 Open Access
Open Access
REVIEW
Right Axillary Thoracotomy Should Be the Standard of Care for Repair of Non-Complex Congenital Heart Defects in Infants and Children
1 Division of Pediatric and Adult Congenital Cardiac Surgery, Department of Surgery, Maria Fareri Children’s Hospital, Westchester Medical Center, New York Medical College, New York, NY 10595, USA
2 Department of Cardiothoracic Surgery, Faculty of Medicine, Alexandria University, Alexandria, 5372066, Egypt
* Corresponding Author: Sameh M. Said. Email:
Congenital Heart Disease 2024, 19(4), 407-417. https://doi.org/10.32604/chd.2024.055636
Received 03 July 2024; Accepted 30 September 2024; Issue published 31 October 2024
Abstract
Minimally invasive approaches for cardiac surgery in children have been lagging in comparison to the adult world. A wide range of the most common congenital heart defects in infants and children can be repaired successfully through a variety of non-sternotomy incisions. This has been shown to be associated with superior cosmetic results, shorter hospital stays, and rapid return to full activity compared to sternotomy. These approaches have been around for decades, but they have not been widely adopted for a variety of reasons. Right axillary thoracotomy is one of these approaches that we believe should be the new standard for the repair of a wide variety of heart defects in children and will be the focus of our current review.Keywords
Abbreviations
| ASDs | Atrial septal defect(s) |
| AXC | Aortic cross clamp |
| CHDs | Congenital heart defects |
| CPB | Cardiopulmonary bypass |
| NIRS | Near infra-red spectroscopy |
| PAPVC | Partial anomalous pulmonary venous connections |
| VRAT | Vertical right axillary thoracotomy |
| VSDs | Ventricular septal defects(s) |
The majority of heart centers around the world have reached a level of competence and achieved excellent results in repairing many of the most common congenital heart defects in infants and children. These include atrial (ASD) and ventricular septal defects (VSDs), partial anomalous pulmonary venous connections (PAPVC), and many others. The outcomes have been excellent with very low morbidity, reoperation rates, and almost no mortality for the majority of these defects [1].
The majority of these defects have been approached via standard median sternotomy, which provides the surgeon with a sense of a high level of safety due to the ease of exposure of the majority of mediastinal structures, ease of cannulation for cardiopulmonary bypass (CPB) and readiness for any intraoperative surprises. However, this approach has been associated with several drawbacks that may not be limited to the obvious scar, postoperative pain, and time needed for recovery with a longer period of restricted activity that varies from one patient to another. There are also cases of sternal wound complications and/or sternal deformity as the child grows. The psychological burden of the scar is also something that should not be overlooked, especially in young girls.
Therefore, there has been a great need for alternate approaches to sternotomy. Several non-sternotomy approaches have been around for a long time but have not been widely adopted [2]. These include mini-sternotomy [3,4], anterolateral thoracotomy [5], and both horizontal and vertical axillary thoracotomy [6,7].
While the concerns with these approaches were mostly related to safety and exposure, several studies have shown that these approaches are no different than standard sternotomy and may be associated with superior cosmetic results [8,9].
We have adopted the vertical right axillary thoracotomy (VRAT) at our institution in the past few years and it has been our standard approach for repair of the majority of non-complex heart defects in infants and children. There are, however, several important considerations that are mostly related to patients’ selection to ensure maintenance of excellent outcomes and this will be the focus of the current article.
2 Technical Aspects of Right Axillary Thoracotomy
All photos in the current manuscript were obtained with patients’/parents’ approval. An institutional review board (IRB) at Westchester Medical Center and New York Medical College approval was obtained to use these photos in the current review (IRB#21396, Approval Date 18 June 2024).
Our anesthetic techniques have evolved over time in terms of perioperative pain management in these patients. Our initial protocol involved routine placement of epidural or erector spinae catheter (Fig. 1) prior to incision which was used for the management of postoperative pain and was followed by a specialized pain management team during that time.
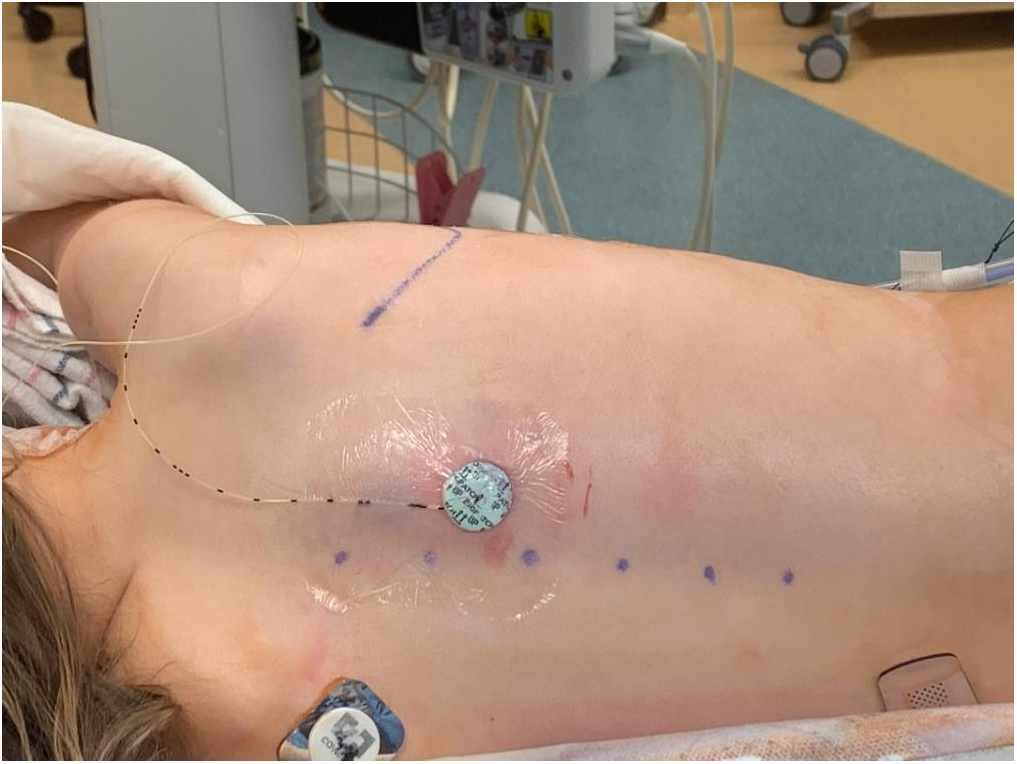
Figure 1: Erector spinae catheter inserted after induction of general anesthesia was our initial routine for the management of postoperative pain
Currently, we use routine spinal morphine combined with erector spinae block without leaving any catheters. This has been significantly associated with less requirement for narcotic medications in the postoperative period.
In addition to routine monitoring lines, bilateral cerebral near infrared spectroscopy (NIRS) is used in all patients and all are routinely extubated in the operating room at the end of the procedure.
2.2 Incision Marking and Patient Positioning
The incision is marked in all patients while they are in the supine position and prior to final positioning to ensure the proper location of the incision.
The patient is positioned in a modified left lateral decubitus with the right side up and the right arm is abducted above the right shoulder (Fig. 2A) and is supported (based on the patient’s size) to a metallic stand that is secured to the operating table (Fig. 2B).
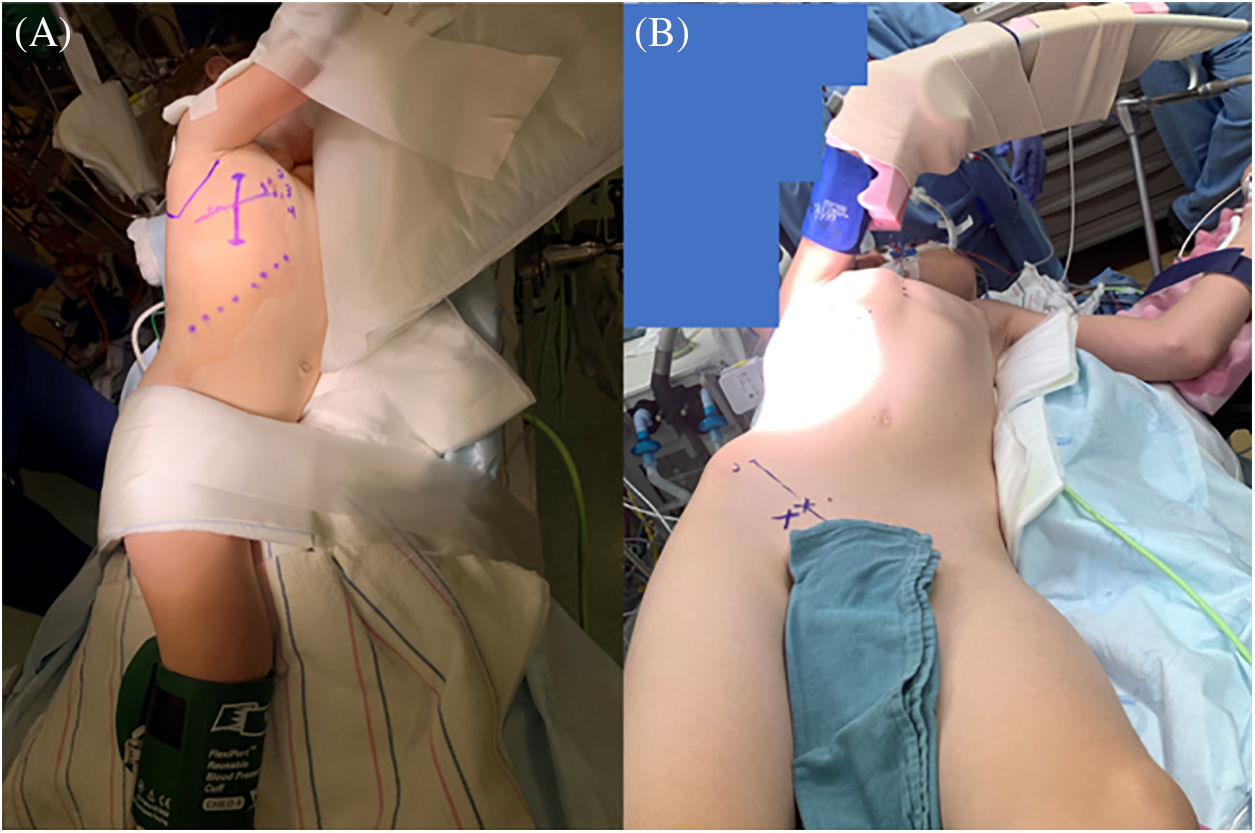
Figure 2: Intraoperative photos showing the position of the patient prior to the incision. (A) All marks are done prior to turning the patient, and (B) an arm stand may be used to secure the right arm above the shoulder in older children
In patients over 45 kg, the right groin is marked and is exposed in case groin cannulation is required. In these patients, the aorta tends to be further away from the chest wall and arterial cannulation can be challenging.
The incision is a 4–5 cm vertical incision in the right mid-axillary line and it extends from the second to the fifth intercostal spaces usually (Fig. 3).
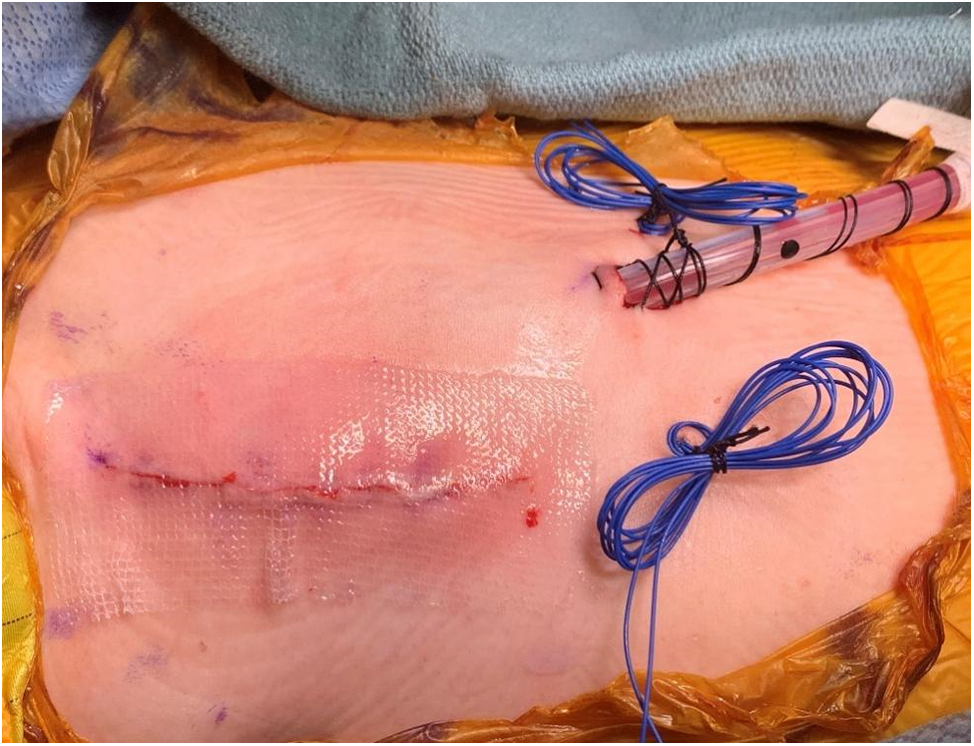
Figure 3: Operative photo showing the right axillary thoracotomy incision after completion of the procedure. Note the placement of a single chest drain and temporary epicardial pacemaker wires
A key step in this approach is the creation of generous skin and subcutaneous flaps which will facilitate exposure while maintaining the cosmetic aspect of the technique. It is important to be aware of the location of the long thoracic neurovascular bundles during mobilization of the posterior flap to avoid their injury with subsequent winging deformity of the scapula.
This incision is a muscle-sparing thoracotomy and the site of entry to the chest is between the fibers of the serratus anterior muscle. The rib space is determined based on the type of defect that needs to be repaired.
For ASDs (Fig. 4), PAPVCs, and subaortic membrane (Fig. 5), the third intercostal space is preferred, while for VSDs (Fig. 6) and mitral (Fig. 7)/tricuspid valve cases, the fourth space is better.
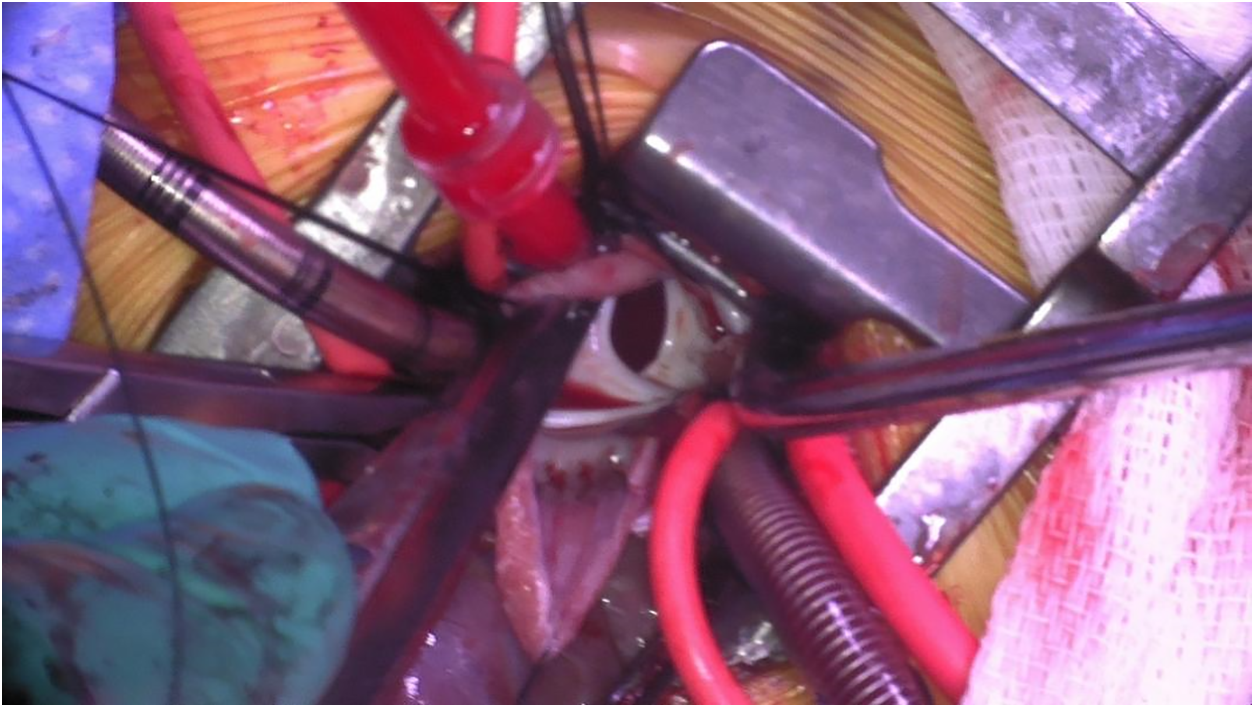
Figure 4: Intraoperative photo through a right axillary thoracotomy showing large secundum atrial septal defect as seen through the opened right atrium
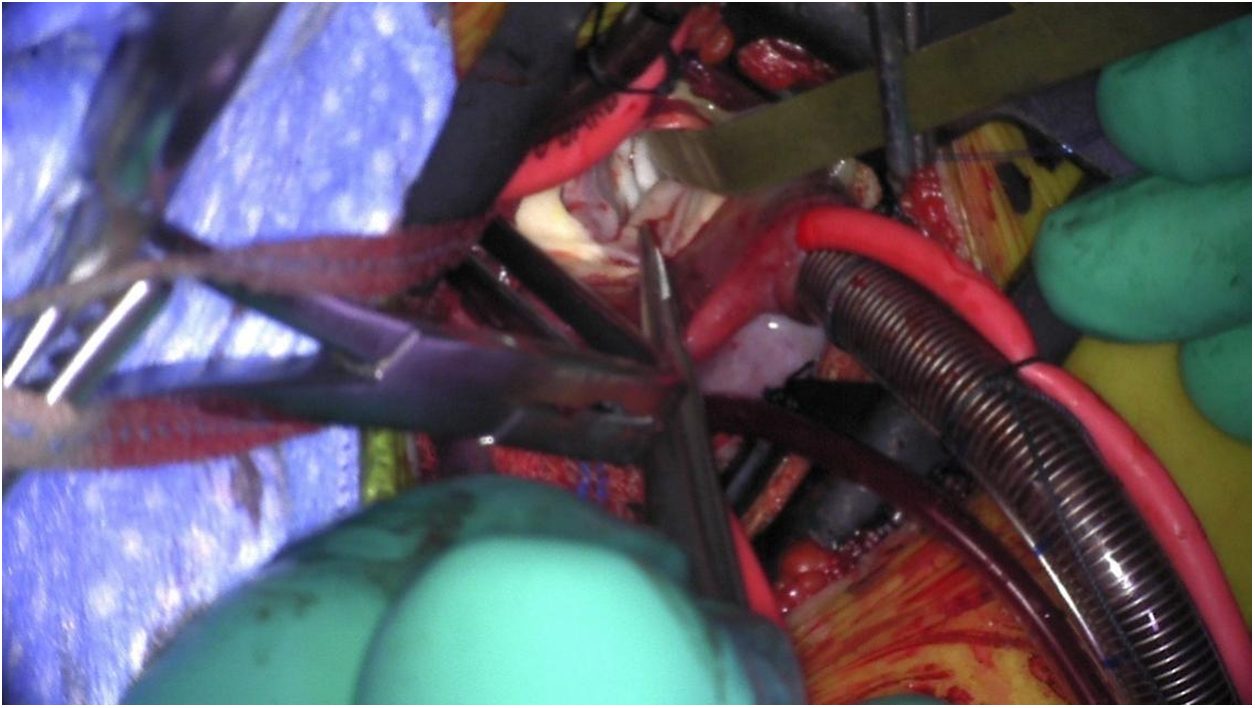
Figure 5: Intraoperative photo showing through a right axillary thoracotomy a discrete subaortic membrane as seen through the ascending aorta. Note aortic valve leaflets are retracted to expose the membrane
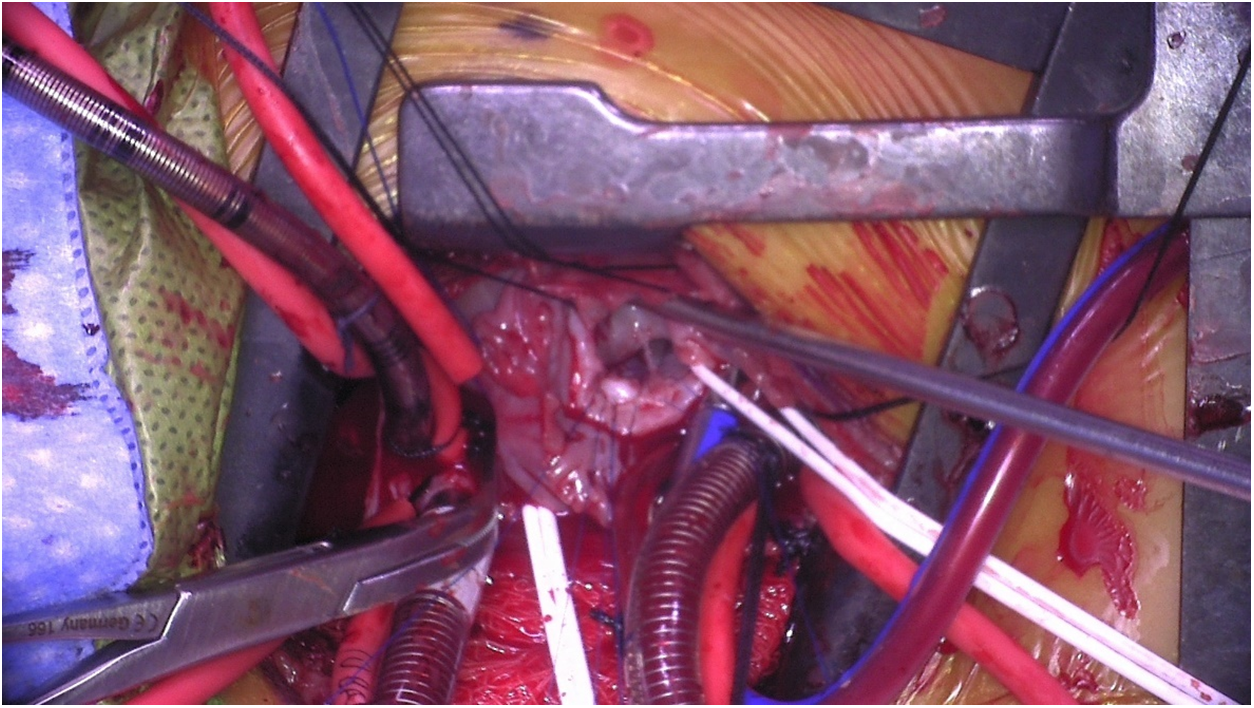
Figure 6: Intraoperative photo showing a large membranous ventricular septal defect as seen through the right atrium from a right axillary thoracotomy
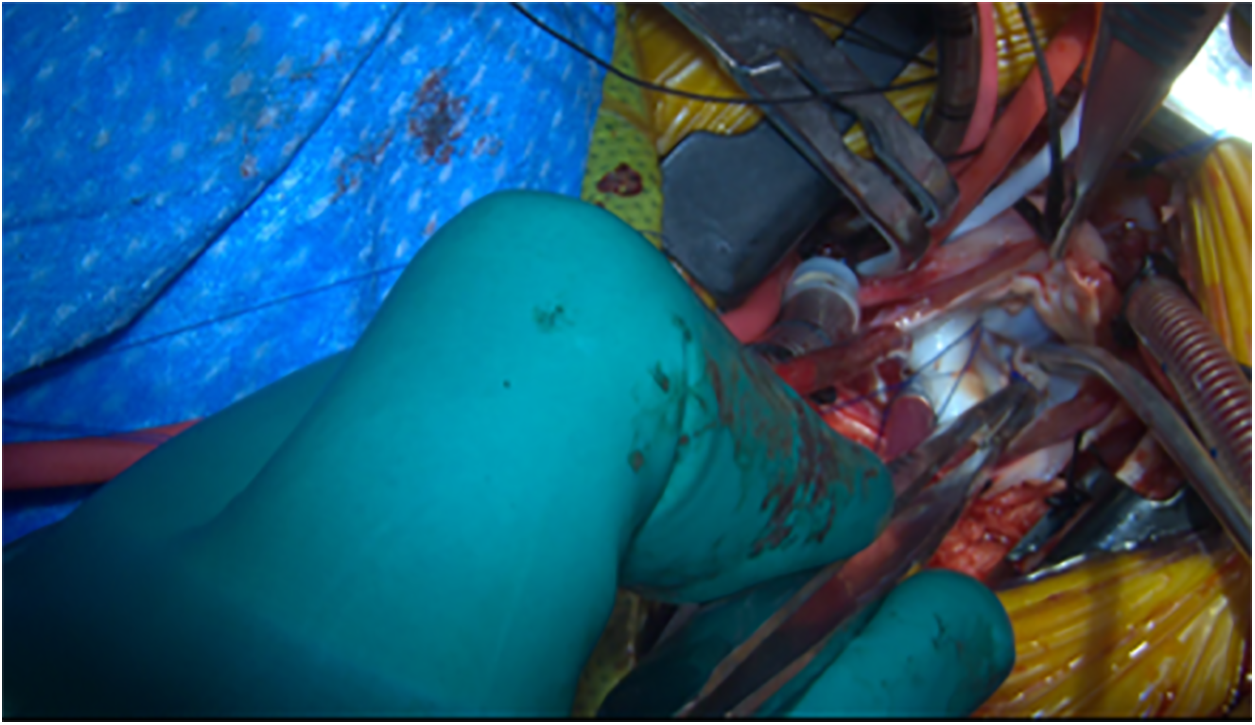
Figure 7: Intraoperative photo showing a competent mitral valve with saline testing after repair. The valve is visualized through the left atrium from a right axillary thoracotomy
Our goal is central cannulation for CPB whenever is feasible which minimizes risks related to groin vessel cannulation in young patients. The repair technique is no different from standard sternotomy which is another key step to ensure the continued excellent outcomes in these anomalies.
Once the repair is completed and the patient is weaned off CPB, transesophageal echocardiographic confirmation of satisfactory repair is followed by heparin reversal and incision closure in the standard fashion. It is important, however, to close the rib spaces without overlapping the ribs especially in infants and growing children as this could lead to long-term scoliosis deformity.
These patients follow an enhanced rapid recovery protocol which starts from extubation in the operating room, followed by minimal narcotic use and early mobilization. Typical hospital discharge occurs in the next 24–48 h postoperatively.
3 Current Outcomes (Table 1)
3.1 Said et al. [10]
In this study, Said et al. [10] assessed the safety and efficacy of the VRAT in 37 children with a mean age and weight of 6.5 years and 25.7 kg, respectively. The most common diagnoses were ASDs, VSDs, and PAPVCs. All patients except one underwent central cannulation for CPB and they were extubated in the operating room, with excellent results. There were no early or late mortality and no reoperation. One patient required permanent pacemaker implantation due to sinus node dysfunction five months postoperatively. The average length of hospital stay was 3.3 days. The follow-up was complete with a mean of 7 months. No wound-related complications were observed.
3.2 Atalay et al. [11]
The aim of this study was to assess the postoperative morbidity and cosmetic results of right infra-axillary thoracotomy. A total of 59 patients (45 (76%) were females, and the mean weight and age were 22.38 kg and 6.5 years, respectively) were included in a three-year timeframe. Most cases [47(79.6%)] were secundum ASDs. All patients were centrally cannulated and the mean CPB and aortic cross clamp (AXC) times were 47, and 26 min, respectively. No mortality was reported. A wound infection was reported in 8 (13.5%) patients. The authors concluded that axillary thoracotomy is an important surgical alternative to median sternotomy in selected cases with certain pathologies. It can be performed safely in various congenital heart anomalies and provides excellent functional and cosmetic results [11].
3.3 Analysis of Percutaneous Cannulation for Axillary Thoracotomy
Heinisch et al. [12] reported their experience with axillary thoracotomy using percutaneous femoral venous cannulation for CPB in children. They included 110 patients with a median age of 2.3 (2–16) years and a median weight of 11 (3–47) kg. Half of these patients were females 55 (50%). Bypass was established with aortic/bi-caval cannulation, and in patients weighing >5 kg (38; 35% patients), a percutaneous femoral venous cannula was used. The authors utilized fibrillatory arrest for patients with ASDs and PAPVCs.
Of note, the optimal calculated pump flow was achieved when the femoral cannula size was equal to or smaller than the superior vena cava cannula size. Five patients developed femoral vein thrombosis at the cannulation site, and all were treated with heparin with subsequent resolution.
The median hospital length of stay was 7 (4–31) days. There was no mortality. The follow-up time was 14.4 (0.8–47.19) months and was complete. One complete atrioventricular canal patient needed a permanent pacemaker implantation. Reoperation was necessary for a patient due to severe left atrioventricular valve regurgitations and it was done through the same incision.
The authors concluded that axillary thoracotomy is a feasible and effective approach for the treatment of Congenital heart diseases (CHDs) with cosmetically favorable results. Peripheral femoral cannulation can be applied even in smaller infants, but early postoperative heparin prophylaxis is recommended.
In an analysis of 1672 patients [13] who underwent right axillary thoracotomy, the most common procedure was the closure of VSDs (971; 58.1%), followed by ASDs (607; 36.3%). The median age was 2.3 (1.3–3.4) years, with more than half of the patients being females (919; 55%). The median weight was 12.5 (5–34) kg. There was no mortality or conversion to median sternotomy. There were 5 (0.3%) early reoperations through the same incision (postoperative bleeding in three, coarctation secondary to cannulation site in one, and significant residual shunt in another).
Follow-up data were available for 91.6% of the patients, with a median of 3.2 (0.2–4.9) years. There was no reported thoracic wall deformity or breast asymmetry during the follow-up. Patient satisfaction was assessed in a randomly selected 100 patients and all patients as well as their parents were satisfied with the cosmetic results.
3.5 Comparative Analysis between Sternotomy and Axillary Thoracotomy
Lo Rito et al. [14] compared the safety and clinical outcomes between axillary thoracotomy (78 patients) and conventional sternotomy (138 patients). The median age was 6.1 (4–14.5) years, and 128 (59.3%) patients were females. The most frequent diagnoses were ASDs (149; 68.9%), followed by PAPVCs (54; 25%), and partial atrioventricular septal defects (AVSDs) in 13 (6.01%) patients [14].
The patients of the thoracotomy group were significantly younger, but there was no significant difference between the groups in terms of the CPB and the AXC times. There was no difference in the hospital stay between the two groups. Of note, the sternotomy group had more pericardial effusion compared to the thoracotomy group (18.8% vs. 2.6%, respectively, p = 0.001). There was no mortality or re-operation in both groups. The authors concluded that surgical repair of simple congenital heart anomalies can be achieved through axillary thoracotomy without increasing surgical risk.
3.6 Retrospective Multicenter Data Analysis
Between 2013 and 2021 in three different centers, Dodge-Khatami et al. [15] reported the outcomes of axillary thoracotomy in 116 patients (median age of 4.3 (0.17–17) years, and median weight of 18.6 (4.8–74.4) kg). There was no mortality or a need for approach conversion. Most procedures were the repair of VSDs (44 patients) and ASDs (33 patients).
CPB time was 104.6 (25–386) min, and the AXC time was 59.4 (1–225) min. On-table extubation was possible in 97 patients. The median hospital stay was 3.9 (2–18) days. Early reoperations were required in 5 (4.3%) patients: one patient developed a complete heart block and diaphragmatic paralysis and underwent pacemaker placement and diaphragmatic plication, two patients with baffle leaks after repair of low sinus venosus defects required revision during the same admission, another revision was needed in one patient with scimitar syndrome due to scimitar vein stenosis, and one patient with Warden procedure required repair of superior vena caval stenosis. All revisions were done through the same incision. The authors concluded that “while providing obvious cosmetic advantage, the minimally invasive right axillary thoracotomy approach yields excellent results and is safe compared to the benchmark median sternotomy approach”.
The axillary approach represents a useful alternate approach to median sternotomy for patients with CHDs.
Several studies have shown the safety and efficacy of right axillary thoracotomy for repair of non-complex CHDs [16]. The advantages go beyond superior cosmetic results in comparison to sternotomy [17]. We have noticed that these patients require much less-to-no narcotics in the postoperative period. The length of hospital stay is significantly shorter compared to sternotomy and the return to full unrestricted activities is much faster. Indeed, we believe VRAT represents the true face of enhanced recovery after pediatric cardiac surgery.
Despite the above-mentioned advantages, the technique is not widely adopted by many surgeons/centers, especially in the United States. Many have raised concerns related to safety, limitations to exposure, and/or potential long-term breast asymmetry/disfigurement, especially in young prepubescent girls and scoliosis and/or musculoskeletal deformity [18].
While these are valuable concerns, these potential limitations have not been proven by any of the many studies with a large number of patients that are reported [19].
The surgical technique has evolved, and several technical modifications helped improve outcomes [20,21]. The technical aspects of entering the chest and closing the rib spaces after are critical to avoid future musculoskeletal deformities and long-term scoliosis. As we described, it is important not to overlap the ribs when closing the incision, especially in infants and growing children.
We prefer the vertical incision compared to the horizontal version of the right axillary thoracotomy for a few reasons. We believe this centers the incision away from the breast tissue and minimizes the risk of future breast asymmetry in prepubescent girls. It also adds the potential advantage of a double thoracotomy technique through the same skin incision if difficulties are encountered in cannulation or performing the repair due to the limitation of the rib spaces. This also may help avoid groin cannulation in older patients by performing the cannulation through the third intercostal space and the repair through the fourth. We have followed this strategy in a few patients.
Another important technical step is to avoid injury to the long thoracic nerve during mobilization of the posterior flap of the incision as this can lead to winging deformity of the scapula due to paralysis of the serratus anterior muscle. By following these technical tips, the cosmetic superiority of the incision is maintained (Fig. 8), and breast/musculoskeletal deformities are minimized if not totally avoided.
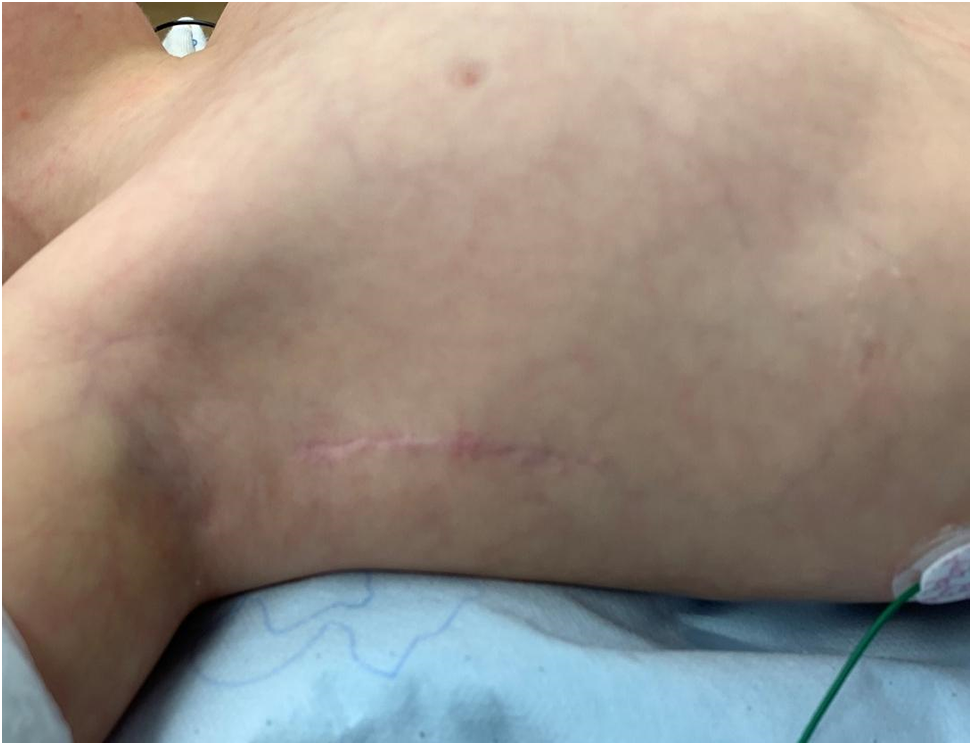
Figure 8: Postoperative photo, three weeks after right axillary thoracotomy showing the completely healed incision which is barely visible. Note that the right arm has to be abducted to allow visualization of the incision
Although some centers have used groin vessels for initiation of CPB and others used fibrillatory arrest, we have not made this our routine. We also do not believe there is a specific well-defined weight for peripheral cannulation and many of the proposed weight limits depend solely on different authors and institutional experiences. We believe what matters will be the size of the peripheral vessels intended to be cannulated to avoid the well-known complications related to this process.
Our goal is to avoid peripheral cannulation as possible and there are multiple tips that can be used to obtain central cannulation, especially in younger patients where peripheral vessel cannulation can be problematic.
A useful peripheral vessel to consider in selective cases is the jugular vein. This may be needed especially in older patients or those where high cannulation is required such as when repairing high anomalous pulmonary venous connections to the superior vena cava but we have not used it so far in any of our patients as it is always possible to cannulate the superior vena cava.
In our experience, all aspects of the procedure can be performed through the same thoracotomy incision with the same conventional surgical instruments that we use for median sternotomy. We believe it is always feasible to apply the AXC and achieve cardioplegic arrest, thus minimizing the slightest risks of air embolism and other neurological risks. Maintaining the same techniques of repairs ensures maintenance of the same excellent outcomes for these simple heart defects. Several studies have shown no significant difference in CPB and AXC times between axillary thoracotomy and conventional sternotomy [22,23]. Follow-up data confirmed satisfactory surgical results, no significant residual shunts and comparable reoperation rates as well.
In conclusion, the vertical right axillary muscle-sparing thoracotomy is a safe alternative to conventional sternotomy in the treatment of most non-complex CHDs. Like any procedure, there is a learning curve, however, by following the previously mentioned technical tips and maintaining the standard repair techniques, this approach will eventually be the standard of care in these selected CHDs due to the obvious cosmetic superiority, shorter length of hospital stays and repair return to unrestricted activities.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Yasin Essa: initial draft, and literature review; Sameh M. Said: supervision, final draft and figures. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data are available upon request to the corresponding author.
Ethics Approval: All photos in the current manuscript were obtained with patients’/parents’ approval. An institutional review board (IRB) at Westchester Medical Center and New York Medical College approval was obtained to use these photos in the current review (IRB#21396, Approval Date 18 June 2024).
Conflicts of Interest: The corresponding author Sameh M. Said is a consultant for Artivion. The author Yasin Essa has no conflict of interest to report regarding the present study.
References
1. Jacobs JP, He X, MayerJr JE, AustinIII EH, Quintessenza JA, Karl TR, et al. Mortality trends in pediatric and congenital heart surgery: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. 2016;102(4):1345–52. doi:10.1016/j.athoracsur.2016.01.071. [Google Scholar] [PubMed] [CrossRef]
2. Gupta S, McEwen C, Eqbal A, Haller C. Minimally invasive surgery for congenital heart disease. Ann Thorac Surg. 2023. doi:10.1016/j.athoracsur.2023.11.032. [Google Scholar] [PubMed] [CrossRef]
3. Vo AT, Vu TT, Nguyen DH. Ministernotomy for correction of ventricular septal defect. J Cardiothorac Surg. 2016;11(1):71. doi:10.1186/s13019-016-0475-2. [Google Scholar] [PubMed] [CrossRef]
4. Seipelt RG, Popov A, Danner B, Paul T, Tirilomis T, Schoendube FA, et al. Minimally invasive partial inferior sternotomy for congenital heart defects in children. J Cardiovasc Surg. 2010;51(6):929–33. [Google Scholar]
5. Vida VL, Tessari C, Fabozzo A, Padalino MA, Barzon E, Zucchetta F, et al. The evolution of the right anterolateral thoracotomy technique for correction of atrial septal defects: cosmetic and functional results in prepubescent patients. Ann Thorac Surg. 2013;95(1):242–7. doi:10.1016/j.athoracsur.2012.08.026. [Google Scholar] [PubMed] [CrossRef]
6. Yang X, Hu Y, Dong J, Huang P, Luo J, Yang G, et al. Right vertical axillary incision for atrial septal defect: a propensity score matched study. J Cardiothorac Surg. 2022;17(1):256. doi:10.1186/s13019-022-01999-0. [Google Scholar] [PubMed] [CrossRef]
7. Dodge-Khatami J, Dodge-Khatami A. Advantages of a mini right axillary thoracotomy for congenital heart defect repair in children. Cardiol Young. 2022;32(2):276–81. doi:10.1017/S1047951121001979. [Google Scholar] [PubMed] [CrossRef]
8. Jung JC, Kim KH. Minimally invasive cardiac surgery versus conventional median sternotomy for atrial septal defect closure. Korean J Thorac Cardiovasc Surg. 2016;49(6):421–6. doi:10.5090/kjtcs.2016.49.6.421. [Google Scholar] [PubMed] [CrossRef]
9. Ding C, Wang C, Dong A, Kong M, Jiang D, Tao K, et al. Anterolateral minithoracotomy versus median sternotomy for the treatment of congenital heart defects: a meta-analysis and systematic review. J Cardiothorac Surg. 2012;4,7:43. doi:10.1186/1749-8090-7-43. [Google Scholar] [PubMed] [CrossRef]
10. Said SM, Greathouse KC, McCarthy CM, Brown N, Kumar S, Salem MI, et al. Safety and efficacy of right axillary thoracotomy for repair of congenital heart defects in children. World J Pediatr Congenit Heart Surg. 2023;14(1):47–54. doi:10.1177/21501351221127283. [Google Scholar] [PubMed] [CrossRef]
11. Atalay A, Yilmaz M, Turkcan BS, Ecevit AN, Ozler B, Azak E, et al. Can right infra-axillary vertical thoracotomy make a big difference in surgical technique preference? Heart Lung Circ. 2022;31(10):1419–24. [Google Scholar] [PubMed]
12. Heinisch PP, Bartkevics M, Beck MJ, Erdoes G, Glöckler M, Humpl T, et al. Right axillary thoracotomy in congenital cardiac surgery: analysis of percutaneous cannulation. Ann Thorac Surg. 2021;112(6):2047–53. doi:10.1016/j.athoracsur.2020.10.011. [Google Scholar] [PubMed] [CrossRef]
13. An K, Li S, Yan J, Wang X, Hua Z. Minimal right vertical infra-axillary incision for repair of congenital heart defects. Ann Thorac Surg. 2022;113(3):896–902. [Google Scholar] [PubMed]
14. Lo Rito M, Brindicci YCM, Moscatiello M, Varrica A, Reali M, Saracino A, et al. Minimally invasive surgery for simple congenital heart defects: preserving aesthetics without jeopardizing patient safety. J Cardiovasc Dev Dis. 2023;10(11):452. doi:10.3390/jcdd10110452. [Google Scholar] [PubMed] [CrossRef]
15. Dodge-Khatami J, Noor R, Riggs KW, Dodge-Khatami A. Mini right axillary thoracotomy for congenital heart defect repair can become a safe surgical routine. Cardiol Young. 2022;18:1–4. doi:10.1017/S1047951122000117. [Google Scholar] [PubMed] [CrossRef]
16. Heinisch PP, Wildbolz M, Beck MJ, Bartkevics M, Gahl B, Eberle B, et al. Vertical right axillary mini-thoracotomy for correction of ventricular septal defects and complete atrioventricular septal defects. Ann Thorac Surg. 2018;106(4):1220–7. doi:10.1016/j.athoracsur.2018.05.003. [Google Scholar] [PubMed] [CrossRef]
17. Prêtre R, Kadner A, Dave H, Dodge-Khatami A, Bettex D, Berger F. Right axillary incision: a cosmetically superior approach to repair a wide range of congenital cardiac defects. J Thorac Cardiovasc Surg. 2005;130(2):277–81. doi:10.1016/j.jtcvs.2005.03.023. [Google Scholar] [PubMed] [CrossRef]
18. Bleiziffer S, Schreiber C, Burgkart R, Regenfelder F, Kostolny M, Libera P, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg. 2004;127(5):1474–80. doi:10.1016/j.jtcvs.2003.11.033. [Google Scholar] [PubMed] [CrossRef]
19. Dave HH, Comber M, Solinger T, Bettex D, Dodge-Khatami A, Prêtre R. Mid-term results of right axillary incision for the repair of a wide range of congenital cardiac defects. Eur J Cardiothorac Surg. 2009;35(5):864–9. doi:10.1016/j.ejcts.2009.01.022. [Google Scholar] [PubMed] [CrossRef]
20. Konstantinov IE, Kotani Y, Buratto E, Schulz A, Ivanov Y. Minimally invasive approaches to atrial septal defect closure. JTCV Tech. 2022;14:184–90. doi:10.1016/j.xjtc.2022.02.037. [Google Scholar] [PubMed] [CrossRef]
21. Kobayashi Y, Kasahara S, Kotani Y. Right vertical infra-axillary thoracotomy approach in simple congenital heart diseases. Op Tech Thorac Cardiovasc Surg. 2022;27(3):294–301. doi:10.1053/j.optechstcvs.2022.06.008. [Google Scholar] [CrossRef]
22. Hong ZN, Chen Q, Lin ZW, Zhang GC, Chen LW, Zhang QL, et al. Surgical repair via submammary thoracotomy, right axillary thoracotomy and median sternotomy for ventricular septal defects. J Cardiothorac Surg. 2018;13(1):47. doi:10.1186/s13019-018-0734-5. [Google Scholar] [PubMed] [CrossRef]
23. Yaliniz H, Topcuoglu MS, Gocen U, Atalay A, Keklik V, Basturk Y, et al. Comparison between minimal right vertical infra-axillary thoracotomy and standard median sternotomy for repair of atrial septal defects. Asian J Surg. 2015;38(4):199–204. doi:10.1016/j.asjsur.2015.01.008. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF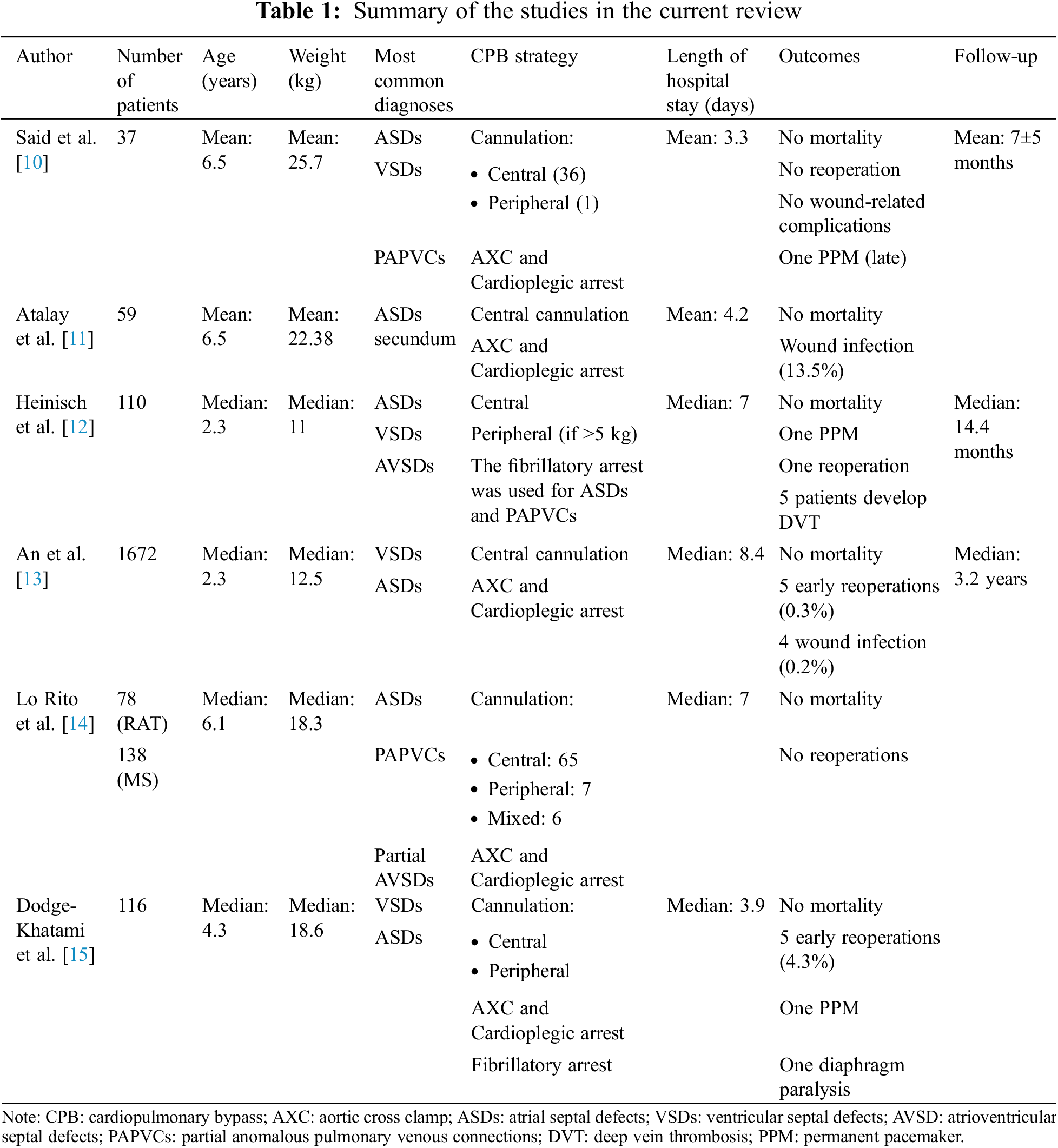
 Downloads
Downloads
 Citation Tools
Citation Tools