 Open Access
Open Access
LETTER
Distinguish Rare Dual Left Anterior Descending Artery with Anomalous Origins in Asymptomatic Patients
1 Key Laboratory of Birth Defects and Related Diseases of Women and Children of Ministry of Education (MOE), West China Second University Hospital, Sichuan University, Chengdu, 610000, China
2 Department of Ultrasonic Medicine, West China Second University Hospital, Sichuan University, Chengdu, 610000, China
3 Department of Radiology, West China Second University Hospital, Sichuan University, Chengdu, 610000, China
4 Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, 610000, China
* Corresponding Authors: Hong Luo. Email: ; Yifei Li. Email:
Congenital Heart Disease 2024, 19(4), 399-406. https://doi.org/10.32604/chd.2024.055178
Received 19 June 2024; Accepted 09 August 2024; Issue published 31 October 2024
Abstract
This article has no abstract.Supplementary Material
Supplementary Material FileCongenital coronary artery anomalies (CAAs) are characterized by abnormalities in the origins, course, or termination of the three main epicardial coronary arteries [1,2]. These anomalies can result in a spectrum of clinical manifestations, from chest pain and angina to more severe conditions such as microcirculation disorders, heart failure, or even sudden cardiac death. Among CAAs, the duplication of the left anterior descending artery (LAD) is particularly rare and usually misdiagnosed [3]. Typically, the LAD originates from the left main coronary artery (LMCA) and courses along the anterior interventricular groove. Since 2014, only five cases of anomalous origin of the left anterior descending artery from the pulmonary artery (ALADCAPA) with dual LAD have been documented [4–8]. Herein, we present the sixth and seventh cases of ALADCAPA with dual LAD in the youngest patients reported to date. Additionally, we provide potential diagnostic clues to differentiate this condition from a left coronary artery-pulmonary artery (LCA-PA) fistula. Finally, we consolidate existing literature on ALADCAPA with dual LAD to discuss clinical outcomes and surgical indications.
This study was approved by the Ethics Committee of the West China Second Hospital, Sichuan University (Approval No. 2023-039). We also obtained written informed consent from adults involved in this research for the publication of related clinical data.
The first case was a 31-month-old female who initially presented an increased heartbeat. Then a routine electrocardiogram (ECG) was performed which demonstrated sinus tachycardia, and an abnormal q wave was observed in I and aVL leads (Fig. 1A). Although serum levels of B-type natriuretic peptide (85.00 pg/mL; n.v. < 100 pg/mL) and troponin I (0.02 µg/L, n.v. < 0.06 µg/L) were normal, the abnormal coronary circulation was suspected due to abnormal q wave had been noticed. The echocardiography demonstrated LMCA arising normally from the aorta sinus, left circumflex artery (LCX) and slender LAD originated from the LMCA, while an abnormal blood shunting flowing into PA and a dilated collateral originated from LMCA coursing to the septum. Thus, the differential diagnosis should be completed among the suspected anomalous origin of the coronary artery, small coronary artery-PA fistula, and coronary artery-right ventricle fistula (Fig. 1B,B′). Then computed tomography angiography (CTA) revealed a dominant functional LCX and a high origin of the right coronary artery (RCA) above the right coronary sinus (RCS). The LMCA originated from the left coronary sinus (LCS) and emitted a short LAD, LCX, and several collateral coronary arteries with a long margining artery coursing in the interventricular groove, originating from PA (Fig. 1C). After that, the transcatheter angiography demonstrated that the short LAD perfused a set of communicating branches to the septum myocardium, and the long LAD draining a few volumes of blood into PA. Besides, there was an extensive network of blood vessels at the apex of the heart connecting the long LAD with LCX and the abnormal collateral coronary arteries, then reversing perfused the short LAD (Fig. 1D,D′, Video S1).
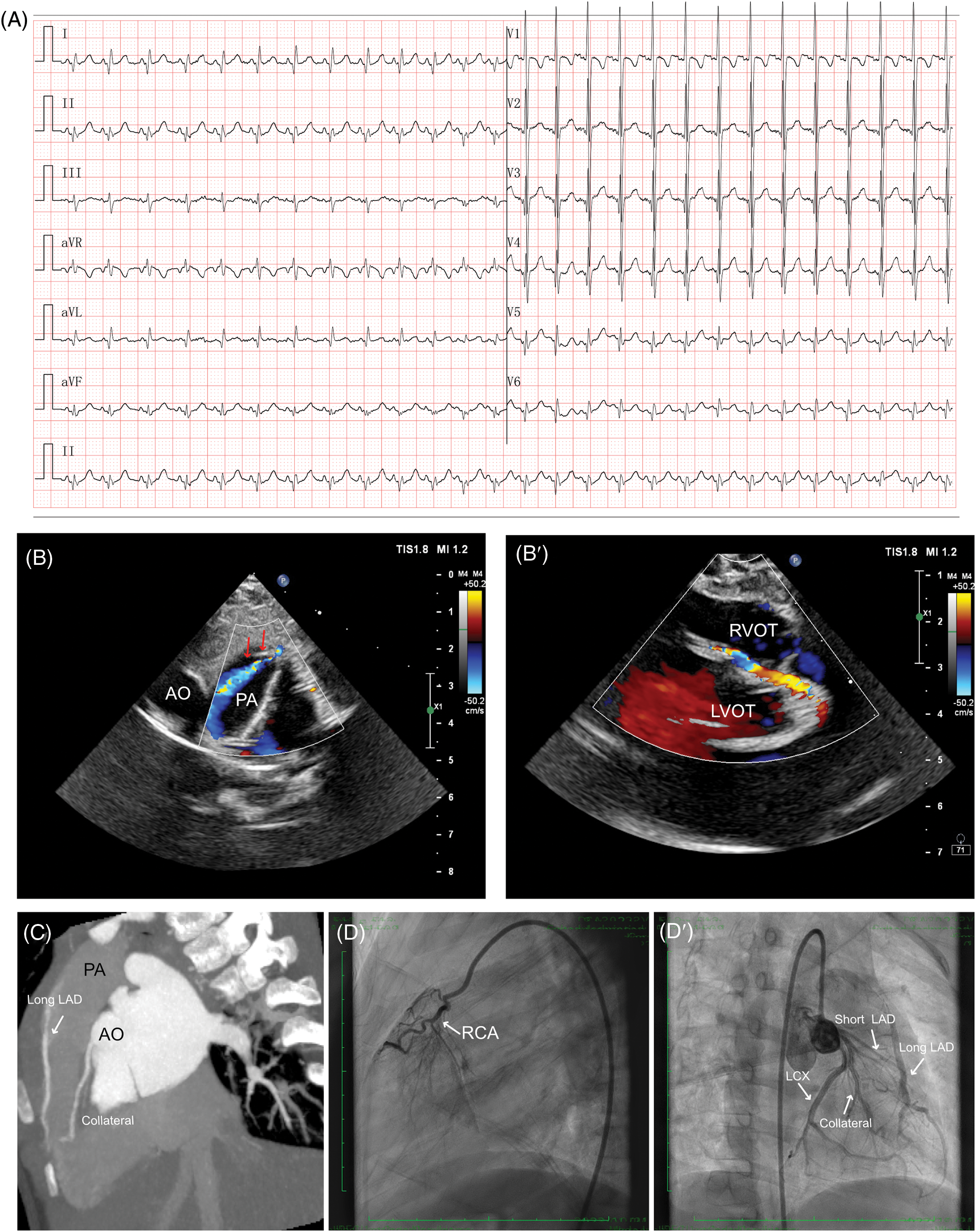
Figure 1: Clinical manifestation of the case I. (A) ECG findings showed sinus tachycardia and abnormal q wave could be observed in I and aVL leads. (B, B′) Echocardiography indicated a suspected anomalous origin of the coronary artery, a small coronary artery-PA fistula (red arrows), and a dilated collateral originating from LMCA coursing to the septum. (C) CTA demonstrated a dual LAD structure, as short LAD arising from LMCA and long LAD arising from PA. (D, D′) Transcatheter angiography revealed a short LAD perfused a set of communicating branches to the septum myocardium and the long LAD which was draining a few volumes of blood into PA. CTA, cardiac computed tomography angiography; ECG, electrocardiogram; RCA, right coronary artery; LMCA, left main coronary artery; LAD, left ascending coronary artery; LCX, left circumflex artery; PA, pulmonary artery; AO, aorta; RVOT, right ventricular outflow tract; LVOT, left ventricular outflow tract
The second case was a 38-month-old female who was admitted to our cardiac department for transcatheter closure for patent ductus arteriosus (PDA). Despite the absence of chest pain symptoms, a routine ECG was performed, demonstrating sinus rhythm without any abnormalities, especially for ST-T segment changes and q waves (Fig. 2A). A detailed echocardiography was conducted, revealing a residual shunt between the aorta and PA was demonstrated, indicating a PDA lesion with a 4 mm diameter. The echocardiography also showed LMCA normally arising from the aortic sinus with a mild dilation of 2.6 mm, LCX, and slender tortuous coursing LAD originated from the LMCA. At the same time, abnormal blood shunting flowed into the right ventricle. Additionally, another abnormal blood shunt draining into PA. These non-invasive assessments suggested differential diagnoses among PDA, small coronary artery-PA fistula, and coronary artery-right ventricle fistula (Fig. 2B,B′). A CTA examination was then conducted to illustrate the three branches of coronary arteries, which revealed dominant functional LCX from LMCA and the RCA’s typical origin in RCS. The LMCA originated from the LCS and emitted a short LAD, while a long margining LAD coursed in the interventricular groove, originating from PA (Fig. 2C). However, the CTA did not reveal multiple collateral branches between the short and long LADs. After that, the transcatheter angiography was performed before PDA closure, which demonstrated a long LAD originating from PA coursing to the apex of the left ventricle in the interventricular groove. The short LAD was given by the LMCA, with several collateral communicating branches connecting the RCA and long LAD. Besides, some small vessels drained blood into the right ventricle at the septal myocardium (Fig. 2D,D′, Video S2).
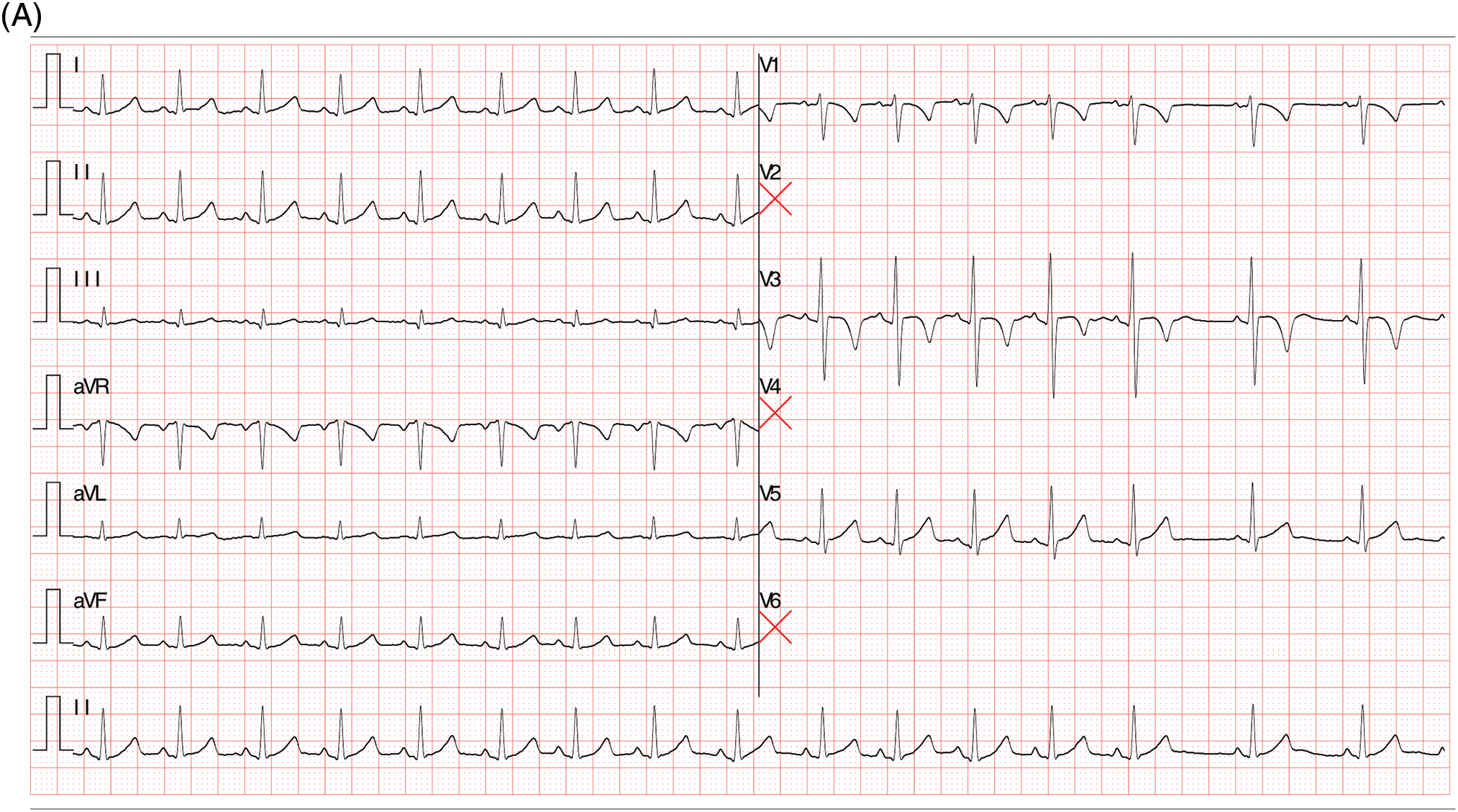
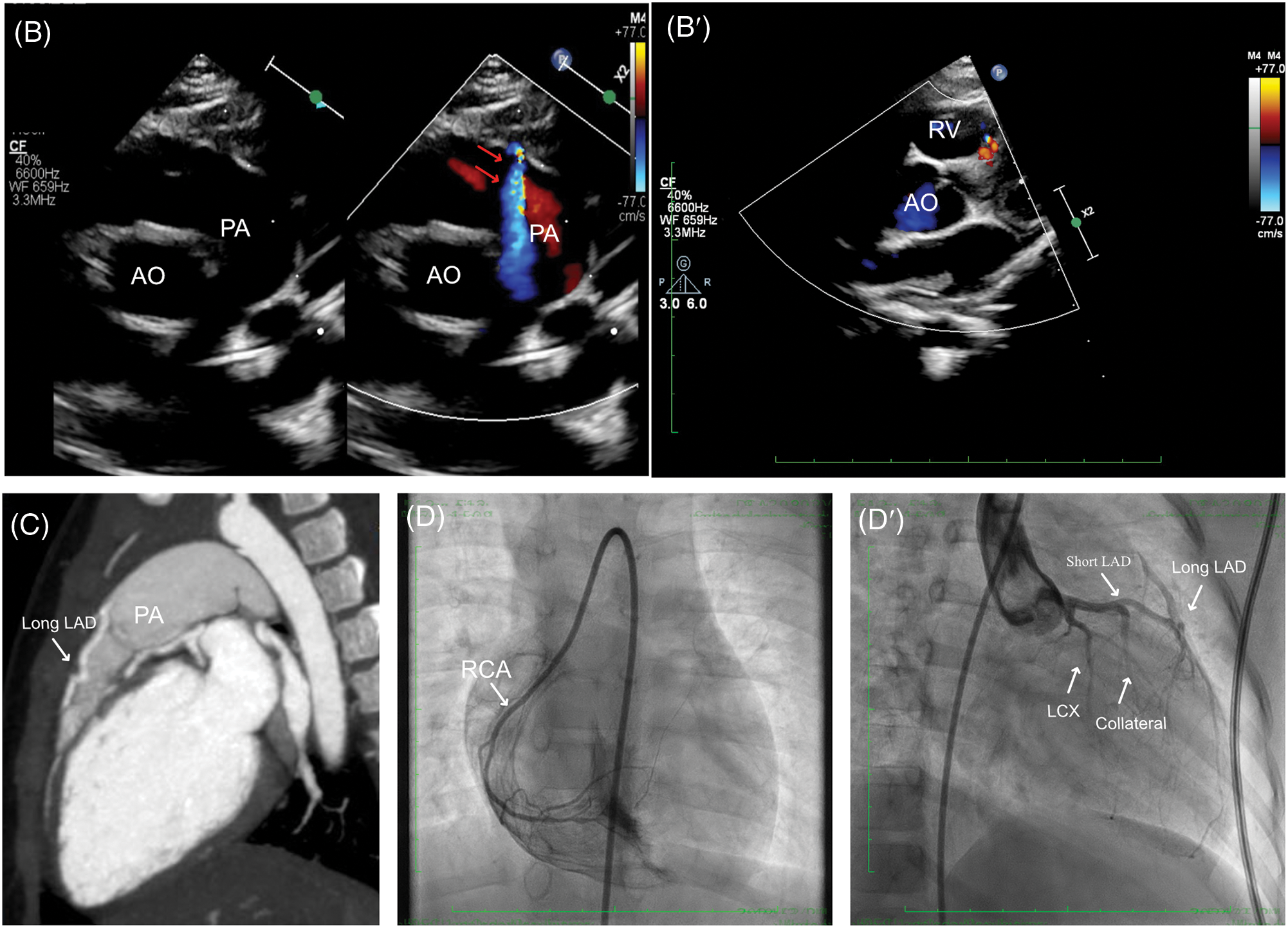
Figure 2: Clinical manifestation of the Case II. (A) Sinus rhythm without any abnormalities. (B, B′) Echocardiography indicated a malformation of small coronary artery-PA fistula (red arrows), and coronary artery-right ventricle fistula. (C) CTA demonstrated LMCA originated from the LCS and emitted a short LAD, while a long margining LAD coursing in the interventricular groove which originated from PA. (D, D′) Transcatheter angiography revealed a short LAD perfused a set of communicating branches to the septum myocardium and the long LAD which was draining a few volumes of blood into PA. CTA, cardiac computed tomography angiography; ECG, electrocardiogram; LAD, left ascending coronary artery; LMCA, left main coronary artery; PA, pulmonary artery; AO, aorta
ALADCAPA with dual LAD artery, a rare kind of congenital CAA, often presents no early-life clinical symptoms in patients (Table 1). However, the potential for developing ischemic myocardial disease in later years underscores the critical necessity for early detection [5]. ALADCAPA with dual LAD can be easily misdiagnosed as an LCA-PA fistula [9]. The presence of multiple LCA-right ventricle fistulas and significant collateral communicating branches should alert clinicians to the potential of ALADCAPA with dual LAD. In such cases, it’s important to consider alternative diagnostic methods such as coronary CTA or transcatheter angiography should be considered for patients with unusual coronary artery to pulmonary artery fistula (CFAD). Furthermore, the use of single photon emission computed tomography (SPECT) for risk stratification in assessing myocardial ischemia is strongly recommended.
Our understanding of the outcome of surgical intervention for this rare condition is still limited. However, we believe that the best surgical treatment decisions are those that take into account the patient’s clinical presentation, with a focus on the risk and severity of potential ischemic myocardial disease. The surgical options include ligation of LAD from PA with re-implantation to the aorta or grafting with saphenous-venous or left internal mammary graft [6,10,11]. The Takeuchi operation is an option for anomalous left coronary artery from the pulmonary artery (ALCAPA) treatment when direct coronary re-implantation is not feasible [12]. Surgery becomes necessary when patients start showing symptoms of myocardial ischemia, such as chest pain, angina pectoris, and ischemic changes on ECG. However, the decision to treat patients who are absent any symptoms with surgery is a topic of debate. Some experts argue that ALADCAPA carries a relatively low risk of myocardial ischemia as compared to ALCAPA, due to the less myocardium in jeopardy from retrograde myocardial perfusion [5], especially in the presence of well-collateralized territories perfused by these anomalous vessels. However, ALADCAPA with dual LAD also carries the risk of developing silent myocardial ischemia, left ventricular adverse remodeling and dysfunction, mitral insufficiency, and even sudden cardiac death. To prevent such cardiovascular adverse events, most cardiologists believe that once diagnosed, it is necessary to correct the anomalous origin of the LAD from the pulmonary artery in time, even in child cases [11]. The cardiologist in our cardiac center also recommended surgery for these two patients; unfortunately, their parents refused the surgery suggestion, highlighting the ongoing controversy in this field.
Acknowledgement: None.
Funding Statement: This work was supported by grants from the National Natural Science Foundation of China (82270249). The funding did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author Contributions: Wen Zhang and Yifei Li were the patient’s physicians. Wen Zhang and Ting Wu analyzed the echocardiography images. Xijian Chen analyzed the CTA images of the reported patients. Yifei Li performed catheter angiography of the reported patients. Wen Zhang and Yifei Li reviewed the literature and contributed to manuscript drafting; Hong Luo and Yifei Li conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. Yifei Li and Hong Luo were responsible for the revision of the manuscript for important intellectual content. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sets used in this study are available upon reasonable request by the corresponding author.
Ethics Approval: This study was approved by the Ethics Committee of the West China Second Hospital, Sichuan University (Approval No. 2023-039). The written informed consent from parents of participants who were under the age of 16 to participate in this study for publication of this case report and any accompanying images. We also obtained written informed consent from adults involved in this research for the publication of related clinical data. A copy of the written consent is available for review by the Editor of this journal.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2024.055178.
References
1. Gentile F, Castiglione V, De Caterina R. Coronary artery anomalies. Circulation. 2021;144(12):983–96. doi:10.1161/CIRCULATIONAHA.121.055347. [Google Scholar] [PubMed] [CrossRef]
2. Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–455. [Google Scholar] [PubMed]
3. Sidhu NS, Wander GS. Prevalence and characteristics of dual left anterior descending artery in adult patients undergoing coronary angiography. Future Cardiol. 2019;15(6):425–35. doi:10.2217/fca-2019-0052. [Google Scholar] [PubMed] [CrossRef]
4. Alizade E, Guler A, Acar G, Sahin M, Karabay CY, Turkmen MM. Dual left anterior descending coronary artery with partial anomalous origin from the pulmonary artery. Heart Lung Circ. 2012;21(11):743–4. doi:10.1016/j.hlc.2012.03.014. [Google Scholar] [PubMed] [CrossRef]
5. Vohra A, Narula H. Dual left anterior descending artery with anomalous origin of long LAD from pulmonary artery-rare coronary anomaly detected on computed tomography coronary angiography. Indian J Radiol Imaging. 2016;26(2):201–5. doi:10.4103/0971-3026.184423. [Google Scholar] [PubMed] [CrossRef]
6. Nakamae K, Ichihara Y, Morita K, Niinami H. Surgical management for dual left anterior descending artery with anomalous origin of left coronary artery from pulmonary artery: a case report. Gen Thorac Cardiovasc Surg. 2021;69(1):94–6. doi:10.1007/s11748-020-01377-4. [Google Scholar] [PubMed] [CrossRef]
7. Kakarla S, Sasikumar D, Valakkada J. Dual left anterior descending artery with anomalous left anterior descending artery from pulmonary artery: double trouble. Eur J Cardiothorac Surg. 2022;63(1):ezac567. doi:10.1093/ejcts/ezac567. [Google Scholar] [PubMed] [CrossRef]
8. Hao M, An Q, Luo S. Congenital dual anterior interventricular artery. Cardiol Young. 2024;10:1–2. doi:10.1017/S1047951123003785. [Google Scholar] [PubMed] [CrossRef]
9. Maggialetti N, Greco S, Lorusso G, Mileti C, Sfregola G, Brunese MC, et al. The role of coronary CT angiography in the evaluation of dual left anterior descending artery prevalence and subtypes: a retrospective multicenter study. J Pers Med. 2023;13(7):1127. doi:10.3390/jpm13071127. [Google Scholar] [PubMed] [CrossRef]
10. George KG, Gobu P, Selvaraj R, Balachander J. Anomalous left anterior descending artery from pulmonary artery: an extremely rare coronary anomaly. Indian Heart J. 2013;65(1):88–90. doi:10.1016/j.ihj.2012.12.013. [Google Scholar] [PubMed] [CrossRef]
11. El Habbal MM, de Leval M, Somerville J. Anomalous origin of the left anterior descending coronary artery from the pulmonary trunk: recognition in life and successful surgical treatment. Br Heart J. 1988;60(1):90–2. doi:10.1136/hrt.60.1.90. [Google Scholar] [PubMed] [CrossRef]
12. Juan YH, Saboo SS, Keraliya A, Khandelwal A. Coronary strictures, intraluminal thrombus and aneurysms: unreported imaging appearance of ALCAPA syndrome post Takeuchi procedure. Int J Cardiol. 2015;186:291–3. doi:10.1016/j.ijcard.2015.03.242. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF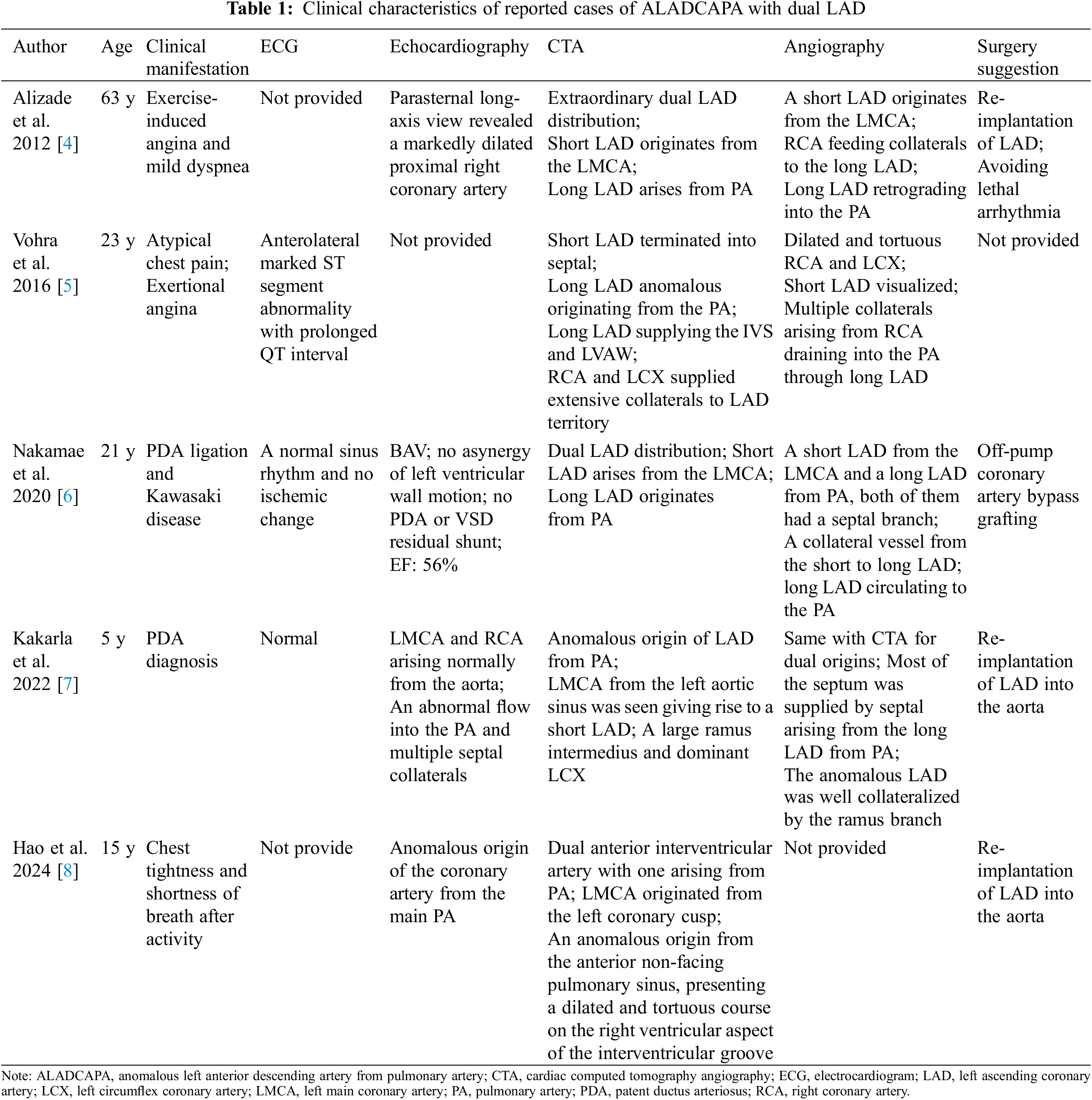
 Downloads
Downloads
 Citation Tools
Citation Tools