 Open Access
Open Access
ARTICLE
Evaluation of Post-Operative Atrial Fibrillation after Cardiac Surgery for Adult Congenital Heart Disease
1Internal Medicine-Pediatrics Residency, University of Colorado, Aurora, CO 80045, USA
2 Adult Congenital Heart Disease Fellowship, University of Colorado, Aurora, CO 80045, USA
3 School of Medicine, University of Colorado, Aurora, CO 80045, USA
4 Department of Pediatrics, University of Colorado, Aurora, CO 80045, USA
5 Division of Pediatric Cardiology, Electrophysiology, University of Colorado, Aurora, CO 80045, USA
6 Division of Pediatric Cardiology, University of Colorado, Aurora, CO 80045, USA
7 Division of Cardiology, University of Colorado, Aurora, CO 80045, USA
* Corresponding Author: Jonathan S. Taylor-Fishwick. Email:
Congenital Heart Disease 2024, 19(5), 457-472. https://doi.org/10.32604/chd.2024.057151
Received 09 August 2024; Accepted 29 September 2024; Issue published 31 December 2024
Abstract
Background: Post-operative atrial fibrillation (POAF) frequently occurs after cardiac surgery. Although adult congenital heart disease (ACHD) patients have higher rates of arrhythmia than the general population, there is scant literature on POAF in ACHD patients. Objectives: Identify key risk factors associated with post-operative atrial fibrillation and evaluate the short- and mid-term significance of developing POAF. Methods: A retrospective cohort study was conducted of ACHD patients from 2013–2021 at the University of Colorado Hospital and Children’s Hospital of Colorado. The institutional Society of Thoracic Surgeons (STS) surgical registry was used to identify patients ≥18-year-old with congenital heart disease who underwent cardiac surgery during the study period. Results: A total of 168 patients (48% female) were included. The median age was 36 years (IQR 28–48). One-hundred and fifty patients (90%) had moderate ACHD anatomical complexity, and 10 patients (6%) had severe ACHD anatomical complexity based on initial ACHD diagnosis. POAF occurred in 40 (24%) patients. Older age, history of supraventricular tachycardia, intra-operative arrhythmia, and post-operative hypokalemia independently predicted POAF. POAF was associated with an increased length of stay (8 vs. 5 days, p < 0.001) and recurrence of atrial fibrillation (46% vs. 21%, OR 3.35, p = 0.002) but did not predict mortality, stroke, or bleeding event. Conclusion: Atrial fibrillation is a common complication after cardiac surgery in the ACHD population. Older age, history of supraventricular tachycardia, intra-operative arrhythmia, and post-operative hypokalemia independently predicted POAF. Further investigation is needed to understand the long-term impacts of POAF.Keywords
Post-operative atrial fibrillation (POAF) is a common complication following cardiac surgery in adults, occurring about 17%–34% of the time with higher risk reported following valvular surgeries [1]. Risk factors that have been associated with POAF include increased age, left atrial enlargement, mitral valve disease, congestive heart failure, and past history of atrial fibrillation [2]. Importantly, the presence of POAF has been associated with increased length of stay, stroke, in-hospital mortality and 30-day mortality [1]. Given this burden, multiple national and international guidelines have been developed to provide evidence-based recommendations on the prevention and treatment of POAF [3–5]. While there is robust data regarding POAF in the general adult population following cardiac surgery, evidence and guidelines concerning POAF in adults with congenital heart disease (ACHD) is less robust.
ACHD have an increased incidence of atrial and ventricular arrhythmias when compared to the general population, with one study finding a 22-fold increased risk [6,7]. The previously described risk factors that apply to the general public such as left atrial enlargement, mitral valve disease, and congestive heart failure are also common among this population, especially those with previous interventions. Additionally, certain congenital lesions have an even higher lifetime incidence of atrial fibrillation, including secundum atrial septal defects, atrioventricular septal defects, tetralogy of Fallot, D-transposition of the great arteries with atrial switch, and hypoplastic left heart syndrome [6].
Importantly, ACHD patients who develop atrial tachyarrhythmias are at increased risk for adverse clinical events [8]. In one study, patients with atrial arrhythmias had a 50% increase in mortality, two-fold increase in stroke or onset of heart failure, and three-fold increased risk of requiring surgical or catheter-based intervention [9]. In a cohort of patients with a history of atrial switch procedure for transposition of the great arteries, atrial arrhythmia led to decreased systemic ejection fraction and worse right ventricular function [10]. Furthermore, a study of patients with tetralogy of Fallot identified atrial arrhythmia as a predictor of mortality and subsequent ventricular tachycardia [11].
A few prior studies have evaluated POAF specifically in the ACHD population. In one study of 239 patients, age greater than 60 years, pre-operative pulmonary hypertension, mitral valve intervention, and post-operative inotropic support independently increased the risk of POAF [12]. In a second study of 1598 patients, older age, hypertension, left atrial reserve strain, right atrial dysfunction, and non-systemic atrioventricular valve regurgitation were associated with POAF [13]. The incidence of POAF in both studies was 21%. Furthermore, one study showed POAF was associated with a longer total length of stay while both studies showed POAF was not associated with mortality [12,13].
Given these limited data, this study sought to understand the incidence, risk factors, and outcomes associated with POAF in the ACHD surgical population at our institution, which includes both an adult and a children’s hospital. Given the increasing numbers of ACHD surgeries being performed at children’s hospitals and the unique physiological and demographic differences when compared to the general adult cardiac population, these data offer the chance to improve understanding of surgical risks and outcomes in POAF ACHD patients.
A single-center, retrospective cohort study was performed of adult patients (age ≥ 18 years old) who underwent congenital cardiac surgery at either the University of Colorado Hospital or Children’s Hospital of Colorado at the University of Colorado-Anschutz Medical Campus between January 2013 and December 2021. Data was extracted from the Society of Thoracic Surgery (STS) congenital surgery registry and enriched with manual chart review of individual charts. Data collected included demographics (i.e., age, body mass index (BMI), sex, ACHD anatomical classification), echocardiography reports (i.e., atrial enlargement, ventricular enlargement or dysfunction, valvular stenosis or regurgitation), historical risk factors (i.e., pre-operative oxygen use, hypertension, pulmonary hypertension, supraventricular tachycardia, coronary artery disease, thyroid disease, obstructive sleep apnea, dyslipidemia, and pacemaker use), surgical interventions (i.e., STAT (The Society of Thoracic SurgeonsEuropean Association for Cardio-Thoracic Surgery) score, prior sternotomies, intra-operative arrhythmia, bypass and cross-clamp time), surgeries performed (i.e., intervention on atrioventricular valves or atria), post-operative risk factors (i.e., pericarditis, electrolytes disturbances, inotrope use), management (i.e., post-operative medication and anticoagulant use, discharged on anticoagulation or rate or rhythm control medication), and outcomes (i.e., mortality, stroke, bleeding events, length of stay, atrial fibrillation recurrence). Patients were included if they carried a formal congenital heart disease diagnosis and underwent congenital cardiac surgery. Patients were excluded who underwent heart transplant, catheter-based procedures, or cardiac surgery not requiring bypass. POAF was defined by progress note and/or discharge summary documentation for atrial fibrillation onset during hospitalization.
ACHD anatomic classification was determined per the 2018 American Heart Association/American College of Cardiology guidelines for the Management of Adults with Congenital Heart Disease [14]. Atrial dilation was recorded as dilated vs. normal by echocardiography report. Valvular stenosis and regurgitation were considered positive if determined to be moderate or severe by echocardiography report. Ventricular dilation was determined to be significant if mild or greater by echocardiography report. Patients were determined to have a history of supraventricular tachycardia if they carried a history of any atrial arrhythmia including ectopic atrial tachycardia, atrial fibrillation, atrial flutter, atrioventricular re-entrant, and atrioventricular node re-entrant tachycardias. STAT score, which predict risk of mortality associated with surgical repair of congenital heart disease, was determined per the 2020 STS Surgical Congenital Heart Disease Mortality Categories [15]. Hypokalemia and hypomagnesemia were determined by values below the reference range of 3.5 mmol/L and 1.6 mg/dL, respectively, during the post-operative period. This study was approved by the University of Colorado Multiple Institutional Review Board (COMIRB 22-0503).
Initial demographic data was stratified by atrial fibrillation status and p-values were calculated using the Wilcoxon Rank Sum test for numeric variables and the Fisher’s exact test for categorical variables. Univariate logistic regression was performed to identify variables associated with atrial fibrillation. Variables that were significant at the univariate level were placed into a multivariate logistic regression model. Using Akaike information criterion (AIC) and the Likelihood Ratio Test, backward selection was used to remove non-significant variables to determine the final model. Significance level was set to an
A total of 168 patients (48% female) were included in this study. Of these patients, the median age was 36 years (Inter-quartile range (IQR) 28–48), and the median BMI was 25.6 kg/m2 (IQR 22.5–30.1) (Table 1). The majority of patients had moderate anatomic complexity (90%, n = 151), 4% (n = 7) with mild anatomic complexity, and 6% (n = 10) with severe anatomic complexity. Over half of the surgical procedures (52%, n = 87) were a STAT score of 1, 31% (n = 51) were a STAT score of 2, 10% (n = 16) were STAT score of 3, and 8% (n = 14) were a STAT score of 4. Fifty-one percent (n = 85) of patients underwent an intervention within the atria (e.g., atrial septal defect (ASD) or partial anomalous pulmonary venous return (PAPVR) repair), 11% (n = 18) of patients underwent an intervention on the systemic atrioventricular valve, and 8% of patients (n = 14) underwent an intervention on the non-systemic atrioventricular valve. A summary of all procedures performed and patient’s fundamental congenital diagnosis is included in Appendix A.
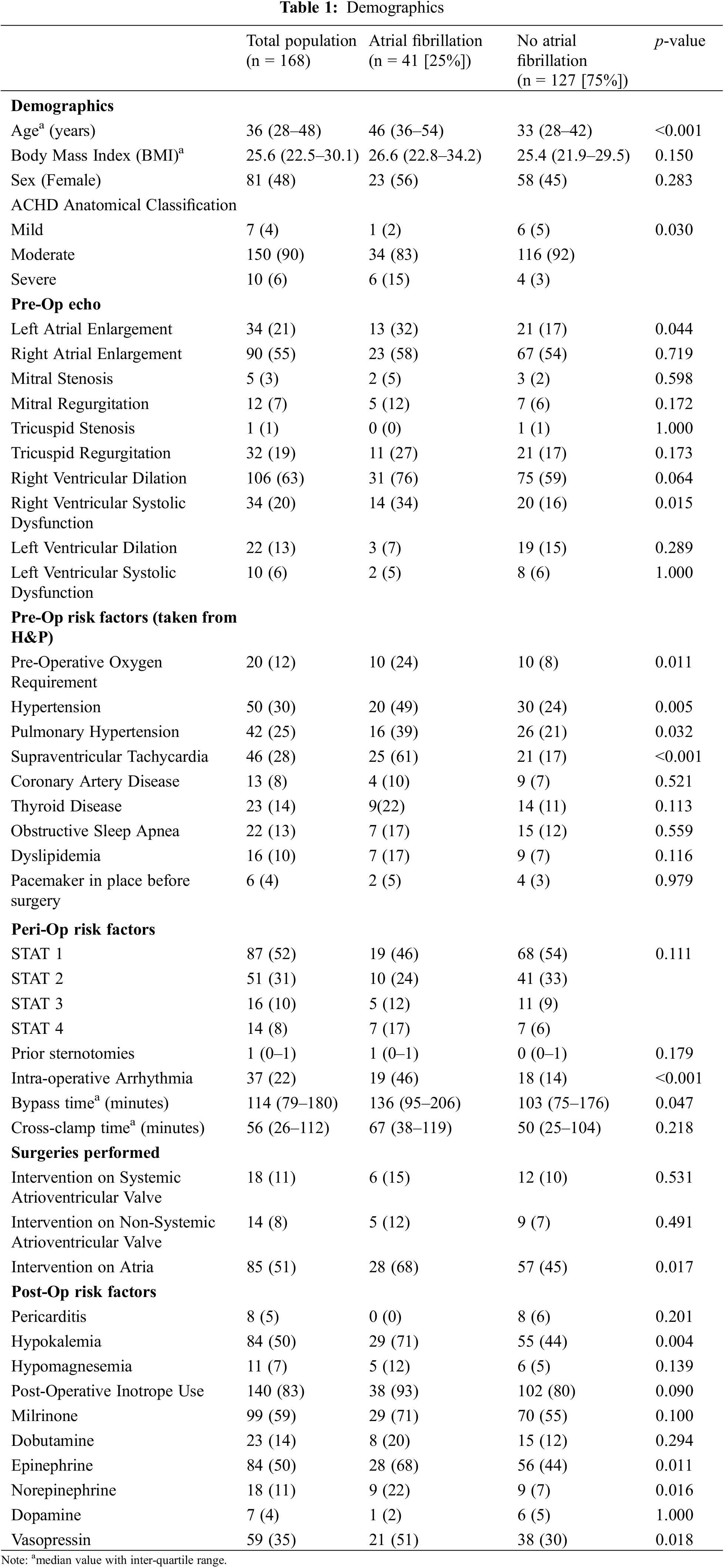
3.2 Risk Factors Associated with Atrial Fibrillation
The incidence of POAF was 24%. Patients who developed POAF were significantly older, with a median age of 46 years (IQR 36–54) in comparison to 33 years (IQR 28–42, p < 0.001, Table 1). There were no significant differences in sex (56% vs. 45% female, p = 0.283) nor BMI (26.6, IQR 22.8–34.2 vs. 25.4, IQR 21.9–29.5, p = 0.150). Patients who developed POAF had a higher proportion of severe anatomic complexity (14.6% vs. 3.1%), and lower proportions of moderate (82.9% vs. 92.1%) and mild (2.4% vs. 4.7%) anatomic complexities (p = 0.030). There was not a significant difference in STAT score distribution between groups (p = 0.111).
Analyzing the predictive value of pre-operative echocardiographic parameters at the univariate level, reduced right ventricular systolic function was associated with atrial fibrillation (p = 0.013, OR 2.77 CI 1.23–6.19, Table 2), while right ventricular dilation, left ventricular dilation, and left ventricular dysfunction were not. Left atrial enlargement was associated with POAF (p = 0.038, OR 2.38 CI 1.03–5.26) while right atrial enlargement was not. Valvular stenosis and valvular regurgitation were not associated with POAF.
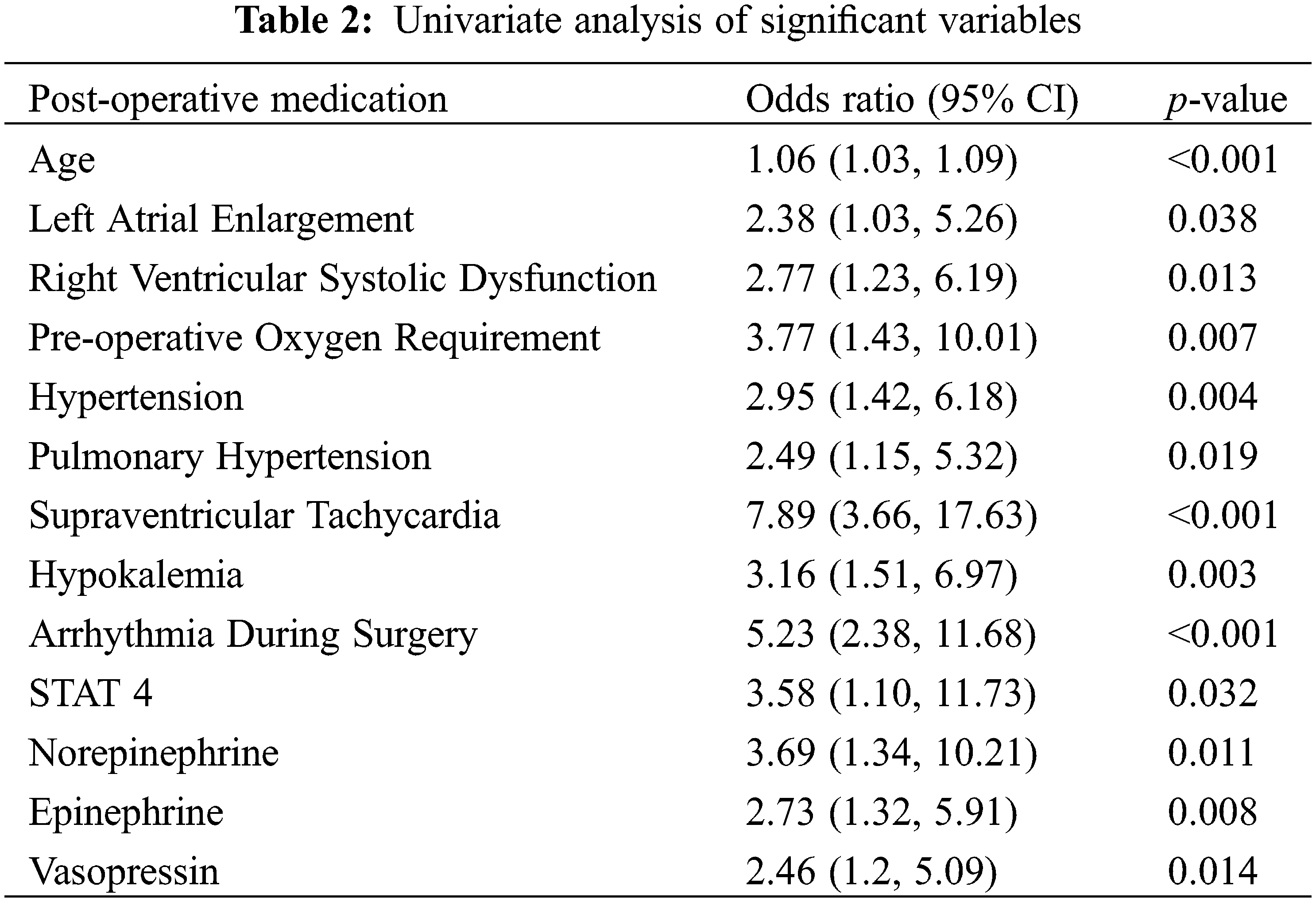
By historical risk factors, pre-operative oxygen use (p = 0.007, OR 3.77 CI 1.43–10.01), systemic hypertension (p = 0.004, OR 2.95 CI 1.42–6.18), pulmonary hypertension (p = 0.019, OR 2.49 CI 1.15–5.32), and history of supraventricular tachycardia (p < 0.001, OR 7.89 CI 3.66–17.63) were all associated with POAF at the univariate level. Thyroid disease, obstructive sleep apnea, dyslipidemia, coronary artery disease and prior pacemaker implantation were not associated with POAF.
Surgical factors associated with POAF at the univariate level included a STAT score of four in comparison to a STAT score of one (p = 0.032, OR 3.58 CI 1.10–11.73). Post-operatively, hypokalemia (p = 0.003, OR 3.16 CI 1.51–6.97) and use of epinephrine (p = 0.008, OR 2.73 CI 1.32–5.91), norepinephrine (p = 0.011, OR 3.69 CI 1.34–10.21), and vasopressin (p = 0.014, OR 2.46 CI 1.2–5.09) were associated with POAF, while milrinone (p = 0.100), dobutamine (p = 0.294), and dopamine (p = 1.000) were not associated with POAF.
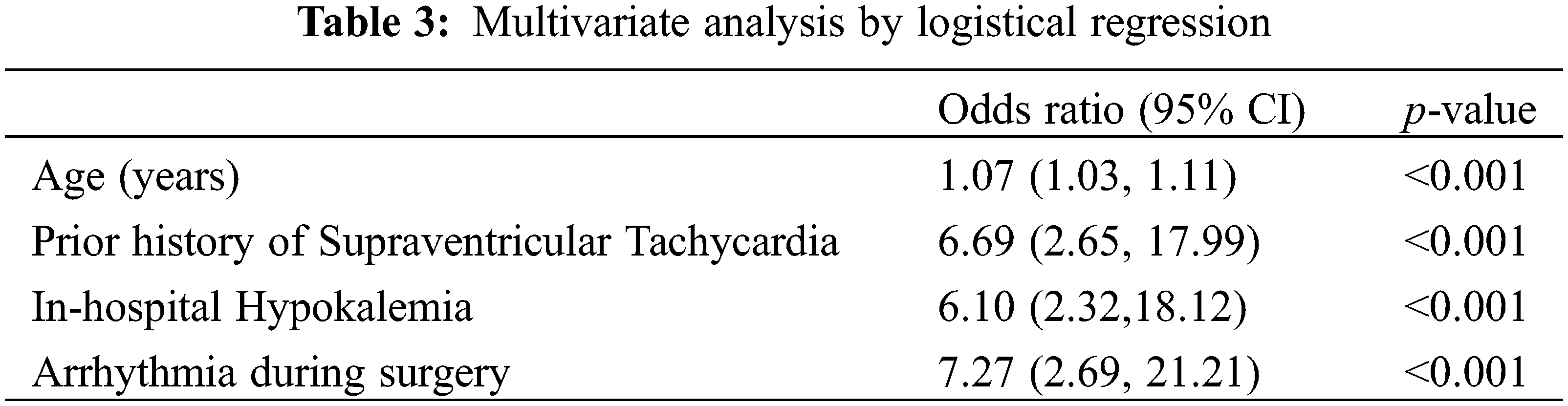
Subsequent multivariate analysis of factors shown to be significant during univariate analysis determined that age, history of prior supraventricular tachycardia, intra-operative arrhythmia and post-operative hypokalemia were significantly associated with odds of POAF (Table 3).
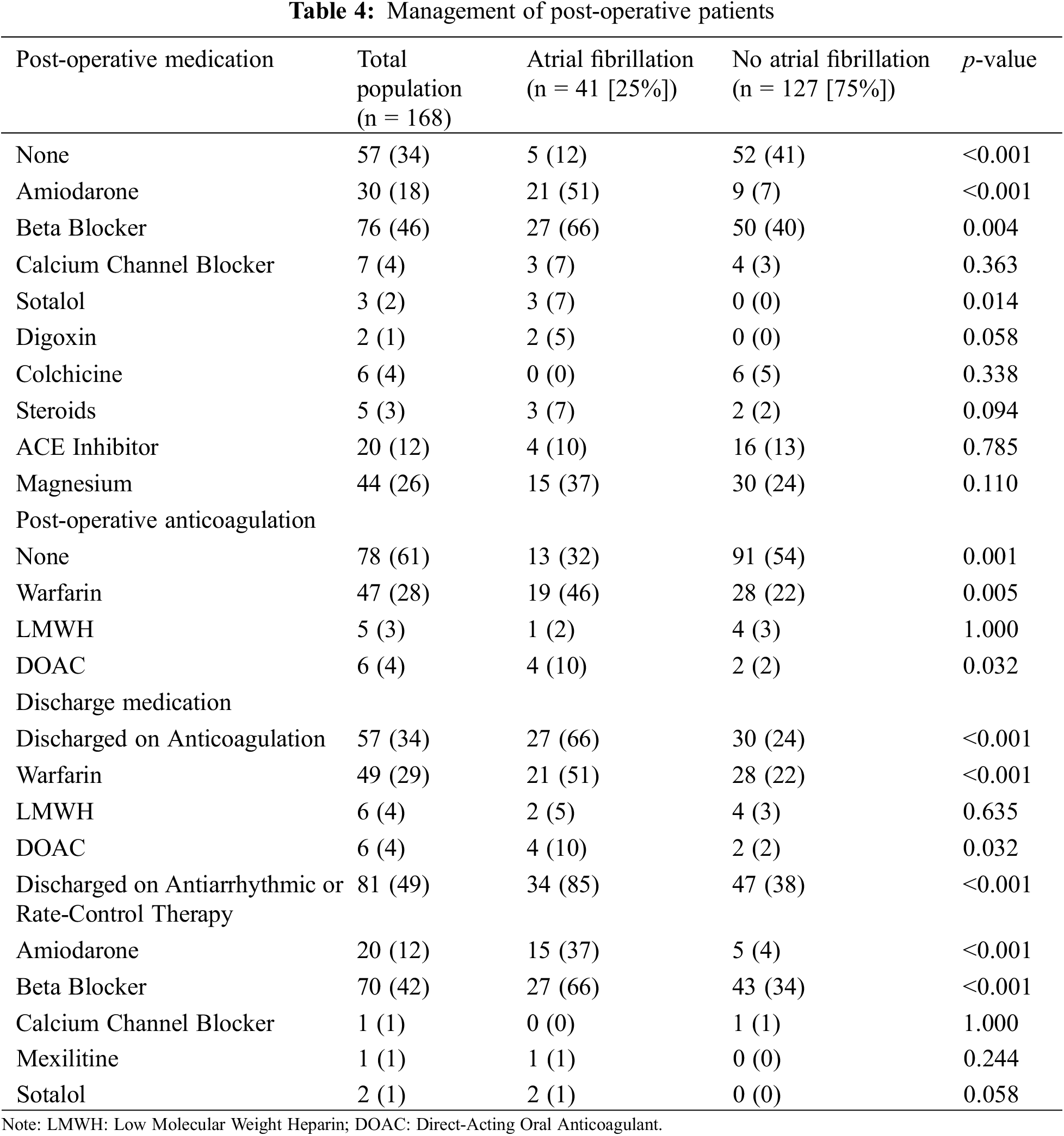
Patients who developed POAF were treated predominantly with amiodarone (51%, n = 21) and beta blockers (66%, n = 27) (Table 4). Seventeen of the patients (34%) who developed POAF were on beta blockers prior to admission. Fifteen (37%) patients with POAF were discharged on amiodarone and 27 patients (66%) were discharged on beta blockers. For patients with POAF, warfarin was the most common anticoagulant used at discharge (n = 21, 51%) followed by direct oral anticoagulants (n = 4, 10%) and low molecular weight heparin (n = 2, 5%) (Table 4). At discharge, patients who developed POAF, when compared to those who did not develop POAF, were more likely to be discharged on anticoagulation (66%, n = 27 vs. 24%, n = 30, p < 0.001) and antiarrhythmics or rate-control therapy (85%, n = 34, vs. 38%, n = 47, p < 0.001).
3.4 Outcomes and Recurrence of Atrial Fibrillation
POAF was associated with increased length of stay (8 days IQR 6–10 vs. 5 IQR 4–6) with the odds of length of stay of at least seven days being 7.81 (95% CI 3.64–17.73, p < 0.001) in comparison to patients who did not develop POAF (Table 5). The relationship between POAF and length of stay remains significant after adjusting for age, STAT score and ACHD complexity. After adjustment, the odds for POAF patients to have a length of stay of at least seven days is 5.11 times higher than patients that did not develop POAF (95% CI 2.15–12.73, p < 0.001). There was no statistically significant difference in mortality, stroke, nor bleeding events for patients who developed POAF.
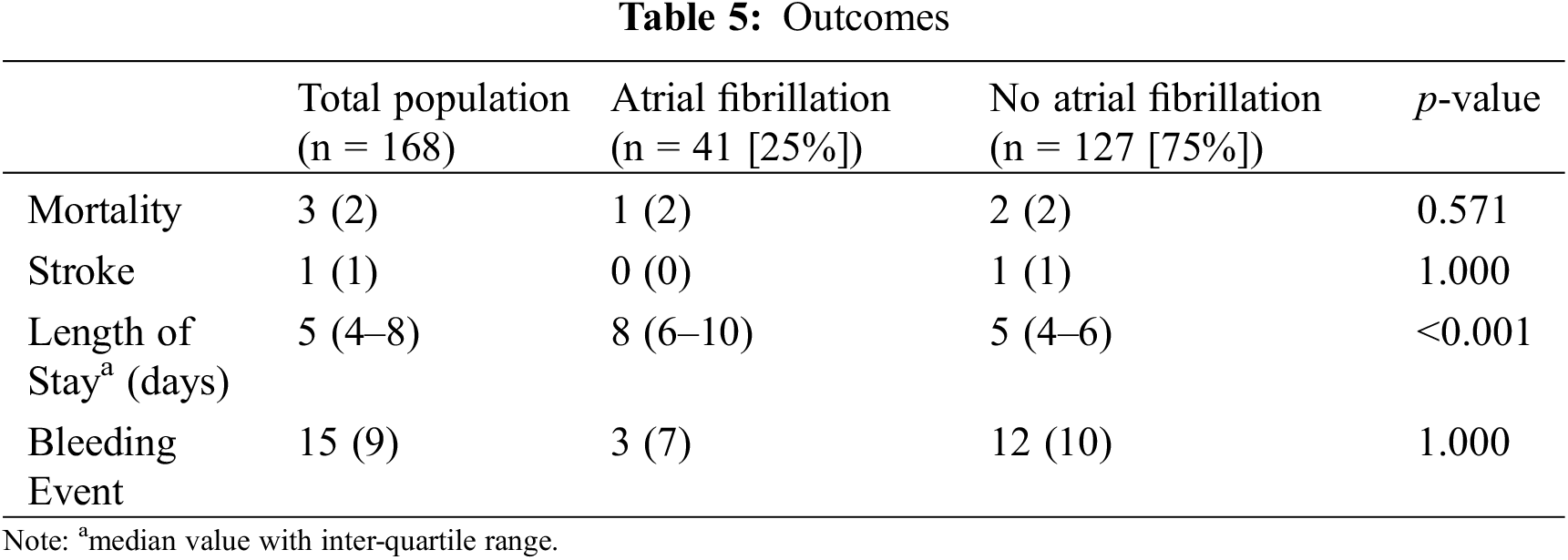
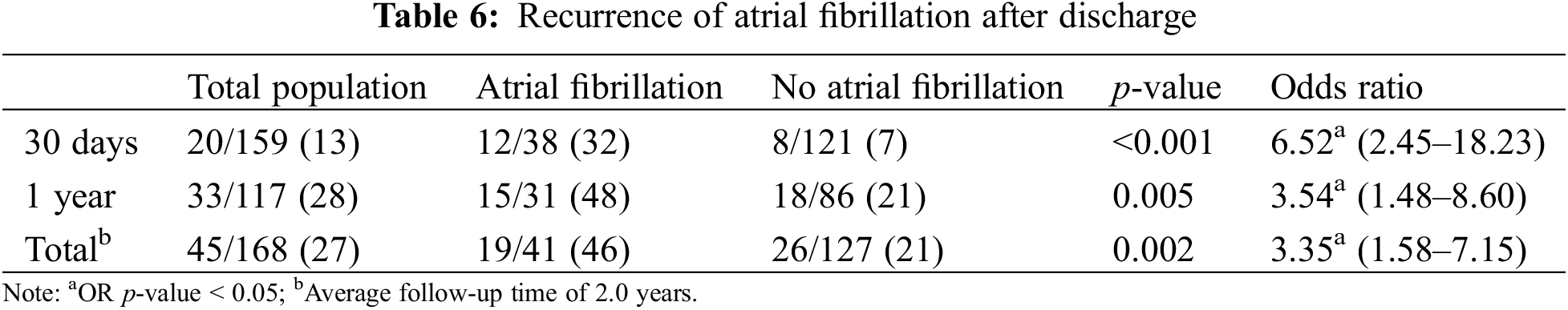
POAF was significantly associated with increased risk of out of hospital recurrence of atrial fibrillation (p < 0.001, OR 3.35 CI 1.58–7.15, Table 6) over a median follow up of 2.0 years (IQR 2.4 months–4 years). Among patients followed at least thirty days (n = 159) and one year (n = 117), POAF rates were 13% and 28%, respectively. POAF was associated with an increased risk of POAF at 30 days (32% vs. 7%, p < 0.001, OR 6.52 CI 2.45–18.23), and one year (48% vs. 21%, p = 0.005, OR 3.54 CI 1.48–8.60).
This study found POAF is a common complication following cardiac surgery in the ACHD population, occurring in 24% of our patients. Older age, history of supraventricular tachycardia, intra-operative arrhythmia, and post-operative hypokalemia were all independently associated with POAF, and patients who developed POAF had significantly longer hospital stays and increased risk of future recurrence of atrial fibrillation. Given the significant long-term outcomes of having atrial fibrillation, these data highlight the importance of identifying ways to predict, prevent, and optimally manage POAF [16].
To date, there is limited data available on the risk factors, management, and outcomes of POAF in the ACHD population. Two prior studies have sought to address this data gap and have identified an incidence of 21% for POAF, similar to our study’s incidence of 24%. These studies similarly identified older age as an independent predictor of POAF, but had not evaluated history of supraventricular tachycardia, intra-operative arrhythmia and post-operative hypokalemia which present novel risk factors for POAF. Prior identified risk factors, including hypertension, pulmonary hypertension, and certain postoperative inotropes were significant in univariate analysis but not multivariate analysis in this study. Conversely, mitral valve intervention and non-systemic atrioventricular valve regurgitation did not predict POAF in this population [12,13]. These data add important information and reiterate prior reported risk factors to help strengthen the understanding of predicting POAF in the ACHD population.
These results support prior studies findings regarding the higher incidence of POAF with older age. While the typical ACHD patient is younger than the general adult cardiac patient (average age of 30s vs. 60s), adults with congenital heart disease continue to live longer and thus it is important to recognize age as a significant risk factor [1,17,18]. Additionally, several novel risk factors were identified, including a history of supraventricular tachycardia, intra-operative arrhythmia, and post-operative hypokalemia. While this study did not distinguish between types of supraventricular tachycardia, it highlights the importance of prior history of supraventricular tachycardia when considering the odds of POAF were 7.89 times greater for those who had a history of supraventricular tachycardia in comparison to those who did not. This is consistent with prior adult literature which has shown the risk for history of atrial fibrillation causing POAF [19,20]. Specific to the ACHD population, history of paroxysmal atrial fibrillation was previously evaluated as significant in univariate analysis in one study but was not included in the subsequent multi-variate analysis [8]. Current guidelines for the management of atrial fibrillation include a 2a recommendation for the short-term prophylactic use of beta blockers or amiodarone in patients at high-risk for POAF [21,22]. Given these findings, further studies are needed to assess the indications for preventative medications in ACHD patients with a history of supraventricular tachycardia.
Regarding intra-operative arrhythmia, prior studies have not evaluated this variable and its significance may help highlight patients who need closer monitoring for POAF after leaving the operating room. Furthermore, these findings may highlight patients who may benefit most from prophylactic post-operative antiarrhythmic therapy. Finally, post-operative hypokalemia was found to be significantly associated with POAF in multivariate analysis. Many centers, including our own, have electrolyte repletion protocols to minimize electrolyte abnormalities; however, these protocols are typically responsive to lab values and do not completely eliminate electrolyte imbalances. While no prior study has assessed this factor in ACHD patients, given the known increased risk of atrial fibrillation due to hypokalemia, this identifies an important modifiable risk factor which may significantly impact rates of POAF and may warrant more aggressive or prophylactic treatment in the post-operative period [23].
While not significant in multivariate analysis, the significance of pre-operative oxygen use in univariate analysis may indicate the potential role of pre-operative hypoxemia in causing POAF. In the general adult surgery population, hypoxia has been described as contributing to POAF through ischemia of myocardial atria cells increasing ectopy and reducing atrial refractoriness [24–26]. It is plausible that pre-operative hypoxemia may increase the risk of POAF in the ACHD population through a similar mechanism. Given the significant altitude of our center, it is possible that the elevation may have led to a higher incidence of pre-operative hypoxemia than similar centers at sea level.
Conversely, there were several variables that were associated with POAF in prior studies that were not reproduced in this study. Hypertension and pulmonary hypertension were significant in univariate analysis but not multivariate analysis, though this may be limited by the low power of the study. As hypertension is a known risk factor for both atrial fibrillation and POAF in the general population, it is plausible that this variable is associated with POAF in this population and should be studied further [27,28]. A similar connection between pulmonary hypertension and POAF has been described in the general population [29]. Additionally, non-systemic valve regurgitation was positive in prior studies but was not positive in univariate analysis in our study. As non-systemic valve regurgitation frequently accompanied pulmonary hypertension, is it possible that these variables are inter-related [30].
Of note, left atrial enlargement was predictive of atrial fibrillation in univariate analysis in this study but not multi-variate analysis. However, left atrial enlargement is a prior described risk factor for atrial fibrillation in the general public [31–33]. Proposed mechanisms include disturbed impulse propagation caused by alterations in the functional and structure of the atria, including increased atrial stretch creating heterogeneous refractory periods within the atria which predisposes to atrial fibrillation [31,33,34]. Furthermore, studies have shown that residual left atrial enlargement is a risk factor for inability to maintain sinus rhythm after successful cardioversion as well as the need for repeat ablation of atrial fibrillation [33,35]. Therefore, it is reasonable that left atrial enlargement would precipitate atrial fibrillation in the ACHD population and may be significant in larger sample sizes.
Overall, as a class post-operative inotropes were not predictive of developing POAF; however, specific inotropes, including norepinephrine, epinephrine, and vasopressin, were associated in developing POAF. While Brock et al. suggested a significant impact of post-operative inotropes on POAF, it was unclear which type of inotropes were used [12]. Therefore, these data suggest further investigation is needed to determine if specific classes of inotropes are more likely to contribute to the development of POAF.
Our data shows a similar finding to other studies in which POAF is not associated with increased mortality, stroke, or bleeding events. While in the general population, POAF is associated with increased risk of stroke and mortality, the much lower prevalence of adults with congenital heart disease undergoing cardiac surgery limits our ability to study this potential complication [1]. Similar to Brock et al., we found an increased length of stay for patients with POAF [12]. As increased length of stay can be associated with increased complications, financial burden, and morbidity, these data emphasize the importance of close surveillance throughout the hospitalization [36]. A prior meta-analysis in the general adult cardiac population showed the impact of prophylactic pharmacological and non-pharmacological intervention on preventing post-operative atrial fibrillation and reduced hospital length of stay [37]. As the incidence of POAF is quite high in the ACHD population, these data suggest the importance of future studies evaluating methods to prevent POAF and the potential of inpatient POAF protocols in institutions treating ACHD patients [12,13].
There were several limitations of our study. First, the small sample size may preclude the ability to determine the significance of several variables. Additionally, this study was conducted at a single center. As each study conducted to date has been a single-center study, there may be factors that are significant only to specific centers and regional variability which may confound the identification of nationwide risk factors. While each patient chart was rigorously assessed, relying on documentation alone may introduce error into the identification of atrial fibrillation.
Atrial fibrillation is a common complication after cardiac surgery in the ACHD population and is associated with prolonged length of hospital stay and increased risk of atrial fibrillation recurrence. Several risk factors, including older age, history of supraventricular tachycardia, intra-operative tachycardia, and post-operative hypokalemia were predictive of POAF in multivariable analysis. These results reinforce the importance of close surveillance in the postoperative period, and given the high incidence of POAF in this population, argue for further pre-operative risk assessment tools as well as inpatient POAF management protocols.
Acknowledgement: Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources [38].
Funding Statement: The authors received no specific funding for this study.
Author Contributions: All authors have thoroughly reviewed and contributed to the manuscript. Specifically, study conception and design: Jonathan S. Taylor-Fishwick, Nicholas Holzemer, Johannes C. von Alvensleben, Amber Khanna; data collection: Jonathan S. Taylor-Fishwick, Vivian Duarte, Brandon Middlemist; analysis and interpretation of results: Jonathan S. Taylor-Fishwick, Amber Khanna, Kaitlin E. Olson, Megan SooHoo, Nicholas Holzemer; draft manuscript preparation: all authors. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Deidentified data can be requested through the corresponding author.
Ethics Approval: This study was approved by the University of Colorado Multiple Institutional Review Board (COMIRB 22-0503) and did not require direct contact with human subjects. The requirement for informed consent was waived due to the retrospective nature of the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. EP Europace. 2012;14(2):159–74. doi:10.1093/europace/eur208. [Google Scholar] [PubMed] [CrossRef]
2. Mayson SE, Greenspon AJ, Adams S, Decaro MV, Sheth M, Weitz HH, et al. The changing face of postoperative atrial fibrillation prevention: a review of current medical therapy. Cardiol Rev. 2007;15(5):231–41. doi:10.1097/CRD.0b013e31813e62bb. [Google Scholar] [PubMed] [CrossRef]
3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–51. doi:10.1161/CIR.0000000000000665. [Google Scholar] [PubMed] [CrossRef]
4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. doi:10.1093/eurheartj/ehw210. [Google Scholar] [PubMed] [CrossRef]
5. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, et al. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34(11):1371–92. doi:10.1016/j.cjca.2018.08.026. [Google Scholar] [PubMed] [CrossRef]
6. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric And Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACCthe American Heart Association (AHAthe European Heart Rhythm Association (EHRAthe Canadian Heart Rhythm Society (CHRSand the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11:e102–65. doi:10.1016/j.hrthm.2014.05.009. [Google Scholar] [PubMed] [CrossRef]
7. Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson PO, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137(9):928–37. doi:10.1161/CIRCULATIONAHA.117.029590. [Google Scholar] [PubMed] [CrossRef]
8. Karbassi A, Nair K, Harris L, Wald RM, Roche SL. Atrial tachyarrhythmia in adult congenital heart disease. World J Cardiol. 2017;9(6):496–507. doi:10.4330/wjc.v9.i6.496. [Google Scholar] [PubMed] [CrossRef]
9. Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120(17):1679–86. doi:10.1161/CIRCULATIONAHA.109.866319. [Google Scholar] [PubMed] [CrossRef]
10. Gatzoulis MA, Walters J, McLaughlin PR, Merchant N, Webb GD, Liu P. Late arrhythmia in adults with the mustard procedure for transposition of great arteries: a surrogate marker for right ventricular dysfunction? Heart. 2000;84(4):409–15. doi:10.1136/heart.84.4.409. [Google Scholar] [PubMed] [CrossRef]
11. Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100(3):247–53. doi:10.1136/heartjnl-2013-304958. [Google Scholar] [PubMed] [CrossRef]
12. Brock MA, Coppola JA, Reid J, Moguillansky D. Atrial fibrillation in adults with congenital heart disease following cardiac surgery in a single center: analysis of incidence and risk factors. Congenit Heart Dis. 2019;14(6):924–30. doi:10.1111/chd.12857. [Google Scholar] [PubMed] [CrossRef]
13. Egbe AC, Miranda WR, Anderson JH, DeSimone CV, Andi K, Goda AY, et al. Outcome of new-onset postoperative atrial fibrillation after cardiac surgery in adults with congenital heart disease. JACC: Clinical Electrophysiol. 2022;8(11):1407–16. doi:10.1016/j.jacep.2022.08.033. [Google Scholar] [PubMed] [CrossRef]
14. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139:e698–800. doi:10.1161/CIR.0000000000000603. [Google Scholar] [PubMed] [CrossRef]
15. The Society of Thoracic Surgeons—European Association for Cardio-Thoracic Surgery. The Society of Thoracic Surgeons—European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (STAT Mortality Categories). 2020. https://www.sts.org/sites/default/files/CHSD%20Appendix%20C%20-%20STAT%20Categories.pdf. [Accessed 2024]. [Google Scholar]
16. Lip GY, Lim HS. Atrial fibrillation and stroke prevention. The Lancet Neurol. 2007;6(11):981–93. doi:10.1016/S1474-4422(07)70264-8. [Google Scholar] [PubMed] [CrossRef]
17. Mandalenakis Z, Giang KW, Eriksson P, Liden H, Synnergren M, Wåhlander H, et al. Survival in children with congenital heart disease: have we reached a peak at 97%? J Am Heart Assoc. 2020;9(22):e017704. doi:10.1161/JAHA.120.017704. [Google Scholar] [PubMed] [CrossRef]
18. Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56. doi:10.1161/CIRCULATIONAHA.113.008396. [Google Scholar] [PubMed] [CrossRef]
19. Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–9. doi:10.1001/jama.291.14.1720. [Google Scholar] [PubMed] [CrossRef]
20. Deliargyris EN, Raymond RJ, Guzzo JA, Dehmer GJ, Smith SC, Weiner MS, et al. Preoperative factors predisposing to early postoperative atrial fibrillation after isolated coronary artery bypass grafting. Am J Cardiol. 2000;85(6):763–4+A8. doi:10.1016/S0002-9149(99)00857-7. [Google Scholar] [PubMed] [CrossRef]
21. Pires LA, Wagshal AB, Lancey R, Huang SK. Arrhythmias and conduction disturbances after coronary artery bypass graft surgery: epidemiology, management, and prognosis. Am Heart J. 1995;129(4):799–808. doi:10.1016/0002-8703(95)90332-1. [Google Scholar] [PubMed] [CrossRef]
22. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149(1):e1–e156. doi:10.1161/CIR.0000000000001193. [Google Scholar] [PubMed] [CrossRef]
23. Krijthe BP, Heeringa J, Kors JA, Hofman A, Franco OH, Witteman JC, et al. Serum potassium levels and the risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol. 2013;168(6):5411–5. doi:10.1016/j.ijcard.2013.08.048. [Google Scholar] [PubMed] [CrossRef]
24. Diallo EH, Brouillard P, Raymond JM, Liberman M, Duceppe E, Potter BJ. Predictors and impact of postoperative atrial fibrillation following thoracic surgery: a state-of-the-art review. Anaesthesia. 2023;78(4):491–500. doi:10.1111/anae.15957. [Google Scholar] [PubMed] [CrossRef]
25. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nature Reviews Cardiol. 2019;16(7):417–36. doi:10.1038/s41569-019-0166-5. [Google Scholar] [PubMed] [CrossRef]
26. Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemostasis: JTH. 2015;13(Suppl 1):S304–12. doi:10.1111/jth.12974. [Google Scholar] [PubMed] [CrossRef]
27. Lopes LA, Agrawal DK. Post-operative atrial fibrillation: current treatments and etiologies for a persistent surgical complication. J Surgery Res. 2022;5(1):159–72. doi:10.26502/jsr.10020209. [Google Scholar] [PubMed] [CrossRef]
28. Bidar E, Bramer S, Maesen B, Maessen JG, Schotten U. Post-operative atrial fibrillation—pathophysiology, treatment and prevention. J Atr Fibrillation. 2013;5(6):781. doi:10.4022/jafib.781. [Google Scholar] [PubMed] [CrossRef]
29. Funk M, Richards SB, Desjardins J, Bebon C, Wilcox H. Incidence, timing, symptoms, and risk factors for atrial fibrillation after cardiac surgery. Am J Critical Care: Official Publication, Am Assoc Critical-Care Nurses. 2003;12(5):424–35. [Google Scholar]
30. Mutlak D, Aronson D, Lessick J, Reisner SA, Dabbah S, Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest. 2009;135(1):115–21. doi:10.1378/chest.08-0277. [Google Scholar] [PubMed] [CrossRef]
31. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The framingham heart study. Circulation. 1994;89(2):724–30. doi:10.1161/01.CIR.89.2.724. [Google Scholar] [PubMed] [CrossRef]
32. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61. doi:10.1161/01.CIR.96.7.2455. [Google Scholar] [PubMed] [CrossRef]
33. Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467–75. doi:10.4065/76.5.467. [Google Scholar] [PubMed] [CrossRef]
34. Satoh T, Zipes DP. Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. J Cardiovasc Electrophysiol. 1996;7(9):833–42. doi:10.1111/j.1540-8167.1996.tb00596.x. [Google Scholar] [PubMed] [CrossRef]
35. Kong Q, Shi L, Yu R, Long D, Zhang Y, Chen Y, et al. Biatrial enlargement as a predictor for reablation of atrial fibrillation. Int J Med Sci. 2020;17(18):3031–8. doi:10.7150/ijms.47568. [Google Scholar] [PubMed] [CrossRef]
36. Mathew PJ, Jehan F, Kulvatunyou N, Khan M, O’Keeffe T, Tang A, et al. The burden of excess length of stay in trauma patients. Am J Surg. 2018;216(5):881–5. doi:10.1016/j.amjsurg.2018.07.044. [Google Scholar] [PubMed] [CrossRef]
37. Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. The Cochrane Database Syst Rev. 2013;2013(1):CD003611. doi:10.1002/14651858.CD003611.pub3. [Google Scholar] [PubMed] [CrossRef]
38. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi:10.1016/j.jbi.2008.08.010. [Google Scholar] [PubMed] [CrossRef]


Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools