 Open Access
Open Access
ARTICLE
Standardized Management of Acute Pulmonary Hemorrhage after Percutaneous Pulmonary Vein Intervention
1 Department of Pediatric Cardiology, University of Colorado, The Heart Institute, Children’s Hospital Colorado, Aurora, CO 80045, USA
2 Department of Pharmacology, Children’s Hospital Colorado, Aurora, CO 80045, USA
3 Department of Anesthesia, University of Colorado, Children’s Hospital Colorado, Aurora, CO 80045, USA
* Corresponding Author: Jenny E. Zablah. Email:
Congenital Heart Disease 2024, 19(4), 389-397. https://doi.org/10.32604/chd.2024.055121
Received 18 June 2024; Accepted 09 September 2024; Issue published 31 October 2024
Abstract
Introduction: Pulmonary hemorrhage (PHm) is a life-threatening complication that can occur after catheter-based interventions in patients with pulmonary vein stenosis (PVS). Inhaled racemic epinephrine (iRE) and tranexamic acid (iTXA) have been used in other conditions, but a standardized approach in PVS has not been described. We aimed to describe the current management of PHm after PVS catheter-based interventions. Methods: We present a retrospective review of episodes of PHm from July 2022 to February 2024. PHm was defined as frank blood suctioned from the endotracheal tube including blood-tinged secretions and >3% decrease in saturations and/or ventilatory changes with or without acute chest X-ray changes. Each individual episode of PHm was considered a separate event. Incidence was calculated based on the total number of PVS interventions during the study period. Results: Eleven episodes of PHm were identified out of 108 PVS interventions, resulting in an incidence of 10.2%. Five (45.5%) had primary PVS, and seven (63.6%) had bilateral PVS. The median age at PHm was 23 months (3–91 months). Four episodes were treated with iRE, five with both iRE and iTXA, and two with only iTXA due to a history of suprasystemic right ventricular pressures. Median time on mechanical ventilation after PHm was 24 h (15–72 h) and a median ICU stay of 2 days (1–8 days). Hemostasis was achieved in all events. There were no adverse events after iTXA, however, transient hypertension was observed after iRE which was dose-related. Conclusions: The implementation of a standardized protocol for the treatment of PHm in PVS has the potential to improve procedural planning, has a wider availability of medications, and greater awareness by the providers involved, possibly leading to earlier detection of PHm and appropriate treatment.Keywords
Nomenclature
| PVS | Pulmonary Vein Stenosis |
| PHm | Pulmonary Hemorrhage |
| iRE | Inhaled Racemic Epinephrine |
| iTXA | Inhaled Tranexamic Acid |
| PEEP | Positive End Expiratory Pressure |
| ETT | Endotracheal Tube |
Pulmonary hemorrhage (PHm) is a life-threatening complication that can occur in a wide variety of conditions [1]. Patients with pulmonary vein stenosis (PVS) usually warrant recurrent transcatheter interventions for balloon or stent angioplasty, with a higher risk of procedural complications [2–4]. In addition to the intrinsic endothelial disorder that characterizes these patients, the need for recurrent interventions with drug-eluting stents, cutting balloons, and progressive balloon dilation of previously placed stents poses a significant risk for the development of PHm during these procedures [5–7]. Besides the risk of direct vascular injury, these patients may have an associated interstitial or parenchymal issue, to a different degree depending on the mechanism of PVS, that may contribute to the chronic state of pulmonary arterial and venous hypertension [5]. Chronically elevated pulmonary vascular pressures, or an acute change in the capillary hydrostatic pressures, may result in higher capillary wall stress resulting in PHm [8]. Moreover, the acute change in the distribution of flow after intervening on stenosed pulmonary veins, in the context of elevated intravascular pressures, contributes to the additional risk of acute reperfusion injury resulting in PHm. For this reason, prompt identification of PHm and initiation of the appropriate therapy is crucial to decrease post-procedural risks in this complex patient population.
There are several pharmacologic strategies used in the management of PHm in other conditions, however, a specific therapy in the context of PVS catheter-based interventions has not been described. Current treatment strategies in other conditions include inhaled or endotracheal administration of tranexamic acid (iTXA) [9–11]. iTXA is a lysine analog that acts by blocking the conversion of plasminogen to plasmin, preventing plasmin from binding to fibrin which results in a more stable fibrin matrix [10,11]. iTXA has been used in the treatment of hemoptysis in adults, as well as in children after congenital heart surgery, trauma or neurosurgery [9].
Direct endotracheal administration of epinephrine has been used to control acute bleeding during bronchospcopic interventions or acute PHm due to pulmonary vascular malformations [12,13]. On the other hand, the use of inhaled racemic epinephrine (iRE) in children has traditionally been linked to the management of acute upper respiratory infections through the sympathomimetic vasoconstriction effect of epinephrine to reduce airway edema [14]. Due to the fast availability of iRE, the relatively low cost when compared to iTXA and the alveolar-capillary vasoconstriction in the context of PHm is thought to offer direct control of the source of bleeding, however, with limited reports in neonatal PHm and no reports in PVS [15–17]. Finally, a transient increase in positive end-expiratory pressure (PEEP) has also been described as adjuvant therapy in acute PHm, especially in critically ill patients both in pediatric and neonatal populations [16,18].
With this in mind and considering the lack of evidence in the management of PHm in PVS interventions, in the last two years our center has implemented a standardized protocol for the management of PHm with iRE and/or iTXA. Our aim was to describe the current management of PHm in patients after PVS catheter-based interventions.
We reviewed a retrospective cohort of patients with the following inclusion criteria: at least one episode of PHm that were treated with iRE and/or iTXA at Children’s Hospital Colorado from July 2022 to February 2024. PHm was defined as frank blood suctioned from the endotracheal tube (ETT) during the case, including blood-tinged secretions, angiographic or fluoroscopic evidence of extravasated blood in the lung parenchyma or to the airway +/− one or more of the following: (1) >3% decrease in saturations, (2) acute ventilatory changes or (3) acute localized chest X-ray changes. No exclusion criteria were considered. The retrospective review was approved by our institutional review board (Colorado Multiple Institutional Review Board #20-2811). As it was a retrospective review, no individual patient consent was sought.
The following standardized institutional protocol has been implemented since 2022 for the management of acute PHm in patients with PVS undergoing catheterization procedures using iRE and iTXA. All patients are presented weekly prior to the scheduled procedure in a multidisciplinary case conference discussion. At this point, the team decides if each patient qualifies for iRE or iTXA in the case of PHm. If the patient is planning to undergo a bronchoscopy prior to the catheterization procedure, pulmonary/ENT is included in the discussion.
iTXA needs to be ordered at least one hour prior to the procedure so it is available in case of a PHm event. Racemic epinephrine has been stocked in the catheterization laboratory since this protocol was implemented. This process might vary between institutions. Additionally, the drug of choice is mentioned during routine time-out before the procedure. At our institution, all patient’s PVS interventions are conducted under general anesthesia with ETT.
Fig. 1A shows the current algorithm for managing acute PHm. In case of a focal injury that needs bronchoscopy for bleeding control, epinephrine through the ETT should be administered at a dose of 2 mL (two aliquots of 0.1 mg/mL). In the case of PHm without focal injury, iRE should be administered at a dose of 0.05 to 0.1 mL/kg diluted in normal saline (see dosing chart, Fig. 1B). This can be repeated every 20 min, up to two doses. In the absence of adequate control, inhaled iTXA should be considered (≤25 kg 250 mg, ≥25 kg 500 mg). In both cases, the Aerogen® Nebulizer system and ETT adapter are used. If the patient has systemic or suprasystemic right ventricular (RV) pressure, moderately or severely depressed right or left ventricular function, has a history of untreated arrhythmias, or is currently receiving inotropic therapy, iTXA should be used as the first measure for bleeding control. A dosing chart is readily available in the catheterization suite for anesthesia to refer to as needed (Fig. 1B).
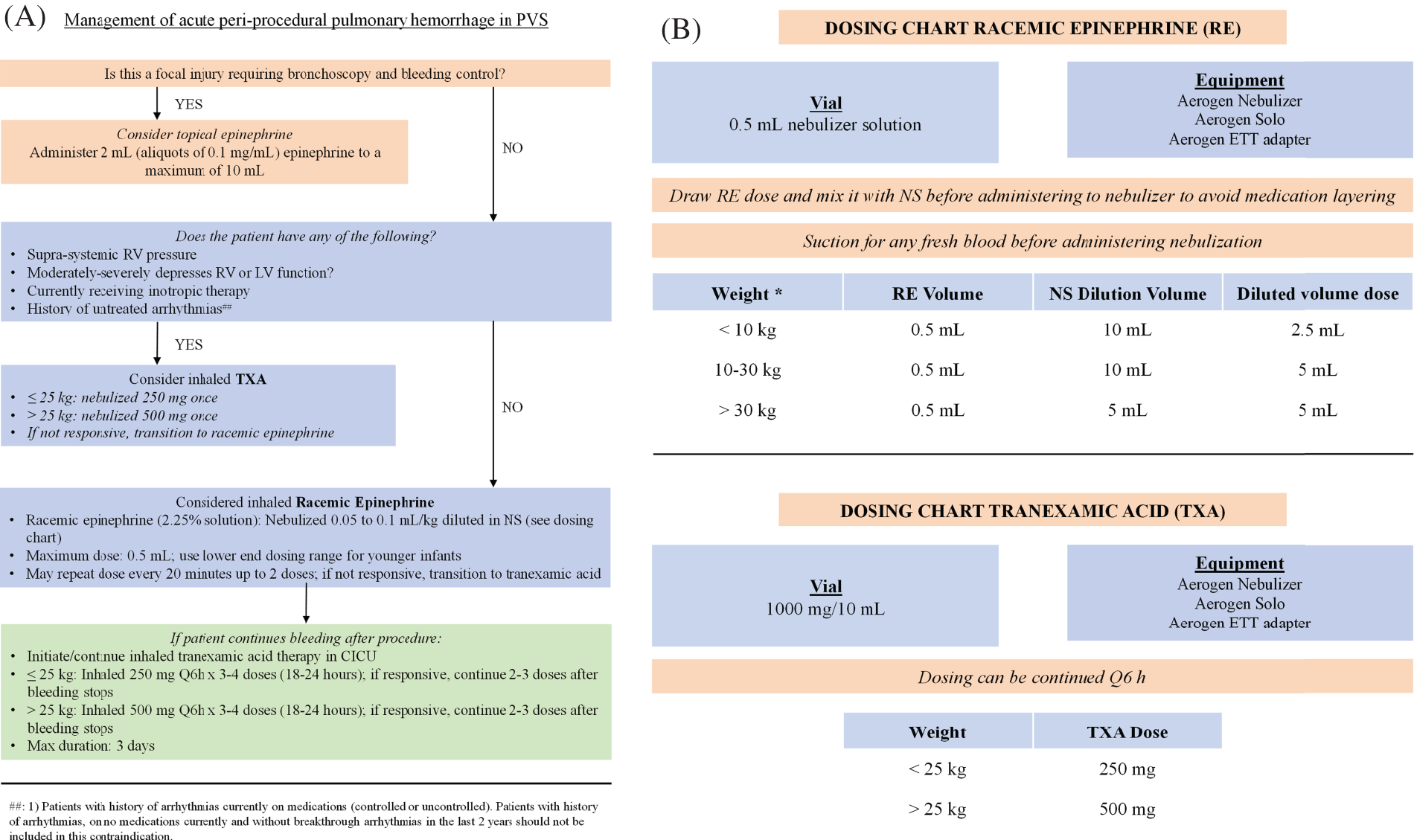
Figure 1: (A). Management of acute pulmonary hemorrhage in pulmonary vein stenosis (PVS). ##: 1) Patients with a history of arrhythmias currently on medications (controlled or uncontrolled). Patients with a history of arrhythmias, on no medications currently and without breakthrough arrhythmias in the last 2 years should not be included in this contraindication. RV: right ventricle, LV: left ventricle, TXA: tranexamic acid, NS: normal saline, CICU: cardiac intensive care unit. (B). Dosing Chart Available in the Catheterization Suite. RE: racemic epinephrine, NS: normal saline, TXA: tranexamic acid. *Dosing for racemic epinephrine should be based in ideal body weight to decrease the risk of adverse effects
It is important to note that as part of the general PVS protocol, a complete blood count is routinely performed prior to the procedure in order to rule out the presence of significant anemia or thrombocytopenia. Anemia is proactively treated as part of our multidisciplinary approach, and hematocrit is monitored intra-procedurally in case an acute change is noticed. Additionally, sirolimus levels are continuously checked during the overall follow-up of PVS patients within our program, as well as before catheterization procedures. Finally, as per institutional protocol, all patients are routinely anticoagulated on heparin for PVS interventions to aim for an activated clotting time (ACT) >250 s. ACT is subsequently checked every 15 min until an ACT of 250 s is reached, then every 30 min.
Each individual episode of PHm was treated as a separate event. To calculate the incidence of PHm, events of PHm during catheter-based procedures for PVS were recorded in relation to the total number of catheter-based PVS interventions performed during the study period. Descriptive statistics of the cohort were analyzed using IBM SPSS v29 (Chicago, IL, USA). Data was tested for normality with a Shapiro-Wilk test. Non-parametric distribution was considered, thus, measures of central tendency for continuous variables are reported as median and range and categorical variables as absolute numbers and percentages.
During the study period, eleven episodes of PHm were identified, for an incidence of 10.2% (11 episodes out of 108 PVS catheterization procedures in a total of 36 patients). Demographic and clinical characteristics of patients with PHm are presented in Table 1. Of these, six were female (54.5%), four had a history of prematurity, and 5 (45.5%) had primary PVS without underlying anomalous pulmonary venous return. Two patients had a repeated episode of PHm. Overall, the median gestational age was 37 weeks (range 25–39 weeks) and birth weight ranged from 400 to 4554 grams (median 2690 grams). The median age at PVS diagnosis was 2.5 months (range 1–4 months). Pulmonary arterial hypertension was present in 6 patients (54.5%). Only one patient had single vessel disease, seven (63.6%) had bilateral disease and five (45.5%) had four vessel disease. Five patients were receiving systemic sirolimus at the time of the bleeding episode.
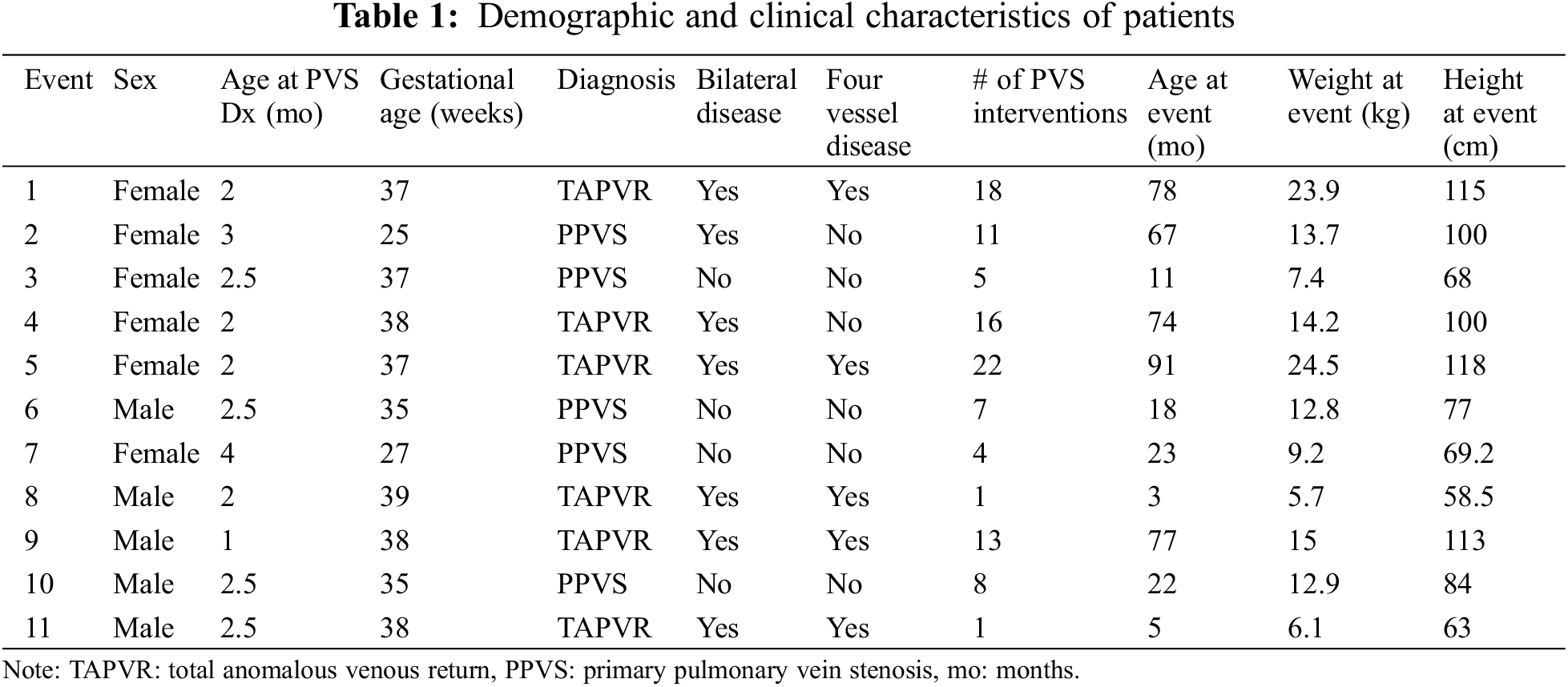
The median age at bleeding was 23 months (range 3–91 months). Four out of the eleven episodes were treated with only iRE, five with both iRE and iTXA, and two were treated only with iTXA due to a history of suprasystemic RV pressure. Cutting balloons had been used in five patients (45.5%) at the location of the bleed.
In terms of the hemodynamics at the time of PHm episode, the median left atrial pressure was 7 mmHg (range 4–12 mmHg). The median RV systolic pressure was 45.6 mmHg (range 23–75 mmHg), and four patients had an RV pressure that was more than 60% of the systemic pressure (from 67.6% to 125%). The mean pulmonary artery (PA) pressure ranged from 15–55 mmHg (median 26 mmHg) and the pulmonary vascular resistance ranged from 3–13 mmHg (median 5.7 mmHg). The gradient in the veins where the PHm episode occurred ranged from 4–24 mmHg (median 11 mmHg). After intervention the decrease in gradient ranged from 2–19 mmHg (median 8 mmHg). Finally, during balloon angioplasty at the site of hemorrhage, the maximum atmosphere balloons were inflated to range from 14–40 atm (median 20 atm). The latter in the context of a high-pressure balloon for previous stent fracture. None of the events presented required a bronchoscopy for bleeding control.
Seven patients required ventilatory support after the procedure, three were extubated in a catheterization suite and one had tracheostomy with ventilator at baseline with no changes in ventilatory settings after the procedure. The median time on mechanical ventilation was 24 h (range 15–72 h). Nine patients were admitted to the ICU, with a length of stay that ranged from 1–8 days (median 2 days) and a total hospital length of stay that ranged from 1–13 days (median 2 days). Six patients had record of a transient increase in PEEP during the bleeding episode, with a median increase of 3 cmH20 (range 2–5) for a median of 4.3 h (range 0.75–11 h). No patients died during the study period.
No adverse events were observed after iTXA. However, after iRE transient systemic hypertension was observed in two patients lasting <10 min. Event #8 had a significant increase in systemic and pulmonary arterial pressures that prompted to suspend the iRE. The increase in mean pulmonary pressures was from 40 to 60 mmHg (from two thirds systemic to suprasystemic) and an increase in systemic pressure to a systolic of 101 mmHg (percentile 95 +12 mmHg for this patient). This resolved after iRE was suspended. Additionally, event #4 had a significant increase in systemic blood pressure (a systolic up to 145 mmHg, percentile 95 + 12 mmHg for this patient), which resolved without treatment. Two patients (18%) had evidence of an upper respiratory infection around the time of the PHm event. In both, symptoms became clinically evident after the procedure, in one patient, respiratory syncytial virus was identified. No arrhythmias were noted after the administration of iRE.
Fig. 2 shows the distribution of patients with PHm and the relationship of specific characteristics; from their initial diagnosis (primary PVS vs. PVS after anomalous venous return correction), the presence of bilateral or single vessel disease, an elevated RV systolic pressure, and the chosen treatment for PHm.
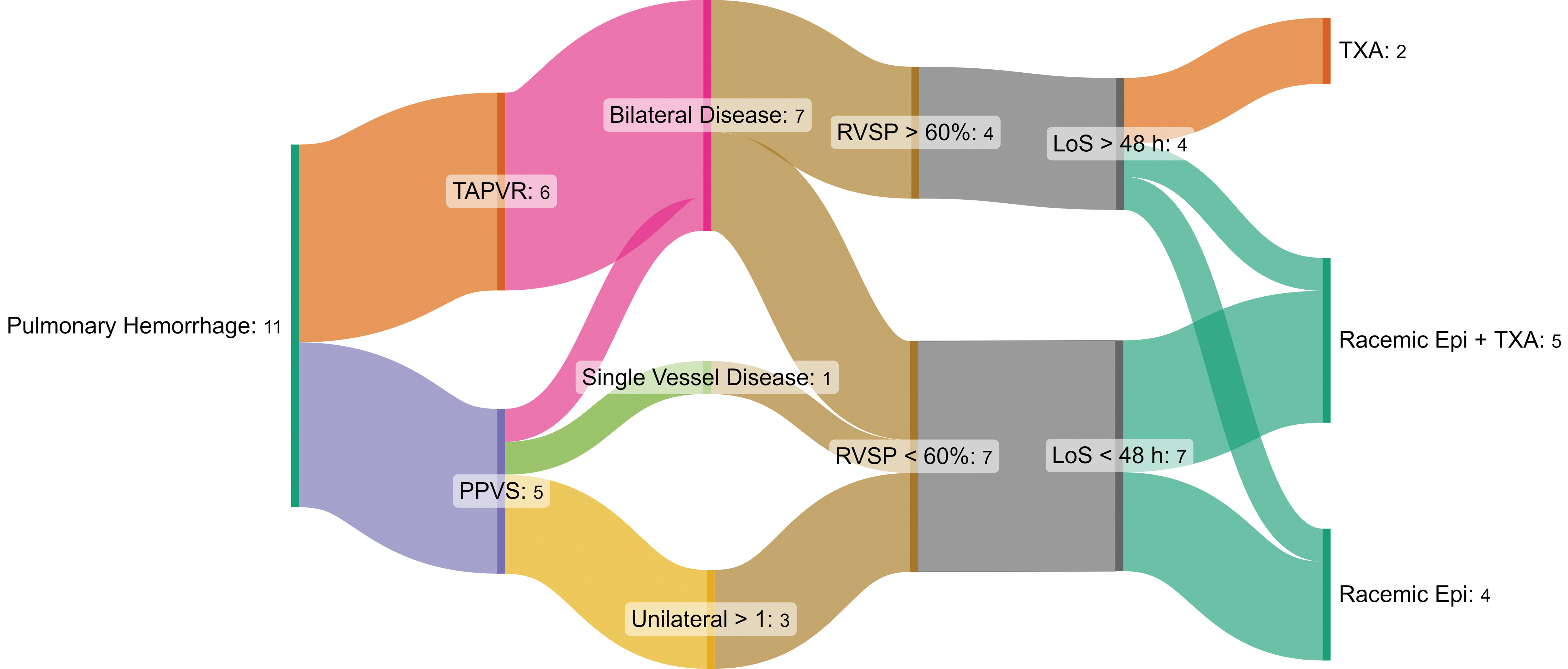
Figure 2: Sankey Diagram. It shows the relationship between the episodes of pulmonary hemorrhage, the presence of primary vs. secondary pulmonary vein stenosis (PVS), the distribution of disease and an elevated right ventricular systolic pressure (RVSP) as markers of severity of disease. This, in relation to the in-hospital length of stay and the treatment that was chosen for each event. TAPVR: total anomalous venous return, PPVS: primary pulmonary vein stenosis, LoS: length of stay. TXA: tranexamic acid
We present a single center-experience on the management of PHm after PVS intervention with a standardized protocol. Here we present a varied group of patients with both primary and secondary PVS. It is important to note the presence of known markers of severity in PVS that can be found in the patients that had one or more episodes of PHm, such as the presence of bilateral disease or four-vessel disease, with only one of our patients having a single vessel involved. Additionally, all but two patients had a significant number of catheter-based PVS interventions in their lifetime (ranging from 4–22). The need for more frequent interventions may suggest a higher degree of endothelial progression with more severe disease, which in turn can suggest a chronic state of pulmonary capillary hypertension and a higher propensity to bleeding episodes, as well as a higher chance for direct vascular injury during repeated balloon angioplasty [6,7,19,20]. Although a larger sample size is needed to identify specific risk factors for PHm, it appears that it is more common in patients with a more severe disease, with higher number of lifetime catheter-based interventions and bilateral disease.
This is the first report, to our knowledge, of the use of iRE and iTXA regularly in the management of acute PHm in PVS interventions. In our institution, the establishment of a specialized PVS Program in 2019 led to the creation of standardized protocols for the diagnosis and treatment of patients with PVS; from follow-up imaging to the frequency and timing of catheter-based interventions and pharmacologic treatment [3,21]. The closer these patients are followed, the sooner re-stenosis is identified which results in more frequent interventional procedures to ensure vessel patency, possibly a higher complexity of the patient population treated and a speculated higher risk of PHm during interventions. Further data is being gathered to accurately describe the results of the PVS Program in our overall PVS patient population.
The lack of current evidence for the management of PHm with iRE and iTXA specifically in PVS interventions, as well as the wide variety of conditions in which they have been used separately, makes it challenging to compare our results with those published [8,11]. Nonetheless, considering the mentioned pharmacologic mechanism of both iRE and iTXA and comparing this to the known complications of PVS interventions, the most relevant direct outcomes from this acute intervention can be summarized with the following questions: Is this intervention (1) providing adequate hemostasis, (2) impacting the duration of mechanical ventilation after intervention, and (3) impacting the need for ICU admission and prolonged in-hospital stay? In our series, all events were adequately controlled with the chosen management. Three patients were extubated after the procedure and without major chest X-ray changes, one continued their baseline home tracheostomy ventilatory settings, and seven required additional ventilatory support for a median time of 24 h. Three patients were not admitted to ICU, and those who were stayed for a median of 2 days.
The dosing for iRE used in our protocol was derived from the management of Uniform Resource Identifier (URI) and for iTXA, it was based on data regarding the management of diffuse alveolar hemorrhage [8,14]. Currently, there are no reported adverse events associated with the use of nebulized iTXA [9,11]. However, iRE comes with a theoretical risk of sympathomimetic stimulation including a potential risk for hypertension, tachycardia, or arrhythmia [14,17]. For these reasons, and as mentioned within the protocol, specific risk factors have been identified to contraindicate the use iRE to reduce these risks. This was clearly observed in two of our patients, with transient, but significant pulmonary and arterial hypertension. Additionally, the use of a modified dosing chart based on ideal body weight (Fig. 1B) has decreased the risk of this sympathomimetic effect.
Other adjuvant strategies that can provide prompt hemostasis are protamine reversal and transient increase in PEEP. Regarding the first, and given the short half-life of heparin, we have rarely encountered the need to consider protamine reversal in our population, possibly due to how closely we monitor the ACTs during the cases. Moreover, due to the complexity of these interventions and the risk of stent thrombosis in a low-flow state with relatively small stents, protamine reversal needs to be cautiously considered, which is why it is not included in our PHm protocol. Finally, the transient increase in PEEP is part of the standard management of PHm in other scenarios and is routinely performed by anesthesia and ICU specialists as part of PHm management. This adjuvant strategy as acute management of PHm is variable and the duration may depend on the patient’s ventilatory status and lung function and time until the bleed is controlled, which is outside of the scope of the protocol described in this manuscript.
The implementation of this standardized protocol has allowed for better procedural planning, wider availability of medications, and greater awareness by the providers involved, possibly leading to earlier detection of PHm and appropriate treatment. Although further evidence is needed, qualitative assessment suggests that this has permitted an improvement in overall outcomes after a PHm episode.
Limitations
The small patient population, as well as the lack of a control group, limits our capacity to directly measure the impact of the presented treatment strategy. Additionally, the implementation of this specialized protocol may carry the risk of confirmation bias with the risk of overtreating earlier stages of PHm by early identification.
Frequent interventions in patients with PVS, as well as the increasing complexity, appear to carry a higher risk of PHm. Early detection and increased awareness about PHm are recommended to improve outcomes, thus, establishing institutional standardized protocols is potentially a good way to start. However, more studies with a larger sample size are needed to directly measure the true presence and size of these associations.
Acknowledgement: Paige Sheaks PA-C for being critical to the Pulmonary Vein Stenosis (PVS) Program as the clinical coordinator, Dr. Todd M. Wine for proactively bringing up solutions for pulmonary hemorrhage from the pediatric otolaryngology perspective, Sonia Hidalgo for her support in determining the best equipment and administration techniques for iRE and iTXA in the Cath Lab. To all the families and patients from our PVS program for their support and trust to our team.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Catalina Vargas-Acevedo, Jenny E. Zablah, Rhynn Soderstrom, Richard Ing and Nicholas Houska; data collection: Catalina Vargas-Acevedo; analysis and interpretation of results: Catalina Vargas-Acevedo, Jenny E. Zablah and Gareth J. Morgan; draft manuscript preparation: Catalina Vargas-Acevedo, Jenny E. Zablah and Gareth J. Morgan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data was reviewed from institutional electronic medical records.
Ethics Approval: The retrospective review was approved by our institutional review board (Colorado Multiple Institutional Review Board #20-2811). As it was a retrospective review, no individual patient consent was sought.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. De Silva C, Mukherjee A, Jat KR, Lodha R, Kabra SK. Pulmonary hemorrhage in children: etiology, clinical profile and outcome. Indian J Pediatr. 2018;86(1):7–11. doi:10.1007/s12098-018-2725-x. [Google Scholar] [PubMed] [CrossRef]
2. Vanderlaan RD, Rome J, Hirsch R, Ivy D, Caldarone CA. Pulmonary vein stenosis: treatment and challenges. J Thorac Cardiovasc Surg. 2020;161(6):2169–76. doi:10.1016/j.jtcvs.2020.05.117. [Google Scholar] [PubMed] [CrossRef]
3. Shorofsky MJ, Morgan GJ, Mejia E, Rodriguez SA, Greene M, Sheaks P, et al. Management of complex pulmonary vein stenosis at altitude combining comprehensive percutaneous interventional treatment with sirolimus, pulmonary hypertension medications and intraluminal imaging with optical coherence tomography. Pediatr Cardiol. 2023 Jun 1;44(5):1125–34. doi:10.1007/s00246-023-03102-3. [Google Scholar] [PubMed] [CrossRef]
4. McLennan DI, Solano ECR, Handler SS, Lincoln J, Mitchell ME, Kirkpatrick EC. Pulmonary vein stenosis: moving from past pessimism to future optimism. Front Pediatr. 2021 Oct 5;9:747812. doi:10.3389/fped.2021.747812. [Google Scholar] [PubMed] [CrossRef]
5. Zablah JE, Ing RJ. Which pediatric patients require postoperative intensive care after cardiac catheterization for pulmonary veins stenosis? J Cardiothorac Vasc Anesth. 2022 Aug 1;36:2509–10. doi:10.1053/j.jvca.2022.04.012. [Google Scholar] [PubMed] [CrossRef]
6. Kovach AE, Magcalas PM, Ireland C, McEnany K, Oliveira AM, Kieran MW, et al. Paucicellular fibrointimal proliferation characterizes pediatric pulmonary vein stenosis: clinicopathologic analysis of 213 samples from 97 patients. Am J Surg Pathol. 2017;41(9):1198–204. doi:10.1097/PAS.0000000000000892. [Google Scholar] [PubMed] [CrossRef]
7. Masaki N, Adachi O, Katahira S, Saiki Y, Horii A, Kawamoto S, et al. Progression of vascular remodeling in pulmonary vein obstruction. J Thorac Cardiovasc Surg. 2020 Sep 1;160(3):777–790.E5. doi:10.1016/j.jtcvs.2020.01.098. [Google Scholar] [PubMed] [CrossRef]
8. Saha BK, Chong WH. Diffuse alveolar hemorrhage in cardiac diseases. Lung. 2021;199:103–12. doi:10.1007/s00408-021-00433-x. [Google Scholar] [PubMed] [CrossRef]
9. Nishijima DK, Monuteaux MC, Faraoni D, Goobie SM, Lee L, Galante J, et al. Tranexamic acid use in US children’s hospitals. J Emerg Med. 2016 Jun 1;50(6):868. doi:10.1016/j.jemermed.2016.02.004. [Google Scholar] [PubMed] [CrossRef]
10. Martin Juan J, Romero BR, Montes Worboys A, Marquez Martin E, Gonzalez Vergara D, Arellano E. Evaluación del efecto de la administración endobronquial de ácido tranexámico en el sangrado de la vía aérea. Revista Española de Patología Torácica. 2014;26(3):181–8 (In Spanish). [Google Scholar]
11. O’Neil ER, Schmees LR, Resendiz K, Justino H, Anders MM. Inhaled tranexamic acid as a novel treatment for pulmonary hemorrhage in critically ill pediatric patients: an observational study. Crit Care Explor. 2020 Jan 24;2(1):e0075. doi:10.1097/CCE.0000000000000075. [Google Scholar] [PubMed] [CrossRef]
12. Sidman JD, Wheeler WB, Cabalka AK, Soumekh B, Brown CA, Wright GB. Management of acute pulmonary hemorrhage in children. Laryngoscope. 2001;111(1):33–5. doi:10.1097/00005537-200101000-00006. [Google Scholar] [PubMed] [CrossRef]
13. Fekri MS, Hashemi-Bajgani SM, Shafahi A, Zarshenas R. Comparing adrenaline with tranexamic acid to control acute endobronchial bleeding: a randomized controlled trial. Iran J Med Sci. 2017 Mar 1;42(2):129–35. [Google Scholar]
14. Bjornson C, Russell KF, Vandermeer B, Durec T, Klassen TP, Johnson DW. Nebulized epinephrine for croup in children. Cochrane Database Syst Rev. 2013 Oct 10;(10):CD006619. doi:10.1002/14651858.CD006619.pub3. [Google Scholar] [PubMed] [CrossRef]
15. Proença-Módena JL, Acrani GO, Snider CB, Arruda E. Respiratory viral infections. In: Tropical infectious diseases: principles, pathogens and practice, Third Ed Elsevier Inc.; 2011. p. 378–91. doi:10.1016/B978-0-7020-3935-5.00058-6. [Google Scholar] [CrossRef]
16. Abou Zahr R, Ashfaq A, Marron-Corwin M. Neonatal pulmonary hemorrhage. Neoreviews. 2012;13(5):302–6. doi:10.1542/neo.13-5-e302. [Google Scholar] [CrossRef]
17. Ledwith CA, Shea LM, Mauro RD. Safety and efficacy of nebulized racemic epinephrine in conjunction with oral dexamethasone and mist in the outpatient treatment of croup. Ann Emerg Med. 1995;25(3):331–7. doi:10.1016/S0196-0644(95)70290-3. [Google Scholar] [PubMed] [CrossRef]
18. Barnes ME, Feeney E, Duncan A, Jassim S, MacNamara H, O’Hara J, et al. Pulmonary haemorrhage in neonates: systematic review of management. Acta Paediatr. 2022 Feb;111(2):236–44. doi:10.1111/apa.16127. [Google Scholar] [PubMed] [CrossRef]
19. Frank DB, Levy PT, Stiver CA, Boe BA, Baird CW, Callahan RM, et al. Primary pulmonary vein stenosis during infancy: state of the art review. J Perinatol; 2021;41:1528–39. doi:10.1038/s41372-021-01008-7. [Google Scholar] [CrossRef]
20. Vanderlaan RD. Commentary: targeting neointimal lesions in pulmonary vein stenosis: fact or fiction? J Thorac Cardiovasc Surg. 2020 Sep 1;160(3):794–5. doi:10.1016/j.jtcvs.2020.02.021. [Google Scholar] [PubMed] [CrossRef]
21. O’callaghan B, Zablah JE, Weinman JP, Englund EK, Morgan GJ, Dunbar Ivy D, et al. Computed tomographic parenchymal lung findings in premature infants with pulmonary vein stenosis. Pediatr Radiol. 2023;53:1874–84. doi:10.1007/s00247-023-05673-y. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools