pace. Thus, the complex defects began to increase in adults, and this attracted the attention of some pediatric and adult cardiologists who showed interest in following this population [12].
Currently, we know that the ACHD profile at the major cardiovascular centers in countries like Mexico, Colombia, Chile, Argentina and Brazil consists of moderate and complex ACHDs in more than 60% of cases [13].
Following the international guidelines for ACHD care [14], the ACHD specialty has begun to be officially implemented in LATAM and Caribbean countries over the last few years.
4 The Importance of Transition and Transfer Based on the Severity of Congenital Heart Disease
According to the 2001 Bethesda classification, repaired and unrepaired CHDs in adults were classified in simple, moderate and complex. Expert recommendations until a few years ago were:
Simple CHDs could be treated by general cardiologists
Moderate CHDs could be treated together with a cardiologist specializing in congenital heart defects
Complex CHDs could be treated only cardiologists specializing in ACHD [15]
However, this concept is now being challenged.
The American Heart Association/American College of Cardiology (AHA/ACC) Guidelines for congenital heart disease (CHD) in 2018 developed the anatomic and physiologic classification (APC) [(anatomy of repaired or unrepaired CHD integrate with American Heart Association (NYHA) functional class) and associated to other clinical variables (hypoxemia; pulmonary hypertension/pulmonary arterial hypertension; hemodynamically significant shunt; venous and arterial stenosis; exercise capacity; end-organ dysfunction; concomitant acquired valvular disease; arrhythmia; and aortopathy)]. The classification establishes four physiologic stages (Table 1). According to the guidelines, adults in APC: IB-D, IIA-D, and IIIA-D should be managed in collaboration with an ACHD cardiologist, with only those classified as IA being left to the care of general cardiologists [16,17].
Table 1: Physiological stage classification.
| A | - NYHA functional class (FC) I - no hemodynamic or anatomic sequelae - no arrhythmias - normal exercise capacity - normal renal/hepatic/pulmonary function |
| B | - NYHA FC II - mild hemodynamic sequelae (mild aortic enlargement, mild ventricular enlargement, mild ventricular dysfunction) - mild valvular disease - trivial or small shunt (not hemodynamically significant) - arrhythmia not requiring treatment - abnormal objective cardiac limitation to exercise |
| C | - NYHA FC III - significant (moderate or greater) valvular disease; moderate or greater ventricular dysfunction (systemic, pulmonary, or both) - moderate aortic enlargement - venous or arterial stenosis - moderate aortic enlargement - venous or arterial stenosis - mild or moderate hypoxemia/cyanosis - hemodynamically significant shunt - arrhythmias controlled with treatment - pulmonary hypertension (less than severe) - end-organ dysfunction responsive to therapy |
| D | - NYHA FC IV - severe aortic enlargement - arrhythmias refractory to treatment - severe hypoxemia (almost always associated with cyanosis) - severe pulmonary hypertension - Eisenmenger syndrome - refractory end-organ dysfunction |
| Adaptated from: Stout et al. [16]. |
5 Current State of Transition and Transfer of Adults with Congenital Heart Disease in Latin America and the Caribbean
Globally, the rate of ACHD losses to follow-up is 30–60%. This varies widely in the European Union countries, and in some countries, it is even lower than 10% (6.5% average), compared to the United States where it is 34% [18]. These differences are due to the implementation of very effective transition and transfer programs in Europe, which are still being developed in America.
The problem of losses to follow up begins, in most cases, during adolescence. The transition from pediatric to adult care is a critically vulnerable time for adolescents. During this stage of life, the adolescents’ physical and psychological changes cause them emotional stress. Many minimize or eliminate their symptoms, and they often stop taking medications or going to medical appointments.
During adolescence, losses to follow up exceed 70%. Wacker et al., at the German cardiac center in Munich, evaluated the rate and outcomes of ACHDs lost to follow-up. The patients were selected from the CHD program registry (n > 10,500). Loss to follow-up was defined as the inability of patients to return to follow-up visits at their center for more than five years. The investigators found that more than 76% of the patients were lost to follow-up [19].
The outlook for LATAM and the Caribbean countries is bleaker and, based on the previously explained observations, it is estimated that less than 10% of ACHDs are under specialized follow-up and, of these, more than 90% did not have a prior transition (Fig. 2).
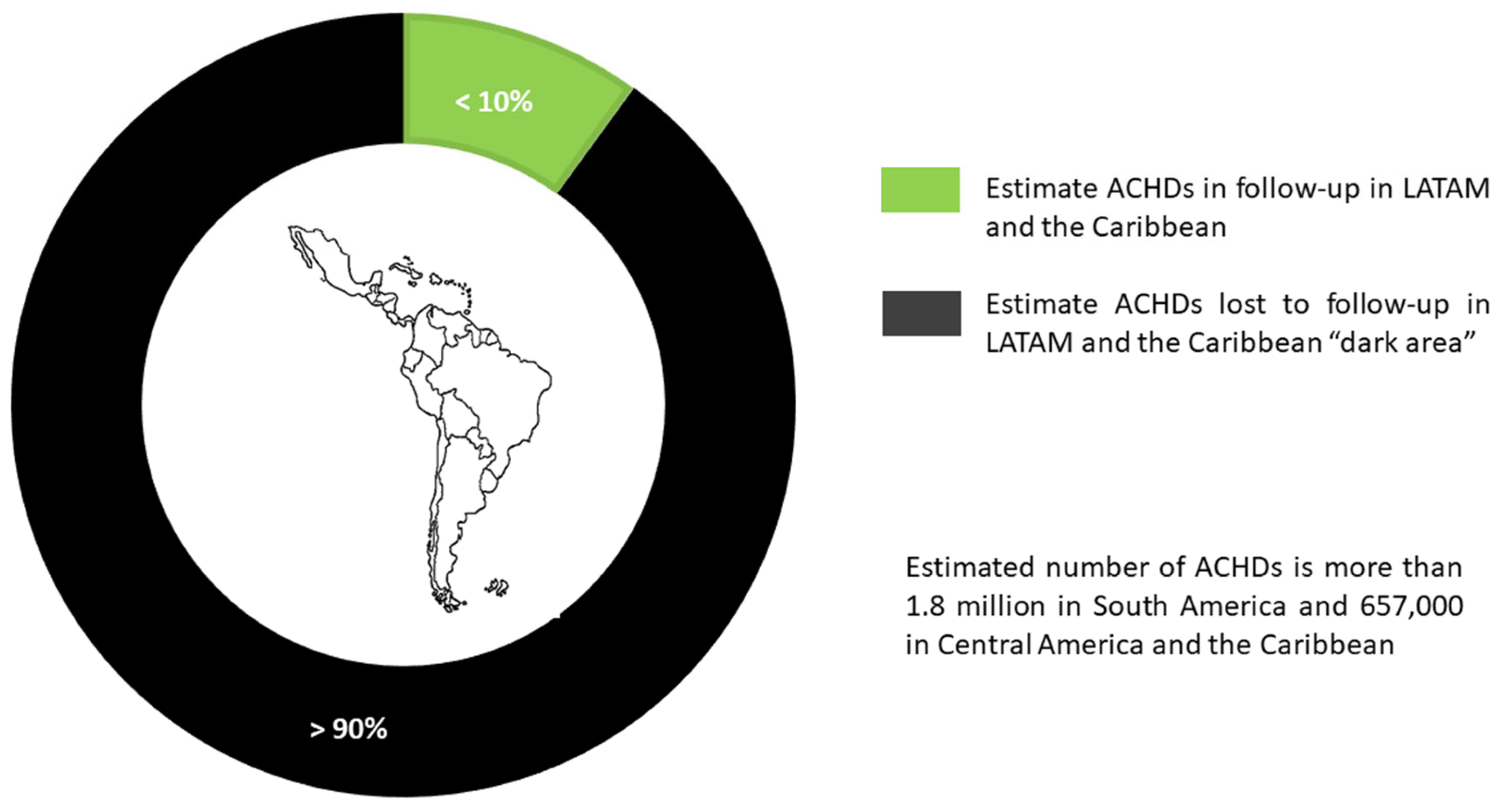
Figure 2: Current status of care for adults with congenital heart disease in Latin America and the Caribbean countries.
Efforts to develop transition and transfer programs have not shown very positive results yet. Studies in Argentina have shown that the rate of successful transition and transfer is less than 5% [20].
In Chile, Soto et al. showed that 30 to 40% of the young adolescents with CHD surveyed revealed gaps regarding their CHD, particularly the harmful effects of tobacco, alcohol or illegal drugs. A third of female patients stated that they did not know if pregnancy was safe, considering their CHD; 50% of the patients did not know how to find their medical specialist, and 57% stated that they did not know how their follow-up would be in the future [21].
These figures are concerning and a great challenge for the cardiologists responsible for following and managing ACHDs, as a large part of the work will consist in recovering the population lost to follow-up and improving outcomes. This will undoubtedly be a task for the cardiologists who are beginning to establish specialized ACHD centers in LATAM and the Caribbean.
6 General Concepts of Transition and Transfer
Transition, in CHDs, involves an active process of training and empowerment regarding the individual’s illness, to prepare him/her to leave parent-dependent pediatric care and advance to adult care. This stage is characterized by independence from parent or caregiver management, thus achieving an individual who is empowered regarding his/her illness and self-sufficient in managing his/her health care.
The most important and influential consensus on transition and transfer was published by Moons P, et al. in 2021 (“Transition to adulthood and transfer to adult care of adolescents with congenital heart disease: A global consensus statement”). Eleven scientific societies from around the world participated in this consensus [22].
The necessary and important concepts are summarized below, based on this consensus:
a. Adults with congenital heart disease: the population that has reached adulthood (over 18 years of age) and has some congenital heart disease (regardless of its complexity), either repaired or unrepaired.
b. Transition: a step from one stage of life, physical condition or social role to another, resulting in a temporary disconnection from the normal way of life, which requires an adjustment in the person and his/her surroundings.
The Society of Adolescent Medicine defined the standard of transition as “a purposeful, planned process that addresses the medical, psychological and educational/vocational needs of adolescents and young adults with chronic physical and medical conditions as they move from child-centered to adult-oriented health care systems” [23]. According to the American Academy of Pediatrics, the goal of this process is to optimize lifelong function through high-quality and uninterrupted services [24].
c. Timing of transition: the ideal time of life for a person with CHD to begin training and empowering activities regarding his/her disease. It should be personalized according to the individual’s current condition and functional capacity to understand the training instructions. According to the American College of Cardiology/American Heart Association guidelines on the management of adults with congenital heart disease, the transition process should start at 12 years of age in order to prepare the patient for the adult care provider by the late teenage years or the end of college.
d. Transfer: an event or series of events through which adolescents and young adults with chronic physical and medical conditions shift their care from a pediatrician to an adult physician.
For CHDs, transfer is the act of moving, shifting or changing from a pediatric CHD care setting to adult CHD care. In concrete terms, it is a conscious transfer from a pediatric to an adult area.
e. Transition program: a group of coordinated transition care interventions that are provided in a structured but personalized manner to support the process and achieve the transition outcomes.
f. Losses to follow-up: temporary lapses in patients’ medical care or follow-up. The inability of patients to return to specialized CHD follow-up appointments. These lapses may last for months or even years.
Yeung et al. found that 60% of the patients with a long lapse in care had a new hemodynamically significant diagnosis, which included hemodynamically significant valvular regurgitation (41%), obstructive lesions (21%), ventricular dysfunction (18%), new anatomical lesions (11%), pulmonary hypertension (5%) and obstructive coronary lesions (4%) [25].
The international guidelines for ACHD management and follow-up provide written recommendations according to the complexity of the CHD, with regular follow-up periods recommended depending on the patients’ clinical condition.
Taking these recommendations into account, loss to follow-up can be simplified as follows:
- simple congenital heart disease: lapses in monitoring or follow-up lasting more than five years.
- moderate heart disease: lapses in monitoring lasting more than two years.
- complex heart disease: lapses in follow-up or monitoring lasting more than six months to one year.
g. Follow-up program for adults with congenital heart disease: a set of healthcare, diagnostic, treatment, rehabilitation and care activities, as well as health promotion, healthcare education and disease prevention activities directed as specialized care to the ACHD population.
This also includes the concept of adult congenital heart disease units (ACHD Units).
h. Adult congenital heart disease units: specialized tertiary care centers with suitable professional staff, multidisciplinary teams, and physical and structural resources, able to respond to and provide solutions for the healthcare needs of ACHDs. An ACHD Unit is a place in which specialized ACHD care and follow-up programs are run.
7 Main Challenges for Latin America and the Caribbean
After a patient with CHD leaves pediatric care, he/she must be transferred to the ACHD follow-up program, and a proper transfer requires a prior adequate transition. This point is fundamental and critical for the successful follow-up of new ACHDs.
Although there are many different reasons that try to explain such high losses to follow-up, we now know that it is not enough to recognize that there is a serious problem; we also need to know how to deal with it, and what solutions can be proposed to reduce these losses.
The main recognized challenges include:
a. a lack of specialized adult congenital heart disease centers:
The centralization of adults with CHD in ACHD Units marks a big difference in follow-up and long-term outcomes. The ACHD Units have suitable professional staff, multidisciplinary teams, and physical and structural resources that can respond to and solve this population’s healthcare needs.
The development of ACHD Units is a challenge for LATAM and Caribbean countries, due to their high implementation cost and limited number of ACHD specialists. These units are being developed, and with their implementation the prognosis and survival of this population will no doubt change.
b. a lack of transition programs:
Just as there are few ACHD units, official transition and transfer programs are also scarce. Thus, these units are developing parallel to transition and transfer programs.
Globally, it is estimated that adolescents and emerging adults at a transition age account for 15 to 20% of the overall CHD population [26]. Considering the estimated number of children and ACHDs, we could infer that there are 615,000–820,000 adolescents and emerging adults at a transition age in South America.
c. training pediatricians in transitioning:
Another challenge is educating the pediatric cardiologists responsible for providing specialized care to understand that their job is not limited to medical follow-up, but rather should include a CHD educator role. This is a crucial point, since raising awareness in pediatric cardiologists to develop transition programs will result in successful transfer to ACHD Units.
d. a lack of specialists in adult congenital heart disease:
As explained previously, the clinical profile of adults with noncongenital cardiovascular disease is different from that of ACHDs, in which the underlying CHD is now combined with adult diseases. Therefore, these new cardiovascular patients need a trained cardiologist with all the necessary tools and knowledge to respond to their health problems.
e. separate cardiovascular hospitals:
This point refers to centers that only have pediatric cardiology, and not adult cardiology. Thus, when the patients conclude pediatric care, they are forced to find a hospital with the capacity to care for adults with CHD, and there is no proper transition process or formal transfer during this change of hospital.
f. economic barriers:
In general, LATAM and Caribbean countries have developing economies. Some are better established, like Chile, Mexico or Brazil. A nationally subsidized healthcare system will never be sufficient to meet the needs of CHD patients: the pediatric population on the one hand and new ACHDs on the other. Therefore, resource distribution must be analyzed in line with national and local health policies that can provide efficient solutions and responses. This is an even greater problem for the rest of the countries in the region, whose financial resources are limited and are unable to cover even the needs of the pediatric population alone. So, how to distribute resources for the new ACHD population is a question that remains unanswered.
Clearly, good cardiovascular health in the pediatric population will result in healthy adults and fewer patients. For CHDs, the timeliness principle would apply in care and repair. In other words, children cared for in a timely fashion and CHDs repaired promptly will result in postoperative adults in better conditions, which will reduce the burden of unrepaired complex CHDs, with fewer sequelae in adulthood.
g. conceptual barriers in congenital heart disease:
In past decades, it was believed that CHDs were exclusively pediatric conditions and their existence in adults was very rare and almost exotic. In America, from the 50s to the 80s, children who were born with complex CHDs had three possibilities:
- to be born with CHD and die due to its severity.
- to be born with CHD, survive naturally for a few years and later die from its hemodynamic repercussions.
- to be born with CHD, undergo surgical repair and survive.
In the United States, in the 70s, only 15% of children with complex CHDs survived to age 18, and very little was known about the natural course of the disease in adults. Therefore, the existence of ACHD was not considered in American countries, and CHD care was limited to surgical repair during childhood and discharge, with the idea that the patients were cured.
This mistaken concept of surgical cure is another reason that has led to losses to follow-up in CHD. Hundreds of thousands of successful pediatric surgical repair cases were discharged and not followed appropriately.
Changing the paradigm of cure in cardiologists from previous generations who are still in active practice is another challenge for LATAM and Caribbean countries, as it must be underscored that CHDs are not cured with surgery, but rather repaired.
h. social and cultural barriers:
This is not an easy point to deal with, due to cultural beliefs. Families’ mistrust of new cardiology professionals who will be responsible for following the new adults with CHDs continues to be a barrier between the family, the patient and the cardiologist, as, for years, the trusted cardiologist has been the pediatric cardiologist. Now, the family and patient must understand that the new adult needs to leave the pediatric setting. This causes uncertainty and apprehension in the family and new adult, so they prefer to abandon follow-up.
8 A Strategy of Specialized Care Models for Adults with Congenital Heart Disease to Reduce Losses to Follow-Up
We know that the levels of health care which can be provided for ACHDs are related to the level of complexity of the hospitals and cardiovascular centers, as well as the level of specialized training of the medical staff at these centers.
Knowledge and understanding of CHDs in adults requires a detailed and specific analysis for making treatment decisions, especially in complex cases. Pediatric cardiologists understand CHD and are familiar with the development of the heart and the behavior of CHD in childhood. However, the pathophysiological disorders in adulthood differ from those of the child. The inherent changes in the adult heart’s physiology make functional interpretations of the heart confusing for a pediatric cardiologist unfamiliar with the assessment of adults. Adults with congenital heart disease are mostly postoperative patients with anatomic and functional changes not only due to the prior surgical repair, but also due to the natural disease progression and to diseases acquired in adulthood. Disregarding the changes and evolution of an ACHD leads to treatment errors with serious repercussions. Likewise, adult cardiologists understand the adult semiological approach, anatomy, physiology, study interpretation, cardiovascular treatment and diseases. However, CHDs are a disease spectrum with which they are not familiar.
Generally, and in order of complexity, there are three levels of ACHD care (Table 2).
Table 2: levels of specialized care.
| Level | Activities | Strategy | |
|---|---|---|---|
| I | Basic | Cardiologists with basic adult cardiology training | Care be limited to following low-complexity ACHDs, and, if possible, that follow-up be shared with a pediatric cardiologist. This level of care must be able to decide when to refer these patients to specialized care. |
| II | Expert | Pediatric or adult cardiologists who have undergone some additional training in ACHD to acquire experience in managing the whole spectrum of patients | Care be limited to following low-complexity and some stable moderate-complexity ACHDs, and, as much as possible, that follow-up be shared with a pediatric cardiologist. |
| III | Specialist | Pediatric or adult cardiologists who have received complete ACHD training. | Responsible for caring for complex and decompensated moderate ACHDs, as well as for ACHD research and teaching. |
Traditionally, ACHDs who are followed in cardiology services have arrived with no transition process. This is known as the abrupt transfer model, which is nothing more than an arbitrary jump from pediatric CHD care to adult cardiology care which is not specialized in CHD. This care model is not the correct one.
In an ideal scenario, young ACHDs should have a progressive, staged specialized care model, consisting of the introduction to transition which generally begins in childhood, before age 12, followed by a pre-transition phase which occurs between 12–14 years of age. This process continues with the formal transition, which extends a little beyond the age of majority (18 years), usually two to three years more. Finally, the young adults are completely discharged to ACHD care. This is known as the programmed transfer model with a transition process (Fig. 3).
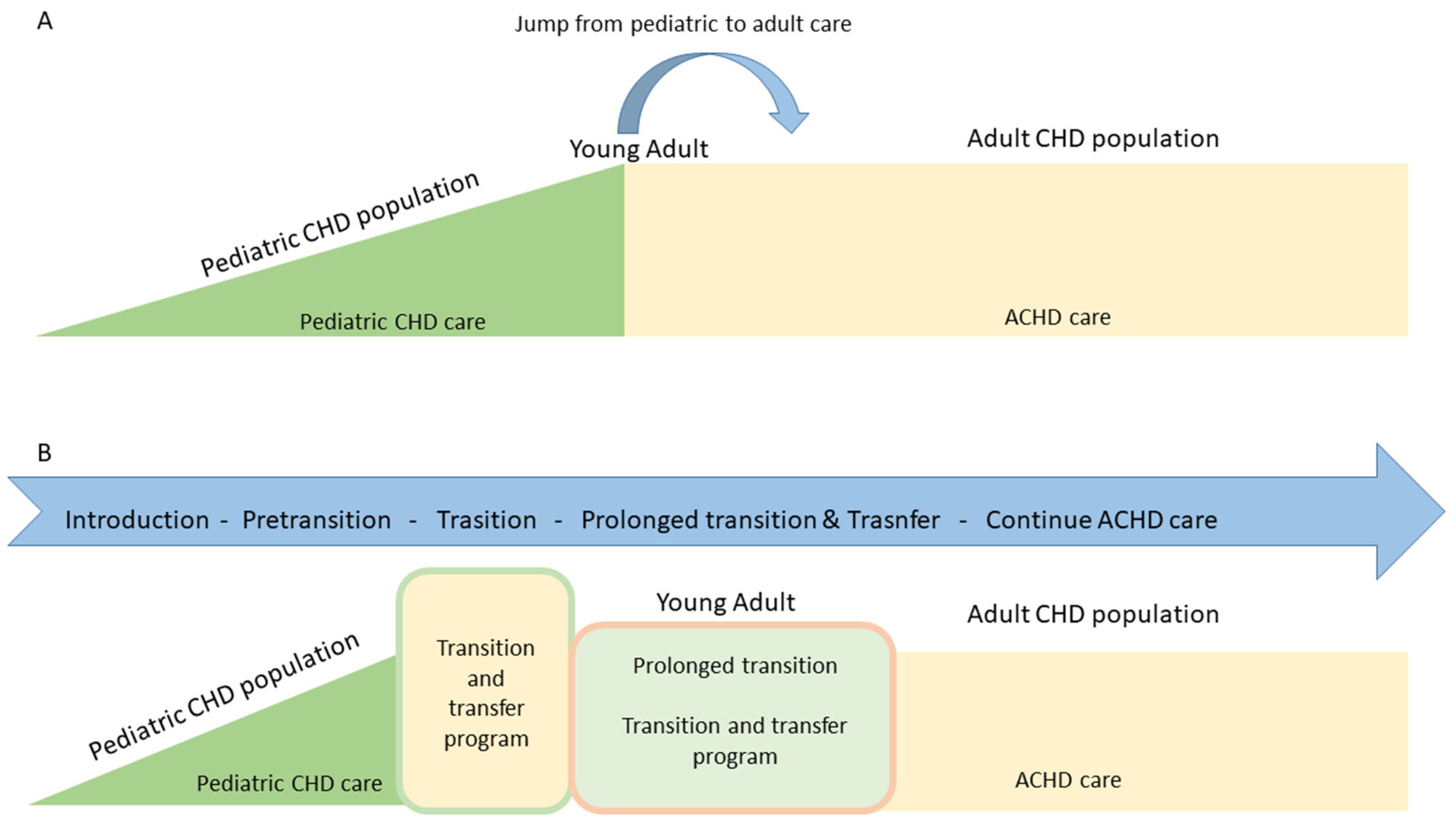
Figure 3: Models of care for adults with congenital heart disease. (A): abrupt transfer model, without transition process; (B): programmed transfer model, with transition process.
Meanwhile, until transition and transfer programs are developed parallel to ACHD Units, two alternative models of specialized care for young ACHDs are proposed which, while not ideal, do allow follow-up of young ACHDs to be maintained in LATAM and Caribbean countries.
A. The extended pediatric model: this ACHD care and treatment model involves activities for which the pediatric cardiologist is responsible, generally not extending beyond the third decade of life. Its objective is also to decrease losses to follow-up and strengthen transition and transfer.
B. The dual or shared model: this alternative involves simultaneous and shared activities between the pediatric and adult cardiologists. It generally begins in late adolescence and continues throughout the rest of the ACHD’s life. In addition to reducing losses, it can potentiate knowledge and support in ACHD management between the two specialists.
These alternative ACHD care models help provide a timely response to this population and reduce losses to follow-up (Fig. 4).
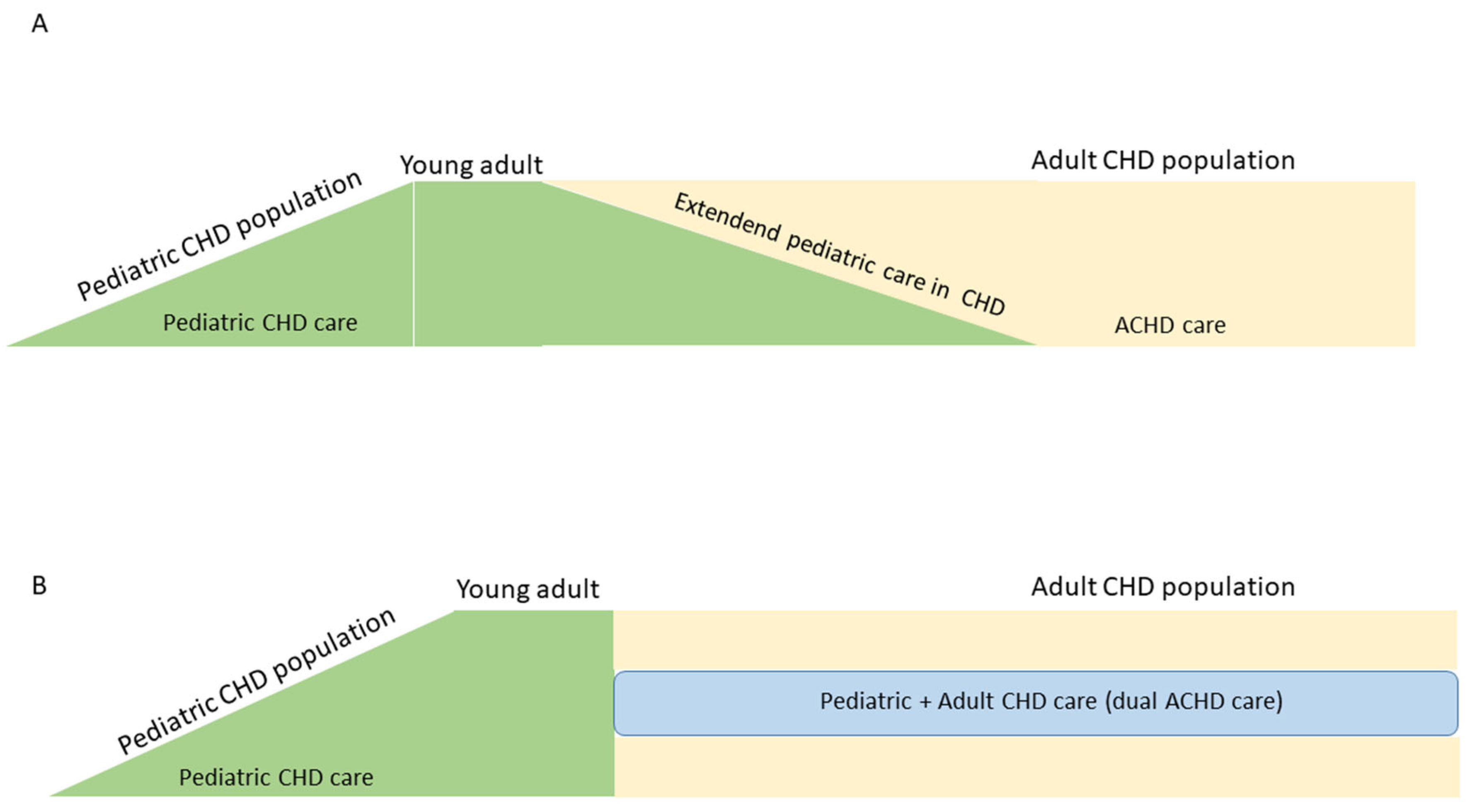
Figure 4: Alternative models of specialized care in adults with congenital heart disease. (A): extended pediatric model; (B): dual or shared model.
9 Strategies for Developing Transition and Transfer Programs
These programs are being developed in tandem with ACHD Units in LATAM and the Caribbean, and efforts over the last few years have focused on this point. The Interamerican Society of Cardiology (SIAC in Spanish) functions in this region, integrating 28 societies from the American continent. It is divided into 17 councils, with specialized work chapters within these councils [27].
Looking to face major challenges, the ACHD Chapter was founded in mid-2018, as part of the SIAC’s Pediatric Cardiology Council. This chapter consists of several CHD specialists from almost all countries in the continent and seeks to integrate all the cardiologists responsible for ACHD care. Its organizational structure also includes spokespeople or regional representatives in each country who are in direct contact with the chapter leaders [28]. Its main objectives are to:
a. promote educational and cooperative activities between the different countries in the continent.
b. foster ACHD research.
c. integrate and cooperate with other international ACHD care societies like the International Society for Adult Congenital Heart Disease (ISACHD) and the European Society of Cardiology Working Group on Adult Congenital Heart Disease (Euro ACHD).
The first guidelines for implementing ACHD Units were published in 2023 [29]. This strategy is helping to structure centers of excellence for ACHD care which are implementing advanced transition and transfer programs.
The first phase of the ACHD Unit development program is currently being implemented through a strategic program known as the “ROCK” pathways:
R: Recognize the need to organize and structure ACHD units
O: Organize to deal with great challenges
C: Centralize the facilities and specialists
K: Knowledge: create a multidisciplinary team of ACHD specialists and subspecialists. Foster the development of research and knowledge.
This program (currently being implemented) has provided an understanding of the ACHD reality of the hospitals and cardiovascular centers, and helped organize and classify them according to levels of complexity. Level III centers are those that have a duly structured ACHD Unit and a transition and transfer program. (Unpublished data) have identified:
Argentina: 4
Colombia: 3
Chile, Brazil, Mexico, and Dominican Republic: 1 each.
The second phase of the program will include a training course on transition and transfer (currently being structured).
The growing number of ACHDs and greater survival of this population around the world is a reality that is also occurring in LATAM and Caribbean countries. The lack of specialized ACHD centers and almost total absence of transition and transfer programs lead to high losses to follow-up. The barriers to transition and transfer must be addressed, but the definitive solutions to the problem are the development and implementation of transition and transfer programs, along with the development of ACHD Units. Until these objectives are achieved, hospitals in LATAM and Caribbean countries can implement specialized care alternatives to reduce and mitigate the repercussions.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study..
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The author declares that he is the founder and leader of the Adult Congenital Heart Disease Chapter of the Interamerican Society of Cardiology. The author has remained neutral in writing this article.
References
1. Gilboa S, Devine O, Kucik J, Oster M, Riehle-Colarusso T, Nembhard W, et al. Congenital heart defects in the United States estimating the magnitude of the affected population in 2010. Circulation. 2016;134(2):101–9. [Google Scholar]
2. Webb G, Mulder B, Aboulhosn J, Daniels J, Elizari A, Hong G, et al. The care of adults with congenital heart disease across the globe: Current assessment and future perspective: A position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2015;195:326–33. [Google Scholar]
3. Liu Y, Chen S, Zühlke L, Black G, Choy M, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48:455–63. [Google Scholar]
4. Van der Linde D, Konings E, Slager M, Witsenburg M, Helbing W, Takkenberg J, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. [Google Scholar]
5. Torres-Cosme J, Rolón-Porras C, Aguinaga-Ríos M, Acosta-Granado P, Reyes-Muñoz E, Murguía-Peniche T, et al. Mortality from congenital heart disease in Mexico: A problem on the rise. PLoS One. 2016;11:e0150422. [Google Scholar]
6. Ruiz J. Cardiopatías congénitas del adulto: residuos, secuelas y complicaciones de las cardiopatías congénitas operadas en la infancia. Rev Esp Cardiol. 2003;56(1):73–88. (In Spanish). [Google Scholar]
7. Araujo J. The New 2020 European society of cardiology guidelines for the management of adults with congenital heart disease: A Perspective of the interamerican adult congenital heart disease council of the interamerican society of cardiology (ACHDC-IASC). Ann Public Health Rep. 2020;4(1):108–14. [Google Scholar]
8. Araujo J. Adults with congenital heart disease in south america: where are we today and what will the immediate future look like? Ann Public Health Rep. 2024;8(1):328–31. [Google Scholar]
9. Perloff J. Pediatric congenital cardiac becomes a postoperative adult. The changing population of congenital heart disease. Circulation. 1973;47:606–19. [Google Scholar]
10. Van der Bom T, Mulder B, Meijboom F, Van Dijk A, Pieper P, Vliegen H, et al. Contemporary survival of adults with congenital heart disease. Heart. 2015;101:1989–95. [Google Scholar]
11. Stout K, Valente A, Bartz P, Cook S, Gurvitz M, Saidi A, et al. Task force 6: Pediatric cardiology fellowship training in adult congenital heart disease. Circulation. 2015;132:91–8. [Google Scholar]
12. Araujo J. Building an adult congenital heart disease unit in Colombia, a comprehensive model of specialized care for a growing population. Int Arch Public Health Community Med. 2023;7(1):088. 10.23937/2643-4512/1710088. [Google Scholar] [CrossRef]
13. Morós C, Otero M, Grippo M, Rubio M, Nicolosi L. Need for palliative, corrective, and second-corrective surgery in adult patients with congenital cardiopathy classified according to disease complexity. Int J Ssi Res. 2014;3(11):329–32. [Google Scholar]
14. Baumgartner H, De Backer J, Babu-Narayan S, Budts W, Chessa M, Diller G, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. [Google Scholar]
15. Webb G, Williams R. 32nd Bethesdaconference: Care of the adult with congenital heart disease. J Am Coll Cardiol. 2001;37:1161–98. [Google Scholar]
16. Stout K, Daniels C, Abulón J, Bozkurt B, Broberg C, Colman J, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494–563. [Google Scholar]
17. Araujo J. Units for transitioning pediatric cardiology to adult care with congenital heart disease: why, when and how? J Cardiol Catheter. 2019;1:37–45. [Google Scholar]
18. Moons P, Skogby S, Bratt E, Zühlke L, Marelli A, Goossens E. Discontinuity of cardiac follow-up in young people with congenital heart disease transitioning to adulthood: a systematic review and meta-analysis. J Am Heart Assoc. 2021;10(6):e019552. [Google Scholar]
19. Wacker A, Kaemmerer H, Hollweck R, Hauser M, Deutsch M, Brodherr-Heberlein S, et al. Outcome of operated and unoperated adults with congenital cardiac disease lost to follow-up for more than five years. Am J Cardiol. 2005;95(6):776–9. [Google Scholar]
20. Mouratian M, Villalba C, Ramos A, Lafuente M, Farall Y, Munzón M, et al. El adolescente con cardiopatía congénita: transición y transferencia. Med Infant. 2019;26(2):130–9. (In Spanish). [Google Scholar]
21. Soto M, Zubarew T, Arancibia-Galilea M. Adolescentes y jóvenes portadores de cardiopatías congénitas en etapa de transferencia a la atención médica de adultos. Revista Chilena Pediatr. 2020;91(3):339–46. (In Spanish). [Google Scholar]
22. Moons P, Bratt E, De Backer J, Goossens E, Hornung T, Tutarel O, et al. Transition to adulthood and transfer to adult care of adolescents with congenital heart disease: a global consensus statement of the ESC association of cardiovascular nursing and allied professions (ACNAP), the ESC working group on adult congenital heart disease (WG ACHD), the association for European paediatric and congenital cardiology (AEPC), the pan-african society of cardiology (PASCAR), the asia-pacific pediatric cardiac society (APPCS), the inter-American society of cardiology (IASC), the cardiac society of Australia and New Zealand (CSANZ), the international society for adult congenital heart disease (ISACHD), the world heart federation (WHF), the European congenital heart disease organisation (ECHDO), and the global alliance for rheumatic and congenital hearts (global ARCH). Eur Heart J. 2021;42(41):4213–23. [Google Scholar]
23. Said S, Driscoll D, Dearani J. Transition of care in congenital heart disease from pediatrics to adulthood. Semin Pediatr Surg. 2015;24:69–72. [Google Scholar]
24. Hays L. Transition to adult congenital heart disease care: a review. J Pediatr Nurs. 2015;30(5):e63–9. [Google Scholar]
25. Yeung E, Kay J, Roosevelt G, Brandon M, Yetman A. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol. 2008;125(1):62–5. [Google Scholar]
26. John A, Jackson J, Moons P, Uzark K, Mackie A, Timmins S, et al. Advances in managing transition to adulthood for adolescents with congenital heart disease: A Practical approach to transition program design: a scientific statement from the American heart association. J Am Heart Assoc. 2022;11(7):e025278. [Google Scholar]
27. Reseña y Mesa Directiva. Sociedad Interamericana de Cardiología [Internet]. [cited 2025 Jan 15]. Available from: https://www.siacardio.com/siac/. [Google Scholar]
28. Consejo de Pediatría. Sociedad Interamericana de Cardiologia [Internet]. [cited 2025 Jan 15]. Available from: https://www.siacardio.com/pediatria/. [Google Scholar]
29. Araujo J, Rodríguez-Monserrate C, Elizari A, Yáñez-Gutiérrez L, Mouratian M, Amaral F, et al. Position statement for the development of adult congenital heart disease units in Latin America and the caribbean: recommendations by the adult congenital heart disease chapter and pediatric cardiology council of the interamerican society of cardiology endorsed by the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2023;13:100461. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools