 Open Access
Open Access
LETTER
Hybrid Procedure for Interruption of Aortic Arch Associated with Bicuspid Aortic Valve Stenosis in an Infant
1 Department of Cardiac Intensive Care Unit, Children’s Hospital Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, 310052, China
2 Department of Cardiac Surgey, Children’s Hospital Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, 310052, China
* Corresponding Authors: Shanshan Shi. Email: ; Xiangming Fan. Email:
# Jiajun Xu and Zhuo Shi contributed equally to this manuscript
Congenital Heart Disease 2024, 19(5), 535-540. https://doi.org/10.32604/chd.2024.057558
Received 21 August 2024; Accepted 06 November 2024; Issue published 31 December 2024
Abstract
Interrupted Aortic Arch (IAA) combined with aortic stenosis (AS) is a rare and complex congenital cardiac anomaly that presents significant challenges in clinical management. In this letter, we aim to share our experience in performing hybrid procedures on an infant diagnosed with IAA combined with bicuspid AS. This child exhibited significant recovery during follow-up.Keywords
A male newborn, born at 35 + 2 weeks of gestation and weighing 2300 g, was diagnosed with IAA, ventricular septal defect (VSD), atrial septal defect (ASD), and patent ductus arteriosus (PDA) (Fig. 1) at two days old. He underwent aortic arch reconstruction, VSD and ASD repair, and PDA ligation under cardiopulmonary bypass. No aortic override was found during surgery. Immediately after the operation, the echocardiography confirmed that there was no left ventricular outlet obstruction (LVOTO) or narrowing. However, the infant had difficulty weaning off extracorporeal circulation, leading to the initiation of extracorporeal membrane oxygenation (ECMO) support, which was discontinued on the fifth postoperative day. However, echocardiography revealed a previously undiagnosed bicuspid aortic valve with severe stenosis, showing a peak flow velocity of 4.3 m/s at the valve orifice. The gradient across the aortic valve was measured 73 mmHg, with a left ventricular ejection fraction (EF) of 0.64, and the aortic ring diameter was 6 mm. On the 28th postoperative day, transapical aortic valvuloplasty was performed (Fig. 2). A small incision was made on the left anterior chest wall, approaching the 5th intercostal space, and a purse-string suture was placed at the apex. Under transesophageal ultrasound guidance, a 0.35 mm hardened guidewire was inserted through the apex, across the aortic valve, and into the descending aortic arch, successfully creating a track. The aortic valve was then dilated using 5 and 6 mm balloons. After the procedure, the aortic valve area significantly improved, with a reduced peak flow velocity of 1.9 m/s. Post-balloon valvuloplasty echocardiography showed no aortic valve regurgitation. The gradient across the valve was 34 mmHg, the left ventricular EF was 0.79, and the aortic ring diameter increased to 8 mm. The infant was successfully weaned from the ventilator 16 days after the hybrid procedure and discharged 30 days post-operation. During follow-up, echocardiography and computed tomographic angiography (CTA) scans revealed the bicuspid aortic valve remained stenotic without regurgitation (Fig. 3), with the peak aortic valve flow velocity increasing to 5.3 m/s, as well as secondary hypertrophy and stenosis of the left ventricular outflow tract. Ten months after the hybrid procedure, the infant underwent surgical aortic valvuloplasty and left ventricular outflow tract reconstruction. A meticulous inverted L-shaped incision was made in the ascending aorta. Intraoperative findings included left ventricular hypertrophy, a bicuspid aortic valve in an anterior-posterior orientation with a fish-mouth shape, and an irregular right commissure (post-balloon angioplasty). Additional findings included adhesion of the left commissure, thickened and slightly curled valve margins with adequate coaptation, a 5 mm valve orifice, subvalvular fibrosis, severe muscle hypertrophy, and tubular stenosis of the outflow tract. The surgery involved incising the left commissure, thinning the valve leaflets, excising the hyperplastic subvalvular tissue, and wedging hypertrophic fibrous muscle bundles to widen the left ventricular outflow tract. The right commissure was also reshaped. Post-procedure, the aortic valve orifice accommodated a 9 mm bougie. Extracorporeal circulation lasted 107 min, with aortic cross-clamp time of 65 min. Intraoperative transesophageal ultrasound confirmed unobstructed flow through the left ventricular outflow tract, with a flow rate of 1.5 m/s at the aortic valve orifice. The infant was weaned from ventilation on the first postoperative day and discharged on the ninth day post-surgery. At the six-month follow-up, the aortic valve ring diameter was measured 10.7 mm, the valve orifice was 6.7 mm, and the flow rate was 1.0 m/s. This research was approved by the institutional review board in Children’s Hospital Zhejiang University School of Medicine and the approval number is 2024-IRB-0236-P-01 and informed consent was obtained.
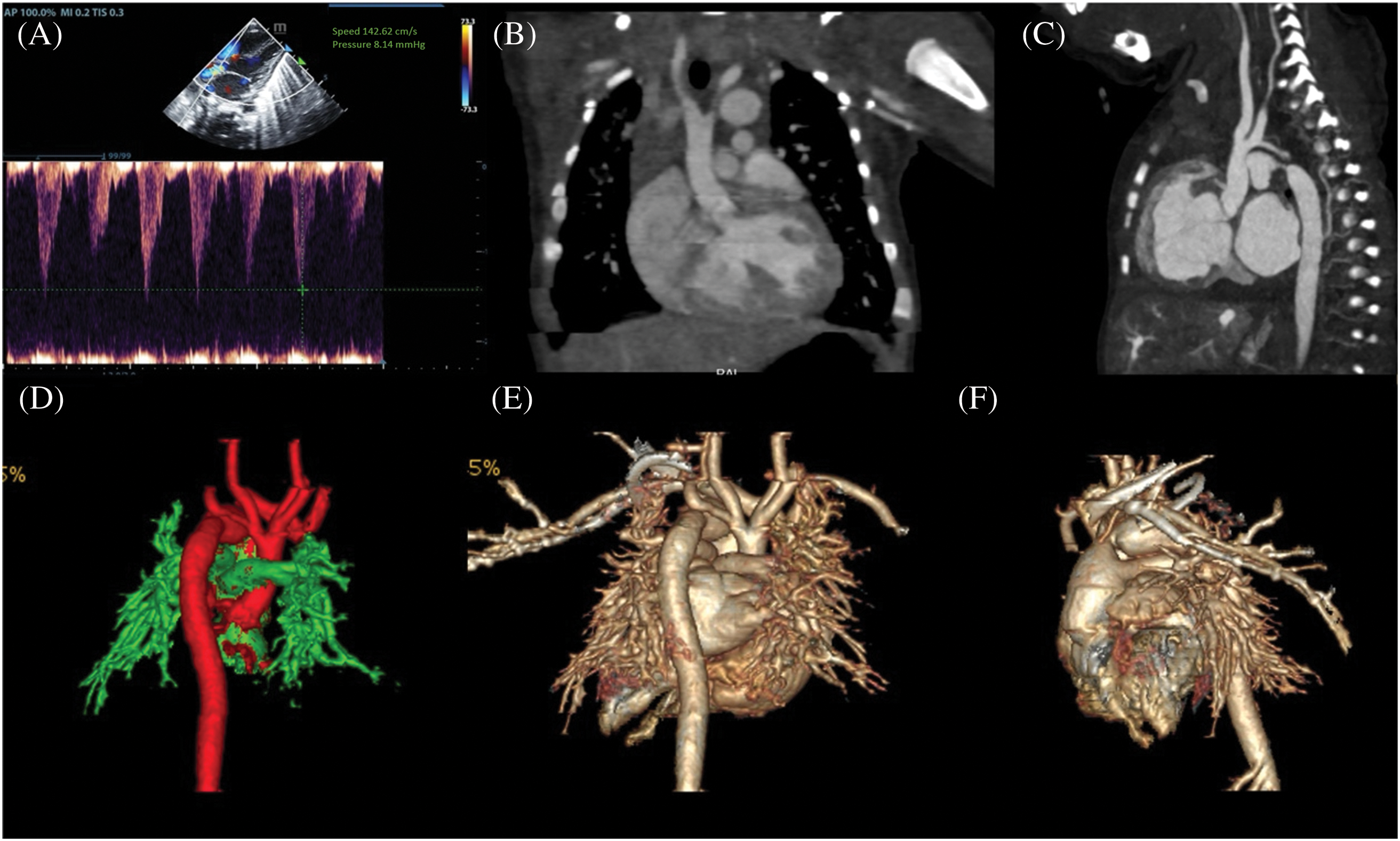
Figure 1: (A) depicts the aortic valve flow rate of 1.4 m/s prior to IAA correction. (B) illustrates the CTA left ventricular outflow tract and aortic valve section. (C) depicts the CTA aortic arch section, which demonstrates that there is no continuation of the ascending aorta. The descending aorta is connected to the pulmonary artery via a PDA. The CTA 3D reconstruction (D–F) demonstrates that there is no continuation of the ascending aorta after it sends out a branch, and that the descending aorta is connected to the pulmonary artery via a PDA
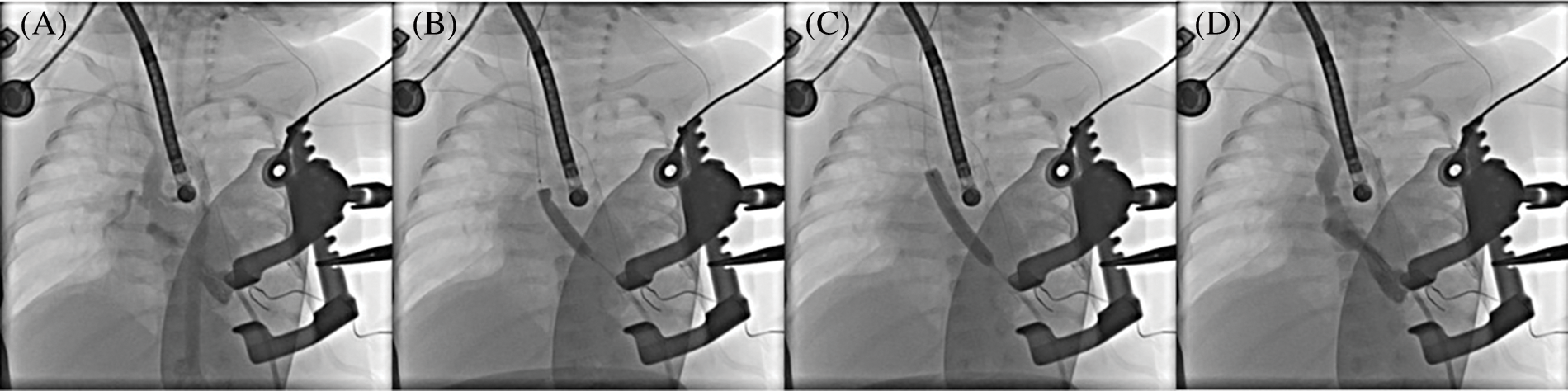
Figure 2: Aortic valvular balloon valvuloplasty. (A) Aortic valvular stenosis and dome-like changes in the valve orifice before balloon valvuloplasty; (B) 5 mm balloon valvuloplasty; (C) 6 mm balloon valvuloplasty; (D) Significant improvement in aortic valve stenosis after balloon valvuloplasty
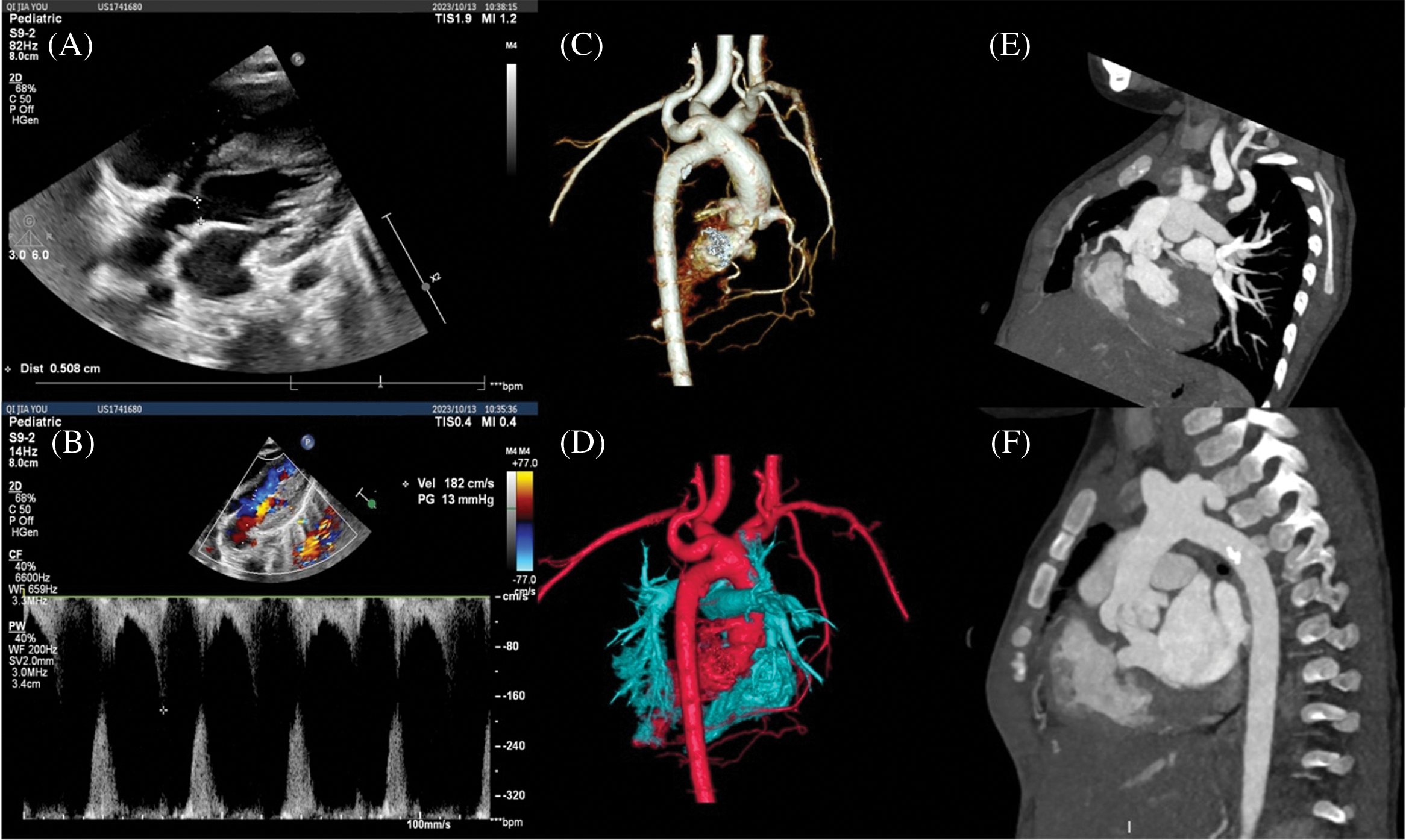
Figure 3: CTA images at 10 months after balloon valvuloplasty. (A, B) Echocardiography showed that the diameter of the aortic valve orifice was 5 mm, and the flow velocity at the beginning of the left ventricular outflow tract was 1.8 m/s; (C, D) Three-dimensional reconstruction of CTA showed that there was no obvious stenosis of the aortic arch and anastomosis after IAA correction; (E, F) Stenosis of the left ventricular outflow tract and aortic valve orifice, and dome-like changes
Currently, there are limited reports on surgical experiences in treating IAA combined with AS. Previous researchers reported four cases of children undergoing Yasui surgery to correct IAA combined with VSD and either AS or aortic atresia (AA) [1]. They suggested that Yasui surgery is a viable option for biventricular correction of IAA/VSD/AS (or AA), with satisfactory early outcomes. However, this procedure is complex and high-risk, involving prolonged operation and extracorporeal circulation time. Careful consideration is necessary when determining the indications for the Yasui procedure. IAA/VSD/AA represents an absolute indication, and aortic valve ring diameters smaller than (body weight + 1) mm, or a Z value less than −4, may be suitable criteria for Yasui surgery. In cases involving ascending aortic hypoplasia, the indication becomes even stronger [1]. We proposed a hybrid procedure to reduce surgical risks and trauma, followed by aortic valvuloplasty after the infant gains weight and is in better condition. The infant in our case had a birth weight of only 2300 g, and the bicuspid AS was undiagnosed preoperatively. Therefore, the second-stage transapical balloon aortic valvuloplasty became a last resort. However, this approach provided valuable insights: for children with IAA and bicuspid AS in poor condition, a phased surgical strategy, incorporating hybrid procedures, can minimize the duration of radical surgery, reduce trauma, and improve survival outcomes. Once IAA is diagnosed, early surgical intervention is essential. In neonates, particularly those with complex cardiac malformations, timely surgery is crucial for improving survival, despite the inherent surgical risks [2]. Individualized surgical plans should be based on the location and extent of arch interruption as well as the accompanying intracardiac malformations. In cases with concurrent AS, simultaneous aortic valve surgery may be necessary [3]. Had critical AS been diagnosed preoperatively in our case, we would have recommended a one-stage procedure involving aortic arch repair and surgical aortic valvuloplasty to reduce left heart afterload and prevent left heart failure since he had difficulty in weaning from mechanical ventilation. This infant had bicuspid AS, which has been shown to be a significant risk factor for valve-related reintervention following one-stage repair for aortic arch obstruction [4]. Neonates with a bicuspid aortic valve may require future reinterventions, underscoring the importance of lifelong cardiac follow-up. However, there is another treatment option for palliative surgery in IAA/VSD. Researches indicated that for critically ill neonates diagnosed with IAA and LVOTO whose condition were not suitable for early one stage surgical repair, bilateral pulmonary artery banding (bPAB) and PDA stenting is an alternative first-line approach used to move the patient to an age and body weight for which the risks are lower for early one stage repair [5]. Another study performed a hybrid procedure in a newborn with critical aortic valve stenosis with acute pulmonary edema in the first hour after birth, demonstrating that for neonates in critical illness who are not suitable for surgical correction under extracorporeal circulation, hybrid procedure may be a excellent choice [6]. However, a selective management strategy customized to the degree of aortic valve and subaortic area narrowing [7].
In this case, immediately after surgery, echocardiographic examination for several days demonstrated that there was no LVOTO caused by VSD repair. LVOTO is a common complication after IAA/VSD repair [8]. Before IAA/VSD correction, cardiac surgeons should be aware of the presence or absence of LVOTO or left ventricular hypertrophy. Surgical repair of the VSD should be performed in such a way as to avoid compromising the patency of the left ventricular outflow tract while the presence of LVOTO should be closely monitored in the postoperative period. VSD repair may result in alterations to the anatomy of the ventricles, affecting the morphology and function of the left ventricular outflow tract. Additionally, it may lead to changes in interventricular pressure relationships and blood flow patterns, which could impact the hemodynamics of the left ventricular outflow tract. Furthermore, surgical manipulation within the ventricles may result in the formation of scar tissue, which could subsequently affect ventricular function and potentially develop into an aneurysm. When repairing a VSD, special care must be taken to avoid compromising the anatomy of the left ventricular outflow tract. The location and size of the defect should be carefully evaluated, and appropriate repair materials and techniques should be selected to ensure that left ventricular outflow tract stenosis does not result. An intraoperative echocardiography evaluation is also essential, and care should be taken to avoid overcorrection in the repair of aortic arch and VSD, which may result in stenosis of the left ventricular outflow tract. One latest research showed that both Yasui and Ross-Konno operations effectively mitigate late LVOTO risk [9].
The femoral artery of this infant was only 1.5 mm, which the cardiologists argued that the diameter of femoral artery was not qualified for percutaneous aortic valve balloon dilation. Finally, we selected the approach by using a transapical incision. The treatment of neonatal AS remains a challenge, with ongoing debate over whether balloon aortic valvuloplasty or surgical aortic valvuloplasty should be the initial treatment. Studies show both techniques effectively relieve obstruction and improve survival, but multivariate analysis has identified balloon valvuloplasty as an independent risk factor for reintervention, with a significantly increased risk of reoperation [10]. Additionally, some research has demonstrated the safety and efficacy of percutaneous balloon aortic valvuloplasty in treating congenital AS in children, although long-term attention is required for the potential risk of significant aortic valve regurgitation [11]. Balloon aortic valvuloplasty is emerging as a novel method for treating low birth weight, severe congenital AS in neonates and infants [12]. While it is the preferred method for isolated AS, surgical intervention is often necessary for complex cases, such as restenosis after balloon valvuloplasty or multi-level stenosis [13]. However, the risks and complications of aortic valve balloon valvuloplasty must not be overlooked. Major risks include damage to the cardiac conduction system, aortic valve injury, and embolization, while common complications include arrhythmias, aortic coarctation, aortic regurgitation, and vascular issues. In our case, restenosis of the aortic valve, subaortic stenosis, and subsequent left ventricular hypertrophy were observed during a 10-month follow-up after the hybrid procedure. Consequently, surgical aortic valvuloplasty and left ventricular outflow tract reconstruction were performed under extracorporeal circulation. Despite the restenosis after balloon valvuloplasty, based on our surgical experience, we believe that hybrid therapy for IAA combined with AS is feasible, though more cases are needed to assess long-term outcomes. To date, we have monitored this child for one year following surgical valvuloplasty, during which time we observed good cardiac function with no signs of aortic regurgitation or stenosis.
In conclusion, neonatal IAA associated with AS and other intracardiac malformations can be effectively managed with a hybrid procedure, which reduces the risks and duration of simultaneous correction. However, compared to surgical aortic valvuloplasty, balloon valvuloplasty is associated with a higher incidence of restenosis and an increased risk of reintervention. While we still recommend a one-stage surgical approach for cases involving both aortic arch dissection and aortic valve stenosis, this case demonstrates the potential feasibility of hybrid procedures for managing complex conditions.
Acknowledgement: I would like to express my deepest gratitude to the team of heart center, for their continuous support, patience, and insightful guidance throughout my research. Their expertise and encouragement have been invaluable to the completion of this work.
Funding Statement: Supported by Zhejiang University Scientific Research Development Project (2021FZZX005-06).
Author Contributions: Jiajun Xu and Zhuo Shi contributed equally to this manuscript. Jiajun Xu: writing, conceptualization, review editing and funding acquisition. Zhuo Shi: conceptualization, review editing. Shanshan Shi and Xiangming Fan: supervision and review. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors will supply the relevant data in response to reasonable requests.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and National research Committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This research was approved by the Institutional Review Board in Children’s Hospital Zhejiang University School of Medicine and the approval number is 2024-IRB-0236-P-01 and informed consent was acquired.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Pan YJ, Luo K, Yin M, Zhu HB, Zhu ZQ, Zhang HB, et al. Yasui procedure for surgical repair of interruption of aortic arch and ventricular septal defect associated with aortic stenosis or aortic atresia. Chin J Thorac Cardiovasc Surg. 2021;37(10):591–4 (In Chinese). [Google Scholar]
2. Shi G, Chen H, Zheng J, Zhang H, Zhu Z, Liu J. Primary complete repair of interrupted aortic arch with associated lesions in infants. J Card Surg. 2014;29(5):686–91. doi:10.1111/jocs.12401. [Google Scholar] [PubMed] [CrossRef]
3. Jacobs ML, Chin AJ, Rychik J, Steven JM, Nicolson SC, Norwood WI. Interrupted aortic arch. Impact of subaortic stenosis on management and outcome. Circulation. 1995;92(9 Suppl):II128–31. [Google Scholar] [PubMed]
4. Rahatianpur M, Bakhtiary F, Vázquez-Jiménez J, Dähnert I, Kostelka M. The association of bicuspid aortic valve on long-term outcome following one-stage repair of aortic arch obstruction associated with ventricular septal defect. Cardiol Young. 2023;33(2):227–34. doi:10.1017/S104795112200049X. [Google Scholar] [PubMed] [CrossRef]
5. Toprak MHH, Yakut K, Yilmaz N, Tan Recep BZ, Tüzün B, Ozturk E, et al. Results of a hybrid approach for high risk term newborn patients with interrupted aortic arch (IAA) with left ventricular outflow tract obstruction. Medicine. 2024;103(5):e37121. doi:10.1097/MD.0000000000037121. [Google Scholar] [PubMed] [CrossRef]
6. Vitaliy S, Vladimir Z, Nikolay P, Olga T, Michail K. Emergency hybrid correction in a newborn with critical aortic valve stenosis with acute pulmonary edema in the first hour after birth. Congenit Heart Dis. 2023;18(1):57–65. doi:10.32604/chd.2023.025522. [Google Scholar] [CrossRef]
7. Alsoufi B, Schlosser B, McCracken C, Sachdeva R, Kogon B, Border W, et al. Selective management strategy of interrupted aortic arch mitigates left ventricular outflow tract obstruction risk. J Thorac Cardiovasc Surg. 2016;151(2):412–20. doi:10.1016/j.jtcvs.2015.09.060. [Google Scholar] [PubMed] [CrossRef]
8. McMullen HL, Harrington JK, Blitzer D, Pasumarti N, Levasseur S, Bacha E, et al. Clinical outcomes and echocardiographic predictors of reintervention after interrupted aortic arch repair. Pediatr Cardiol. 2024;45(5):967–75. doi:10.1007/s00246-024-03419-7. [Google Scholar] [PubMed] [CrossRef]
9. Luo S, Schoof PH, Hickey E, Morgan C, Korsuize NA, Grotenhuis HB, et al. The fate of the left ventricular outflow tract following interrupted aortic arch repair. World J Pediatr Congenit Heart Surg. 2024 Jul 25;15(5):562–70. doi:10.1177/21501351241236742. [Google Scholar] [PubMed] [CrossRef]
10. Zhu YF, Jiang Q, Zhang W, Hu RJ, Yu XF, Zhang HB. Comparison of surgical repair and transcatheter balloon dilatation for congenital aortic stenosis. Chin J Thorac Cardiovasc Surg. 2021;37(10):586–90 (In Chinese). [Google Scholar]
11. Han Y, Li JJ, Zhang ZW, Qian MY, Wang SS. Long-term outcome of percutaneous balloon aortic valvuloplasty for children with congenital aortic valve stenosis. Chin J Cardiol. 2020;48(10):853–8 (In Chinese). [Google Scholar]
12. Pan XB, Zhang H, Hu ST, Chen ZR, Ma K, Ouyang WB, et al. Efficacy of hybrid balloon valvuloplasty via sternotomy for treating low-body weight infants with severe congenital valvular aortic stenosis. Chin J Cardiol. 2012;40(08):681–3 (In Chinese). [Google Scholar]
13. Mehrotra P, Jansen K, Flynn AW, Tan TC, Elmariah S, Picard MH, et al. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J. 2013;34(25):1906–14. doi:10.1093/eurheartj/eht094. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools