 Open Access
Open Access
REVIEW
Unmet Needs in Pediatric and Congenital Heart Surgery: A Review
1 Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, M5T 3M6, Canada
2 Division of Cardiac Surgery, University of Toronto, Toronto, ON, M5G 2C4, Canada
3 Division of Cardiovascular Surgery, Hospital for Sick Children, University of Toronto, Toronto, ON, M5G 1X8, Canada
4 Institute of Medical Science, University of Toronto, Toronto, ON, M5S 3H2, Canada
5 Blalock-Taussig-Thomas Pediatric and Congenital Heart Center, Johns Hopkins Hospital, Baltimore, MD 21202, USA
6 School of Medicine, University of Health & Allied Sciences, Ho, PMB31, Volta Region, Ghana
7 National Cardiothoracic Centre, Korle Bu Teaching Hospital, Korle-Bu, Accra, KB846, Ghana
* Corresponding Author: Dominique Vervoort. Email:
Congenital Heart Disease 2024, 19(5), 499-511. https://doi.org/10.32604/chd.2024.057749
Received 26 August 2024; Accepted 29 November 2024; Issue published 31 December 2024
Abstract
Pediatric and congenital heart disease (PCHD) affects millions of children worldwide, including over one million babies born with congenital heart disease (CHD) each year and 300,000 children dying from rheumatic heart disease (RHD) yearly. Although the vast majority of children born with CHD in high-income countries now reach adulthood and RHD is nearly eradicated in these countries, most of the world cannot access the necessary care to prevent or mitigate PCHD. In low- and middle-income countries, over 90% of children with PCHD cannot receive the care they need, as over 100 countries and territories lack local cardiac surgical capacity. The unmet needs for PCHD are large, albeit still poorly quantified, resulting in a considerable socioeconomic impact at the individual and societal levels. This review highlights the extensive opportunities to improve access to and scale PCHD care by strengthening research, clinical care delivery, capacity-building, advocacy, health policy, and financing. We discuss global disparities in access to congenital heart surgery, the socioeconomic impact of untreated PCHD, and propose strategies for scaling pediatric and congenital cardiac care. Our recommendations focus on enhancing research and data collection, expanding training programs, improving healthcare infrastructure, advocating for policy changes, leveraging technological innovations, fostering international collaborations, and developing comprehensive care models.Keywords
Abbreviations
| ASD | Atrial septal defect |
| CHD | Congenital heart disease |
| CHS | Congenital heart surgery |
| CI | Confidence interval |
| DALY | Disability-adjusted life year |
| HIC | High-income country |
| HLHS | Hypoplastic left heart syndrome |
| HR | Hazard ratio |
| ICU | Intensive care unit |
| IQIC | International Quality Improvement Collaborative |
| LMIC | Low- and middle-income country |
| PCHD | Pediatric and congenital heart disease |
| QALY | Quality-adjusted life year |
| RACHS | Risk adjustment for congenital heart surgery |
| RHD | Rheumatic heart disease |
| UI | Uncertainty interval |
| UMIC | Upper-middle-income country |
| VSD | Ventricular septal defect |
| YLD | Years lost to disability |
Pediatric and congenital heart disease (PCHD) affects tens of millions of children worldwide [1]. Every year, over one million children are born with congenital heart disease (CHD), presenting a range of conditions from minor defects that resolve spontaneously to major forms requiring immediate life-saving surgery [2]. It is estimated that one-quarter to one-third of CHD is critical, requiring surgical or interventional care within the first year of life, whereas up to 49% of CHD cases require procedural care at least once in a lifetime [3]. Rheumatic heart disease (RHD) typically manifests in childhood, with clinical presentations often occurring in adolescence or early adulthood, leading to valve dysfunction and, ultimately, heart failure that requires timely surgical intervention. However, the exact needs and rates of surgery for PCHD are poorly defined.
Access to pediatric and congenital heart surgery (CHS) varies significantly across the globe. In high-income countries (HICs), advances in detection, referrals, and surgical and perioperative care have resulted in over 95% of children born with CHD surviving into adulthood, whereas RHD is nearly eradicated outside marginalized populations and indigenous peoples [1]. In contrast, low- and middle-income countries (LMICs) face substantial challenges in providing adequate care for children with PCHD. In these regions, 93% of children with CHD cannot access the care they need, and less than one in ten individuals with RHD requiring cardiac surgery will undergo the necessary procedures [4].
The vast majority of children born with CHD in LMICs are unable to receive essential care due to limited local cardiac surgery capacity, with over 100 countries and territories lacking sufficient resources [1]. This unmet need for PCHD care results in significant socioeconomic impacts at both the individual and societal levels. Untreated PCHD leads to high infant mortality rates and considerable years lost to disability [5,6]. There are significant disparities in CHS expertise worldwide, a situation which is exacerbated by regional variations in CHD prevalence, with the highest rates seen in Asia and the lowest overall rates in Africa, partly due to underdiagnosis in certain areas [7,8].
Addressing the global burden of PCHD requires a multifaceted approach that encompasses research, capacity-building, organization of care, policy, and financing. Improved data collection and research efforts are crucial for understanding the true extent of PCHD needs and guiding policy development.
2 Pediatric and Congenital Heart Surgery Needs
Globally, CHD has a prevalence of over 1780 per 100,000 births and is one of the top ten leading causes of death in infancy [5]. Over 90% of patients with CHD in LMICs either cannot receive care or experience suboptimal care [1]. This lack of access results in nearly 200,000 preventable pediatric deaths from CHD each year, with over 90% of this mortality considered excess [6]. Surgery or interventional care is the definitive treatment for CHD; however, there are significant geographic disparities in surgical expertise and resources worldwide. The number of pediatric cardiac surgeons per million pediatric population varies from 0.08 in sub-Saharan Africa to 2.08 in North America [7]. This disparity highlights the profound global inequity in accessing safe, affordable, and timely pediatric cardiac care. Regional variation in CHD prevalence further complicates this issue [8].
Socioeconomic factors play a significant role in the accessibility and outcome of CHD [1]. Children in LMICs are disproportionately affected by CHD due to limited healthcare infrastructure, insufficient resources, and a lack of trained healthcare professionals. A meta-analysis of 260 international studies from 1970 to 2017 showed an increasing prevalence of mild lesions, such as ventricular septal defect (VSD), atrial septal defect (ASD), and patent ductus arteriosus, likely owing to early and improved postnatal detection. Conversely, the decrease in lesions with left ventricular outflow tract obstruction, such as hypoplastic left heart syndrome (HLHS), can be explained by higher rates of prenatal diagnosis resulting in pregnancy termination in high-income countries; in LMICs, the general absence of prenatal diagnosis results in children born with HLHS but dying during infancy. Of the CHD subtypes, ASD and VSD account for the majority of cases, with a global prevalence rate of over 470 per 100,000 infants (Fig. 1) and is responsible for over 75% of pediatric CHD-related mortality [5]. In 2017, the global mortality from CHD for patients under 19 years of age was estimated to be 235,239 deaths, of which the most vulnerable patient population was less than one year of age and from LMICs (Fig. 2).
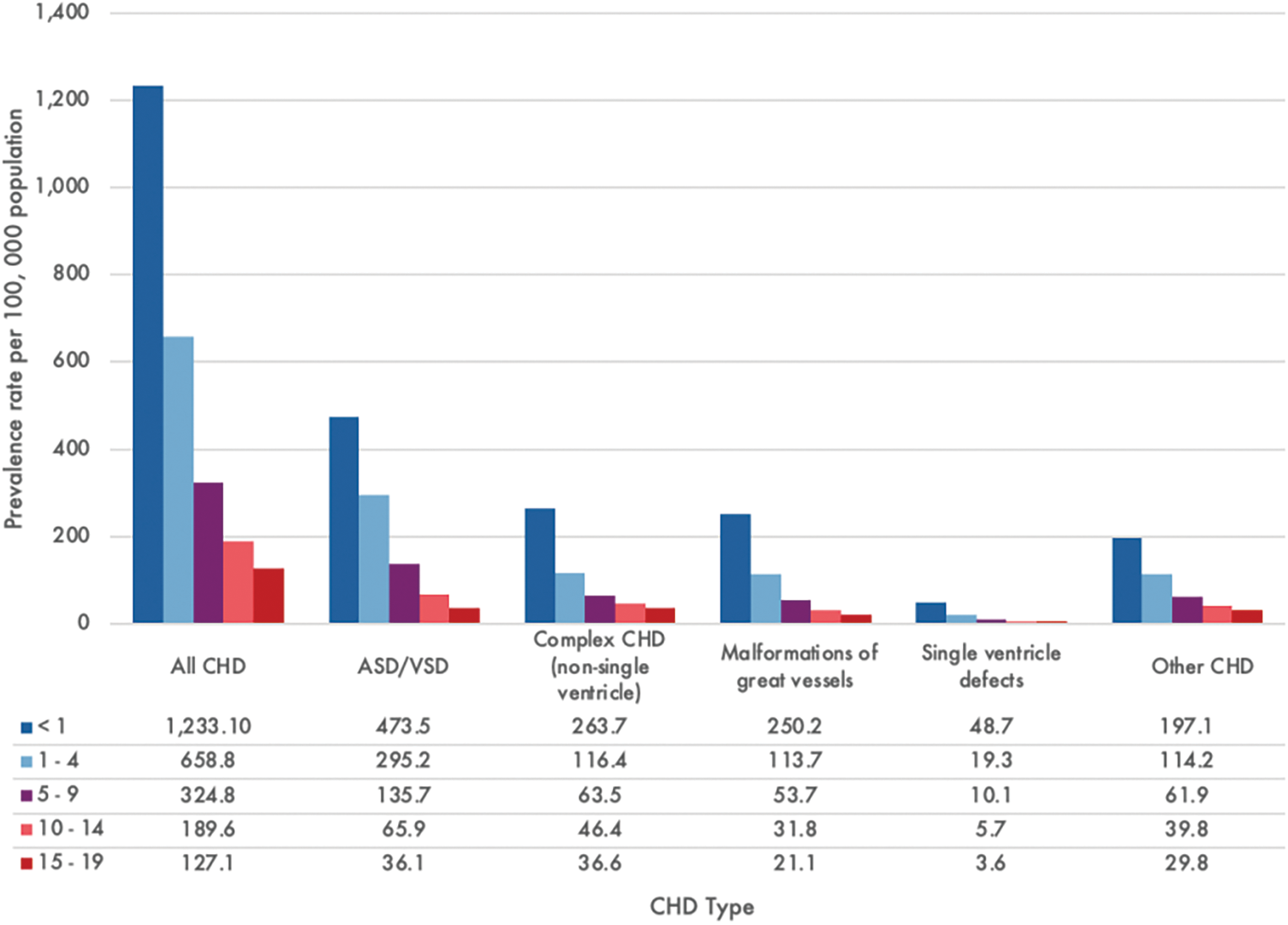
Figure 1: Global congenital heart disease (CHD) prevalence rate by subtype and patient age in 2017. Graph constructed using data from Zimmerman et al. [5]
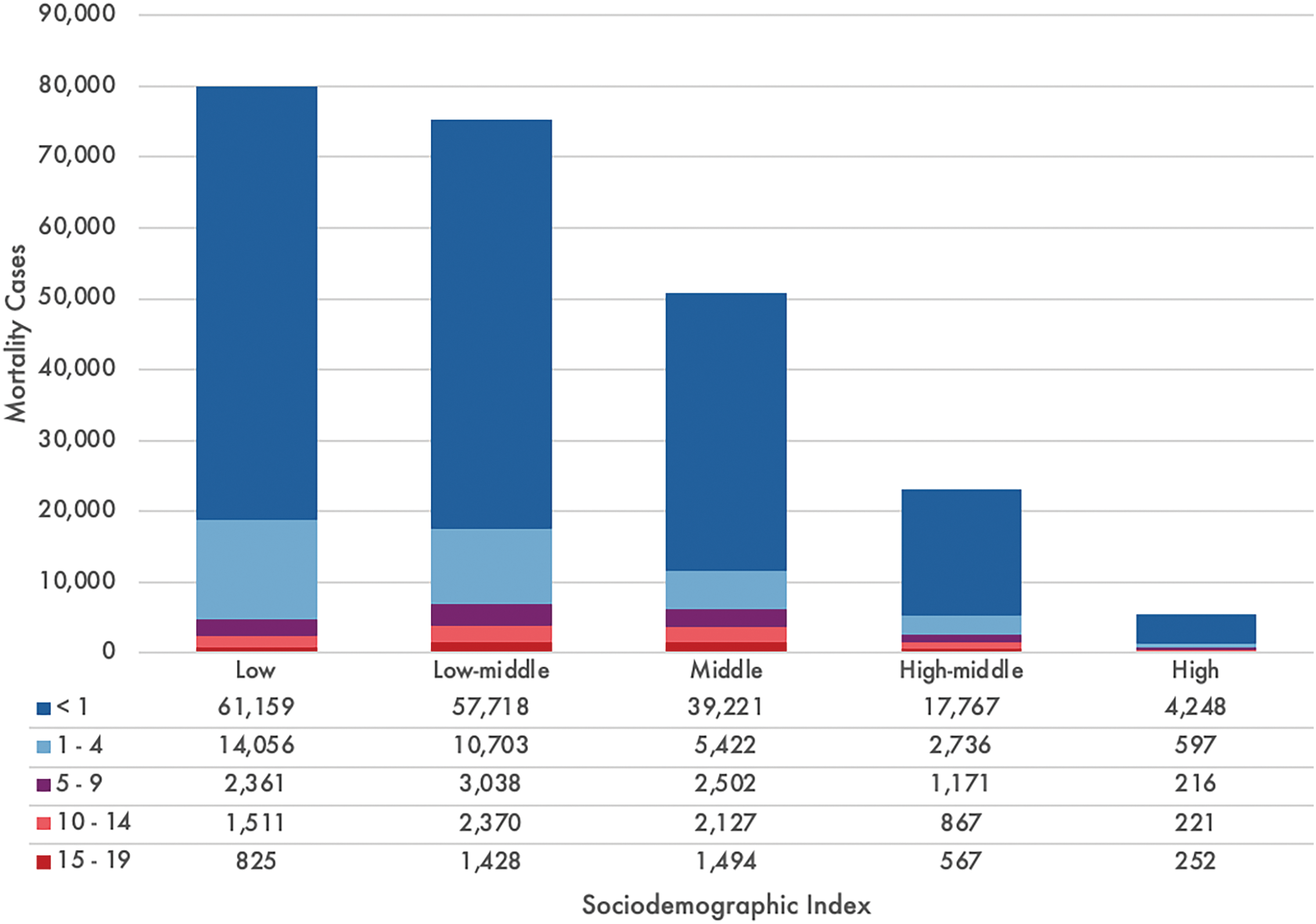
Figure 2: Number of congenital heart disease deaths by countries’ sociodemographic index and age in 2017. The sociodemographic index encompasses the composite of normalized values of a location’s income per capita, the average years of schooling in the population of 15 years and over, and the total fertility rate under 25 years, for which countries and territories are subsequently grouped into five quintiles. Graph constructed using data from Zimmerman et al. [5]
Patients living with untreated CHD experience significant years lost from disability (YLD). In 2017, the total YLD due to CHD was estimated to be 589,479, comparable to that of attention-deficit/hyperactivity disorder, lower respiratory infections, and neonatal jaundice [5]. The YLD per 100,000 individuals was 7 in high sociodemographic index countries compared to 14 in low sociodemographic index countries. Infants in Western, Central, and Eastern sub-Saharan Africa, Central and Southeast Asia, and some provinces of China and India experience the highest YLD, estimated at 50–55 for 100,000 population.
Unlike CHD, RHD is a disease process originating in childhood with clinical manifestations that tend to occur in early adulthood. RHD disproportionately affects patients between 20–49 years of age and carries a high morbidity burden. Although mortality from RHD is decreasing, the global prevalence of RHD was estimated to be 33.4 million in 2015, with a cumulative YLD of 10.5 million [9]. Regions where RHD is considered endemic, such as Oceania, South Asia, and Central sub-Saharan Africa face particularly high burdens with RHD mortality of at least 0.15 deaths per 100,000 population among children five to nine years of age. The REMEDY (Global Rheumatic Heart Disease Registry) study of 3343 patients from 14 LMICs found 16.9% mortality over a two-year follow-up, with a median age of 28.7 years among non-survivors [10]. Patients from low- and lower-middle-income countries had significantly higher age- and sex-adjusted mortality than patients from upper-middle-income countries (UMICs), reflecting disparities in accessing surgical care. A recent prospective study of 13,696 adult patients in 24 RHD-endemic LMICs reported a mortality rate of 15% over a 3.2-year follow-up period, with 67.5% of deaths presumed to be cardiac-related [11]. Only 4.4% of patients underwent surgical intervention during the study period, despite evidence showing that corrective valve surgery and valvuloplasty were associated with significantly lower mortality after adjusting for patient-level factors. The considerable survival benefit of valve interventions for RHD is evident, yet the volume of cases continues to outpace surgical capacity, particularly in LMICs.
Tackling the silent epidemic of RHD begins with early detection. Outreach programs in New Caledonia [12] and Fiji [13] have demonstrated that focused cardiac ultrasound screening for early RHD in school-aged children can be performed accurately by trained nursing staff. A randomized study in Nepal showed the benefit of coupling surveillance with secondary prevention [14]. Children who underwent echocardiographic screening and received antibiotic prophylaxis if imaging suggestive latent RHD had significantly reduced odds of developing RHD at a median follow-up of 4.3 years. Early detection and systematic community screening combined with secondary antibiotic prophylaxis can circumvent the need for operative management altogether, presenting a potential strategy to address the unmet needs of RHD.
3 Pediatric and Congenital Heart Surgery Volumes Worldwide
The global landscape of pediatric heart surgery is marked by significant disparities in surgical volumes. The Lancet Commission on Global Surgery recommends at least 5000 annual surgical procedures per 100,000 population for all types of surgical conditions, but it does not specify by condition or subspecialty [15]. Meanwhile, the World Health Organization sets a target of 400 cardiac surgeries per million population per year, which the Pan-African Society for Cardiothoracic Surgery reduced to 40 per million due to capacity constraints [16].
In HICs, the average annual CHS volume is approximately 7.9 per 100,000 population, though it varies significantly from 1.2 in the United Kingdom to 18.2 in Singapore [17]. The stark contrast between HICs and LMICs in CHS volumes underscores the global inequity in healthcare access. In HICs, surgical volumes align closely with the demand for CHS, enabled by adequate resources, funding, and trained personnel. For example, CHS constitutes 6.4% (123.2 procedures per 100,000) of all cardiac surgeries in HICs. Conversely, in LMICs, data are scarce, but target volumes are adjusted to relative PCHD burdens, estimated at 13.0 procedures per 100,000 population and representing 23.6% (55.1 procedures per 100,000) of the overall cardiac surgical volume [17]. However, actual CHS volumes in LMICs are often far below these targets due to resource limitations and workforce constraints. A study of 37 institutions in 17 LMICs found a median CHS volume of 361 procedures annually, despite an estimated 312–32,285 children born with moderate to severe PCHD per country each year [18]. Approximately 37% of those institutions achieved less than half of their target volumes [18]. This discrepancy reflects broader limitations in healthcare infrastructure, including limited operating room availability, insufficient intensive care unit (ICU) beds, and ongoing workforce shortages [19].
3.1 Surgical Coverage, Procedural Complexity, and Resource Constraints
There is a clear relationship between national income and surgical volumes. In South America, HICs, specifically Chile and Uruguay, have the lowest poverty rates and the highest percentage of national surgical coverage [20]. In these countries, 80%–90% of children with PCHD received surgery, compared to less than 20% in UMICs like Ecuador and Paraguay [20]. Chile and Uruguay also reported 76 CHS cases per million population, achieving volumes closest to the HIC target of 79 cases per million and significantly exceeding the continental average of 44 cases per million.
PCHD complexity, quantified by the Risk Adjustment for Congenital Heart Surgery score (RACHS), represents another crucial determinant of CHS volumes and prognoses. In LMICs, there is often a greater prioritization and feasibility of less complex procedures, with only 30% of CHS in the International Quality Improvement Collaborative (IQIC) registry involving complex cases (RACHS score 3–6) [21]. Treatment of HLHS exemplifies a resource-intensive situation that is often underreported in LMICs due to the high demands of its management. It requires comprehensive diagnostic work-ups, hybrid surgical techniques, multiple staged procedures, and prolonged ICU stays, costing, on average, almost double that of other complex congenital heart procedures [22,23]. Consequently, the true incidence of HLHS is often underreported in LMICs, making accurate volume targets difficult to estimate and discern. The IQIC registry reported that 1%–16% of institutional CHS volumes and 2543 total operations from 2010–2014 involved single ventricle palliation, made up mainly of stage 1 Norwood palliation (5%, 127), the Glenn procedure (53%, 1358), and Fontan completion (27%, 687) [21]. These constraints in access to more complex procedures are also applicable to surgery for RHD. For instance, rheumatic mitral valve repair has superior outcomes and comparable freedom from re-operation as valve replacement [24,25], but the added complexity of reparative technique (such as artificial chordal implantation) requires highly experienced surgical teams which are often lacking in LMICs. Consequently, many patients end up receiving valve replacements, despite the higher risks and significant concerns related to anticoagulation compliance, which are more pronounced in LMICs [25,26].
3.2 Timing of Detection and Intervention
The timing of detection and intervention has significant impact on outcomes. Procedures such as the Glenn and Fontan are performed later in LMICs than in HICs, presumably due to delays in work-ups, referrals and the long-distance transport of patients to capable centers [22]. Specifically, while the Glenn procedure is typically performed between 4–6 months in HICs, 57% of Glenn procedures in LMICs are performed beyond 12 months [21,23]. These delays promote disease progression, resulting in patients being more susceptible to adverse perioperative outcomes or succumbing to the natural history of disease. Thus, while CHS volumes and procedural breakdowns are necessary to establish precise and informed targets, they are often shaped by the availability of resources rather than adherence to evidence-based standards of care.
4 Socioeconomic Impact of Treating Pediatric and Congenital Heart Disease
CHD is a leading cause of infant and childhood mortality in LMICs, with up to 200,000–250,000 neonatal and infant deaths each year attributed to the lack of early intervention [6,27]. Addressing the unmet surgical needs through capacity-building and improved healthcare infrastructure can have profound socioeconomic benefits, reducing preventable mortality and morbidity, and promoting long-term economic growth. Regions such as North Africa and the Middle East, which showed the largest burden of surgically avertable disability-adjusted life years (DALY) for PCHD, particularly stand to benefit [28]. Surgical care was previously a neglected priority in global health efforts, in part due to the resource intensity of surgery [29]. However, there has been a paradigm shift in the last decade upon recognizing the comparable cost-effectiveness of basic surgical interventions to that of oral rehydration, breastfeeding promotion, and antiretroviral therapy [30]. Surgery for CHD is now acknowledged as an “essential pediatric surgical procedure,” based on the criteria of health burden, rate of surgical success, and cost-effectiveness, joining the same category as inguinal hernia repair, trichiasis surgery, cleft lip and palate repair, circumcision, and orthopedic procedures [31]. Although society’s investment in pediatric care is arguably also driven by pathos and the higher value placed on the statistical life of a child [32], the long-term economic benefits of CHS should not be overlooked. An international, multicenter analysis of children undergoing CHS in LMICs through humanitarian assistance trips demonstrated an operative survival of 92% and cost-effectiveness of $171 per DALY averted [27]. For each survivor, 39.9 DALY were averted and 3.5 years of schooling and $159,533 in gross national income per capital were gained during the patient’s extended lifetime. If these benefits are achievable through temporary external CHS expertise, the socioeconomic impacts would likely be greater and more sustainable by developing local CHS capacity. Establishing domestic surgical infrastructure and competencies would also eliminate the annual healthcare expenditure of sending patients from LMICs abroad for cardiac surgical care. Although time and philanthropic support are needed for autonomous, financially independent cardiac surgical programs to become established in lower-resource settings, excellent clinical outcomes can eventually be achieved, comparable to those of established centers in HICs [33].
Unlike CHS, the cost-effectiveness of managing RHD in many LMICs lies primarily in prevention rather than surgery, as RHD is inherently preventable [34,35]. However, given the extensive burden of RHD, and the fact that primary and secondary prevention measures are often undertaken too late or not at all, scaling up tertiary care is both critical and economically appropriate [36]. A strategic and feasible scale-up of RHD prevention and management in the African Union is predicted to prevent 74,000 deaths from 2021 to 2030, achieving a 30.7% deduction in age-standardized RHD mortality by 2030 [36]. The 2030 estimated benefit-cost ratio for primary prevention scale-up was low for primary prophylaxis alone (0.2 [95% uncertainty interval <0.1–0.4]), compared to integrated secondary and tertiary care (4.7 [2.9–6.3]). This indicates that investing in cardiac surgical care offers a significant return on investment. Among the surgical techniques, a cost-utility analysis from India found repair ($2530, 9.7 quality-adjusted life years [QALY] gained) to be less expensive and more effective than mechanical mitral valve replacement ($3220, 6.2 QALYs) or the standard of care ($2990, 8.7 QALYs), which involves both repair and replacement [37]. Bioprosthetic valve replacement ($3190, 10.1 QALYs) was borderline cost-effective depending on the country’s per-capita gross domestic product. Optimizing the cost-benefit balance of RHD management depends on a comprehensive public health approach, with tertiary surgical intervention playing a crucial role.
5 Scaling Pediatric and Congenital Cardiac Care
Although the global gaps in pediatric and congenital cardiac care are significant, numerous opportunities exist to improve access to care for individuals living with PCHD. These opportunities can be broadly categorized into research, capacity-building, organization of care, and policy and financing areas (Fig. 3).
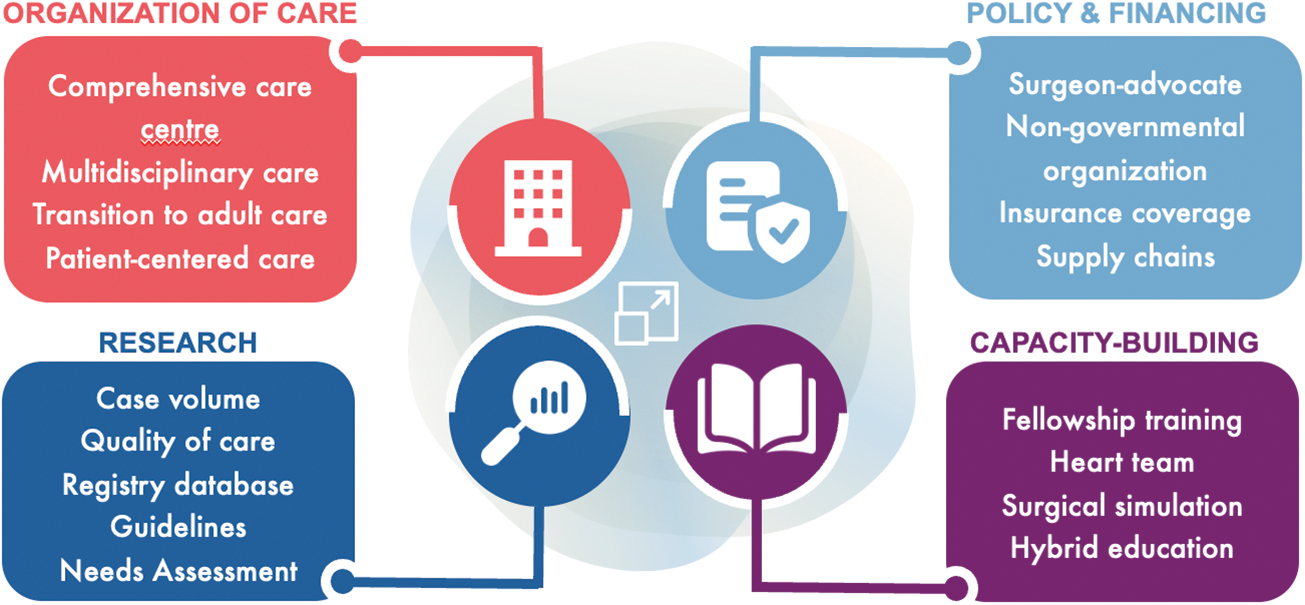
Figure 3: Opportunities in scaling pediatric and congenital cardiac care
“One cannot manage what one does not measure,” illustrating the critical interrelationship between clinical practice, research, and policy. Monitoring and evaluating process measures (e.g., surgical volumes and healthcare utilization) and quality (e.g., perioperative outcomes and readmissions) provide insights into the strength and growth of programs compared to their past performance and peers. Developing databases to track the prevalence, cases, and outcomes with longitudinal follow-up is essential. For instance, the IQIC, launched in 2008, collects CHS and interventional cardiology outcomes from over 70 hospitals in more than 25 LMICs. This effort could be expanded to other existing and emerging centers worldwide and should be supported by the relevant professional societies and governments. Similarly, countries should ensure consistent data collection of workforce numbers, procedural volume, and longitudinal health outcomes, among other data [17]. Data collection, tracking, and analysis are critical in introducing and scaling pediatric cardiac programs globally, ranging from initial site and baseline assessments to evaluate programmatic feasibility, to the gradual growth of programs in terms of capacity, procedural complexity, and iterative quality improvement.
Expert consensus recommendations and guidelines should be developed contextually rather than relying solely on institutional practices or guidelines from North America or Europe. Data-driven needs assessments provide insights into the current capacities of countries. For example, a cardiac surgery needs assessment tool has been developed and piloted in Namibia, Uganda, and Zambia [38], and later adapted for Ethiopia [39]. These findings can assist with policy development and prioritization at national and international levels [40]. Lastly, research requires the necessary expertise and support, including time and resources. Institutions and governments should recognize the return on investment associated with enabling healthcare professionals and researchers to conduct high-quality research [41].
Scaling the PCHD workforce will require considerable time and resources due to the lengthy and complex training involved. The current number of training programs in CHS worldwide is poorly defined but is small and primarily located in HICs and UMICs. For instance, in the United States and Canada, there are only 15 and 4 fellowship programs for CHS, respectively [42]. Expanding these programs across countries is essential not only to increase the number of pediatric cardiac surgeons globally but also to attract and retain expertise, thereby reducing international brain drain. Similar needs exist among all pediatric heart team members, including cardiologists, anesthesiologists, intensivists, pediatricians, nurses, and others.
Efforts to support surgeons in expanding and maintaining their skill sets are equally important. Cardiac surgeons in LMICs report poor availability of continuing medical education [43]. Facilitating short-term training abroad (e.g., mini-fellowships) or domestically (e.g., visiting teams, simulation training, online training) should be better prioritized. For international training, structured programs, financial support, and reduced administrative barriers are essential. Initiatives like the Hands-On Surgical Training (HOST) workshop hosted by the Hospital for Sick Children in Toronto, Canada, allow surgeons to learn and practice CHS on 3D-printed or silicone models, with pilot experiences in Brazil, China, Ecuador, Thailand, and Vietnam. Furthermore, online, open-access educational platforms can enable entire teams to benefit from remote continuing medical education. For example, Heart University is dedicated to (mostly non-surgical) PCHD care, while SURGhub, established by the Global Surgery Foundation and the United Nations Institute for Training and Research, offers surgical and anesthesia courses and resources [1]. The World University for Pediatric and Congenital Heart Surgery, a collaboration of several professional societal organizations, runs monthly curriculum webinars focusing on PCHD, promoting access to education of surgeons and allied professionals involved in the care of these patients. The growth of virtual reality and the advent of freely accessible artificial intelligence applications present additional opportunities for remote training in the near future.
In addition to the pediatric cardiac surgical workforce, efforts must prioritize the concurrent capacity-building of other members of pediatric heart teams, including pediatric cardiologists and dedicated pediatric cardiac anesthesiologists and intensivists. This ensures that patients are appropriately triaged, managed interventionally or surgically as appropriate, and receive optimal post-procedural and follow-up care.
Although specialty care is typically limited to tertiary and quaternary hospitals (i.e., children’s hospitals), the care for individuals with PCHD inherently involves the entire health system. Initially, PCHD care in LMICs tends to follow a regionalized model due to resource constraints [44]. This model may be modified by establishing comprehensive care centers vs. essential care centers, as seen in HICs [45]. In Europe, regionalization of CHS largely developed organically within publicly funded health systems. Recent experiences, such as Sweden’s shift to regionalization, have demonstrated increased healthcare savings and improved outcomes. In contrast, the United States has nearly three times as many congenital cardiac programs as necessary, leading to significant variation in volumes, charges, and outcomes, with minimal benefit in geographical access for patients [46–48]. Deliberate regionalization models, such as those in South Korea and Mexico, have shown feasibility and success when contextually adopted [44]. Regardless of the form of regionalization, PCHD care in any country should include multidisciplinary care with considerations for neurodevelopmental, exercise, and reproductive health, as well as appropriate transition to adult care [49]. It is crucial to recognize the lifelong care needs of individuals with PCHD, as many procedures, even those touted as cures, are essentially palliative and several are not amenable to surgical or interventional therapy. Individuals with PCHD often have a higher prevalence of comorbidities, procedural needs, neurodevelopmental delay, and poor mental health compared to individuals without PCHD [1].
Research and clinical experiences ultimately influence policy and healthcare financing through advocacy, which can be considered as evidence-based messaging. Advocacy begins at the grassroots level and is generally driven by clinicians (e.g., surgeon-advocates), patients and families (e.g., organized patient-family groups), and civil society (e.g., non-governmental organizations) [50]. Advocacy is particularly impactful when it relies on contextual evidence and experiences and is directed toward the appropriate policymakers, politicians, and government agencies. Therefore, healthcare professionals specializing in PCHD play a critical role in engaging with policy developments and processes. Such engagement can occur through personal involvement, including lobbying and running for office, or through indirect involvement, such as outreach to and collaboration with policymakers or participation in policy-oriented events like the World Health Organization’s annual World Health Assembly. Additionally, understanding national policy processes ensures more effective engagement in ongoing or upcoming policy development and implementation, thereby more likely securing a seat at the table.
One key area of policy is healthcare financing and budget allocations. Cardiac surgery and PCHD care are expensive and nearly unaffordable for patients and families without health insurance or external financial support. To overcome such financial barriers, countries should increase financing for PCHD care by including it in basic healthcare insurance packages. These packages may involve broad, population-wide coverage (e.g., forms of universal health coverage) or smaller-scale schemes (i.e., microinsurance) that benefit those in greatest need. The former has been observed in Latin America, where public health insurance has become increasingly inclusive in most countries. In contrast, Nepal has shown leadership in the latter through its Poor Patients Relief program, established by the government to subsidize cardiac surgical care for children, the elderly, and those under the poverty threshold.
Addressing the global burden of PCHD requires a multifaceted approach encompassing research, capacity-building, organization of care, and policy and financing. Research and documentation form the basis for evaluating surgical volumes, outcomes, and epidemiological trends; and will remain pivotal in identifying regional disparities, informing surgical intervention targets, and guiding resource allocation. While PCHD care generally trends with resource availability, the global distribution of met and unmet needs is more nuanced, necessitating timely diagnosis and intervention.
Going forward, capacity-building efforts must prioritize expanding training opportunities for pediatric cardiac specialists and supporting ongoing skill development to meet increasing global demands. PCHD care should be organized in comprehensive centers with multidisciplinary teams to ensure smooth transitions from pediatric to adult services. Simultaneously, policy advocacy will be crucial for shaping healthcare financing, requiring policymakers to recognize the long-term economic benefits of locally sustainable PCHD care models.
By collectively advancing these global, regional, and national efforts, stakeholders can help bridge the gap between surgical needs and volumes, improve overall health and socioeconomic outcomes, and promote more equitable access to life-saving cardiac interventions for children worldwide.
Acknowledgement: Dominique Vervoort and Mimi X. Deng are supported by the Canadian Institutes of Health Research (CIHR) Vanier Canada Graduate Scholarship.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Dominique Vervoort; data collection: Dominique Vervoort, Mimi X. Deng, Aliya Izumi; analysis and interpretation of results: Dominique Vervoort, Mimi X. Deng, Aliya Izumi; draft manuscript preparation: Dominique Vervoort, Mimi X. Deng, Aliya Izumi, Shelby Kutty, Frank Edwin. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No new data were generated or analyzed for this study.
Ethics Approval: Not applicable.
Conflicts of Interest: Dominique Vervoort serves on the Medical Advisory Board for the Global Alliance for Rheumatic and Congenital Hearts (Global ARCH).
References
1. Vervoort D, Jin H, Edwin F, Kumar RK, Malik M, Tapaua N, et al. Global access to comprehensive care for paediatric and congenital heart disease. CJC Pediatr Congenit Heart Dis. 2023 Dec;2(6):453–63. doi:10.1016/j.cjcpc.2023.10.001. [Google Scholar] [PubMed] [CrossRef]
2. Hoffman JIE, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004 Mar;147(3):425–39. doi:10.1016/j.ahj.2003.05.003. [Google Scholar] [PubMed] [CrossRef]
3. Higashi H, Barendregt JJ, Kassebaum NJ, Weiser TG, Bickler SW, Vos T. The burden of selected congenital anomalies amenable to surgery in low and middle-income regions: cleft lip and palate, congenital heart anomalies and neural tube defects. Arch Dis Child. 2015 Mar;100(3):233–8. doi:10.1136/archdischild-2014-306175. [Google Scholar] [PubMed] [CrossRef]
4. Zheleva B, Atwood JB. The invisible child: childhood heart disease in global health. Lancet. 2017 Jan 7;389(10064):16–8. doi:10.1016/S0140-6736(16)32185-7. [Google Scholar] [PubMed] [CrossRef]
5. Zimmerman MS, Smith AGC, Sable CA, Echko MM, Wilner LB, Olsen HE, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Child Adolesc Health. 2020 Mar 1;4(3):185–200. doi:10.1016/S2352-4642(19)30402-X. [Google Scholar] [PubMed] [CrossRef]
6. Vervoort D, Cardarelli M. The global unmet need of congenital cardiac care: a quantitative analysis of the global burden of disease. Cardiol Young. 2020 Nov;30(11):1688–93. doi:10.1017/S1047951120002577. [Google Scholar] [PubMed] [CrossRef]
7. Vervoort D, Meuris B, Meyns B, Verbrugghe P. Global cardiac surgery: access to cardiac surgical care around the world. J Thorac Cardiovasc Surg. 2020 Mar;159(3):987–996.e6. doi:10.1016/j.jtcvs.2019.04.039. [Google Scholar] [PubMed] [CrossRef]
8. Liu Y, Chen S, Zühlke L, Black GC, Choy M-K, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019 Apr 1;48(2):455–63. doi:10.1093/ije/dyz009. [Google Scholar] [PubMed] [CrossRef]
9. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017 Aug 24;377(8):713–22. doi:10.1056/NEJMoa1603693. [Google Scholar] [PubMed] [CrossRef]
10. Zühlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido-Katya Mauff B, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016 Nov 8;134(19):1456–66. doi:10.1161/CIRCULATIONAHA.116.024769. [Google Scholar] [PubMed] [CrossRef]
11. Karthikeyan G, Ntsekhe M, Islam S, Rangarajan S, Avezum A, Benz A, et al. Mortality and morbidity in adults with rheumatic heart disease. JAMA. 2024 Jun 5;332(2):133. doi:10.1001/jama.2024.8258. [Google Scholar] [PubMed] [CrossRef]
12. Mirabel M, Bacquelin R, Tafflet M, Robillard C, Huon B, Corsenac P, et al. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. 2015 Jan;8(1). doi:10.1161/CIRCIMAGING.114.002324. [Google Scholar] [PubMed] [CrossRef]
13. Engelman D, Kado JH, Reményi B, Colquhoun SM, Carapetis JR, Donath S, et al. Focused cardiac ultrasound screening for rheumatic heart disease by briefly trained health workers: a study of diagnostic accuracy. Lancet Glob Health. 2016 Jun;4(6):e386–94. doi:10.1016/S2214-109X(16)30065-1. [Google Scholar] [PubMed] [CrossRef]
14. Karki P, Uranw S, Bastola S, Mahato R, Shrestha NR, Sherpa K, et al. Effectiveness of Systematic echocardiographic screening for rheumatic heart disease in nepalese schoolchildren: a cluster randomized clinical trial. JAMA Cardiol. 2021 Jan 20;6(4):420. doi:10.1001/jamacardio.2020.7050. [Google Scholar] [PubMed] [CrossRef]
15. Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, Ameh EA, et al. Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015 Aug 8;386(9993):569–624. doi:10.1016/S0140-6736(15)60160-X. [Google Scholar] [PubMed] [CrossRef]
16. Yankah C, Fynn-Thompson F, Antunes M, Edwin F, Yuko-Jowi C, Mendis S, et al. Cardiac surgery capacity in sub-saharan Africa: quo vadis? Thorac Cardiovasc Surg. 2014 Aug;62(5):393–401. doi:10.1055/s-00000085. [Google Scholar] [CrossRef]
17. Vervoort D, Lee G, Ghandour H, Guetter CR, Adreak N, Till BM, et al. Global cardiac surgical volume and gaps: trends, targets, and way forward. Ann Thorac Surg Short Rep. 2023 Dec 9;2(2):320–4. [Google Scholar]
18. Wamala I, Gongwer R, Doherty-Schmeck K, Jorina M, Betzner A, Zheleva B, et al. Infrastructure Availability for the care of congenital heart disease patients and its influence on case volume, complexity and access among healthcare institutions in 17 middle-income countries. Glob Heart. 2021 Oct 21;16(1):75. doi:10.5334/gh.968. [Google Scholar] [PubMed] [CrossRef]
19. Ma X, Vervoort D. Critical care capacity during the COVID-19 pandemic: global availability of intensive care beds. J Crit Care. 2020 Apr 23;58:96–7. doi:10.1016/j.jcrc.2020.04.012. [Google Scholar] [PubMed] [CrossRef]
20. Sandoval N, Kreutzer C, Jatene M, Sessa TD, Novick W, Jacobs JP, et al. Pediatric cardiovascular surgery in South america: current status and regional differences. World J Pediatr Congenit Heart Surg. 2010 Oct;1(3):321–7. doi:10.1177/2150135110381391. [Google Scholar] [PubMed] [CrossRef]
21. Schidlow DN, Gauvreau K, Cherian KM, Du X, Kappanayil M, Kumar RK, et al. Single-ventricle palliation in low- and middle-income countries. J Am Coll Cardiol. 2019 Aug 20;74(7):928–31. doi:10.1016/j.jacc.2019.07.004. [Google Scholar] [PubMed] [CrossRef]
22. Phuc VM, Tin DN, Giang DTC. Challenges in the management of congenital heart disease in Vietnam: a single center experience. Ann Pediatr Cardiol. 2015 Jan–Apr;8(1):44–6. doi:10.4103/0974-2069.149517. [Google Scholar] [PubMed] [CrossRef]
23. Edwin F, Edwin AK, Palacios-Macedo A, Mamorare H, Yao NA. Management of hypoplastic left heart syndrome in low-resource settings and the ethics of decision-making. World J Pediatr Congenit Heart Surg. 2022 Sep;13(5):609–14. doi:10.1177/21501351221103511. [Google Scholar] [PubMed] [CrossRef]
24. Krishna Moorthy PS, Sivalingam S, Dillon J, Kong PK, Yakub MA. Is it worth repairing rheumatic mitral valve disease in children? Long-term outcomes of an aggressive approach to rheumatic mitral valve repair compared to replacement in young patients. Interact Cardiovasc Thorac Surg. 2019 Feb 1;28:191–8. doi:10.1093/icvts/ivy234. [Google Scholar] [PubMed] [CrossRef]
25. Simpson MT, Kachel M, Neely RC, Erwin WC, Yasin A, Patel A, et al. Rheumatic heart disease in the developing world. Struct Heart. 2023 Nov;7(6):100219. doi:10.1016/j.shj.2023.100219. [Google Scholar] [PubMed] [CrossRef]
26. Kumar RK, Antunes MJ, Beaton A, Mirabel M, Nkomo VT, Okello E, et al. Contemporary diagnosis and management of rheumatic heart disease: implications for closing the gap: a scientific statement from the american heart association. Circulation. 2020 Nov 17;142(20):e337–57. [Google Scholar]
27. Cardarelli M, Vaikunth S, Mills K, DiSessa T, Molloy F, Sauter E, et al. Cost-effectiveness of humanitarian pediatric cardiac surgery programs in low- and middle-income countries. JAMA Netw Open. 2018 Nov 2;1(7):e184707. doi:10.1001/jamanetworkopen.2018.4707. [Google Scholar] [PubMed] [CrossRef]
28. Higashi H, Barendregt JJ, Vos T. The burden of congenital anomalies amenable to surgeries in low-income and middle-income countries: a modelled analysis. Lancet. 2013 Jun 17;381:S62. doi:10.1016/S0140-6736(13)61316-1. [Google Scholar] [CrossRef]
29. Farmer PE, Kim JY. Surgery and global health: a view from beyond the OR. World J Surg. 2008 Apr;32(4):533–6. doi:10.1007/s00268-008-9525-9. [Google Scholar] [PubMed] [CrossRef]
30. Grimes CE, Henry JA, Maraka J, Mkandawire NC, Cotton M. Cost-effectiveness of surgery in low- and middle-income countries: a systematic review. World J Surg. 2014 Jan;38(1):252–63. doi:10.1007/s00268-013-2243-y. [Google Scholar] [PubMed] [CrossRef]
31. Saxton AT, Poenaru D, Ozgediz D, Ameh EA, Farmer D, Smith ER, et al. Economic analysis of children’s surgical care in low- and middle-income countries: a systematic review and analysis. PLoS One. 2016 Oct 28;11(10):e0165480. doi:10.1371/journal.pone.0165480. [Google Scholar] [PubMed] [CrossRef]
32. Leung J, Guria J. Value of statistical life: adults versus children. Accid Anal Prev. 2006 Nov;38(6):1208–17. doi:10.1016/j.aap.2006.05.009. [Google Scholar] [PubMed] [CrossRef]
33. Tiwari A, Sharma A, Jaswal S, Kaur SS, Thakur N. Assessing the patient outcomes and performance of a cardiothoracic and vascular surgery (CTVS) unit during its first two years in a tier-2 city in India: a comprehensive audit and analysis. Cureus. 2023 Aug;15(8):e42910. [Google Scholar] [PubMed]
34. Hellebo AG, Zuhlke LJ, Watkins DA, Alaba O. Health system costs of rheumatic heart disease care in South Africa. BMC Public Health. 2021 Jul 3;21(1):1303. doi:10.1186/s12889-021-11314-6. [Google Scholar] [PubMed] [CrossRef]
35. Watkins D, Lubinga SJ, Mayosi B, Babigumira JB. A cost-effectiveness tool to guide the prioritization of interventions for rheumatic fever and rheumatic heart disease control in African Nations. PLoS Negl Trop Dis. 2016 Aug;10(8):e0004860. doi:10.1371/journal.pntd.0004860. [Google Scholar] [PubMed] [CrossRef]
36. Coates MM, Sliwa K, Watkins DA, Zühlke L, Perel P, Berteletti F, et al. An investment case for the prevention and management of rheumatic heart disease in the African Union 2021–30: a modelling study. Lancet Glob Health. 2021 May 10;9(7):e957–66. doi:10.1016/S2214-109X(21)00199-6. [Google Scholar] [PubMed] [CrossRef]
37. Uy J, Ketkar AG, Portnoy A, Kim JJ. Cost-utility analysis of heart surgeries for young adults with severe rheumatic mitral valve disease in India. Int J Cardiol. 2021 Sep 1;338:50–7. doi:10.1016/j.ijcard.2021.05.059. [Google Scholar] [PubMed] [CrossRef]
38. Forcillo J, Watkins DA, Brooks A, Hugo-Hamman C, Chikoya L, Oketcho M, et al. Making cardiac surgery feasible in African countries: experience from Namibia, Uganda, and Zambia. J Thorac Cardiovasc Surg. 2019 Nov;158(5):1384–93. doi:10.1016/j.jtcvs.2019.01.054. [Google Scholar] [PubMed] [CrossRef]
39. Argaw S, Genetu A, Vervoort D, Agwar FD. The state of cardiac surgery in Ethiopia. JTCVS Open. 2023 Jun;14:261–9. doi:10.1016/j.xjon.2023.03.001. [Google Scholar] [PubMed] [CrossRef]
40. Shawar YR, Shiffman J. Generating global priority for addressing rheumatic heart disease: a qualitative policy analysis. J Am Heart Assoc. 2020 Apr 21;9(8):e014800. doi:10.1161/JAHA.119.014800. [Google Scholar] [PubMed] [CrossRef]
41. Dominique V, Grace L, Yihan L, Roberto CRJ, Kudzai K, Noah T. 6 billion people have no access to safe, timely, and affordable cardiac surgical care. JACC: Adv. 2022 Aug 1;1:1–5. [Google Scholar]
42. Oh NA, Blitzer D, Chen L, Guariento A, Fuller S, Subramanyan RK, et al. The impact of congenital cardiac surgery fellowship on training and practice. Ann Thorac Surg. 2023 Dec;116(6):1320–7. doi:10.1016/j.athoracsur.2023.06.018. [Google Scholar] [PubMed] [CrossRef]
43. Marin-Cuartas M, Vervoort D, Contreras JR, Garcia-Villareal OA, Escobar A, Ferrari J, et al. Perspectives in training and professional practice of cardiac surgery in Latin America. Braz J Cardiovasc Surg. 2023 Feb 10;38(1):1–14. [Google Scholar]
44. Ghandour HZ, Vervoort D, Welke KF, Karamlou T. Regionalization of congenital cardiac surgical care: what it will take. Curr Opin Cardiol. 2021 Oct 13;37(1):137–143. doi:10.1097/HCO.0000000000000940. [Google Scholar] [PubMed] [CrossRef]
45. Backer CL, Overman DM, Dearani JA, Romano JC, Tweddell JS, Ram Kumar S, et al. Recommendations for centers performing pediatric heart surgery in the United States. World J Pediatr Congenit Heart Surg. 2023 Sep;14(5):642–79. doi:10.1177/21501351231190353. [Google Scholar] [PubMed] [CrossRef]
46. Welke KF, Diggs BS, Karamlou T, Ungerleider RM. The relationship between hospital surgical case volumes and mortality rates in pediatric cardiac surgery: a national sample, 1988-2005. Ann Thorac Surg. 2008 Sep 1;86(3):889–96. doi:10.1016/j.athoracsur.2008.04.077. [Google Scholar] [PubMed] [CrossRef]
47. Karamlou T, Johnston DR, Backer CL, Roselli EE, Welke KF, Caldarone CA, et al. Access or excess? Examining the argument for regionalized cardiac care. J Thorac Cardiovasc Surg. 2020 Sep;160(3):813–9. doi:10.1016/j.jtcvs.2019.12.125. [Google Scholar] [PubMed] [CrossRef]
48. Welke KF, Pasquali SK, Lin P, Backer CL, Overman DM, Romano JC, et al. Theoretical model for delivery of congenital heart surgery in the United States. Ann Thorac Surg. 2020 Aug 26;111(5):1628–35. doi:10.1016/j.athoracsur.2020.06.057. [Google Scholar] [PubMed] [CrossRef]
49. Moons P, Bratt E-L, De Backer J, Goossens E, Hornung T, Tutarel O, et al. Transition to adulthood and transfer to adult care of adolescents with congenital heart disease: a global consensus statement of the ESC Association of Cardiovascular Nursing and Allied Professions (ACNAPthe ESC Working Group on Adult Congenital Heart Disease (WG ACHDthe Association for European Paediatric and Congenital Cardiology (AEPCthe Pan-African Society of Cardiology (PASCARthe Asia-Pacific Pediatric Cardiac Society (APPCSthe Inter-American Society of Cardiology (IASCthe Cardiac Society of Australia and New Zealand (CSANZthe International Society for Adult Congenital Heart Disease (ISACHDthe World Heart Federation (WHFthe European Congenital Heart Disease Organisation (ECHDOand the Global Alliance for Rheumatic and Congenital Hearts (Global ARCH). Eur Heart J. 2021 Nov 1;42:4213–23. [Google Scholar]
50. Ross S, Verstappen A. The role of congenital heart disease patient organizations in advocacy, resources, and support across the lifespan. CJC Pediatr Congenit Heart Dis. 2023 Dec;2:256–66. doi:10.1016/j.cjcpc.2023.08.006. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools