 Open Access
Open Access
ARTICLE
Impaired Magnetic Resonance Myocardial Strain in Unoperated Ebstein’s Anomaly Is Associated with Reduced Exercise Capacity
1 Department of Cardiology, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, B15 2GW, UK
2 Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, B15 2TT, UK
3 Department of Cardiology, Faculty of Medicine, Minia University, Minia, 61519, Egypt
4 RRPPS, Department of Imaging and Medical Physics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, B15 2GW, UK
* Corresponding Author: Richard P. Steeds. Email:
# Ahmed M. Dardeer and Victoria M. Stoll are joint first authors, both contributed equally to the work
Congenital Heart Disease 2025, 20(1), 27-39. https://doi.org/10.32604/chd.2025.059729
Received 15 October 2024; Accepted 13 February 2025; Issue published 18 March 2025
Abstract
Background: Patients with unrepaired Ebstein’s anomaly experience exercise intolerance, heart failure and premature mortality. Volumetric assessment of right ventricular function is difficult due to the complex anatomy of the right ventricle and tricuspid valve. Myocardial deformation indices are an early marker in other cardiac pathologies of ventricular dysfunction. Objectives: 1. Assess myocardial deformation in unrepaired Ebstein’s compared to healthy controls. 2. Investigate the relationships between myocardial deformation and exercise capacity. Methods: Myocardial deformation parameters (strain) were calculated using feature tracking from standard cardiac magnetic resonance cine images. Cardiopulmonary exercise results were included where available. Results: 36 patients with unrepaired Ebstein’s and 36 matched controls were included. Right ventricular, right atrial, and left ventricular global longitudinal, as well as left ventricular circumferential strain were impaired in Ebstein’s patients compared to controls (p < 0.05). In Ebstein’s patients right atrial peak strain correlated with their percentage predicted VO2 max (r = −0.448, p = 0.022) and VE/VCO2 slope (r = 0.435, p = 0.026). There were no correlations between right ventricular ejection fraction and exercise parameters. When Ebstein’s patients were divided by severity into mild or severe according to the median total right/left index, those with severe demonstrated significantly impaired right ventricular global longitudinal strain compared to those in the mild category (−17.5 ± 5.4% vs. −21.4 ± 4.4%, p = 0.0017). Conclusions: Myocardial deformation parameters for both the right and left ventricle are impaired in patients with unrepaired Ebstein’s compared to healthy controls. Right atrial peak strain is related to impaired exercise capacity and warrants further investigation as an early prognostic marker in this patient cohort.Keywords
Ebstein’s anomaly of the tricuspid valve is a rare congenital cardiac abnormality that accounts for less than 1% of all congenital heart diseases [1,2]. Ebstein’s anomaly consists of a dysplastic tricuspid valve, displaced towards the right ventricular apex, resulting in atrialisation of the right ventricle, with subsequent significant tricuspid regurgitation, right atrial and right ventricular volume overload [2]. Consequently many patients with Ebstein’s anomaly experience significant exercise intolerance, with reduced mean peak oxygen uptake on cardiopulmonary exercise testing, which has been demonstrated to be an independent predictor of outcome in Ebstein’s anomaly patients [3]. Additionally, early mortality occurs within these patients, with more than half of deaths due to heart failure [4,5].
Cardiovascular magnetic resonance (CMR) imaging confers significant advantages in the assessment of Ebstein’s anomaly that has led to its acceptance as the gold-standard imaging technique for assessment of right ventricular volumes and ejection fraction [6]. Previous CMR studies in patients with Ebstein’s anomaly have correlated the atrialised right ventricular end-diastolic volume, total right ventricular end-diastolic volume, and functional right ventricular end-diastolic volume/left ventricular end-diastolic volume ratio with exercise capacity. However, defining these components can be extremely difficult because of variable anatomy of the tricuspid valve including the degree of offsetting, morphology, and malrotation [7]. More recently, a simplified ratio of total right/left volume index has been associated with heart failure biomarkers [8] and in addition to right ventricular ejection fraction, confirmed as a predictor of major adverse cardiovascular events [9]. These measures are however all volume-based and inevitably are affected by tricuspid regurgitation severity. Therefore, a recent study by Steinmetz et al. [10] assessed atrial and ventricular myocardial deformation (strain) in Ebstein’s anomaly patients and found associations with simple heart failure markers, but did not assess any relationships to exercise capacity. Strain imaging quantifies myocardial muscle contraction and is an early marker of ventricular contractile dysfunction in other congenital conditions [11].
Ninety patients with Ebstein’s Anomaly were identified from a local database of the congenital heart disease patients at the Queen Elizabeth Hospital, Birmingham, United Kingdom. Ebstein’s anomaly was defined as the apical displacement of the septal leaflet of the tricuspid valve by >8 mm/m2 relative to the mitral valve leaflet insertion point [12]. Thirty-four of the ninety patients had not undergone CMR (implantable defibrillator or pacemaker; claustrophobia/intolerance; other associated congenital pathology), 16 had undergone tricuspid valve surgery, and 4 had incomplete studies due to problems with breath-holding and arrhythmias. Hence, 36 unoperated Ebstein’s Anomaly patients were eligible for inclusion in this study. This observational retrospective study was approved by the local clinical governance committee (RRK6237) and conformed to the guidelines regarding the principles of Good Clinical Practice. Additionally, 36 age and sex-matched controls, who were healthy donors studied pre kidney donation, were identified from a prospective, observational CMR study (NCT01028703) [13].
2.2 Cardiac Magnetic Resonance Imaging Acquisition
A 1.5T scanner (Magnetom Avanto, Siemens, Germany) was used for CMR imaging. Standard CMR protocols using steady-state free precession cine images to assess cardiac morphology and function were utilised. as per previously validated methodology [14].
2.3 Volumetric Imaging Analysis
Offline image analysis was performed using version 5.3.4 cvi42, Circle Vascular Imaging, Calgary, Canada. Standard contouring of the ventricular short axis stack at end diastole and end systole, as per recognised published guidance, was performed for calculation of ventricular volumes, function, and left ventricular mass [14].
Right atrial volumes were measured using the horizontal long axis view that shows all four cardiac chambers. For the Ebstein’s anomaly patients a smooth endocardial contour encompassing both the anatomical right atrium and the atrialised right ventricle was drawn to calculate the volume of the functional right atrium. These contours were drawn to the insertion point of the displaced tricuspid valve leaflets.
To calculate the total right/left volume index in the patients with Ebstein’s anomaly one smooth endocardial contour was drawn encompassing the functional right atrium (anatomical and atrialised right ventricle) and functional right ventricle in the end diastolic timeframe. An additional smooth endocardial contour was drawn for the left atrium and left ventricle. The total right/left volume index was calculated using the following equation: (anatomical right atrium + atrialised right ventricle + functional right ventricle)/(left atrium + left ventricle) volumes [8]. A similar method was applied to the healthy controls and the total right/left volume index was calculated as follows: (right atrium + right ventricle)/(left atrium + left ventricle) volume. The median value of the total right/left volume index in the Ebstein’s anomaly patients was chosen as a cut-off value to subclassify the Ebstein’s anomaly cohort into mild and severe cases.
2.4 Feature Tracking Image Analysis
Feature tracking analysis was undertaken as per the standard methodology previously utilized by our group [15,16]. A 2-dimensional technique was used to analyse right atrial and right ventricular global longitudinal strain analysis. On the horizontal long axis view smooth end diastolic endocardial and epicardial contours were drawn. In the patients with Ebstein’s anomaly, the smooth endocardial contour encompassing the functional right atrium was utilized for quantification of right atrial global longitudinal strain. For the right ventricular analysis, two datasets were created—one with the inclusion of the septum; free wall plus septum global longitudinal strain (FW + S GLS), and a second with the exclusion of the septum; free wall global longitudinal strain (FW GLS), see Fig. 1. These two datasets reflect the different approaches to the assessment of the septal contribution to right ventricular function.
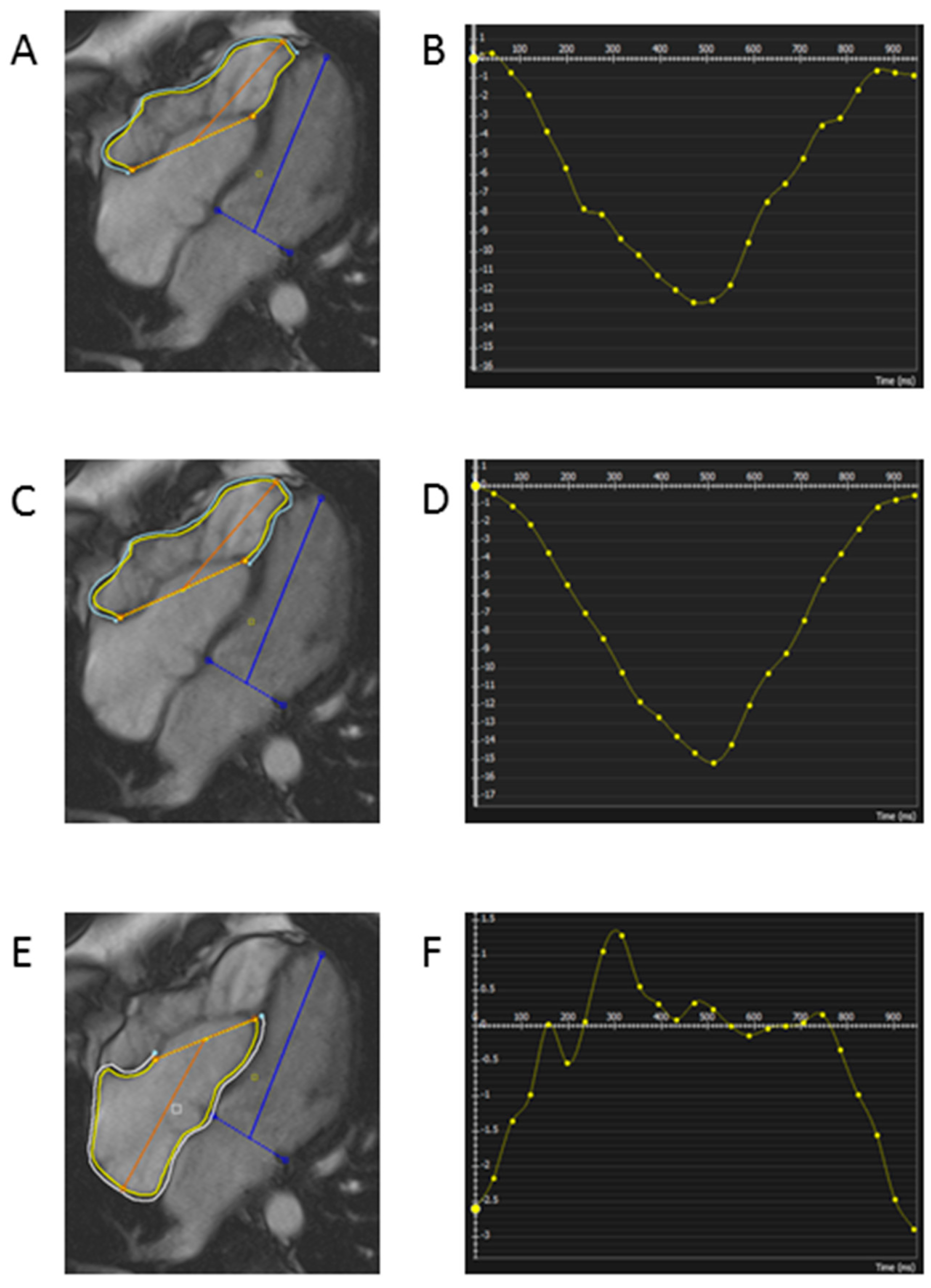
Figure 1: Example of endocardial and epicardial contouring for calculation of right atrial and right ventricular strain parameters. (A) Right ventricular contouring for calculation of RV free wall global longitudinal strain (B) Results for RV free wall global longitudinal strain (C) Right ventricular contouring for calculation of RV free wall plus septum global longitudinal strain (D) Results for RV free wall plus septum global longitudinal strain (E) Right atrial contouring for calculation of right atrial global longitudinal strain (F) Results for right atrial global longitudinal strain Endocardial contour yellow, Epicardial contour blue.
A 3-dimensional technique for assessment of left ventricular strain was performed based on previously published methodology by our group [16]. On all short and long axis slices at end diastole smoothed endocardial and epicardial borders were drawn. The superior right ventricular insertion points within the left ventricle were then defined. From this analysis left ventricular global longitudinal strain (LV GLS), global circumferential strain (LV GCS) and global radial strain (LV GRS) measurements were derived. All strain measurements were taken as the highest strain value calculated irrespective of the timeframe during systole.
2.5 Reproducibility of Myocardial Strain Parameters
To assess intra-observer variability a second analysis of ten randomly selected cases was performed after a six-month interval.
2.6 Cardiopulmonary Exercise Testing
Cardiopulmonary exercise tests were performed using GE Healthcare CASE ES Ergospirometry Testing System combined with a GE treadmill T2100 (GE Healthcare, Kuopio, Finland). Standardized ramped protocols were utilized aiming for 8–12 min of exertion, until the participant became symptom limited and stopped exercising [17]. Cardiopulmonary exercise tests in the Ebstein’s anomaly patients conducted within six months of cardiac magnetic resonance imaging were eligible for inclusion in the study results.
Standard transthoracic echocardiography was undertaken in the Ebstein’s anomaly patients (Philips iE33 Medical Systems, Amsterdam, The Netherlands). Measurements of right ventricular function were recorded as per standard guidelines. The parameters assessed were tricuspid annular plane systolic excursion, right ventricular S velocity, and right ventricular systolic pressure [18]. The degree of tricuspid regurgitation was graded as none, mild, moderate, or severe according to the assessment criteria recommended by Lancellotti et al. [19]. In brief this uses color flow imaging to diagnose tricuspid regurgitation (TR) and then adopts a quantitative approach to grade the severity. Possible quantification techniques include proximal isovelocity surface area (PISA), vena contracta width, systolic hepatic flow reversal, and continuous wave Doppler of the TR jet. Mean pulmonary artery pressure was derived using the TR peak velocity [20].
The presence of a patent foramen ovale was assessed by echocardiogram, and was defined as a dynamic deficit of the inter-atrial septum with intermittent right to left color flow seen on Doppler imaging in the region of the fossa ovalis [21,22]. The presence of an atrial septal defect was also assessed by echocardiogram, as per standard definitions of atrial septal defects with shunting detected across the inter-atrial septum [22].
Statistical analysis was performed using SPSS version 24 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± standard deviations. Normality was assessed using the Shapiro-Wilk test. Independent t-tests were used to compare results between Ebstein’s anomaly patients and controls. Correlation was assessed using the Pearson or Spearman method, as appropriate. Intra-observer agreement was assessed using the intraclass correlation coefficient. p values < 0.05 were considered significant.
3.1 Participant Characteristics
36 patients with Ebstein’s Anomaly (age 36 ± 13 years, 44% male) and 36 controls (age 37 ± 11 years, 44% male) were included in the study. The two groups were well matched for age (p = 0.6 and gender p > 0.99), as well as body mass index (Ebstein’s 25.5 ± 4.2 kg/m2, controls 25.2 ± 6.1 kg/m2, p = 0.8), see Table 1.
3.2 Myocardial Structure and Function
Results for right atrial, right ventricular, and left ventricular volumes and function are summarized in Table 1. As expected the functional right atrial volume was larger in Ebstein’s anomaly patients compared to controls (p < 0.0001). Ebstein’s anomaly patients had dilated right ventricular volumes, with lower ejection fractions than controls (p < 0.0001). Additionally, Ebstein’s anomaly patients had smaller left ventricular end diastolic volumes, with lower ejection fraction than controls (p = 0.0357 and p = 0.0195, respectively). As expected, the total right/left index score was significantly higher in Ebstein’s anomaly patients compared to controls (2.2 vs. 0.67, p 0.002).
Table 1: Participant characteristics, cardiac magnetic resonance ventricular volumes and function for Ebstein’s Anomaly patients vs. controls.
| Ebstein’s Anomaly (n = 36) | Controls (n = 36) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 36 ± 13 | 37 ± 11 | 0.6 |
| Gender (number male, % male) | 16 (44%) | 16 (44%) | >0.99 |
| Body mass index (kg/m2) | 25.5 ± 4.2 | 25.2± 6.1 | 0.8 |
| Imaging parameters | |||
| Functional RA volume (mL/m2) | 116 ± 91 | 41 ± 14 | <0.0001 |
| RVEDVi (mL/m2) | 112 ± 60 | 68 ± 15 | <0.0001 |
| RVESVi (mL/m2) | 49 ± 25 | 23 ± 9 | <0.0001 |
| RVEF (%) | 51 ± 11 | 67 ± 8 | <0.0001 |
| LVEDVi (mL/m2) | 60 ± 14 | 66 ± 12 | 0.0357 |
| LVESVi (mL/m2) | 20 ± 7 | 21 ± 6 | 0.9392 |
| LVEF (%) | 65 ± 8 | 69 ± 5 | 0.0195 |
| LV mass index (g/m2) | 53 ± 13 | 60 ± 13 | 0.0405 |
| Total right/total left index | 2.2 ± 2.6 | 0.67 ± 0.17 | 0.002 |
3.3 Reproducibility of Myocardial Strain Parameters
Right atrial, right ventricular and left ventricular strain parameters demonstrated good intra-observer reproducibility. The intraclass correlation coefficient for the right atrial global longitudinal strain was 0.8, whilst for both the right ventricular free wall plus septum global longitudinal strain and right ventricular free wall global longitudinal strain the intraclass correlation coefficient was 0.84. The intraclass correlation coefficient for the left ventricular strain parameters were as follows: left ventricular global circumferential strain, 0.86; left ventricular global radial strain 0.78 and left ventricular global longitudinal strain 0.84.
3.4 Myocardial Strain Differences between Ebstein’s Anomaly and Controls
Results for myocardial strain are summarised in Fig. 2. Patients with Ebstein’s Anomaly had impaired strain for all strain parameters compared to controls, except for left ventricular global radial strain. Right ventricular strain was reduced in Ebstein’s Anomaly patients compared to controls when both the septum was included and excluded in the analysis. However, right ventricular strain results for Ebstein’s Anomaly patients were not significantly different depending upon whether the septum was included or not in the analysis (p = 0.0741).
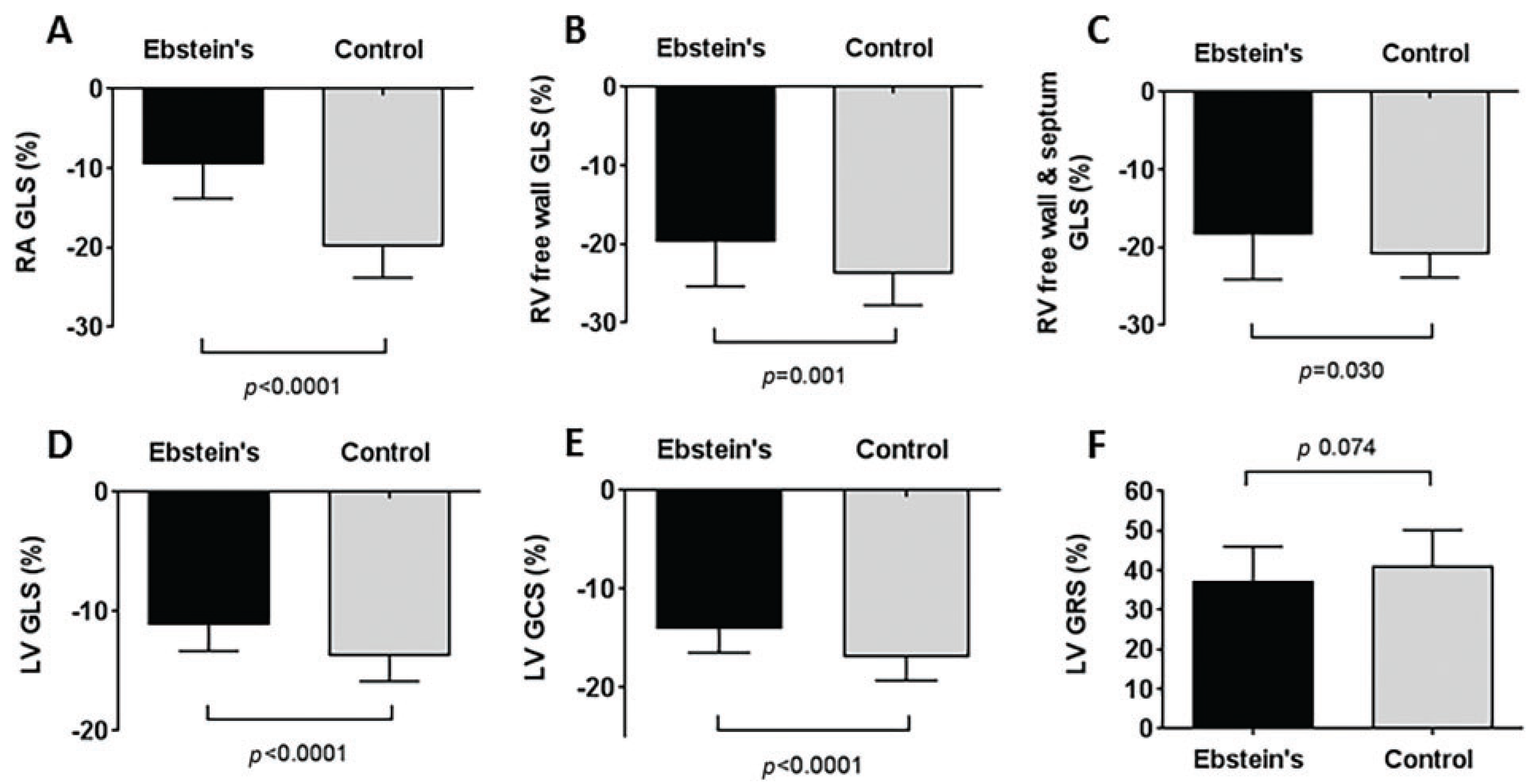
Figure 2: Results for myocardial strain parameters in patients with Ebstein’s Anomaly compared to controls. (A) Right atrial global longitudinal strain, (B) Right ventricular free wall global longitudinal strain, (C) Right ventricular free wall plus septum global longitudinal strain, (D) Left ventricular global longitudinal strain, (E) Left ventricular global circumferential strain, (F) Left ventricular global radial strain. p values calculated using independent t-tests.
3.5 Association of Myocardial Strain with Cardiopulmonary Exercise Parameters
Cardiopulmonary exercise test results within six months of cardiac magnetic resonance imaging were available for 27 (75%) of the Ebstein’s Anomaly patients, with these results shown in Table 2. The mean age of these patients was 36 ± 13 years, with 48% male. Twenty patients (74%) were NYHA Class I, 6 (22%) NYHA Class II, and 1 patient (4%) NYHA Class III. Patients exercised on average for 9 min, achieving a mean respiratory exchange ratio of 1.2 ± 0.1. Mean peak VO2 was 26.9 ± 8.5 mL/kg/min, representing 87 ± 20% age and gender predicted peak VO2. Peak VO2 did not correlate with right ventricular (p = 0.290) or left ventricular ejection fraction (p = 0.302). However, peak VO2 was inversely correlated with the right atrial global longitudinal strain (r = −0.448, p = 0.022). Additionally, right atrial global longitudinal strain positively correlated with the VE/VCO2 slope (r = 0.435, p = 0.026), see Fig. 3, whereas ventricular ejection fraction did not.
Table 2: Clinical, cardiopulmonary and echocardiography parameters for patients with Ebstein’s Anomaly.
| Ebstein’s Anomaly n = 36 (%) | |
|---|---|
| NYHA class, n (%) | |
| I | 26 (72) |
| II | 9 (25) |
| III | 1 (3) |
| Severity of TR by echocardiogram, n (%) | |
| None | 0 |
| Mild | 9 (25) |
| Moderate | 10 (28) |
| Severe | 17 (47) |
| PFO present, n (%) | 5 (14) |
| ASD present, n (%) | 6 (17) |
| CPEX results | |
| Duration of exercise, min | 9.0 ± 2.2 |
| Maximum workload, RER peak | 1.2 ± 0.1 |
| Percentage peak age predicted heart rate, % | 90 ± 13 |
| Peak VO2, mL/kg/min | 26.9 ± 8.5 |
| Percentage age predicted peak VO2, % | 87 ± 20 |
| VE/VCO2 slope | 30.7 ± 6.1 |
| Echo results | |
| RV S velocity, m/s | 14.5 ± 6.6 |
| TAPSE, mm | 26 ± 7 |
| RVSP, mmHg | 37.2 ± 10.7 |
| Mean PAP, mmHg | 24.7 ± 6.5 |
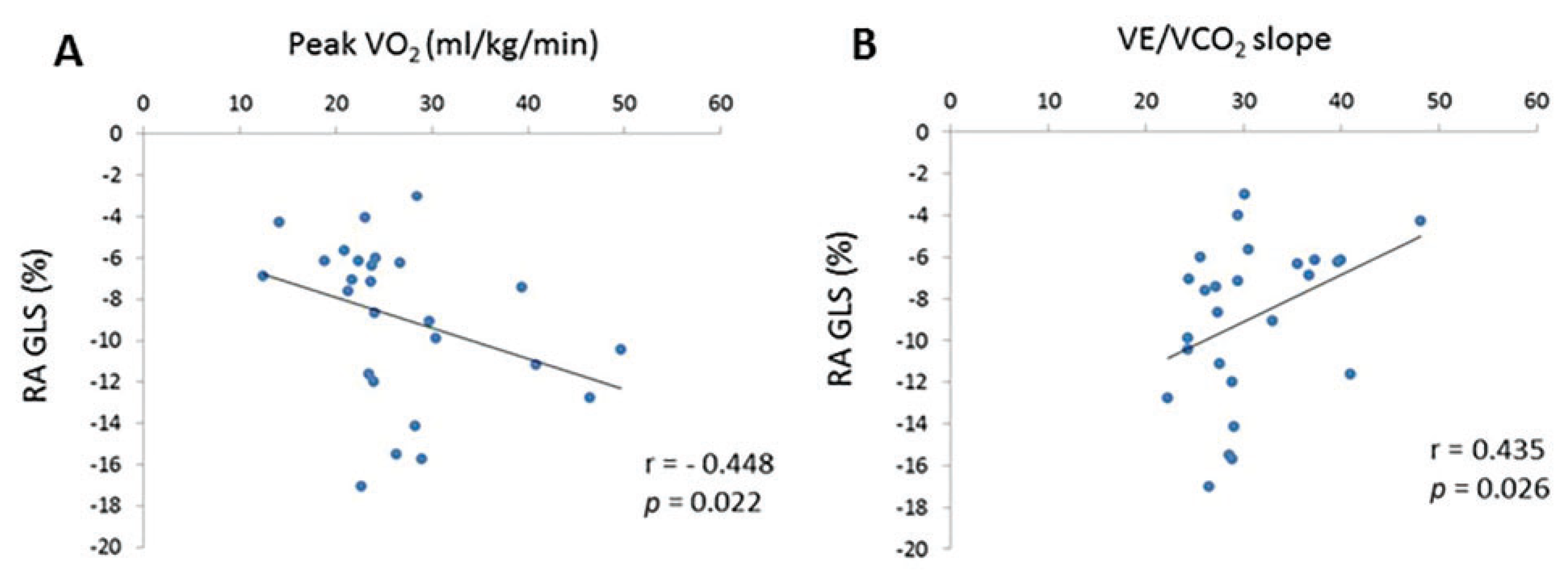
Figure 3: Correlations for patients with Ebstein’s Anomaly between right atrial global longitudinal strain and (A) Peak VO2 max and (B) VE/VCO2 slope. Correlation assessed using the Spearman method.
3.6 Severity of Ebstein’s Anomaly and Relationship to Myocardial Strain, Echocardiography Results and Exercise Capacity
Patients with Ebstein’s Anomaly were split into two groups based on the median total right/left index of 1.3 (range 0.6–12). For the patients classified as mild, the mean right atrial global longitudinal strain was −9.4 ± 3.7%, compared to −9.7 ± 4.8, for the severe patients p = 0.84. For the 18 patients in the severe Ebstein’s Anomaly category the mean right ventricular global longitudinal strain (using the mean composite of the two right ventricular strain parameters, with and without septum) was significantly impaired compared to the Ebstein’s Anomaly patients classified as mild (right ventricular global longitudinal strain −17.5 ± 5.4% in severe category vs. −21.4 ± 4.4% in the mild category, p = 0.0017). For all patients, the average right ventricular global longitudinal strain correlated positively with the total right/left index (r = 0.528, p = 0.001), as well as the VE/VCO2 slope (r = 0.614, p = 0.026).
This study has demonstrated that myocardial deformation indices for the right atrium, right ventricle and left ventricle are impaired in patients with Ebstein’s Anomaly. Additionally, we found that neither right nor left ventricular ejection fraction was associated with peak oxygen uptake during cardiopulmonary exercise testing. However, the right atrial global longitudinal strain was found to be inversely correlated to the peak oxygen uptake during exercise and positively correlated with the VE/VCO2 slope. To our knowledge this is the first study identifying an association between right atrial function and exercise capacity in patients with Ebstein’s Anomaly.
4.1 Left Ventricular Strain Parameters and Clinical Implications in Ebstein’s Anomaly Patients
In keeping with the work of Liu et al. [23], we found left ventricular global longitudinal and circumferential strain parameters to be impaired in patients with Ebstein’s Anomaly. However, unlike Liu et al. [23], we did not find a statistically significant difference for left ventricular global radial strain, although there was a trend towards this being lower in the Ebstein’s Anomaly patient group. Previous studies [9] have found impairment of left ventricular ejection fraction to be associated with adverse cardiovascular outcomes in patients with Ebstein’s Anomaly. Although our patients had lower left ventricular ejection fractions than controls, the values were still within the normal range, suggesting that impairment in left ventricular global longitudinal strain and global circumferential strain may be early prognostic markers that might possibly be of clinical utility in patients with Ebstein’s Anomaly.
4.2 Right Heart Strain and Clinical Implications in Ebstein’s Anomaly Patients
The study by Steinmetz et al. [10] utilized a different analysis technique for strain analysis which likely explains the difference in absolute values obtained between that study and our own results. However, our results are in keeping with this previous work with both studies demonstrating impairment of right ventricular global longitudinal strain and right atrial global longitudinal strain in patients with Ebstein’s Anomaly compared to controls. Additionally, Steinmetz et al. [10] found that both right ventricular and right atrial strain impairment correlated to the patients’ New York Heart Association symptom severity and B-type natriuretic peptide levels, which are both established markers of heart failure severity. They did not however assess whether any right heart strain parameters correlated with an objective measure of exercise capacity, such as we have demonstrated with the association between right atrial global longitudinal strain, peak oxygen uptake capacity, and the VE/VCO2 slope. Peak oxygen uptake has previously been established as an independent predictor of outcome in patients with Ebstein’s Anomaly [3], whilst an increased VE/VCO2 slope was found to be related to a higher risk of death in a noncyanotic congenital heart disease cohort [24].
The importance of right atrial myocardial deformation indices was also demonstrated in an echocardiography study by Prota et al. [25] in pediatric patients with Ebstein’s Anomaly. They found that functional right ventricular fractional area change and right atrium peak systolic strain were lower in patients with Ebstein’s Anomaly experiencing adverse clinical events, compared to those who were stable. These imaging parameters were independent predictors of progressive disease (defined as need for surgery, ventricular tachycardia, heart failure, and/or death) at multivariant analysis.
Studies assessing right atrial strain in the context of severe tricuspid regurgitation may also be pertinent to these patients with unoperated Ebstein’s Anomaly, due to the high prevalence of significant valvular dysfunction. An echocardiography study by Teixeira et al. found in a multivariate linear regression model that the volume of tricuspid regurgitation was independently associated with right atrial strain after adjusting for the RA area, RV longitudinal systolic function and the estimated pulmonary vascular resistance [26]. A study assessing right atrial performance in a different group of congenital heart disease patients; Tetralogy of Fallot, found right atrial longitudinal strain to be abnormal in these patients. However, they did not see any correlation between right atrial longitudinal strain and the degree of tricuspid regurgitation and/or peak oxygen uptake [27].
The right atrium is known to function in a triphasic manner; with a reservoir phase, conduit phase and contractile phase [28]. Impaired atrial function is of increasing interest, both in acquired [29] and congenital heart diseases such as Tetralogy of Fallot [27] as a potential early marker of ventricular dysfunction and disease severity [30]. Right atrial function not only reflects changes in myocardial deformation but is also influenced by right ventricular function, pre-load conditions including tricuspid regurgitation, and afterload conditions including pulmonary artery pressures. This may explain why we found right atrial global longitudinal strain to be the myocardial deformation parameter most closely associated with exercise performance in patients with Ebstein’s Anomaly.
Other MRI parameters including right ventricular/left ventricular end diastolic volume ratio, total right/left volumes and apical septal leaflet displacement have previously been found to be associated with first onset of atrial tachycardia in patients with Ebstein’s Anomaly [9]. Further work is required to identify whether right atrial strain has incremental value to conventional imaging and clinical parameters in predicting disease severity, the need for surgical intervention and prognosis in patients with Ebstein’s Anomaly.
Patients with cardiac devices (pacemakers/defibrillators) were excluded from this study due to the contra-indication for CMR, which may have introduced a patient selection bias for the severity of Ebstein’s Anomaly. Contouring the thin right atrial wall, especially when distorted as may be the case in Ebstein’s anomaly, can be challenging. Our analyses were performed in a single cine plane and in retrospect, clarifying the validity of the strains from multiple planes of the right atrium would have further validated our work. The cut-off of 1.3 for right/left index was chosen based on the median of our results and not any published prognostic value. The clinical utility of thresholds for right/left index still needs to be determined in future longitudinal studies. Only right atrial global longitudinal strain was quantified for assessment of atrial performance in these patients as we felt this was likely to be the parameter that would have the most influence upon a patient’s exercise capacity and be a robust measure with good reproducibility. However, we did not assess the reservoir or conduit function of the RA and these parameters may have given additional insight into the complex mechanics of these abnormally structured right hearts.
This study is a single center and therefore limited to a small cohort size in keeping with the rare nature of this congenital abnormality. No assessment of the stability of strain parameters over time has been possible as only a single CMR study has been analyzed for each participant. A prospective longitudinal study would be of benefit to assess the stability of strain imaging in this patient cohort and the correlation of impairment in strain parameters with adverse clinical events, such as heart failure and arrhythmia.
Myocardial strain indices for the right atrium, right ventricle, and left ventricle are impaired in patients with Ebstein’s Anomaly compared to healthy controls. The right atrial global longitudinal strain was associated with objective measurements of exercise capacity. Further investigation of right atrial global longitudinal strain as a prognostic marker in patients with Ebstein’s Anomaly is warranted in future larger longitudinal studies.
Acknowledgement:
Funding Statement: Victoria Stoll is funded by an NIHR Clinical Lectureship. Ahmed Dardeer is funded by the Egyptian Mission Sector. William Moody was supported by a British Heart Foundation Clinical Research Fellowship (FS/11/17/28700). The CRIB-Donor study (that the healthy controls were recruited to) was performed in the National Institute for Health Research/Wellcome Clinical Research Facility and was also supported by a Queen Elizabeth Hospital Birmingham Charity Grant.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ahmed M. Dardeer, Victoria M. Stoll, Boyang Liu, Colin D. Chue, Roman Wesolowski, Lucy E. Hudsmith and Richard P. Steeds; data collection: Ahmed M. Dardeer, Victoria M. Stoll, Boyang Liu, William E. Moody, Roman Wesolowski, Lucy E. Hudsmith and Richard P. Steeds; analysis and interpretation of results: Ahmed M. Dardeer, Victoria M. Stoll, Boyang Liu, William E. Moody, Paul Clift, Roman Wesolowski, Lucy E. Hudsmith and Richard P. Steeds; draft manuscript preparation: Ahmed M. Dardeer, Victoria M. Stoll, Boyang Liu, William E. Moody, Colin D. Chue, Paul Clift, Roman Wesolowski, Lucy E. Hudsmith and Richard P. Steeds. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets analysed during the current study are not publicly available due to patient confidentiality, but the numerical results generated from the images are available from the corresponding author on reasonable request.
Ethics Approval: This observational retrospective study was approved by the local Clinical Governance Committee (RRK6237) and conformed to the principles of Good Clinical Practice guidelines. Additionally, healthy controls were identified from a national ethics board approved observational CMR study (NCT01028703).
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Pasqualin G, Boccellino A, Chessa M, Ciconte G, Marcolin C, Micaglio E, et al. Ebstein’s anomaly in children and adults: multidisciplinary insights into imaging and therapy. Heart. 2024;110(4):235–44. 10.1136/heartjnl-2023-322420. [Google Scholar] [CrossRef]
2. Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation. 2007;115(2):277–85. 10.1161/CIRCULATIONAHA.106.619338. [Google Scholar] [CrossRef]
3. Radojevic J, Inuzuka R, Alonso-Gonzalez R, Borgia F, Giannakoulas G, Prapa M, et al. Peak oxygen uptake correlates with disease severity and predicts outcome in adult patients with Ebstein’s anomaly of the tricuspid valve. Int J Cardiol. 2013;163(3):305–8. 10.1016/j.ijcard.2011.06.047. [Google Scholar] [CrossRef]
4. Trojnarska O, Szyszka A, Gwizdała A, Siniawski A, Oko-Sarnowska Z, Chmara E, et al. Adults with Ebstein’s anomaly—cardiopulmonary exercise testing and BNP levels exercise capacity and BNP in adults with Ebstein’s anomaly. Int J Cardiol. 2006;111(1):92–7. 10.1016/j.ijcard.2005.07.019. [Google Scholar] [CrossRef]
5. Eckerström F, Dellborg M, Hjortdal VE, Eriksson P, Mandalenakis Z. Mortality in patients with ebstein anomaly. J Am Coll Cardiol. 2023;81(25):2420–30. 10.1016/j.jacc.2023.04.037. [Google Scholar] [CrossRef]
6. Dorfman AL, Geva T, Samyn MM, Greil G, Krishnamurthy R, Messroghli D, et al. SCMR expert consensus statement for cardiovascular magnetic resonance of acquired and non-structural pediatric heart disease. J Cardiovasc Magn Reson. 2022;24(1):44. 10.1186/s12968-022-00873-1. [Google Scholar] [CrossRef]
7. Qureshi MY, O’Leary PW, Connolly HM. Cardiac imaging in ebstein anomaly. Trends Cardiovasc Med. 2018;28(6):403–9. 10.1016/j.tcm.2018.01.002. [Google Scholar] [CrossRef]
8. Hösch O, Sohns JM, Nguyen TT, Lauerer P, Rosenberg C, Kowallick JT, et al. The total right/left-volume index: a new and simplified cardiac magnetic resonance measure to evaluate the severity of Ebstein anomaly of the tricuspid valve: a comparison with heart failure markers from various modalities. Circ Cardiovasc Imaging. 2014;7(4):601–9. 10.1161/CIRCIMAGING.113.001467. [Google Scholar] [CrossRef]
9. Rydman R, Shiina Y, Diller GP, Niwa K, Li W, Uemura H, et al. Major adverse events and atrial tachycardia in Ebstein’s anomaly predicted by cardiovascular magnetic resonance. Heart. 2018;104(1):37–44. 10.1136/heartjnl-2017-311274. [Google Scholar] [CrossRef]
10. Steinmetz M, Broder M, Hösch O, Lamata P, Kutty S, Kowallick JT, et al. Atrio-ventricular deformation and heart failure in Ebstein’s Anomaly—a cardiovascular magnetic resonance study. Int J Cardiol. 2018;257:54–61. 10.1016/j.ijcard.2017.11.097. [Google Scholar] [CrossRef]
11. Rajiah PS, Kalisz K, Broncano J, Goerne H, Collins JD, François CJ, et al. Myocardial strain evaluation with cardiovascular MRI: physics, principles, and clinical applications. Radiographics. 2022;42(4):968–90. 10.1148/rg.210174. [Google Scholar] [CrossRef]
12. Anderson KR, Zuberbuhler JR, Anderson RH, Becker AE, Lie JT. Morphologic spectrum of ebstein’s anomaly of the heart: a review. Mayo Clin Proc. 1979;54(3):174–80. [Google Scholar]
13. Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin ELS, Taylor RJ, et al. Cardiovascular effects of unilateral nephrectomy in living kidney donors. Hypertension. 2016;67(2):368–77. 10.1161/hypertensionaha.115.06608. [Google Scholar] [CrossRef]
14. Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27(23):2879–88. 10.1093/eurheartj/ehl336. [Google Scholar] [CrossRef]
15. Liu B, Dardeer AM, Moody WE, Edwards NC, Hudsmith LE, Steeds RP. Normal values for myocardial deformation within the right heart measured by feature-tracking cardiovascular magnetic resonance imaging. Int J Cardiol. 2018;252:220–3. 10.1016/j.ijcard.2017.10.106. [Google Scholar] [CrossRef]
16. Liu B, Dardeer AM, Moody WE, Hayer MK, Baig S, Price AM, et al. Reference ranges for three-dimensional feature tracking cardiac magnetic resonance: comparison with two-dimensional methodology and relevance of age and gender. Int J Cardiovasc Imaging. 2018;34(5):761–75. 10.1007/s10554-017-1277-x. [Google Scholar] [CrossRef]
17. Wadey CA, Weston ME, Dorobantu DM, Pieles GE, Stuart G, Barker AR, et al. The role of cardiopulmonary exercise testing in predicting mortality and morbidity in people with congenital heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(3):513–33. 10.1093/eurjpc/zwab125. [Google Scholar] [CrossRef]
18. Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, et al. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2015;2(1):G9–G24. 10.1530/ERP-14-0079. [Google Scholar] [CrossRef]
19. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23(5):e171–232. 10.1093/ehjci/jeab253. [Google Scholar] [CrossRef]
20. Jang AY, Shin MS. Echocardiographic screening methods for pulmonary hypertension: a practical review. J Cardiovasc Imaging. 2020;28(1):1–9. 10.4250/jcvi.2019.0104. [Google Scholar] [CrossRef]
21. Kutty S, Sengupta PP, Khandheria BK. Patent foramen ovale: the known and the to be known. J Am Coll Cardiol. 2012;59(19):1665–71. 10.1016/j.jacc.2011.09.085. [Google Scholar] [CrossRef]
22. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr. 2015;28(8):910–58. 10.1016/j.echo.2015.05.015. [Google Scholar] [CrossRef]
23. Liu X, Zhang Q, Yang ZG, Shi K, Xu HY, Xie LJ, et al. Assessment of left ventricular deformation in patients with Ebstein’s anomaly by cardiac magnetic resonance tissue tracking. Eur J Radiol. 2017;89:20–6. 10.1016/j.ejrad.2017.01.013. [Google Scholar] [CrossRef]
24. Inuzuka R, Diller GP, Borgia F, Benson L, Tay ELW, Alonso-Gonzalez R, et al. Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation. 2012;125(2):250–9. 10.1161/CIRCULATIONAHA.111.058719. [Google Scholar] [CrossRef]
25. Prota C, Di Salvo G, Sabatino J, Josen M, Paredes J, Sirico D, et al. Prognostic value of echocardiographic parameters in pediatric patients with Ebstein’s anomaly. Int J Cardiol. 2019;278:76–83. 10.1016/j.ijcard.2018.10.046. [Google Scholar] [CrossRef]
26. Teixeira R, Monteiro R, Garcia J, Baptista R, Ribeiro M, Cardim N, et al. The relationship between tricuspid regurgitation severity and right atrial mechanics: a speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2015;31(6):1125–35. 10.1007/s10554-015-0663-5. [Google Scholar] [CrossRef]
27. Kutty S, Shang Q, Joseph N, Kowallick JT, Schuster A, Steinmetz M, et al. Abnormal right atrial performance in repaired tetralogy of Fallot: a CMR feature tracking analysis. Int J Cardiol. 2017;248:136–42. 10.1016/j.ijcard.2017.06.121. [Google Scholar] [CrossRef]
28. Truong VT, Palmer C, Young M, Wolking S, Ngo TNM, Sheets B, et al. Right atrial deformation using cardiovascular magnetic resonance myocardial feature tracking compared with two-dimensional speckle tracking echocardiography in healthy volunteers. Sci Rep. 2020;10(1):5237. 10.1038/s41598-020-62105-9. [Google Scholar] [CrossRef]
29. Yan P, Sun B, Shi H, Zhu W, Zhou Q, Jiang Y, et al. Left atrial and right atrial deformation in patients with coronary artery disease: a velocity vector imaging-based study. PLoS One. 2012;7(12):e51204. 10.1371/journal.pone.0051204. [Google Scholar] [CrossRef]
30. Inoue K, Smiseth OA. Left atrium as key player and essential biomarker in heart failure. J Cardiol. 2025;85(1):8–16. 10.1016/j.jjcc.2024.07.006. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools