 Open Access
Open Access
ARTICLE
The Norwood with Blalock-Taussig-Thomas Shunt—Worth a Second Look?
1 School of Medicine, University of Missouri-Kansas City, Kansas City, MO 64108, USA
2 Department of Cardiovascular and Thoracic Surgery, Division of Pediatric Cardiothoracic Surgery, West Virginia University, Morgantown, WV 26506, USA
3 Department of Surgery, Parkview Health, Fort Wayne, IN 46845, USA
4 Department of Surgery, Division of Congenital Cardiac Surgery, Children’s Mercy Hospital, Kansas City, MO 64108, USA
* Corresponding Author: Manasa Gadiraju. Email:
Congenital Heart Disease 2024, 19(5), 435-443. https://doi.org/10.32604/chd.2025.059705
Received 15 October 2024; Accepted 17 December 2024; Issue published 31 December 2024
Abstract
Background: Shunt repair for the Norwood procedure with either the right ventricle to pulmonary artery shunt (RVPAS) or the modified Blalock-Taussig-Thomas Shunt (BTTS) varies by institution and surgeon preference. Shunt choice has been informed by landmark trials including the Single Ventricle Reconstruction trial and modern outcomes data may engender future complementary studies. Methods: We conducted a retrospective analysis of all patients who underwent the Norwood procedure from 2014–2022 at a single center to compare outcomes by shunt type. The primary outcome measure was freedom from death or transplant. Secondary outcome measures included hospital length of stay, complications, and unplanned interventions. Results: 93 patients underwent the Norwood procedure at a median age of 7 days (IQR 5, 9) and 39 weeks gestation (IQR 38, 39). 67.7% had hypoplastic left heart syndrome. 39 patients received a BTTS compared to 54 RVPAS. There was no difference in operative mortality (BTTS 12.8%, RVPAS 9.3%, p = 0.58), death or transplant at 1 year (BTTS 15.4%, RVPAS 7.4%, p = 0.31), or between 1 and 3 years (BTTS 0%, RVPAS 5.6%, p = 0.26). There was a significantly higher rate of pulmonary arterial stenting in the RVPAS group (BTTS 1.21/100 patient-years, RVPAS 15.68/100 patient-years, p = 0.01). Conclusions: Similar short- and medium-term survival were seen in BTTS and RVPAS groups with fewer pulmonary artery interventions for BTTS, though our study is underpowered to suggest superior freedom from interventions. These results may serve as a hypothesis-generating study to revisit the SVR trial with a modern cohort in the setting of improved surgical technique and perioperative management.Keywords
Glossary/Nomenclature/Abbreviations
| BTTS | Blalock-Taussig-Thomas Shunt |
| HLHS | Hypoplastic Left Heart Syndrome |
| RVPAS | Right-Ventricle-to-Pulmonary-Artery Shunt |
Since it was first described in 1980, the eponymous Norwood procedure and subsequent two-stage separation of the systemic and pulmonary circulations remain the preferred palliation to improve survival odds in infants with hypoplastic left heart syndrome (HLHS) and other single ventricle variants [1]. However, despite improvements in operative approaches and techniques, surgical critical care, and interstage monitoring in the ensuing decades, three-stage palliation continues to carry high operative and long-term mortality [2]. In 2003, Sano reported remarkable improvement in operative survival with the right ventricle to pulmonary artery shunt (RVPAS). Two years later, the Single Ventricle Reconstruction (SVR) trial initiated enrollment in the seminal randomized control trial [3,4]. The observation of better one-year survival in patients with the RVPAS modification compared to those with a systemic to pulmonary artery Blalock-Taussig-Thomas shunt (BTTS) spurred a significant switch in practice patterns toward the former, with some reports suggesting nearly two-thirds of shunts nationally during the Norwood procedure being the RVPAS [5]. Subsequent manuscripts from the SVR trial and other studies have however demonstrated changing hazard functions that indicate the lasting effects of the RVPAS modification compared to the transient interstage effect of the BTTS shunt. Findings include similar long-term transplant-free survival with increased unintended interventions for the RVPAS groups [4–6]. However, these studies reflect surgeries performed in a different era with novel surgical techniques, evolving perioperative critical care, and the absence of pervasive interstage monitoring processes. There may be a need to revisit the question of shunt selection for Norwood palliation in a contemporary experience. We have begun to address these questions by conducting a retrospective, single-center analysis of patients undergoing the Norwood procedure focusing on survival differences and rates of catheter-based interventions.
A single-center retrospective review was performed for consecutive patients undergoing the Norwood procedure between 01 January 2014 and 31 December 2022. The study was performed in accordance with the principles of the Declaration of Helsinki and ethical approval was obtained under the Children’s Mercy Hospital Institutional Review Board Heart Center Database waiver approval (Protocol #13020045). Data for demographics, imaging studies, intraoperative details, pre and postoperative courses, postoperative cardiac catheterizations, subsequent surgical procedures, and all-cause mortalities were supplied by the Heart Center Database.
93 consecutive patients underwent the Norwood procedure with either BTTS or RVPAS at our institution between 2014 and 2022. Of these patients, 39 received a BTTS and 54 received an RVPAS shunt. Prior to February 2017, all patients (34) received exclusively BTTS. In 2017, a center change was made to implant predominantly RVPAS. This was largely driven by changes in surgical staff and the finding of increased rates of perioperative instability with the BTTS patients over the previous year. Outcomes were compared between these two groups. The primary outcome measure was freedom from death or transplant. Secondary outcome measures included hospital length of stay, incidence of extracorporeal membrane oxygenation (ECMO) and cardiopulmonary resuscitation (CPR), incidence of stroke and seizures after the Norwood procedure in the index admission, and the need for unplanned surgical and catheterization procedures after the Norwood palliation.
Medians and interquartile ranges were derived for continuous variables. Percentages were derived for binary variables. Mann-Whitney U tests were performed to compare continuous variables among two groups. Chi-square tests and Fischer’s exact tests were performed to compare binary variables among two groups. Bivariate Cox regression analysis was performed to compare transplant-free survival among two shunt groups adjusting for ventricular dominance. Unadjusted Kaplan-Meier survival curves were derived for freedom from death or transplant for both shunt groups and compared with a log-rank test. The student’s t-test was performed to compare unintended interventions between two shunt types. Conditional survival at various follow-up time periods was compared by Chi-square test or Fischer’s exact test. All tests were two-sided. A p-value of less than 0.05 was considered statistically significant. Statistical software Stata version 17 was used for statistical analysis [7].
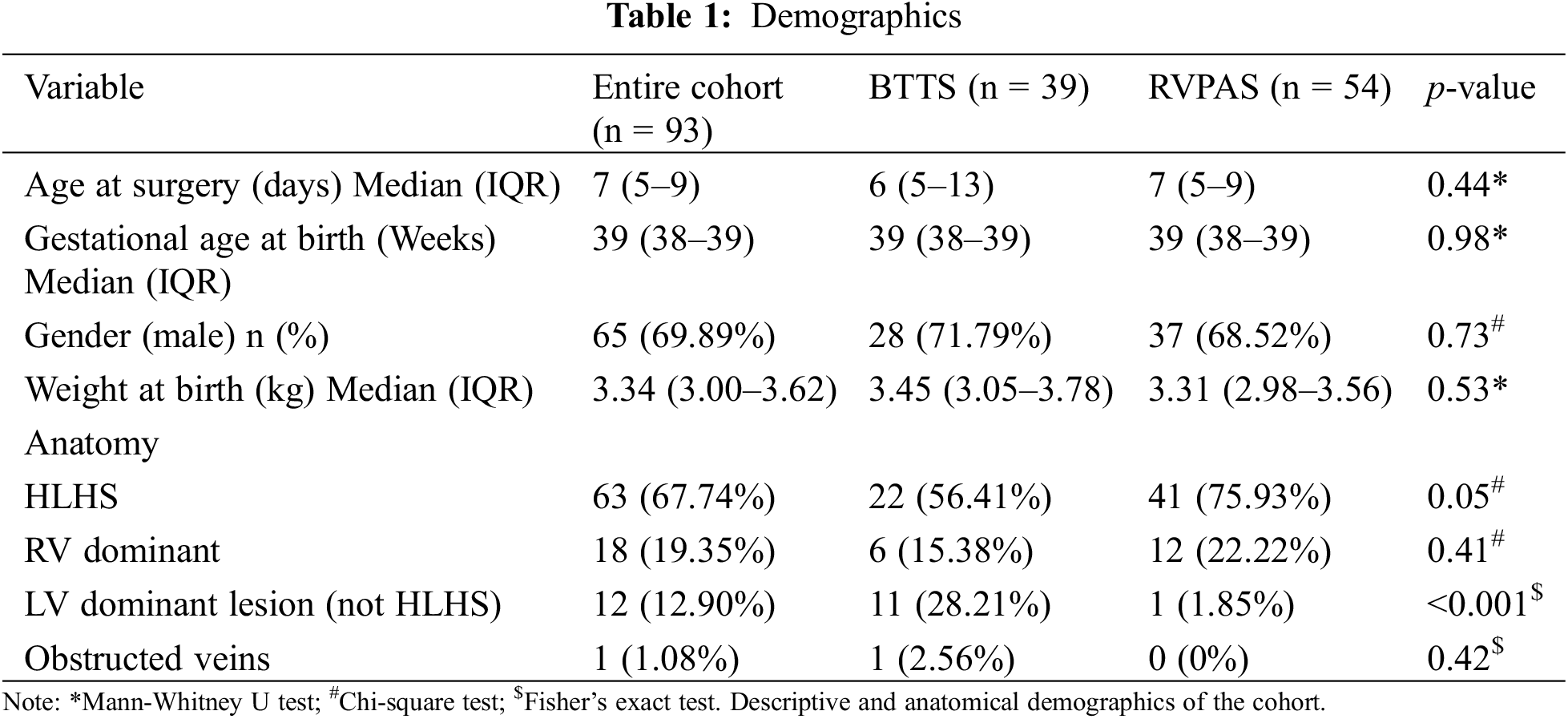
For the cohort, the median age at surgery was 7 days old, the median gestational age was 39 weeks, and the median weight at birth was 3.34 kg. 30% of patients were female. These demographics were similar between shunt groups (Table 1). There were significantly more HLHS patients in the RVPAS group (56.41% BTTS vs. 75.93% RVPAS, p = 0.05) and significantly more non-HLHS left ventricular (LV) dominant lesion anatomy subtypes in the BTTS group (28.21% BTTS vs. 1.85% RVPAS, p < 0.001) (Table 1). Operative mortality was similar between groups (12.82% BTTS vs. 9.26% RVPAS, p = 0.58) (Table 2). Neither median time to extubation (6 days BTTS vs. 7 days RVPAS, p = 0.12), time to delayed sternal closure (4 days BTTS vs. 4 days RVPAS, p = 0.81), nor hospital length of stay (67 days BTTS vs. 61 days RVPAS, p = 0.89) differed between groups (Table 2). Notably, neither CPR (20.51% BTTS vs. 12.96% RVPAS, p = 0.33) nor need for ECMO after the Norwood procedure (23.08% BTTS vs. 18.52% RVPAS, p = 0.59) varied between groups (Table 2).
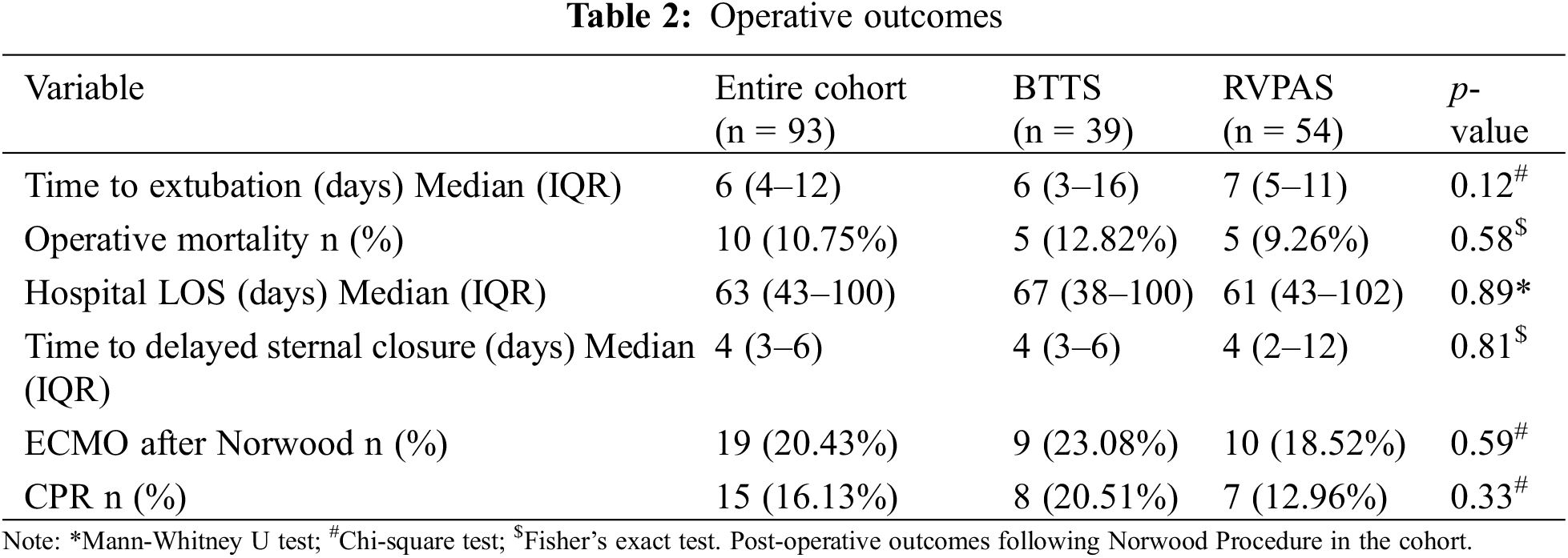
3.1 Unintended Interventions and Complications
There was a higher burden of pulmonary arterial stent angioplasty in the RVPAS group in the follow-up period (15.68 per 100 person-years RVPAS vs. 6.10 per 100 person-years BTTS, p = 0.01). Similarly, balloon intervention on the aortic arch was found to occur at a higher rate in the RVPAS group (40.7 per 100 person-years RVPAS vs. 16.35 per 100 person-years BTTS, p = 0.02). There was a trend towards higher rates of balloon pulmonary angioplasty in the RVPAS group, but this did not achieve significance (p = 0.07). No other difference was found in the evaluated unintended interventions between the groups (Table 3).
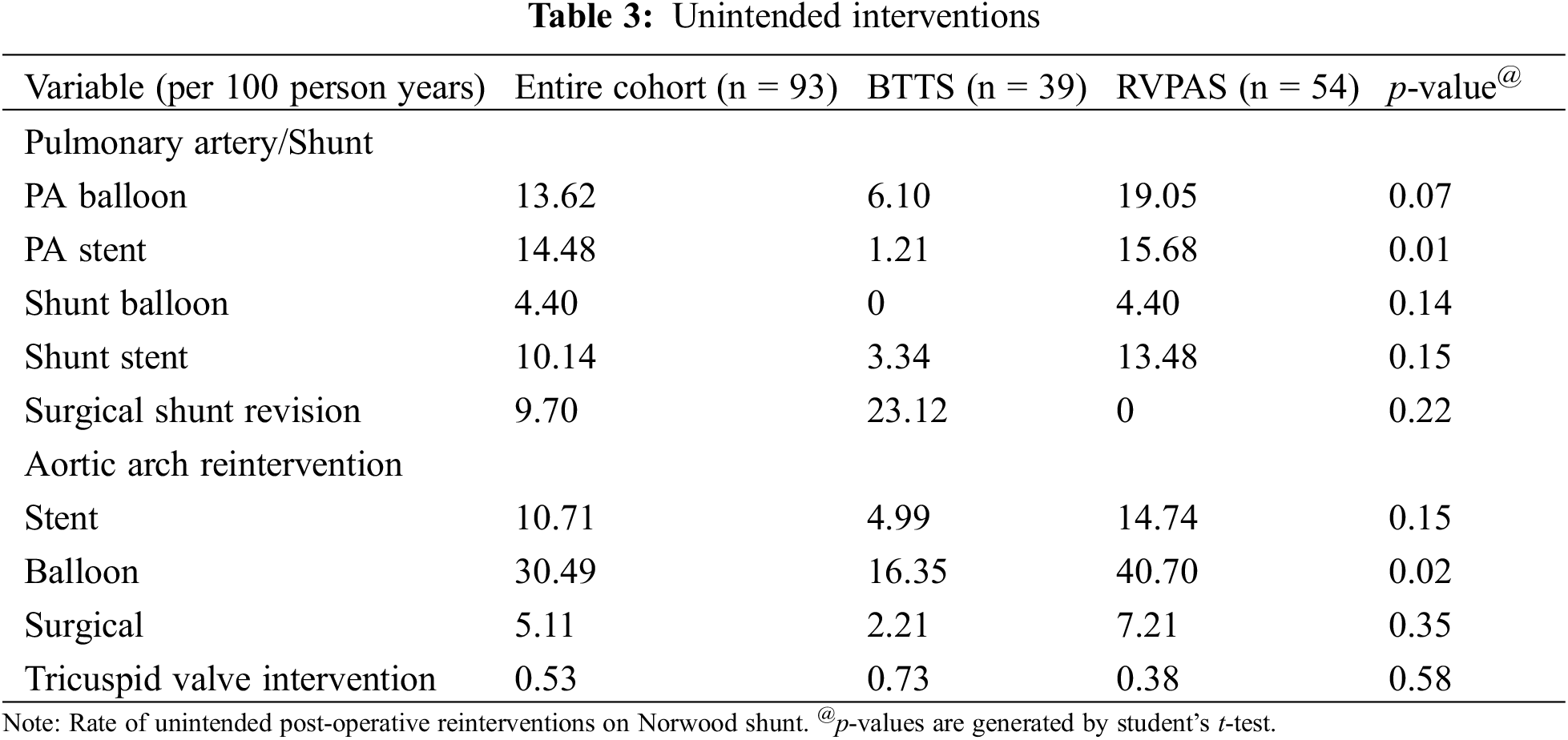
During the period under study, there was no shunt thrombosis in the cohort. Observed complications included a permanent pacemaker implantation rate of 10.8%, stroke rate of 8.6%, and seizure incidence of 7.53%. These rates did not significantly differ between the shunt groups (Table 4).
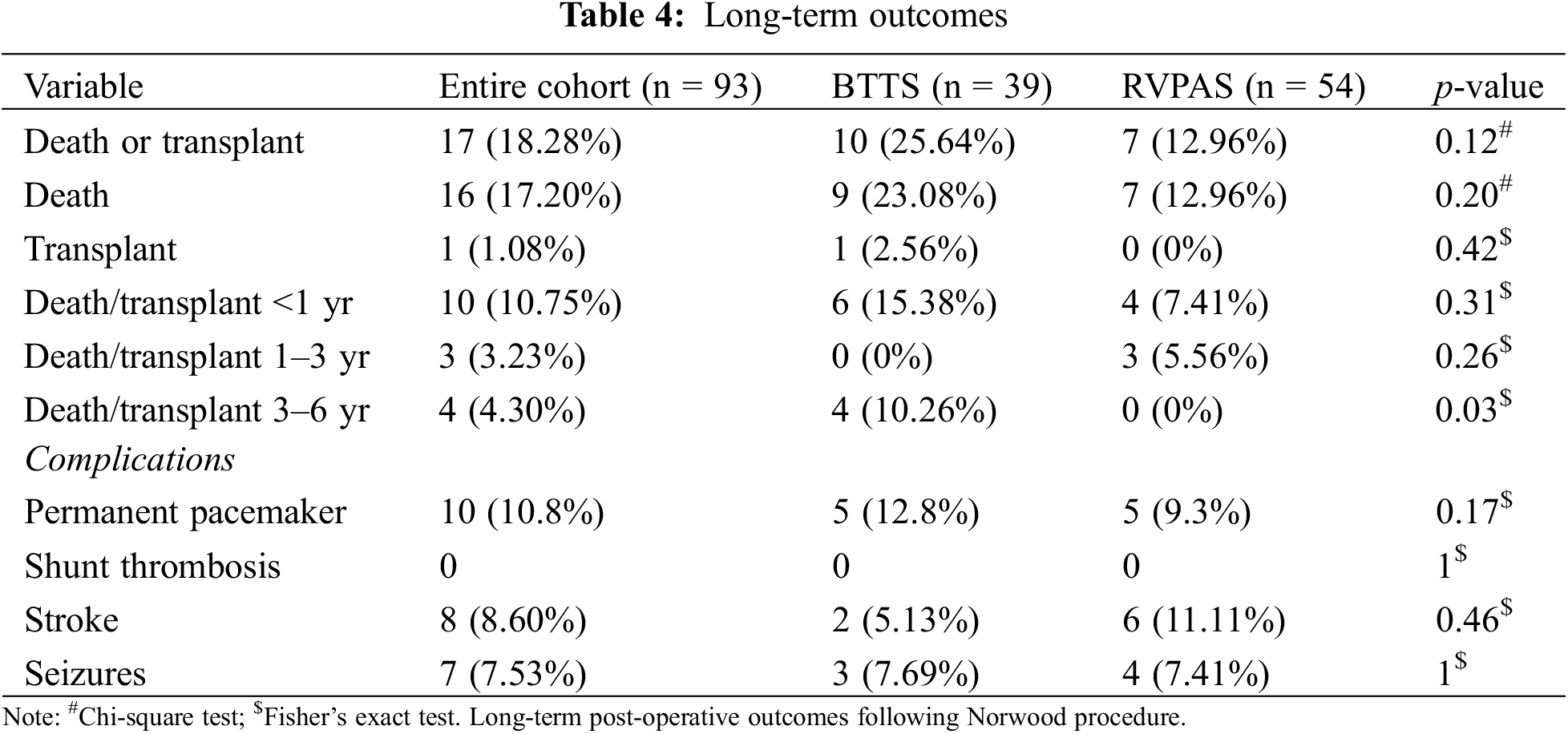
The median follow-up time after discharge from the Norwood operation was 2.8 years. Kaplan-Meier transplant-free survival was similar between the two groups (log-rank p-value = 0.718) (Fig. 1). Given the higher prevalence of LV dominant lesions in the historical BTTS control, the cox-proportional hazards model adjusted for ventricular dominance did not reveal a statistically significant impact on transplant-free survival in the two shunt groups (p = 0.63, HR 1.30, 95% CI 0.45–3.79). There was no difference between groups in early or mid-term mortality. Conditional on surviving the index hospitalization, or surviving to thirty days postoperatively if discharged, there was no difference in death or transplant in the first to third years after surgery. 15.38% of surviving BTTS and 7.41% of surviving RVPAS groups died or received a heart transplant in the first year (p = 0.31); 0% and 5.56% of BTTS and RVPAS respectively died or were transplanted between years 1 and 3 (p = 0.26) (Table 4). Of the ten patients who died within the first year, nine died without discharge from the hospital. Eight were patients who required ECMO perioperatively. Four achieved the Glenn palliation (two in each group). Four of six BTTS, and one of four RVPAS patients died at less than 4 months from surgery. The percentage of patients who died or received a heart transplant was significantly different between years 3 and 6 (10.26% BTTS vs. 0% RVPAS, p = 0.03) (Table 4). However, there were 26 BTTS patients alive without transplant at 3 years while only 9 patients remained in the RVPAS group at this timepoint (Fig. 1).
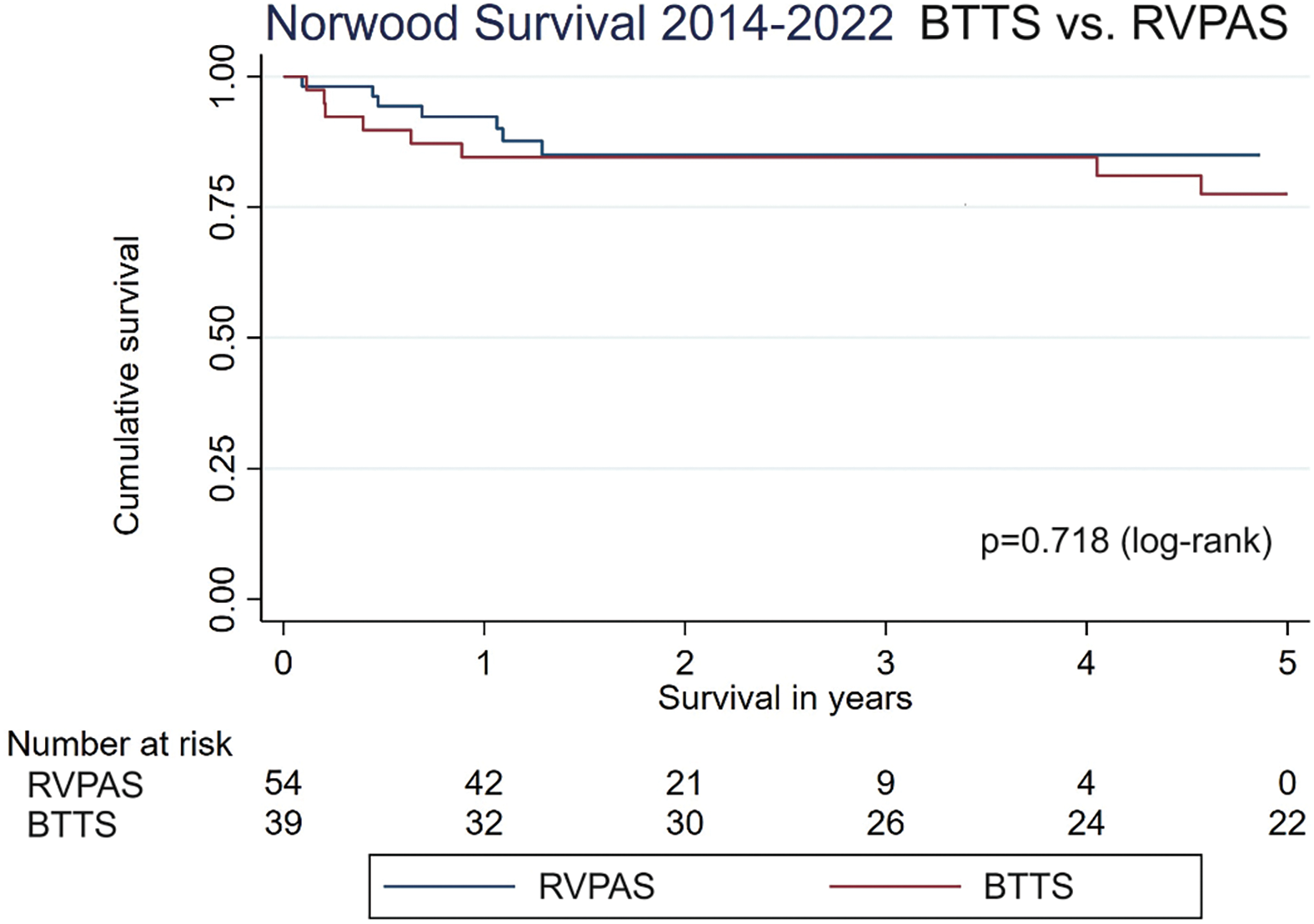
Figure 1: Norwood survival 2014–2022. Kaplan-Meier survival curve for Norwood with BTTS and RVPAS in the cohort
The Norwood procedure with staged pulmonary and systemic circulatory separation is an important palliative pathway for single ventricle physiology that continues to be associated with high interstage mortality and suboptimal postoperative survival and freedom from transplantation [8–12]. The SVR trial and its subsequent follow-up studies noted higher rates of transplant-free survival for the RVPAS only during the first twelve months post-randomization with subsequent changes in the hazard function leading to the eradication of differences by the third year after randomization, a finding which persists post-Fontan palliation [4]. At most recent follow-up at twelve years, the main difference between the groups was the presence of protein-losing enteropathy which was higher in the RVPAS group. The authors ascribed this either to chance, the potential differences in the pulmonary vascular bed from the known burden of pulmonary arterial interventions, or right-ventricular diastolic dysfunction from ventriculotomies for the RVPAS [13].
Other retrospective studies have shown no survival differences between the shunt groups while noting reduced right ventricular ejection fraction in the RVPAS group [2,4,5]. These studies evaluate or include patients from the early era of RVPAS adoption, during which time there was variable shunt size selection, surgical technique, and, in the case of the SVR trial, significant center variability in mortality [14]. In addition, there has been significant improvement in perioperative and intensive care management of patients undergoing the Norwood procedure. However, perioperative outcomes in our study are largely the same and mirror the outcomes of the SVR trial with no shunt group differences in mortality, hospital length of stay, or cardiac arrest requiring CPR. There was also no difference in the fraction of patients requiring ECMO after Norwood. The latter finding is particularly interesting given the conventional belief that the RVPAS provides hemodynamic stability compared to the BTTS [2]. Our findings differ from the SVR trial and others which found significant attrition of the BTTS cohort in the interstage period [3–6,8–10]. This finding exists despite no significant difference in unintended interventions on the atrioventricular valve, a proxy for regurgitation which Ghanayem et al. propose to explain differences in interstage mortality [8]. A possible explanation for the lack of overall interstage attrition may be that there was a higher proportion of in-hospital mortality rather than interstage mortality in our cohort. Of the patients who died while in the hospital, four of six in the BTTS group and one of four in the RVPAS group died before the 4-month mark. This increased attrition of BTTS patients in the pre-Glenn period is consistent with the other reports. However, the data shows no interstage survival difference exists for patients discharged home. This finding may be explained by the diligence of the families and providers in our homegrown home monitoring program, Cardiac Acuity Home Monitoring Program (CHAMP), in supporting patients with BTTS who, in previous eras, would have died prior to the Glenn palliation.
Medium-term transplant-free survival was excellent in our groups at greater than 75% at 3 years for the cohort. This did not differ between our cohorts. We did find higher incidences of pulmonary arterial interventions in the RVPAS cohort. This was surprising, as we had hypothesized that RVPAS survival would be superior to BTTS survival with equal pulmonary arterial intervention rates due to matured surgical technique, improved management of SVR physiology, and the tendency for higher-risk patients to undergo alternative pathways like the hybrid palliation in this modern cohort. No differences were found in the reinterventions of the shunts themselves. We noticed significantly higher rates of aortic arch intervention and pulmonary artery (PA) intervention in the RVPAS group. Different Sano techniques, particularly at the pulmonary artery/Sano connection, have been tried at our institution to address this increase in PA intervention and stenting with no significant change in intervention rates. The switch to Sano shunt at our institution did not coincide with a change in patch material for arch reconstruction, and arch intervention rates did not significantly skew towards one surgeon. It has been suggested that single ventricle patients with LV dominance have improved long-term outcomes compared to those without [15,16]. Our study featured a BTTS cohort with a higher fraction of LV dominant patients than the RVPAS group and differs from other studies in that regard. Interestingly, the BTTS cohort had a higher incidence of transplant or death in the 3–6 years period despite higher incidence of LV dominance. In our study, there were not enough Sano patients at risk to compare, as follow up for the Sano cohort did not significantly extend past 3 years. Additionally, adjusting for LV dominance did not result in an observed difference in overall transplant-free survival for the shunt groups. Though not statistically significant, the higher risk of death or transplantation in the BTTS cohort highlights the necessity of a future prospective study comparing the two strategies in the current era with refined surgical technique, employment of single ventricle monitoring programs, and improved post-operative ICU care.
Limitations of this study include its retrospective, non-randomized, small cohort, single-center design which means its findings may not be generalized to other populations. The follow-up period is short and selection bias may exist in the selection of shunt type, which is largely dependent on the period of Norwood procedure (earlier period had BTTS predominance), surgeon preference or experience, and ventricular dominance. Era effect may also play a role in these results, as the majority of BTTS patients were operated on before 2017, whereas all RVPAS shunts were performed since 2017 and only 5 BTTS in this time period. That said, the present study from 2014 to 2022 is contemporary to earlier Norwood reports, including the cohort for SVR trials, and later than our institution of CHAMP. That many BTTS occurred in an earlier era than RVPAS, while skewed, reflects the utilization of a matured surgical technique whereas RVPAS was still evolving; if a later era were to have positive survival outcomes, we would have expected better outcomes in the RVPAS group compared to the BTTS in this study. Nevertheless, an era effects analysis would be a valuable future step, though is not possible in the present study with too few BTTS in the post-2017 era. On that note, a key limitation is that this study is underpowered and therefore was unable to detect differences. These are limitations better addressed with larger multicenter studies. We believe our results set the foundation to stimulate hypotheses for future randomized, prospective trials.
In the modern era, at our center, the data failed to reveal a significant difference between Norwood palliation with BTTS and RVPAS in their short- and medium-term transplant-free survival. Patients with RVPAS continue to suffer from a high burden of pulmonary arterial interventions which raises concerns regarding future Fontan performance. Our findings prompt the question of whether the Norwood with BTTS may have comparable outcomes, with lower burden of pulmonary arterial intervention, for single ventricle patients requiring palliation in the current era at particular centers. Larger multicenter and randomized studies with longer follow-up are needed to determine if our offered therapies are optimized for this vulnerable population.
Acknowledgement: We would like to acknowledge the efforts of Alvaro Gamarra, Jenny Marshall, Lori Erickson, Amy Ricketts, and other members of the Ward Family Heart Center at Children’s Mercy Hospital who contributed to building the database on which this work was performed.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Edo Kwaku Setsoafia Bedzra, James E. O’Brien Jr.; data collection: Edo Kwaku Setsoafia Bedzra, Manasa Gadiraju, Alexandra Schray; analysis and interpretation of results: Manasa Gadiraju, Alexandra Schray, Dhaval Chauhan, Edo Kwaku Setsoafia Bedzra; draft manuscript preparation: Manasa Gadiraju, Edo Kwaku Setsoafia Bedzra, Dhaval Chauhan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Children’s Mercy Hospital Institutional Review Board Heart Center Database waiver approval (Protocol #13020045).
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45(1):87–91. doi:10.1016/0002-9149(80)90224-6. [Google Scholar] [PubMed] [CrossRef]
2. Piber N, Ono M, Palm J, Kido T, Burri M, Röhlig C, et al. Influence of shunt type on survival and right heart function after the norwood procedure for aortic atresia. Semin Thorac Cardiovasc Surg. 2022;34(4):1300–10. doi:10.1053/j.semtcvs.2021.11.012. [Google Scholar] [PubMed] [CrossRef]
3. Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126(2):504–9. doi:10.1016/S0022-5223(02)73575-7. [Google Scholar] [PubMed] [CrossRef]
4. Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, et al. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137(21):2246–53. doi:10.1161/CIRCULATIONAHA.117.029375. [Google Scholar] [PubMed] [CrossRef]
5. Ono M, Kido T, Wallner M, Burri M, Lemmer J, Ewert P, et al. Comparison of shunt types in the neonatal Norwood procedure for single ventricle. Eur J Cardiothorac Surg. 2021;60(5):1084–91. doi:10.1093/ejcts/ezab163. [Google Scholar] [PubMed] [CrossRef]
6. Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–92. doi:10.1056/NEJMoa0912461. [Google Scholar] [PubMed] [CrossRef]
7. StataCorp. Stata statistical software: release 18. College Station, TX: StataCorp LLC; 2023. [Google Scholar]
8. Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906. [Google Scholar] [PubMed]
9. Castellanos DA, Ocampo EC, Gooden A, Wang Y, Qureshi AM, Heinle JS, et al. Outcomes associated with unplanned interstage cardiac interventions after norwood palliation. Ann Thorac Surg. 2019;108(5):1423–9. doi:10.1016/j.athoracsur.2019.06.041. [Google Scholar] [PubMed] [CrossRef]
10. Handler SS, Chan T, Ghanayem NS, Rudd N, Wright G, Visotcky A, et al. Impact of reintervention during stage 1 palliation hospitalization: a national, multicenter study. Ann Thorac Surg. 2023;115(4):975–81. doi:10.1016/j.athoracsur.2022.10.014. [Google Scholar] [PubMed] [CrossRef]
11. Mahle WT, Hu C, Trachtenberg F, JonDavid M, Steven JK, Anne ID, et al. Heart failure after the Norwood procedure: an analysis of the single ventricle reconstruction trial. J Heart Lung Transplant. 2018;37(7):879–85. [Google Scholar] [PubMed]
12. Kulkarni A, Neugebauer R, Lo Y, Gao Q, Lamour JM, Weinstein S, et al. Outcomes and risk factors for listing for heart transplantation after the Norwood procedure: an analysis of the single ventricle reconstruction trial. J Heart Lung Transplant. 2016;35(3):306–11. doi:10.1016/j.healun.2015.10.033. [Google Scholar] [PubMed] [CrossRef]
13. Goldberg CS, Trachtenberg FJ, Gaynor WJ, Mahle WT, Ravishankar C, Schwartz SM, et al. Longitudinal follow-up of children with HLHS and association between Norwood shunt type and long-term outcomes: the SVR III study. Circulation. 2023;148(17):1330–9. doi:10.1161/CIRCULATIONAHA.123.065192. [Google Scholar] [PubMed] [CrossRef]
14. Shuhaiber JH. Norwood procedure with right ventricle to pulmonary artery shunt: an underestimated technical surgical variable. J Thorac Cardiovasc Surg. 2011;141(5):1329–30. doi:10.1016/j.jtcvs.2011.01.007. [Google Scholar] [PubMed] [CrossRef]
15. Ponzoni M, Azzolina D, Vedovelli L, Gregori D, Di Salvo G, D’Udekem Y, et al. Ventricular morphology of single-ventricle hearts has a significant impact on outcomes after Fontan palliation: a meta-analysis. Eur J Cardiothorac Surg. 2022;62(6):ezac535. doi:10.1093/ejcts/ezac535. [Google Scholar] [PubMed] [CrossRef]
16. Padalino MA, Ponzoni M, Reffo E, Azzolina D, Cavaliere A, Puricelli F, et al. The impact of dominant ventricle morphology and additional ventricular chamber size on clinical outcomes in patients with Fontan circulation. Cardiol Young. 2024:1–10. doi:10.1017/S1047951124026581. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools