 Open Access
Open Access
ARTICLE
Predictors of In-Hospital Mortality and Survival Outcomes in a Paediatric Congenital Cardiac Cohort in South Africa—A 12-Year Review
1 Department of Anaesthesiology, University of the Witwatersrand, Johannesburg, 2193, South Africa
2 Department of Anaesthesiology, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, 2193, South Africa
3 Department of Cardiothoracic Surgery, Nelson Mandela Children’s Hospital, University of the Witwatersrand, Johannesburg, 2193, South Africa
4 Department of Anaesthesiology, Charlotte Maxeke Johannesburg General Academic Hospital, University of the Witwatersrand, Johannesburg, 2193, South Africa
* Corresponding Author: Palesa Mogane. Email:
Congenital Heart Disease 2025, 20(1), 41-53. https://doi.org/10.32604/chd.2025.060382
Received 30 October 2024; Accepted 24 February 2025; Issue published 18 March 2025
Abstract
Background: Congenital cardiac diseases (CCD) are common congenital birth defects that require high-risk surgery. Outcomes following congenital cardiac surgery in children living in high-income countries (HIC) have been documented, but little is known from the African continent. This study aimed to determine factors associated with perioperative mortality in patients who underwent congenital cardiac surgery at our institution. Methods: This retrospective, cross-sectional study was conducted at Charlotte Maxeke Johannesburg Academic Hospital over 12 years (2006–2017). A multivariable regression analysis was performed for the factors which had a p-value of 0.1 and less in the univariable regression analysis. A Cox regression analysis was performed for mortality over time. All hypothesis testing accepted a p-value of <0.05 as statistically significant. Results: There was an 11% (188/1701) in-hospital mortality rate overall. Patients who had a median age of 0.33 years (interquartile range [IQR] 0.13–1.25), weight of 5 kg (IQR 3.45–8.20), longer cardiopulmonary bypass (CPB) of 179 min (16.0–275.5) and aortic cross-clamp of 82.5 min (35–139) were at greater risk of in-hospital mortality. Palliative surgeries had the highest in-hospital mortality rate at 41%. Cardiopulmonary bypass time (p < 0.001), ICU length of stay (p < 0.001), aortic cross-clamp time (p = 0.001) and days on the ventilator (p < 0.001) were independently predictive of perioperative all-cause in-hospital mortality in a multivariable regression model. Current predictive scoring systems had inconsistent performance, with the STAT 2020 mortality score being predictive in a multivariable regression analysis (p = 0.038) and the RACHS-2 score in a Cox regression analysis (p = 0.002). Conclusions: Perioperative mortality following congenital cardiac surgery was higher in our study group compared to HICs. Future prospective studies are required to highlight the need to prioritise and invest in the declining services provided to children with CCD in South Africa’s public sector.Keywords
Supplementary Material
Supplementary Material FileCongenital cardiac diseases are the most common congenital birth defects, with a global incidence of four to eight cases per 1000 live births [1]. In South Africa, Hoosen et al. reported an estimated prevalence of 0.6 to 0.8 cases per 1000 live births, with approximately 11,000 children born each year with congenital heart disease [2]. Approximately 40% of these children require surgical interventions each year [2,3].
With the developments and advances in paediatric cardiac surgical services, several studies have reported good outcomes (90–95% survival to adulthood) in high-income countries (HIC) [4,5]. These reported advances in care are still not available for over 90% of children born with CHD in low- and middle-income countries (LMIC) [6]. Historically, South Africa and Egypt have had better cardiac services than most countries on the African continent. However, disparities still exist, with <40% of the South African population with CHD obtaining surgical management due to low access, particularly in the public sector, with an estimated 50 public cardiac patients per million, as compared to 595 private cardiac patients per million undergoing surgery annually in South Africa [7].
In HIC, perioperative mortality rates have decreased from 8.7% to fewer than 4% [8,9,10]. There is a paucity of perioperative outcome data for paediatric and neonatal congenital cardiac surgery from the African continent and other low- and middle-income countries [11] due to a lack of expertise and resources [12]. Many patients experience delayed referrals due to a lack of specialised centres to perform cardiac surgery, a shortage of trained specialists, high cost and inadequate investment in health [13]. Reports show that only 1–5% of children received care in LMICs [14,15]. The few studies published from upper-middle-income countries (UMIC), such as Brazil and China, reported higher morbidity and mortality than HICs [4,13].
Perioperative mortality is associated with the complexity of surgery and the volume of cases performed at the centre [16]. Surgery for CHD is highly complex and involves many anatomical and physiological variations, making it difficult to compare performance or outcomes among institutions. Thus, clinicians, primarily in North America and Europe, have used multi-institutional research databases to establish a standard system of nomenclature to stratify operative complexity and the effect this may have on outcomes [17]. The risk stratification tools used include the Risk Adjustment for Congenital Heart Surgery (RACHS-1) updated to RACHS-2 [17], the Aristotle Basic Complexity (ABC) levels, and the Society of Thoracic Surgeons (STS)–European Association for Cardio-Thoracic Surgery (EACTS) Congenital Heart Surgery Mortality categories and scores (STS-EACTS Mortality Score and Categories), commonly referred to as STAT Mortality Scores and Categories, updated to STAT 2020 scores and categories [18].
We embarked on a 12-year single-centre retrospective study to determine factors associated with in-hospital, all-cause mortality of paediatric patients presenting for congenital cardiac surgery at the Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) in Johannesburg, South Africa. This is the first report detailing perioperative outcomes in a South African public healthcare centre.
Approval to conduct the study was obtained from the Human Research Ethics Committee (Medical)(HREC) of the University of the Witwatersrand. Ethical principles governed by the South African Guidelines for Good Clinical Practice [19] and the Declaration of Helsinki [20] were strictly followed. This was a retrospective cross-sectional study of all consecutive patients under 18 who underwent congenital cardiac surgery at CMJAH for 12 years (01 January 2006 to 31 December 2017). Patients were excluded if records were incomplete (>50% of data was required; otherwise, the patient was excluded). Furthermore, patients older than 18 who underwent cardiac surgery other than congenital cardiac surgery, were also excluded (Fig. 1).
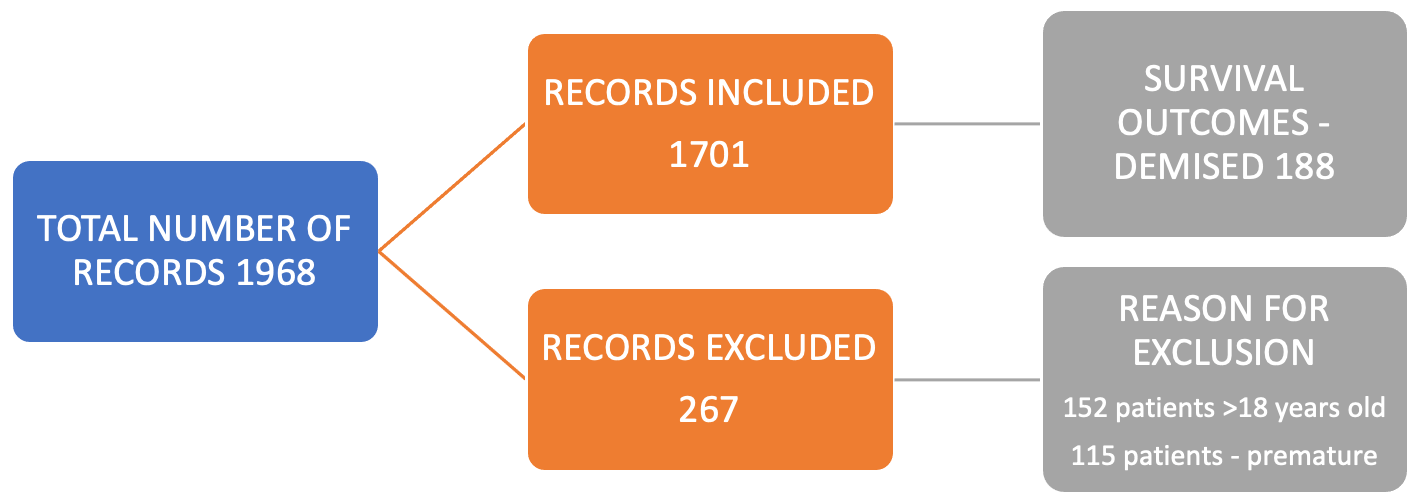
Figure 1: Details of records collected.
CMJAH is a 1088-bed quaternary hospital that serves the public sector. During the study period, CMJAH was one of only five major public centres in South Africa to offer comprehensive paediatric cardiac services [12]. The current study intended to use all available records. However, a minimum adequate sample size was still calculated.
The sample size was calculated as 289. Table S1 (sample size computation parameters) demonstrates all the factors and criteria used to determine the sample. Notably, the nature of the study allowed the researchers to use all available records, which was significantly higher than the minimum required sample size.
Each available patient record was assigned a study number, and the relevant variables were recorded on Microsoft® Excel for Windows. One author (PS) collected all data, and a second reviewer (KV, the originator of the database) assisted in minimising transcription errors from the database to an Excel spreadsheet. The following study definitions were used: paediatric patients—patients under 18 years; mortality—all-cause mortality that occurred during or after the completion of congenital cardiac surgery in-hospital within the same admission. Mechanical support included patients who required extracorporeal life support or ventricular assistive devices. The public sector is part of the healthcare sector controlled by the state or government. South Africa has a two-tiered and highly unequal healthcare system. The public sector caters to the majority (71%) of the population [21]. The private sector refers to that part of the healthcare sector run and controlled by individuals or companies. These usually function to make financial profit. This sector is funded by individual contributions to medical aid schemes or health insurance and serves around 27% of the population [21]. Other definitions are included in Table S2.
The collated data were analysed using STATA 16.0 software (STATA Corp. LP, College Station, TX, USA). Depending on the distribution, continuous variables were reported using means (standard deviation) or medians (interquartile range). Relationships between perioperative in-hospital mortality and variables such as patients’ characteristics and risk stratification scores (RACHS-2, ABC and STAT 2020) were compared using paired and unpaired Student’s t-tests for parametric data or Mann-Whitney and Wilcoxon matched pairs for nonparametric data. A multivariable regression analysis was performed for univariable regression analysis products with a p-value ≤ 0.1. A Cox regression analysis was performed for mortality over time. A p-value of <0.05 was accepted as significant.
The time effect in this study might have resulted in a bias in that events and outcomes of interest were time-related. However, the bias was addressed by using medical reports of surgeries performed by the senior cardiothoracic surgeon, who is a co-author in this paper and supervised two junior cardiothoracic surgeons during this period.
On average, 246 patients with congenital cardiac lesions presented annually, and only 42.6% were operated on that same year due to limited access in the public sector (Table S1). Approximately 12% of patients were either refused surgery or died preoperatively, while 45.4% were not operated on by the end of the year of presentation.
Altogether, 1701 patients underwent congenital cardiac surgical procedures (Fig. 1). The median age of patients was 1.92 years, with a median weight of 10 kg at first recorded surgery (Table 1). A total of 2222 occurred, with 521 of these being repeat operations. Details of the sociodemographic factors, clinical parameters, risk stratification tools and their association with in-hospital mortality are shown in Table 1.
Table 1: Sociodemographic factors, clinical parameters, risk stratification tools and the association with mortality.
| Parameter | Mean (SD)/Median (IQR)/n (%) | ||||
|---|---|---|---|---|---|
| Overall (n = 1701) | In-Hospital Mortality (n = 188) | Survival to Discharge (n = 1513) | p-value | ||
| Age | 1.92 (0.67–4.83) | 0.33 (0.13–1.25) | 2.08 (0.92–5.33) | <0.001 | |
| Weight | 10.0 (7.00–16.0) | 5.00 (3.45–8.20) | 10.3 (7.50–17.7) | <0.001 | |
| Gender | Males | 824 (48.4%) | 79 (51.6%) | 727 (48.1%) | 0.69 |
| Females | 858 (50.4%) | 91 (48.4%) | 767 (51.9%) | ||
| Aortic Cross-Clamp Time | 65.0 (36.0–100.0) | 82.5 (35.0–139.0) | 65.0 (36.5–98.0) | 0.01 | |
| CPB Time* | 116.0 (82.0–159.0) | 179 (16.0–272.5) | 112 (81.0–152.0) | <0.001 | |
| LOS ICU† | 5.0 (3.00–8.00) | 8.0 (3.0–16.5) | 4 (3.0–8.0) | <0.001 | |
| Ventilator Days | 2.0 (1.0–5.0) | 8.0 (3.0–16.0) | 2.0 (1.0–4.0) | <0.001 | |
| Mechanical Life Support | Yes | 58 (3.4%) | 31(16.5%) | 27 (0.02%) | <0.001 |
| No | 1643 (96.7%) | 157 (83.5%) | 1486 (98.2%) | ||
| RACHS-2 | |||||
| 1 | 494 (29.2%) | 16 (3%) | 478 (97%) | <0.001 | |
| 2 | 901 (53.3%) | 63 (7%) | 838 (93%) | <0.001 | |
| 3 | 46 (2.7%) | 8 (17%) | 38 (83%) | 0.07 | |
| 4 | 242 (14.3%) | 64 (26%) | 178 (74%) | <0.001 | |
| 5 | 6 (0.4%) | 5 (83%) | 1 (17%) | <0.001 | |
| ABC Complexity Levels | |||||
| 1 | 399 (23.5%) | 14 (4%) | 385 (96%) | <0.001 | |
| 2 | 832 (49.0%) | 78 (10%) | 754 (90%) | 0.98 | |
| 3 | 380 (22.4%) | 38 (10%) | 342 (90%) | 0.66 | |
| 4 | 88 (5.2%) | 28 (32%) | 60 (68%) | <0.001 | |
| STAT 2020 Mortality Category | |||||
| 1 | 727 (42.9%) | 21 (3%) | 706 (92%) | <0.001 | |
| 2 | 644 (38.0%) | 59 (9%) | 585 (91%) | 0.96 | |
| 3 | 208 (12.3%) | 50 (24%) | 158 (76%) | <0.001 | |
| 4 | 111 (6.5%) | 23(21%) | 88 (79%) | <0.001 | |
| 5 | 6 (0.4%) | 5(83%) | 1 (17%) | <0.001 | |
Most repeat surgeries (Fig. S1) occurred amongst complex and palliative surgeries, followed by tetralogy of Fallot and ventricular septal defect repairs. Of the 521 repeat surgeries, 415 were performed during the same hospital admission (early repeat), whilst 106 were performed at subsequent admissions (late repeat surgeries) within the study period. Patent ductus arteriosus (PDA) ligations were the only procedures involving one-time definitive repair and did not require repeat surgeries.
Palliative surgeries (procedures that improve cardiac function without correcting the underlying defect) had the highest in-hospital mortality rate of 41% (77/188), followed by complex repairs (18.6%, n = 35) and atrioventricular canal repair group (10.6%, n = 20) (Fig. S2). Yearly total in-hospital mortality ranged from 4.7% to 11.4%, with variations in mortality rates from individual procedures per year (Fig. 2). Mortality also varied between surgical categories within a year (Fig. 3).
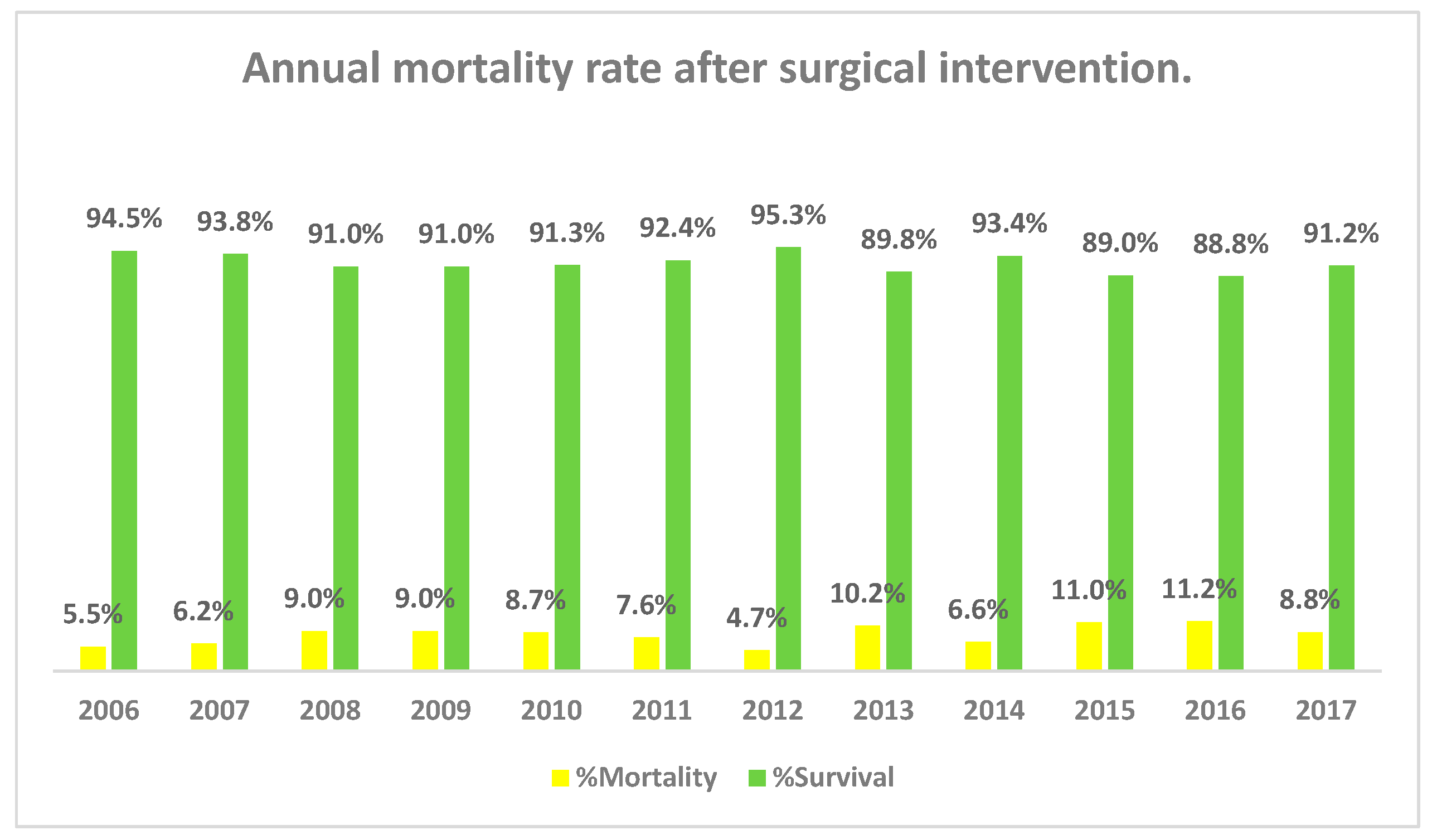
Figure 2: Annual in-hospital mortality rate after surgical intervention (2006–2017).
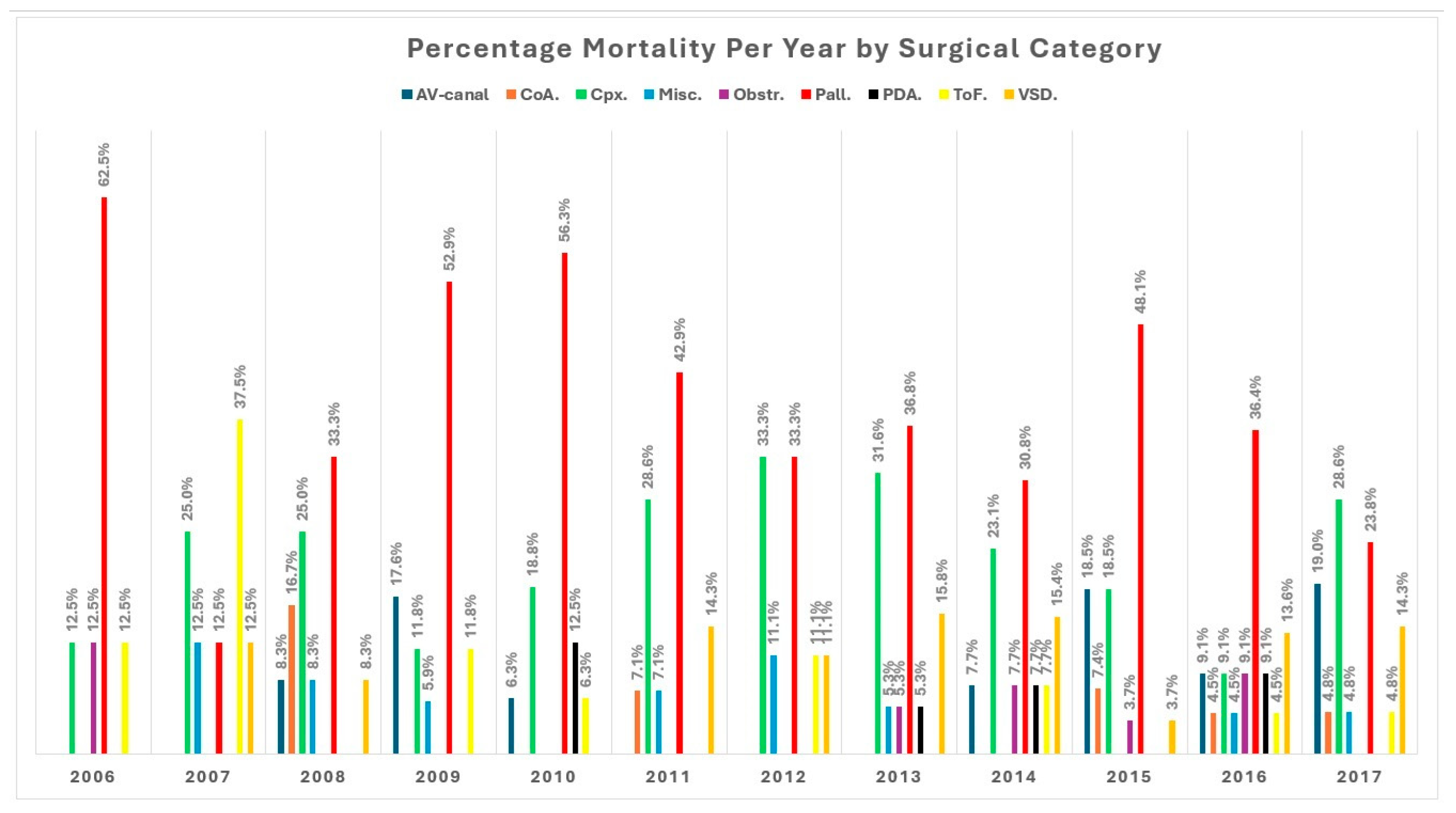
Figure 3: Percentage mortality per year by surgical category.
A relationship between in-hospital mortality and existing predictive scores/categories showed significantly higher mortality with RACHS-2 score category 5 and STAT 2020 category 5 (Table 1). Survival was significantly higher in the RACHS-2 score categories 1, 2, and 4; ABC levels 1 and 4; and STAT 2020 categories 1,3 and 4 (Table 1). The following variables had a univariable association with mortality: younger age, lower weight, longer CPB time, longer aortic cross-clamp time, longer ICU stay, ventilated days, STAT mortality and ABC scores, RACHS-2 categories 2, 3, 4 and 5, STAT 2020 mortality categories 2, 3, 4 and 5, and ABC complexity levels 2, 3 and 4 (Table 2).
Table 2: Regression analysis model for in-hospital mortality.
| Characteristic | N | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | AOR (95% CI) | p-Value | ||
| Age | 1701 | 0.69 (0.61, 0.77) | <0.001 | 0.83 (0.63, 1.05) | 0.2 |
| Weight (kg) | 1701 | 0.82 (0.78, 0.86) | <0.001 | 1.01 (0.90, 1.11) | >0.9 |
| CPB time (minutes) | 1197 | 1.01 (1.01, 1.01) | <0.001 | 1.02 (1.01, 1.02) | <0.001 |
| ICU stay (days) | 1625 | 1.03 (1.02, 1.05) | <0.001 | 0.68 (0.57, 0.79) | <0.001 |
| Aortic cross-clamp time (minutes) | 1219 | 1.01 (1.00, 1.05) | <0.001 | 0.99 (0.98, 0.99) | 0.001 |
| Ventilated days postop | 1533 | 1.06 (1.05, 1.08) | <0.001 | 1.54 (1.32, 1.84) | <0.001 |
| STAT 2020 score | 1701 | 8.33 (5.57, 12.5) | <0.001 | 24.6 (1.11, 512) | 0.04 |
| ABC basic score | 1701 | 1.33 (1.23, 1.45) | <0.001 | 1.13 (0.68, 1.94) | 0.7 |
| Gender (n = 1701) | Female | Baseline | Baseline | ||
| Male | 1.04 (0.75, 1.44) | 0.8 | |||
| RACHS-2 (n = 1690) | 1 | Baseline | Baseline | ||
| 2 | 2.32 (1.36, 4.2) | 0.003 | 1.14 (0.35, 4.16) | 0.8 | |
| 3 | 6.34 (2.44, 15.4) | <0.001 | 2.2 (0.36, 13) | 0.4 | |
| 4 | 10.8 (6.22, 19.7) | <0.001 | 1.85 (0.25, 13.4) | 0.5 | |
| 5 | 151 (22.6, 2977) | <0.001 | 0 (0.00, 6.72) | 0.14 | |
| STAT 2020 mortality category (n = 1701) | 1 | Baseline | Baseline | ||
| 2 | 3.39 (2.07, 5.78) | <0.001 | 1.73 (0.63, 5.03) | 0.3 | |
| 3 | 10.5 (6.24, 18.4) | <0.001 | 0.31 (0.04, 2.34) | 0.3 | |
| 4 | 8.81 (4.68, 16.7) | <0.001 | 0.15 (0.01, 3.56) | 0.2 | |
| 5 | 169 (25.8, 3307) | <0.001 | N/A | N/A | |
| ABC complexity level (n = 1701) | 1 | Baseline | Baseline | ||
| 2 | 2.86 (1.65, 5.33) | <0.001 | 0.66 (0.12, 4.56) | 0.7 | |
| 3 | 3.07 (1.67, 5.96) | <0.001 | 0.68 (0.05, 11) | 0.8 | |
| 4 | 12.9 (6.53, 26.6) | <0.001 | 0.33 (0.01, 11.8) | 0.5 | |
Cardiopulmonary bypass time, ICU length of stay, aortic cross-clamp time and days on the ventilator were independently predictive of perioperative all-cause in-hospital mortality in a multivariable regression model. Extracorporeal life support in the form of ECMO or VAD was associated with a less than 30% survival rate. Most of these cases had complex cardiac lesions or were presenting for palliative procedures. The RACHS-2 score, STAT 2020 mortality categories, and ABC scores and complexity level were not predictive of in-hospital all-cause mortality in a multivariable regression analysis. The STAT 2020 score had a significant association with mortality in the univariable and multivariable analysis, p < 0.001 and p = 0.04, respectively.
The multivariable regression model was highly predictive of mortality and yielded an area under the curve of 0.90 (Fig. S3). A total of 11 patients had an unspecified RACHS 2 score. In a Cox regression model, a unit increase in a RACHS-2 score was associated with a 60% increase in mortality risk, p = 0.002 (Table 3). Prolonged cardiopulmonary bypass time was associated with mortality over time, p < 0.001. Shorter CPB time was strongly associated with survival to discharge.
Table 3: Cox regression analysis of in-hospital mortality.
| Parameter | HR | Std Error | Z | 95% CI | p > |z| |
|---|---|---|---|---|---|
| Age | 0.80 | 0.10 | −1.82 | 0.63, 1.02 | 0.07 |
| Weight (kg) | 1.00 | 0.05 | 0.03 | 0.91, 1.11 | 0.98 |
| ICU stay | 0.97 | 0.03 | −1.07 | 0.91, 1.03 | 0.28 |
| Ventilated days | 1.02 | 0.04 | 0.47 | 0.95, 1.10 | 0.64 |
| CPB time (min) | 1.00 | 0.00 | 5.68 | 1.00, 1.01 | <0.001 |
| AoXCl time (min) | 1.00 | 0.00 | −0.53 | 0.99, 1.00 | 0.59 |
| Gender | 1.18 | 0.26 | 0.75 | 0.76, 1.83 | 0.45 |
| RACHS-2 | 1.40 | 0.16 | 3.04 | 1.13, 1.75 | 0.002 |
| ABC complexity level | 0.91 | 0.16 | −0.52 | 0.65, 1.29 | 0.61 |
| STAT 2020 mortality category | 1.30 | 0.42 | 0.82 | 0.69, 2.46 | 0.42 |
The Global Burden of Disease and Injury Risk Factor (GBDIRF) study found a 4.2% global increase in birth prevalence of congenital cardiac disease (CCD) between 1990 and 2017. In an estimated population of one billion on the African continent, approximately 2500 to 3000 children undergo cardiac surgery annually [22]. For a child who presents with CCD in the South African public sector, affordable, comprehensive cardiac healthcare is restricted due to the limited number of centres that perform surgery in South Africa. Zilla et al. [7] reported 46 (8 public and 38 private) centres, while Roussouw [23] reported a decline to 22 centres in 2021. Limited staff training and retention strategies and a lack of administrative prioritisation of CCD have contributed to this decline [23]. Higashi et al. [24] estimated that only 3% of children who needed surgery in sub-Saharan Africa were operated on.
Mortality data for congenital cardiac surgery in LMIC, and, particularly in Africa, are under-reported. In high-income countries (HIC), perioperative mortality rates (POMR) have reportedly decreased from 8.7% to under 4% [16,25,26]. In a few LMICs where data is available, higher morbidity and mortality were reported, with Cavalcante et al. [4] quoting a POMR of 11.6% [4,13].
Data from our centre showed a perioperative mortality rate of 11%, with mortality variability dependent on cardiac lesion complexity, with 0% amongst ASD surgery and 41% in palliative repairs. The mortality also differed year on year, with some years seeing higher mortality for these non-complex surgeries. The combination of PDA, VSD and AV canals showed the highest fluctuation in mortality (0–21%), which may have been related to personnel changes. There was and still is limited skilled staff and training of junior surgeons. The all-cause, in-hospital mortality rates of simple congenital heart diseases such as ASD, VSD and PDA were higher in our centre collectively compared to HICs, including Europe and China (3.2% compared to 0.99% and 0.43%, respectively).
At its peak, the paediatric cardiothoracic surgical complement at our institution comprised one senior and two junior specialists, as evidenced in an audit by Hoosen et al. [12]. This is far from the need documented by the same authors in 2017 [3]. More palliative and complex cases were also operated on during this period. Our data shows an average of 40% of patients presented per year receiving surgical intervention, with increases in the waiting list per year. The local perioperative team had a low median volume of paediatric congenital heart disease cases, with approximately 105 performed annually. In addition, the same team would operate/assist in procedures on paediatric and adult rheumatic heart disease and grown up congenital disease (GUCH) patients.
Case volumes are described as low when <150 cases are performed, medium when 150–249 cases are performed and high when >250 are performed per year [27]. It has been shown that there is an inverse relationship between the number of congenital cardiac surgeries performed in a centre per year and mortality, with centres that perform large volumes of cases having lower mortality rates [27]. However, this relationship has also been shown to be complex and multifactorial [27]. Brown recommended a differentiation between comprehensive (greater than 200 index cases per year) and essential (a minimum of 75 index cases per year) paediatric cardiac centres [28]. The effect of low-volume centres is magnified in rare high-risk procedures, as is often seen in palliative and complex cases.
Riley et al. [29] found a variety of modifiable (preoperative mechanical ventilation and nutritional status, longer CPB, staffing) and non-modifiable factors (younger age, lower weight and increased surgical complexity) associated with cardiac arrest after paediatric cardiac surgery. In our cohort, sepsis, bleeding and cardiac failure or dysfunction also contributed to mortality. Infants (<1 yr) with CHD have lower body weight, require specialised skills, and have a higher risk of anaesthesia, resulting in higher mortality [10]. This correlation was also observed by Zheng et al. [10]. However, it was in contrast to the study performed by Sharifi et al. [30], which showed no significant relationship between weight, age and mortality in their patient population.
Surgical complexity and CPB duration variables are related, as typically, more complicated surgeries require a longer bypass duration. Children with longer CPB times and complex surgical repairs, as seen in our palliative and complex population, should be viewed as extremely high risk and prone to postoperative complications. In-depth analysis and identification of these risk factors in our population would allow for the institution of timely and effective strategies and management algorithms targeted at the vulnerable population.
Given the extensive range of surgical procedures available to correct congenital heart diseases, a comparison of mortality should follow a risk stratification system. Our study demonstrated higher in-hospital mortality with higher levels of the RACHS-2 score and STAT 2020 categories. This finding contrasts with studies conducted in other middle-income countries and those in HICs [4,31,32], where the RACHS-1 score predicted mortality in their patient populations.
Perioperative outcomes data in Africa are very few and skewed by complex cardiac surgery performed by mission surgical camps or definitive surgical care in countries outside the continent [33,34]. This limits an assessment relating outcomes to local skill sets and resources. Kinsley et al. [35] established a successful private-public partnership through the Walter Sisulu Paediatric Cardiac Centre and documented the need for congenital cardiac surgery in Africa. Hoosen et al. [3] audited paediatric cardiac services in South Africa and noted deficiencies at multiple levels that contributed to the needs of children with CHD not being met.
In Northern Uganda, of the 224 patients who needed surgery, only 57 received definitive intervention, with a proportion of them being operated on in Germany, the Cayman Islands, and India using funding from charity organisations [36]. In Mozambique, Mocumbi et al. [33] reported late presentations among their congenital cardiac patients, with 29% presenting with complications at the time of diagnosis. Their operation rate was 36%, with only 26% having complex lesions. In Rwanda, only 149 open cardiac procedures were performed over 10 years by Team Heart, a humanitarian international effort [22]. Our study is the first to provide insight into the perioperative mortality outcomes of paediatric congenital cardiac surgeries in Africa performed by local surgeons in a public sector facility.
Despite mortality being historically used to evaluate the quality of cardiac surgical services, its use to compare services in the paediatric population may not be suitable for surgical outcomes [3]. Mortality depends on various factors, which may have regional differences amongst high, middle- and low-income countries.
The limitation of the study is its retrospective single-centre nature, and it thus may not be generalisable, even to the South African population. South Africa is meant to have the best services in Africa alongside Egypt, with Johannesburg and Cape Town being the most resourced [7]; however, this is not the case. This discrepancy is evidenced in Hoosen et al. [12], which found outcomes data not being reported. Most centres have one or two paediatric surgeons. Our centre at CMJAH had one resident senior paediatric surgeon for most of the study period, with two new surgeons joining the centre in the latter part. South Africa currently faces challenges with a decline in services. We assume that our findings would represent the challenges in Southern Africa and Africa as a whole.
The cross-sectional nature of the study limits our ability to investigate the causality of the covariates to morbidity and mortality. A second data reviewer (and the originator of the database) assisted in minimising transcription errors from a paper-based database to a Microsoft Excel spreadsheet. Further prospective studies are underway to increase our understanding of the unique variables that may contribute to the higher mortality rates seen in our patient population and to assess morbidity.
This is the first report looking at surgical outcomes after paediatric congenital cardiac surgeries performed at a centre in South Africa by local personnel and not involving mission surgical camps within the African context. Delayed referral of children to specialised centres leads to presentation with a range of associated comorbidities and complications such as renal dysfunction, pulmonary hypertension, systemic infection, poor nutritional status, genetic syndromes, and severe ventricular dysfunction [37]. These factors are likely not as common in higher-income countries where the current risk stratification systems were created.
The cohorts of patient data sets used to develop these scoring systems do not represent patients globally. This area will require further research and analysis and may be used to define contributing factors for the higher mortality rates seen in our population. These data will hopefully pave the way to increase the understanding of the contributing elements to the higher mortality rates in our population and thus assist in improving congenital cardiac surgical services in South Africa and other comparable middle-income nations.
Careful patient selection and medical optimisation of patients aligned with specific expertise at dedicated children’s hospitals may improve the mortality rate. Future studies comparing the outcomes of patients with cardiac disease based on hospital type, surgical volume, and surgical skill may help determine the future of care, including the potential need for regionalisation of paediatric cardiac centres for this vulnerable patient population [38].
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Palesa Motshabi-Chakane, Moses Kebalepile. Data collection: Prathap Sarma, Katharina Vanderdonck. Analysis and interpretation of results: Palesa Motshabi-Chakane, Moses Kebalepile, Prathap Sarma, Katharina Vanderdonck. Draft manuscript preparation: Palesa Motshabi-Chakane, Moses Kebalepile, Prathap Sarma, Katharina Vanderdonck, Palesa Mogane. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials. The dataset generated or analysed during this study is available for review from the corresponding author, provided patient privacy is protected.
Ethics Approval: Approval to conduct the study was obtained from the Human Research Ethics Committee (Medical) (HREC) of the University of the Witwatersrand (M211151). Ethical principles governed by the South African Guidelines for Good Clinical Practice and the Declaration of Helsinki (as revised in 2013) were strictly followed.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2025.060382.
References
1. Davey B, Sinha R, Lee JH, Gauthier M, Flores G. Social determinants of health and outcomes for children and adults with congenital heart disease: a systematic review. Pediatr Res. 2021;89(2):275–94. doi:10.1038/s41390-020-01196-6. [Google Scholar] [CrossRef]
2. Hoosen EM, Cilliers AM, Hugo-Hamman CT, Brown SC, Lawrenson JB, Zühlke L, et al. Paediatric cardiac services in South Africa. S Afr Med J. 2011;101(2):106–7. doi:10.7196/samj.4684. [Google Scholar] [CrossRef]
3. Hoosen EGM, Cilliers AM, Hugo-Hamman CT, Brown SC, Harrisberg JR, Takawira FF, et al. Optimal paediatric cardiac services in South Africa–what do we need? SA Heart. 2017;7(1):10–16. doi:10.24170/7-1-1963. [Google Scholar] [CrossRef]
4. Cavalcante CTMB, de Souza NMG, Pinto Júnior VC, Branco KMPC, Pompeu RG, Teles ACO, et al. Analysis of surgical mortality for congenital heart defects using RACHS-1 risk score in a Brazilian single center. Braz J Cardiovasc Surg. 2016;31(3):219–25. doi:10.5935/1678-9741.20160022. [Google Scholar] [CrossRef]
5. Hasegawa T, Masuda M, Okumura M, Arai H, Kobayashi J, Saiki Y, et al. Trends and outcomes in neonatal cardiac surgery for congenital heart disease in Japan from 1996 to 2010. Eur J Cardiothorac Surg. 2017;51(2):301–7. doi:10.1093/ejcts/ezw302. [Google Scholar] [CrossRef]
6. Vervoort D, Meuris B, Meyns B, Verbrugghe P. Global cardiac surgery: access to cardiac surgical care around the world. J Thorac Cardiovasc Surg. 2020;159(3):987–96. doi:10.1016/j.jtcvs.2019.04.039. [Google Scholar] [CrossRef]
7. Zilla P, Yacoub M, Zühlke L, Beyersdorf F, Sliwa K, Khubulava G, et al. Global unmet needs in cardiac surgery. Glob Heart. 2018;13(4):293–303. doi:10.1016/j.gheart.2018.08.002. [Google Scholar] [CrossRef]
8. Berger JT, Holubkov R, Reeder R, Wessel DL, Meert K, Berg RA, et al. Morbidity and mortality prediction in pediatric heart surgery: physiological profiles and surgical complexity. J Thorac Cardiovasc Surg. 2017;154(2):620–8. doi:10.1016/j.jtcvs.2017.01.050. [Google Scholar] [CrossRef]
9. Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg. 2009;87(1):216–22. doi:10.1016/j.athoracsur.2008.10.032. [Google Scholar] [CrossRef]
10. Zheng G, Wu J, Chen P, Hu Y, Zhang H, Wang J, et al. Characteristics of in-hospital mortality of congenital heart disease (CHD) after surgical treatment in children from 2005 to 2017: A single-center experience. BMC Pediatr. 2021;21(1):521. doi:10.1186/s12887-021-02935-2. [Google Scholar] [CrossRef]
11. Shukla VV, Bobhate P, Mohanty S, Rao S, Joshi P, Joshi V. Early outcomes of neonatal cardiac surgery in India. Indian Pediatr. 2020;57(2):129–32. [Google Scholar]
12. Hoosen EGM, Cilliers AM, Brown SC, Harrisberg JR, Takawira FF, Govendrageloo K, et al. Audit of paediatric cardiac services in South Africa. SA Heart. 2017;7(1):4–9. doi:10.24170/7-1-1962. [Google Scholar] [CrossRef]
13. Saxena A. Congenital cardiac surgery in the less privileged regions of the world. Expert Rev Cardiovasc Ther. 2009;7(12):1621–9. doi:10.1586/erc.09.141. [Google Scholar] [CrossRef]
14. Saxena A. Congenital heart disease in India: A status report. Indian Pediatr. 2018;55(12):1075–82. [Google Scholar]
15. Giamberti A, Rito ML, Stellin G, Karl T, Frigiola A. Editorial: pediatric cardiology and cardiac surgery in developing countries: Current needs and future perspectives. Front Pediatr. 2022;10:1067193. doi:10.3389/fped.2022.1067193. [Google Scholar] [CrossRef]
16. Spiegelhalter DJ. Mortality and volume of cases in paediatric cardiac surgery: retrospective study based on routinely collected data. BMJ. 2002;324(7332):261–3. doi:10.1136/bmj.324.7332.261. [Google Scholar] [CrossRef]
17. Allen P, Zafar F, Mi J, Crook S, Woo J, Jayaram N, et al. Risk stratification for congenital heart surgery for ICD-10 administrative data (RACHS-2). J Am Coll Cardiol. 2022;79(5):465–78. doi:10.1016/j.jacc.2021.11.036. [Google Scholar] [CrossRef]
18. Jacobs ML, Jacobs JP, Thibault D, Hill KD, Anderson BR, Eghtesady P, et al. Updating an empirically based tool for analyzing congenital heart surgery mortality. World J Pediatr Congenit Heart Surg. 2021;12(2):246–81. doi:10.1177/2150135121991528. [Google Scholar] [CrossRef]
19. Department of Health South Africa. Guidelines for good practice in the conduct of clinical trials with human participants in South Africa. 3rd ed. Pretoria, South Africa: Department of Health; 2020. [Google Scholar]
20. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [Google Scholar]
21. Rensburg R. Healthcare in South Africa: how inequity is contributing to inefficiency [Internet]. 2021 [cited 2025 Feb 23]. Available from: https://theconversation.com/healthcare-in-south-africa-how-inequity-is-contributing-to-inefficiency-163753. [Google Scholar]
22. Swain JD, Sinnott C, Breakey S, Hasson Charles R, Mody G, Nyirimanzi N, et al. Ten-year clinical experience of humanitarian cardiothoracic surgery in Rwanda: building a platform for ultimate sustainability in a resource-limited setting. J Thorac Cardiovasc Surg. 2018;155(6):2541–50. doi:10.1016/j.jtcvs.2017.11.106. [Google Scholar] [CrossRef]
23. Rossouw B. Congenital heart disease in Africa threatens sustainable development goals. South Afr J Crit Care. 2021;37(1). doi:10.7196/SAJCC.2021.v37i1.486. [Google Scholar] [CrossRef]
24. Higashi H, Barendregt JJ, Kassebaum NJ, Weiser TG, Bickler SW, Vos T. The burden of selected congenital anomalies amenable to surgery in low and middle-income regions: cleft lip and palate, congenital heart anomalies and neural tube defects. Arch Dis Child. 2015;100(3):233–8. doi:10.1136/archdischild-2014-306175. [Google Scholar] [CrossRef]
25. Thiagarajan RR, Laussen PC. Risk adjustment for congenital heart surgery-1 (RACHS-1) for evaluation of mortality in children undergoing cardiac surgery. In: Pediatric and congenital cardiac care. London, UK: Springer; 2015. p. 327–36. [Google Scholar]
26. Hewitson J, Zilla P. Children’s heart disease in sub-Saharan Africa: challenging the burden of disease. SA Heart. 2017;7(1):18–29. doi:10.24170/7-1-1964. [Google Scholar] [CrossRef]
27. Welke KF, O’Brien SM, Peterson ED, Ungerleider RM, Jacobs ML, Jacobs JP. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg. 2009;137(5):1133–40. doi:10.1016/j.jtcvs.2008.12.012. [Google Scholar] [CrossRef]
28. Brown ML, Nasr VG. The minimum requirements for a pediatric cardiac surgical site: what is needed? J Cardiothorac Vasc Anesth. 2024;38(6):1302–4. doi:10.1053/j.jvca.2024.03.002. [Google Scholar] [CrossRef]
29. Riley CM, Murphy LD, Mastropietro CW. Cardiac arrest in children following cardiac surgery: a scoping review of contributing factors. World J Pediatr Congenit Heart Surg. 2022;13(4):475–81. doi:10.1177/21501351221100791. [Google Scholar] [CrossRef]
30. Sharifi M, Mirzaaghayan MR, Memarian S, Sharifi H, Gharib B. Risk factors of mortality in the intensive care unit following cardiac surgery for congenital heart diseases in children. Iran J Pediatr. 2023;33(4):e132744. doi:10.5812/ijp-132744. [Google Scholar] [CrossRef]
31. Nakayama Y, Shibasaki M, Shime N, Nakajima Y, Mizobe T, Sawa T. The RACHS-1 risk category can be a predictor of perioperative recovery in Asian pediatric cardiac surgery patients. J Anesth. 2013;27(6):850–4. doi:10.1007/s00540-013-1645-1. [Google Scholar] [CrossRef]
32. Oster ME, Strickland MJ, Mahle WT. Impact of prior hospital mortality versus surgical volume on mortality following surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2011;142(4):882–6. doi:10.1016/j.jtcvs.2011.04.011. [Google Scholar] [CrossRef]
33. Mocumbi AO, Lameira E, Yaksh A, Paul L, Ferreira MB, Sidi D. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol. 2011;148(3):285–8. doi:10.1016/j.ijcard.2009.11.006. [Google Scholar] [CrossRef]
34. Djike Puepi YF. Profile and outcome of congenital heart disease in Buea, south west region of Cameroon: A cross-sectional study. J Pediatr Adv Res. 2022;1(1):1–6. doi:10.46889/jpar.2022.1102. [Google Scholar] [CrossRef]
35. Kinsley RH, Edwin F, Colsen PR, Mamorare H, Martin G, Brink J. Paediatric cardiac surgery for a continent–The experience of the Walter Sisulu Paediatric Cardiac Centre for Africa. SA Heart. 2017;8(2):122–9. doi:10.24170/8-2-1907. [Google Scholar] [CrossRef]
36. Aliku T, Beaton A, Lubega S, Dewyer A, Scheel A, Kamarembo J, et al. Profile of congenital heart disease and access to definitive care among children seen at Gulu Regional Referral Hospital in Northern Uganda: A four-year experience. J Congenit Cardiol. 2021;5(1):9. doi:10.1186/s40949-021-00064-0. [Google Scholar] [CrossRef]
37. Sliwa K, Zühlke L, Kleinloog R, Doubell A, Ebrahim I, Essop M, et al. Cardiology-cardiothoracic subspeciality training in South Africa: a position paper of the South Africa heart association. Cardiovasc J Afr. 2016;27(3):188–93. doi:10.5830/CVJA-2016-063. [Google Scholar] [CrossRef]
38. Hu R, Zhu H, Qiu L, Hong H, Xu Z, Zhang H, et al. Association between preoperative factors and in-hospital mortality in neonates after cardiac surgery in China. Front Pediatr. 2021;9:670197. doi:10.3389/fped.2021.670197. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools