 Open Access
Open Access
ARTICLE
Long-Term Follow-Up Study on Electrophysiology Guidance for Transcatheter Closure of Perimembranous Ventricular Septal Defect in Adults
1 Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, Guangzhou, 510000, China
2 Department of Geriatric Intensive Medicine, Guangdong Provincial Geriatrics Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, Guangzhou, 510000, China
* Corresponding Author: Caojin Zhang. Email:
Congenital Heart Disease 2024, 19(5), 445-455. https://doi.org/10.32604/chd.2024.053604
Received 06 May 2024; Accepted 21 November 2024; Issue published 31 December 2024
Abstract
Objectives: To explore the feasibility and efficacy of electrophysiology guidance for transcatheter closure of perimembranous ventricular septal defect (PmVSD) in adults. Methods: Adult patients with PmVSD who underwent transcatheter in Guangdong Provincial People’s Hospital, Guangdong Cardiovascular Institute from February 2016 to January 2018 were selected. The distribution of the His-Purkinje system (HPS) close to the margins of PmVSD in the left ventricle was identified using three-dimensional (3D) electro-anatomic mapping and near-field HPS was further confirmed by different pacing protocols. The follow-up protocol included electrocardiogram (ECG)and transthoracic echocardiography at 6, 24, 72 h, 1, 3, 6 months and 1, 2, 3, 5 years after the procedure. Results: Of the 21 patients in the study, with an average age of 28.1 years, 61.9% were female and 38.1% were male. Eighteen patients (85.7%) successfully underwent transcatheter intervention. The minimum distance between the margins of the PmVSD and near-field HPS ranged from 2.5 ± 0.7 (1.3~3.9) mm. An average follow-up period was 4.1 ± 1.3 (0.25~5) years, median of which was 5 years. 1, 3, 5-year follow-up rate was 95.2%, 90.4% and 52.4%, respectively. At the end of the follow-up, ECG abnormalities were observed in 5 cases (23.8%), including left anterior hemiblock (LAH) in 3 cases (14.3%), incomplete right bundle branch block and LAH in 1 case (4.8%), premature atrial contraction in 1 case (4.8%). Mild tricuspid regurgitation (TR) and mild TR with mild mitral regurgitation with mild aortic regurgitation was observed in 1 case (4.8%), as well as left ventricular diastolic dysfunction. Conclusions: The study indicates that the results have guiding significance for transcatheter closure of PmVSD guided by 3D electro-anatomic mapping technique. However, this method requires a larger amount of clinical research data to support.Keywords
Abbreviation
| ECG | Electrocardiogram |
| HPS | His-Purkinje system |
| 3D | Three-dimensional |
| VSD | Ventricular septal defects |
| AVB | Atrioventricular block |
| CLBBB | Complete left bundle branch block |
| TTE | Trans Esophageal Echocardiography |
| LV | left ventricle |
| LVG | Left ventriculography |
| MC | Mapping catheter |
| NYHA | New York Heart Association |
| SR | Sinus rhythm |
| PAC | Premature atrial contraction |
| LAH | Left anterior branch block |
| IRBBB | Incomplete right bundle branch block |
| CRBBB | Complete right bundle branch block |
| AIJR | Accelerated junctional autonomic rhythm |
Ventricular septal defects (VSD) account for up to 40% of all congenital cardiac disease [1]. The diagnosis encompasses a wide spectrum of anomalies, including isolated defects and those associated with other congenital cardiac malformations [1]. The defects can be categorized based on their anatomical locations into perimembranous, myocardial, intracrural, or substernal, with perimembranous defects being the most prevalent, accounting for approximately 60% to 70% of all VSD cases. Anatomically, the bundle of Hitchcock typically courses to the left ventricular side of the posterior inferior border of the PmVSD, making interventional procedures in this area likely to damage the conduction system [2]. According to the follow-up results after PmVSD closure, the incidence of left bundle branch block ranges from 0%–8.2%, and the incidence of complete atrioventricular block (AVB) ranges from 0%–3.5% [2]. Therefore, the 2008 American Heart Association guidelines for adults with precordial heart disease did not recommend interventional occlusion for PmVSD [3]. Subsequently, the 2010 European Society of Cardiology guidelines for adults acknowledged clinical evidence supporting the use of interventional occlusion in PmVSD but also brought to light significant postoperative challenges, including the emergence of ECG abnormalities [4]. To prevent perioperative AVB following VSD closure, the most commonly used methods include intravenous dexamethasone administration, strict electrocardiograph monitoring, and the use of a loop recorder device post-discharge. If AVB occurs, therapies such as mannitol for dehydration may alleviate myocardial edema and aid in the recovery of the conduction system. However, some cases of delayed complete AVB have been observed years after the interventional closure of VSD [5]. If irreversible complete left bundle branch block (CLBBB) develops post-closure, it can lead to irreversible left ventricular dilatation, severe biventricular electro-mechanical desynchrony, and reduced cardiac ejection fraction, resulting in poor prognosis of left bundle branch block cardiomyopathy [6,7]. Therefore, the long-term efficacy of transcatheter intervention for PmVSD in adults needs objective evaluation.
Recently, researchers have investigated the effects of interventional occlusion of ventricular septal defects on the cardiac conduction system using cardiac electrophysiological techniques. By tracing intracavitary His electrograms via electrophysiological examination both before and after occlusion, changes in A-H, H-V, and A-V intervals were measured and compared to identify predictors of conduction block and to guide occlusion strategies [8,9]. In this study, patients who underwent electrophysiological marker-guided PmVSD interventional closure were followed up for five years. The objective was to investigate the long-term outcomes of electrophysiological marker-guided closure of PmVSD in adults.
The population of our study consisted of 21 patients with PmVSD who underwent interventional treatment at Guangdong Provincial People’s Hospital, Guangdong Institute of Cardiovascular Diseases from February 2016 to January 2018. This study was approved by the Research Ethics Committee of Guangdong General Hospital, Guangdong, China on July 24, 2015 (No. GDREC2015254H(R1)). Data from all participants were de-identified; thus, the need for informed consent was waived. The inclusion criteria were detailed in the previous study [10]. Inclusion criteria: (1) age over 18 years; (2) simple VSD with hemodynamic abnormalities, diameter >3 mm and <14 mm; (3) ultrasound at the position of 9–12 points in the short-axis five-chamber heart section of the great vessels; (4) distance of the upper edge of the VSD from the right coronary valve Angiography of the aorta ≥2 mm, without aortic right coronary valve prolapse into the VSD and aortic valve regurgitation. (5) VSD superior margin <2 mm from the right aortic coronary valve, no aortic right coronary valve prolapse, no combined aortic regurgitation, or combined mild aortic regurgitation. Exclusion criteria: (1) infective endocarditis or intra-cardiac flab, or a combination of serious infectious disease; (2) thrombus at the placement of the blocker or venous thrombosis in the catheter insertion pathway; (3) co-morbid bleeding disorders and thrombocytopenia; (4) significant hepatic or renal abnormalities; (5) previous surgical occlusion of the VSD or valve replacement or repair for co-morbid valve disease; (6) trisomy-21.
2.2.1 Interventional Treatment
The patient was admitted one day before the procedure and underwent preoperative investigations, including urinalysis and complete blood count, liver and kidney function, electrolytes, coagulation parameters, ECG, chest X-ray TTE, etc. Informed consent forms were signed for interventional occlusion and the electrophysiological examination. The interventional treatment was detailed in the previous study [10]. Angiography of the left ventricle (LV) was performed to determine the PmVSD and the aortic valve (Fig. 1A). An occluder was selected based on the diameter of the PmVSD. A mapping catheter (NaviStar 4 mm; Biosense Webster, Diamond Bar, CA, USA) was utilized to conduct 3D electroanatomic mapping of the HPS in the LV (Fig. 1B). The location of the mapping catheter was also confirmed by TTE (Fig. 1C). To ensure that the aortic valve was not compromised, aortography was performed before releasing the occluder (Fig. 1D) [10]. The PmVSD site, the area of distribution of the HPS near the PmVSD, and the left ventricle were rapidly and accurately modeled point-to-point using the 3D electrophysiological scaler Carto system with a scaler catheter. The pacing was performed with different stimulation currents (20, 15, 10, 5 mA) and pulse widths (2, 1.5, 1, 0.5 ms) at the edge of the PmVSD and at the nearest area of the His-Purkinje system, respectively. The criteria for His capture were: 1. QRS wave width less than 120 ms; 2. QRS pattern close to subsinus pattern; 3. S-QRS close to subsinus HV interval. The morphology of the VSD under left ventriculography, the size of the defect, and the distance between the defect and the aortic valve were determined at the end of the marker test. Ventricular septal defect blockers and occluders made by Beijing Starway Medical Technology Incorporated Company (Beijing, China) and Shenzhen Lifetech Company (Shenzhen, China) were selected, each of which was divided into two types: symmetrical and small waist and large side type. If a high degree of atrioventricular block occurs intraoperatively or if a complete left bundle branch block is present, occlusion should be abandoned. The accuracy of the 3DEP/PmVSD map with the Carto system (Biosense Webster, Diamond Bar, CA, USA) is 0.1 mm (Figs. 1 and 2).
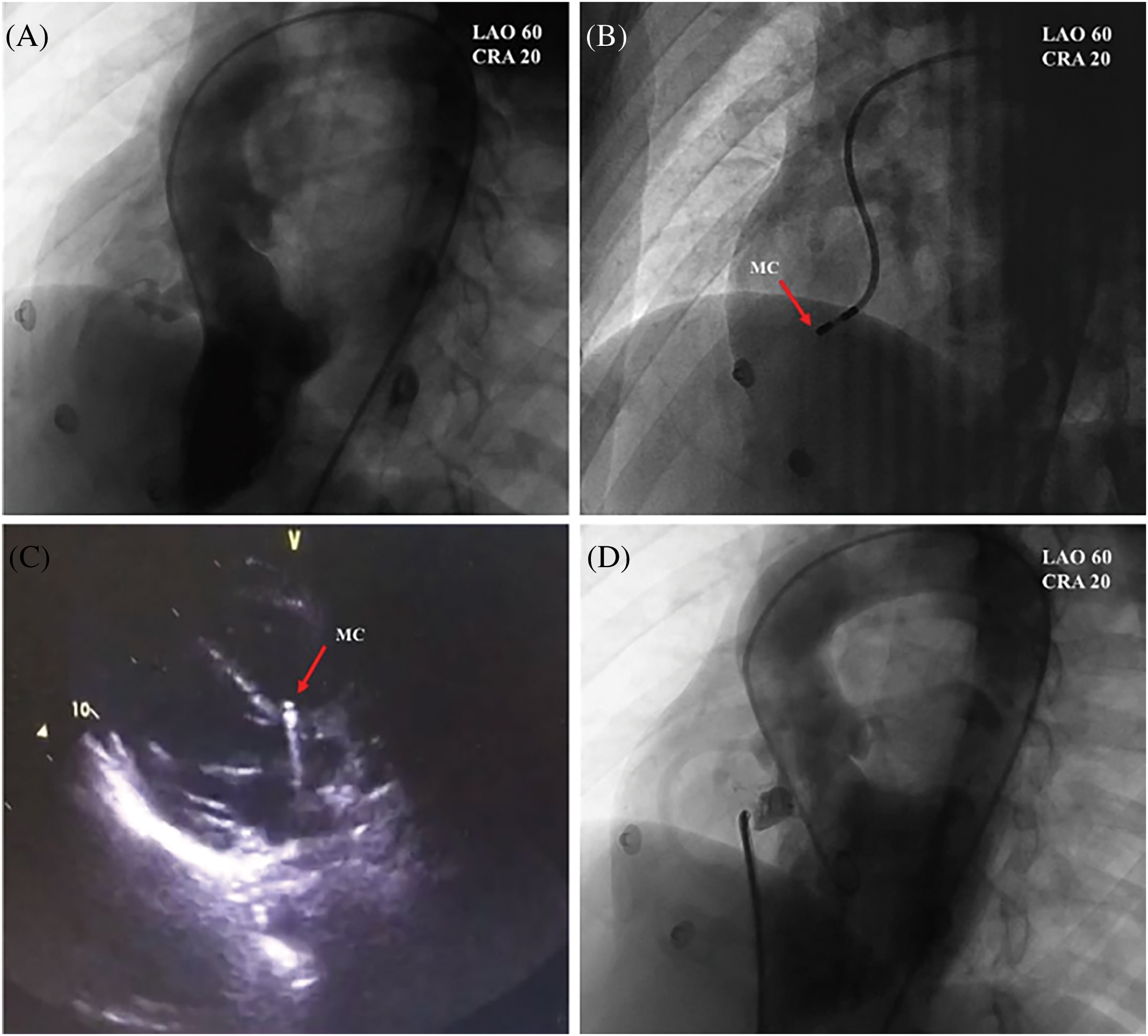
Figure 1: PmVSDs: transcatheter occlusion and three-dimensional mapping. (A) Identification of PmVSD using left ventriculography (LVG). (B) Mapping catheter (MC) placement within a pmVSD. (C) Transthoracic echocardiography verification of MC positioning at PmVSD borders. (D) LVG before the occluder releasing [10]
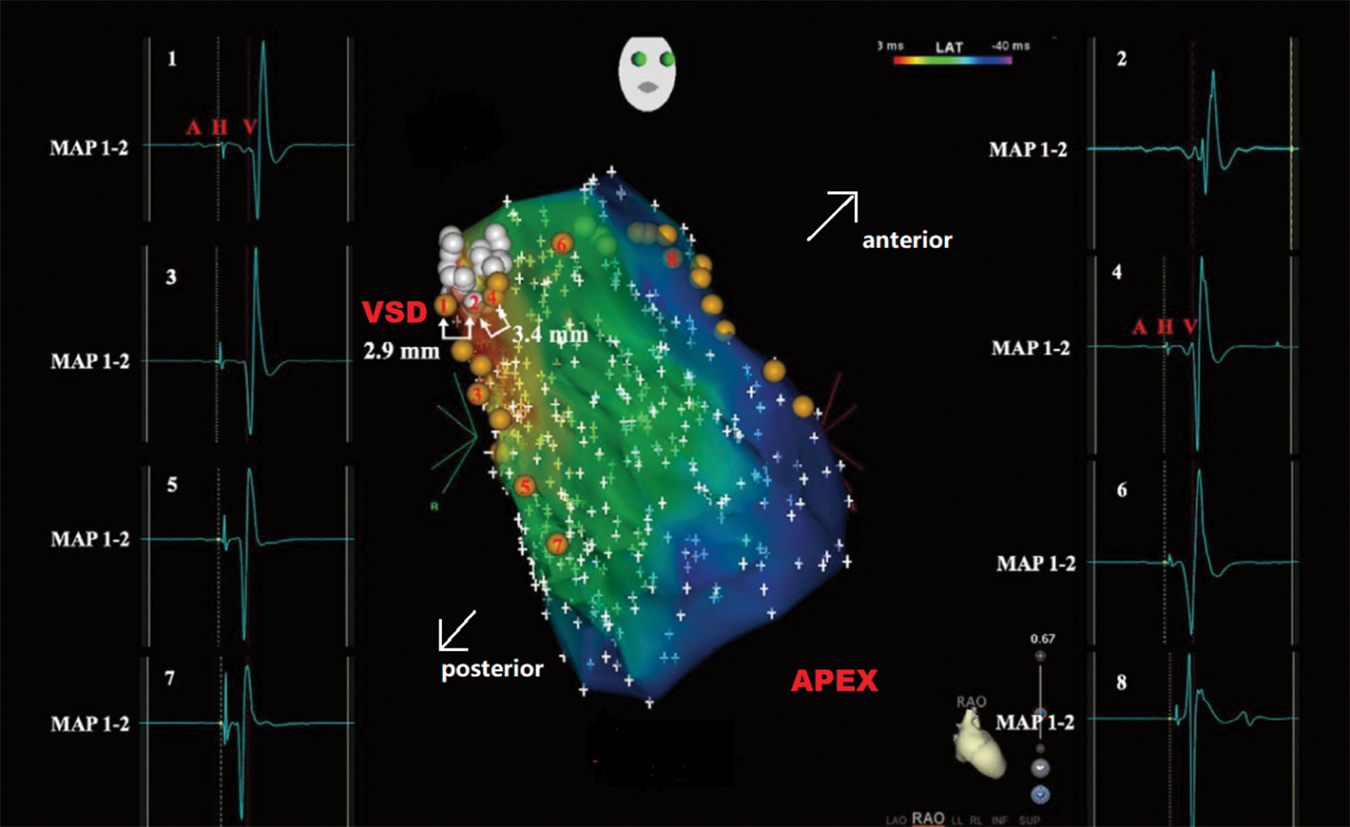
Figure 2: 3D map of His region, the left bundle branch, and PmVSD. White dots indicate the location of PmVSD. Near-field HPS activation can be detected adjacent to the PmVSD (Sites 1 and 4), while there was no Purkinje potential observed within the PmVSD (Site 2). The 3D map also shows Purkinje potentials (Sites 3, and 5–8), representing left anterior fascicular (Sites 3, 5, and 7) as well as left posterior fascicular (Sites 6 and 8) activations. The 3D map measurements reveal distances of 2.9 mm (Sites 1–2) and 3.4 mm (Sites 2–4) between PmVSD margins and near-field HPS
Patients experiencing post-procedure arrhythmic events underwent 24-h bedside ECG monitoring and received corticosteroid therapy to mitigate occluder-related mechanical compression and myocardial edema. All patients were prescribed daily oral aspirin (100 mg) for a six-month period after the procedure.
2.2.2 Postoperative Follow-Up and End-Point
ECG and Cardiac ultrasound were repeated for 6, 24, 72 h, 1 week, 1, 3, 6 months, 1, 2, 3, and 5 years after procedure. The findings at each visit were recorded in an electronic database by well-trained personnel. All patients were regularly followed-up at the outpatient clinic. The primary end-point of the study is the development of severe arrhythmias in patients postoperatively, including complete left bundle branch block, accelerated junctional voluntary rhythm, and complete atrioventricular block.
SPSS 24.0 was used for statistical analysis. Measurement data were expressed in (X±S) and counting data were expressed in frequency (n) and percentage (%); The analysis of the measurement data was carried out by using two samples of independent t-test method, and the comparison of the counting data between the two was using 2 test or Fisher exact probability method, and the difference in p < 0.05 could be considered statistically significant. Statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
8 male and 13 female patients were included in this study, aged between 18 and 48 years. All the patients were classified as New York Heart Association (NYHA) Class I. A septal aneurysm was present with the defect in 14 patients (66.7%) The mean septal defect blocker diameter was 6.4 ± 1.9 mm (5–12 mm). Three-dimensional electrophysiological markers were measured at a distance of 2.5 ± 0.7 mm between the near-field HPS and PmVSD, and 5 patients were measured at a distance of <2 mm (See Table 1). Among them, three patients discontinued the procedure due to severe arrhythmic events during closure (14.3%), two of them developed complete left bundle branch block after the intervention, and one of them developed accelerated junctional autonomic rhythm with incomplete left bundle branch block after termination of conversion to surgical treatment.
By the follow-up endpoint of February 2022, 21 subjects were monitored with a mean follow-up duration of 4.1 ± 1.3 (0.25–5) years. A total of 18 patients were successfully recalled, resulting in a recall rate of 85.7%. The median follow-up time was 5 years. The follow-up rates were 95.2% at 1 year, 90.4% at 3 years, and 52.4% at 5 years.
In this study, we observed that nearly 40% of patients did not experience arrhythmia during the whole procedure oral mid- and long-term follow-up (38.1%). By the respective follow-up endpoint, 5 patients (21.7%) had abnormal ECG. Among them, there were three cases of left anterior branch block, one case of atrial asystole, and one case of left anterior branch block with IRBBB.
3.3.1 Change of Preoperative Abnormal ECG
There were 5 cases (21.7%) of preoperative abnormal ECG. One patient with a left anterior branch block developed into accelerated junctional autonomic rhythm with incomplete left bundle branch block from intraoperative to 6 h after the procedure and the procedure was terminated. 24 h after the procedure, the left anterior branch block was restored, and the patient was transferred to surgery. The rest of the patients had no change until the follow-up endpoint.
3.3.2 Transition of Patients with New ECG Abnormalities from Intraoperative to 24 h Postoperative
Among the patients with normal preoperative ECG, a total of 7 patients developed ECG abnormalities within 24 h postoperatively. Among them, there were 2 cases of left anterior branch block, 2 cases of complete left bundle branch block (both terminated the operation), 2 cases of conversion of left anterior branch block to complete right bundle branch block, and 1 case of conversion of left anterior branch block to IRBBB, all of which returned to normal ECG in 1 month (See Table 2).
3.3.3 Delayed Conduction Block
One patient had a left anterior branch conduction block at the 3-month postoperative follow-up, and no further postoperative ECG abnormalities were found at the continued follow-up and 24-h ambulatory ECG review 1 year after the procedure.
3.4 Echocardiographic Follow-Up Results
Preoperatively, 21 patients had PmVSD confirmed by TTE, among these patients, TTE suggested mild tricuspid regurgitation in 8 patients (38.1%), combined with moderate tricuspid regurgitation in 1 patient (4.8%), combined with mild mitral regurgitation in 1 patient (4.8%), combined with oval foramen non-occlusion in 1 patient (4.8%), and suggested bifid aortic valve with mild stenosis in 1 patient (4.8%).
At the follow-up endpoint, a total of 17 patients completed echocardiography 1 year or more after treatment. Echocardiography was performed one year postoperatively in 11 patients (64.7%), three years postoperatively in two patients (11.8%), four years postoperatively in three patients (17.6%), and five years postoperatively in one patient (5.9%). Residual shunt was not observed in any of the patients, and 1 case had combined mild tricuspid regurgitation (4.8%) and 1 case had combined mild mitral regurgitation with mild tricuspid regurgitation and mild aortic regurgitation (4.8%). A mild aortic regurgitation was seen in 1 patient after the intervention, similar to the pre-intervention comparison. No new aortic regurgitation or aggravation of mild regurgitation of the existing valve, no significant tricuspid valve regurgitation, and no right ventricular outflow tract stenosis were seen at follow-up. Paired-sample t-tests of preoperative and follow-up endpoint echocardiograms showed statistically significant differences in left ventricular end-systolic and right ventricular outflow tract anteroposterior diameters and pulmonary valve orifice flow velocities (p < 0.05) (See Table 3).
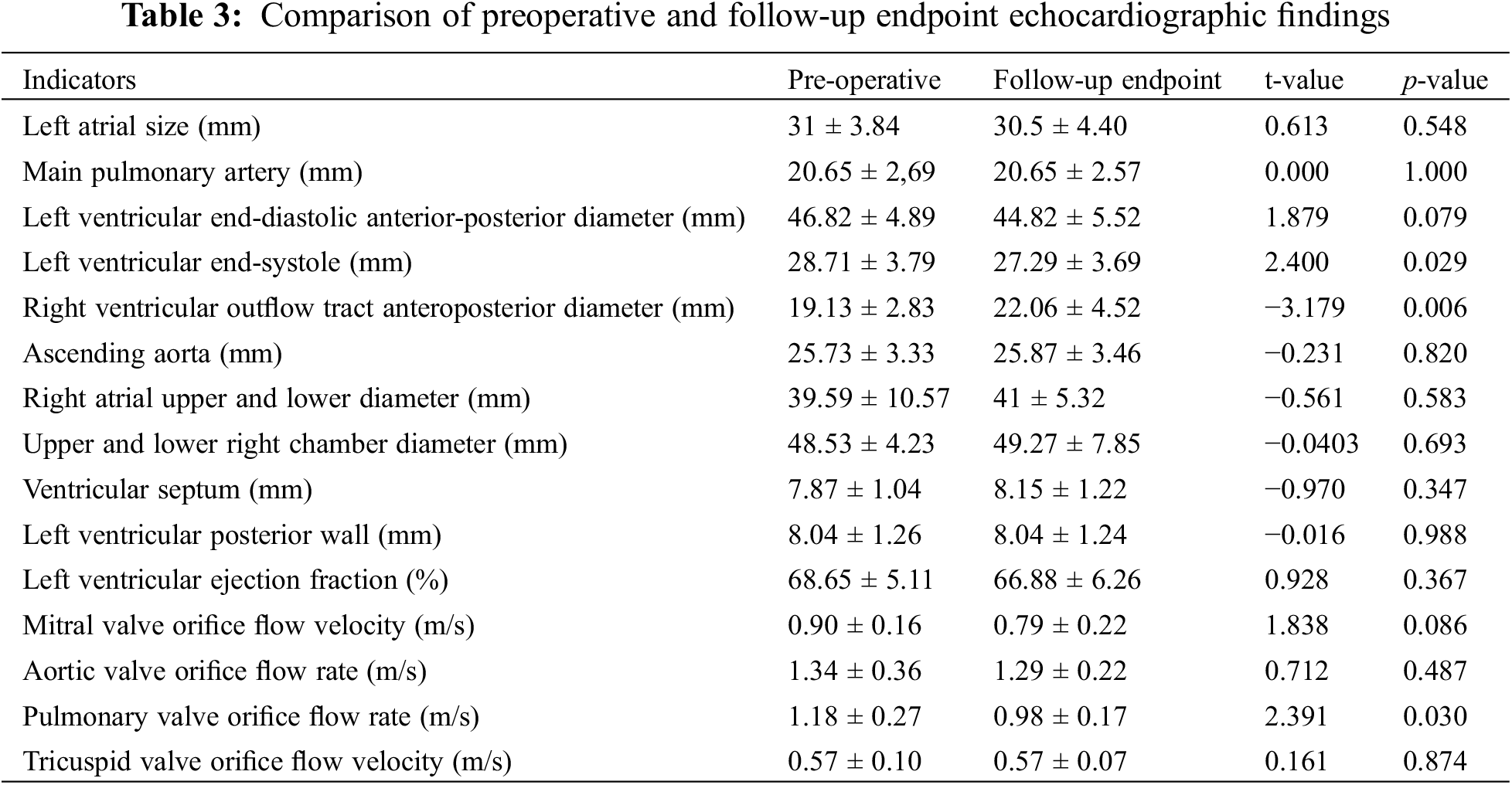
In this study, severe perioperative block occurred in patients with a distance of <2 mm between the edge of PmVSD and the near-field HPS bundle, and no new serious arrhythmic events occurred at follow-up after termination of the procedure. No serious complications such as complete atrioventricular block occurred at the 5-year follow-up. No new aortic regurgitation or mild worsening of existing regurgitation, no significant tricuspid valve involvement, and no right ventricular outflow tract stenosis were observed, as confirmed by echocardiography. In addition, no severe complications such as death, cardiac perforation, infective endocarditis, device-related thrombosis, or occluded placement occurred. These findings suggested that electrophysiological marker technology can effectively guide peri membranous septal defect closure and prevent the occurrence of conduction block.
Arrhythmia is a significant complication of interventional blockade for PmVSD. Previous studies have reported that the incidence of right bundle branch block after interventional blockade for PmVSD can be around 0%–10.5%, left bundle branch block at 0%–8.2%, and complete AV block at 0%–5.8% [11–15]. Based on the timing of arrhythmia onset, the concept of early-onset and late-onset arrhythmias has been proposed to be defined by a time point of 1 month postoperatively [16–18]. Arrhythmias that occur immediately intraoperatively are often caused by repeated stimulation of the ventricular septum during catheterization, are transient, and generally have a good prognosis; in such cases, the patient’s arrhythmia can be resolved by briefly halting the procedure or adjusting the catheter position, with no special treatment required. However, delayed arrhythmias, such as complete left bundle branch block, are often asymptomatic and can be easily overlooked. In the long run, these arrhythmias can cause ventricular contraction asynchrony, leading to delayed left ventricular contraction and then abnormal ventricular wall motion and ejection fraction, progressing to left bundle branch block cardiomyopathy and gradually developing into ventricular dilatation and heart failure [7,8]. At the end of the follow-up, ECG abnormalities were observed in 5 cases (23.8%), including left anterior hemiblock (LAH) in 3 cases (14.3%), IRBBB and LAH in 1 case (4.8%), premature atrial contraction in 1 case (4.8%). Mild tricuspid regurgitation (TR) and mild TR with mild mitral regurgitation with mild aortic regurgitation were observed in 1 case (4.8%), as well as left ventricular diastolic dysfunction. At the end of the follow-up, the results we summarized above have guiding significance for transcatheter closure of PmVSD guided by the 3D electro-anatomic mapping technique.
In this study, no distant complete atrioventricular block events were detected during a mean follow-up period of 4.1 years. Follow-up echocardiography revealed an important reduction in left ventricular end-systolic size, an increase in the anteroposterior diameter of the right ventricular outflow tract, and a significant reduction in pulmonary valve orifice flow velocity (p < 0.05). These results show that the volume load of the left heart system was reduced, which significantly improved the high volume and hyperkinetic state of the left heart system, and the hemodynamic changes were gradually restored. The long-term follow-up results confirmed that ventricular septal defect occlusion guided by electrophysiological marker measurements is safe and effective.
Based on the concern about the occurrence of atrioventricular block after interventional occlusion for PmVSD, international guidelines have not included PmVSD as an indication for interventional occlusion. However, with the help of 3D electro-anatomical landmarking technology, it is expected that the indications for interventional closure can be expanded. For patients with a PmVSD edge less than 2 mm from His before occlusion, an interventional occlusion strategy should be carefully chosen. For patients with a distance greater than 2 mm, the occluder is less likely to damage the conduction system. Combining catheter occlusion with 3Delectroanatomical landmarking techniques and good management of long-term follow-up after occlusion will hopefully reduce the occurrence of postoperative conduction block events.VSD closure using 3D electroanatomical landmarking techniques is a promising therapy that may reduce the incidence of perioperative and long-term conduction block events. Further large-scale clinical trials and detailed studies are necessary to confirm its clinical implications.
Study Limitations: While this research highlighted substantial advantages, it also faced certain constraints. Notably, the study did not employ an ultrasound catheter to enhance understanding of local anatomy. Additionally, the research was constrained by a limited sample size. Second, the small sample size is the limitation of this study. Due to the limited population of adult patients with VSD, reliability might be impacted by this study [10]. Previously, we successfully utilized this mapping method to avoid three potential cases of atrioventricular block. The primary objective of this study is to examine these patients’ long-term outcomes, necessitating larger studies with extended follow-up periods. Furthermore, the edge between the PmVSD and near-field HPS, which could be crucial in predicting procedure-related conduction disturbances, was not assessed. Fourth, patient diversity (age, comorbid conditions) and procedural variability could be confounding factors, which significantly undermine the validity of the results.
The study indicates that the results have guiding significance for transcatheter closure of PmVSD guided by the 3D electro-anatomic mapping technique. Severe perioperative block occurred in patients with a distance of <2 mm between the edge of the PmVSD and the near-field His-Purkinje system bundle, and no new serious arrhythmic events occurred at follow-up after termination of the procedure. However, this method requires a larger amount of clinical research data to support it.
Acknowledgement: We sincerely thank our colleagues in the Department of Cardiac Catheterization.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Caojin Zhang; data collection: Wenrui Li, Ziyang Yang; analysis and interpretation of results: Nanshan Xie, Xianzhang Zhan; draft manuscript preparation: Hezhi Li. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: This study was approved by the Research Ethics Committee of Guangdong General Hospital, Guangdong, China on 24 July 2015 (No. GDREC2015254H(R1)). Data from all participants were de-identified; thus, the need for informed consent was waived.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Song J. Percutaneous transcatheter closure of congenital ventricular septal defects. Korean Circ J. 2023;53(3):134–50. [Google Scholar] [PubMed]
2. Jiang SL. Current status of interventional treatment for congenital heart disease in China. Chin J Practical Intern Med. 2013;33(4):259–62 (In Chinese). [Google Scholar]
3. Houeijeh A, Godart F, Jalal Z, Ovaert C, Heitz F, Mauran P, et al. Transcatheter closure of a perimembranous ventricular septal defect with Nit-Occlud Lê VSD Coil: a French multicentre study. Arch Cardiovasc Dis. 2020;113:104–12. [Google Scholar] [PubMed]
4. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:e714–e833. [Google Scholar] [PubMed]
5. Zheng H, Lin A, Wang L, Xu Y, Zhang Z. The long-term change of arrhythmias after transcatheter closure of perimembranous ventricular septal defects. Cardiol Res Pract. 2021:13(6):1625915. [Google Scholar]
6. Zhao W, Li F, Zhou AQ, Gao W, Yu ZQ, Sun K, et al. Report of two cases of perimembranous ventricular septal defect complicated by delayed complete atrioventricular block after transcatheter occlusion and review of the literature. J Clinic Pediat. 2011;9(7):621–5. [Google Scholar]
7. Vernooy K, Verbeek XA, Peschar M, Crijns HJ, Arts T, Cornelussen RN, et al. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J. 2005;26(1):91–8. doi:10.1093/eurheartj/ehi008. [Google Scholar] [PubMed] [CrossRef]
8. Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, Behar N, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol. 2013;61(10):1089–95. doi:10.1016/j.jacc.2012.10.053. [Google Scholar] [PubMed] [CrossRef]
9. Gao L, Liu J, Hao Y, Tan H, Zheng Q, Deng B, et al. Analysis of 1002 cases of ventricular septal defect treated by domestic blocker intervention. Chin J Pract Intern Med. 2013;1(8):631–4 (In Chinese). [Google Scholar]
10. Tang L, Zhan X, Zhang C, Fang X, Liao H, Liu F, et al. Novel strategy for predicting conduction abnormalities during transcatheter closure of perimembranous ventricular septal defect in adults. Circ J. 2020;84(5):776–85. [Google Scholar] [PubMed]
11. Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. 2007;50(12):1189–95. doi:10.1016/j.jacc.2007.03.068. [Google Scholar] [PubMed] [CrossRef]
12. Xia S, Zhang Z. Efficacy of transcatheter occlusion versus surgery for congenital ventricular septal defect in children and medium and long-term follow-up. Lingnan J Cardiovasc Dis. 2010;16(3):185–9 (In Chinese). [Google Scholar]
13. Zhou D, Pan W, Guan L, Ge J. Transcatheter closure of perimembranous and intracristal ventricular septal defects with the shsmaoccluder. Catheter Cardiovasc Interv. 2012;79(4):666–74. doi:10.1002/ccd.23344. [Google Scholar] [PubMed] [CrossRef]
14. Liu J, You X, Zhao X, Hu J, Cao J, Xu R, et al. Evaluation of the efficacy of domestic blockers in the treatment of congenital periventricular septal defects. Chin J Cardiovasc Dis. 2010;38(4):321–5 (In Chinese). [Google Scholar]
15. Mijangos-Vázquez R, El-Sisi A, Sandoval Jones JP, García-Montes JA, Hernández-Reyes R, Sobhy R, et al. Transcatheter closure of perimembranous ventricular septal defects using different generations of amplatzer devices: multicenter experience. J Interv Cardiol. 2020;33(2):8948249. [Google Scholar]
16. Roymanee S, Su-angka N, Promphan W, Wongwaitaweewong K, Jarutach J, Buntharikpornpun R, et al. Outcomes of transcatheter closure in outlet-type ventricular septal defect after 1 year. Congeni Heart Dis. 2023;18(2):169–81. [Google Scholar]
17. Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol. 2005;96(12A):52–8. [Google Scholar]
18. Wang J, Wang Q, Sheng X, Geng J, Xiao J, Zhu X. Effectiveness and safety of transcatheter closure of various ventricular septal defects using second-generation amplatzer duct occluders. Congeni Heart Dis. 2023;18(2):183–95. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools