 Open Access
Open Access
ARTICLE
Prospective Cohort Research of Aortic Root Dilatation after Surgical Repair in Adults with Tetralogy of Fallot (TRANSIT)
1 Department of Cardiology, Tokyo Metropolitan Children’s Medical Center, Tokyo, 183-8561, Japan
2 Department of Pediatric Cardiology, Sakakibara Heart Institute, Tokyo, 183-0003, Japan
3 Department of Adult Congenital Heart Disease, Chiba Cerebral and Cardiovascular Center, Chiba, 305-8576, Japan
4 Department of Adult Congenital Heart Disease, Chiba Kaihin Municipal Hospital, Chiba, 261-0003, Japan
5 Department of Cardiology, Faculty of Medicine, University of Tsukuba, Tsukuba, 305-8576, Japan
6 Cardiovascular Center, St. Luke’s International Hospital, Tokyo, 104-8560, Japan
7 Department of Cardiology, Tokyo Metropolitan Tama Medical Center, Tokyo, 183-8524, Japan
8 Division of Cardiology, National Center for Child Health and Development, Tokyo, 158-8531, Japan
9 Center for Preventive Medicine, Keio University School of Medicine, Tokyo, 160-0016, Japan
* Corresponding Author: Masaru Miura. Email:
Congenital Heart Disease 2024, 19(4), 351-362. https://doi.org/10.32604/chd.2024.051837
Received 16 March 2024; Accepted 03 June 2024; Issue published 31 October 2024
Abstract
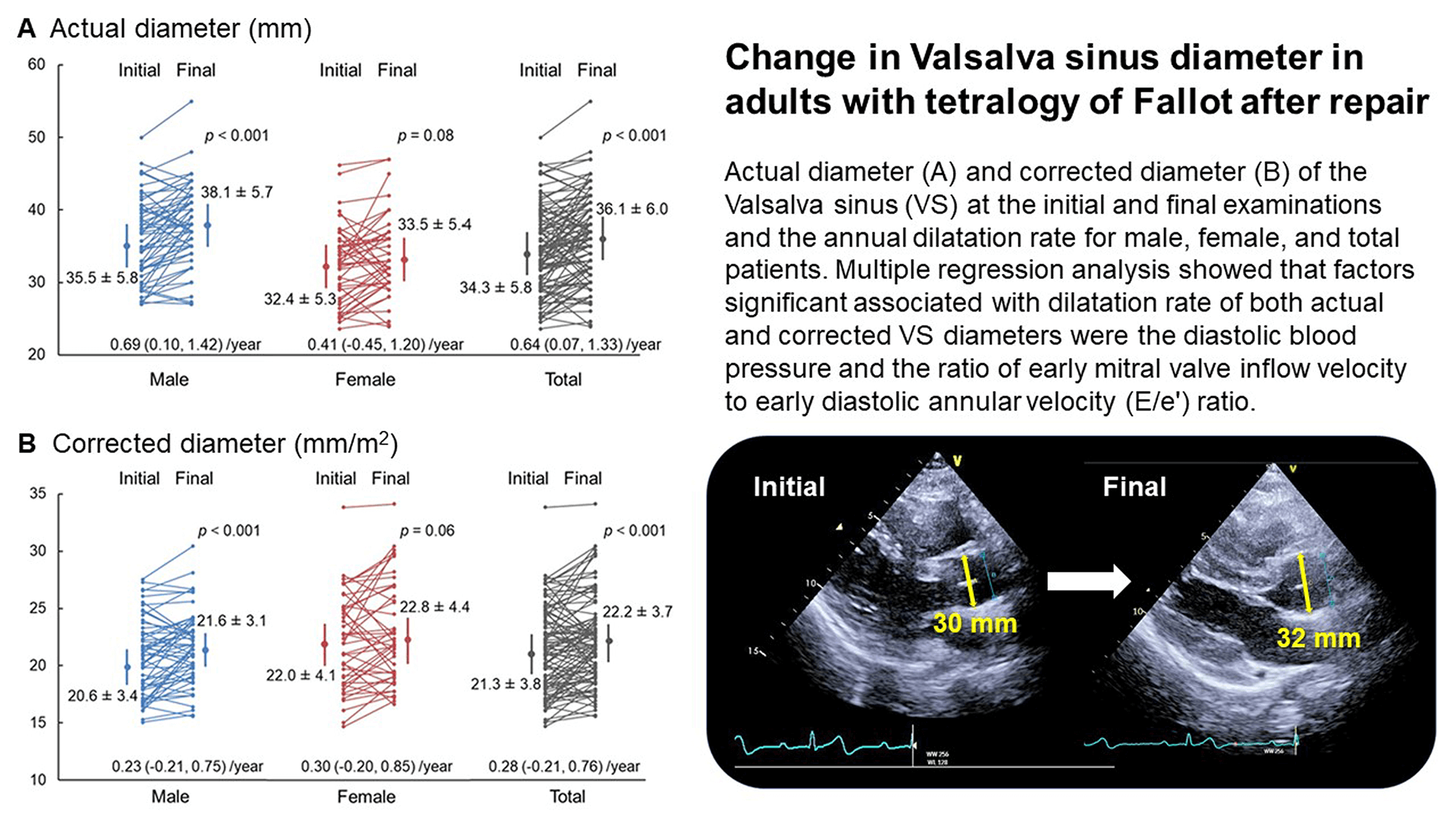
Background: Aortic root dilatation occurs in adults with tetralogy of Fallot (TOF) after surgical repair, but the longitudinal changes are unclear. The main research aim is to determine the annual dilatation rate of aorta in adults with repaired TOF. Methods: The present, multicentric, prospective cohort study assessed the rate of aortic diameter change in adults aged 20 years or older with TOF, including pulmonary artery atresia, who underwent surgical repair. Clinical data, focusing on echocardiograms, were collected at three-year intervals from seven hospitals. Results: In total, 104 patients (58 males; median age: 29 years) were enrolled. The actual Valsalva sinus (VS) diameter was 34.3 ± 5.8 (mean ± standard deviation) and 36.1 ± 6.0 mm at the initial and final examinations, respectively, and the annual dilatation rate was 0.64 (0.07, 1.33) (median, interquartile) mm/year. The corrected diameter at the respective examination was 21.3 ± 3.8 and 22.2 ± 3.7 mm/m2, and the annual dilation rate was 0.28 (−0.21, 0.76) mm/m2/year. Multiple regression analysis showed that factors significant associated with dilatation rate of actual VS diameter were the diastolic blood pressure (standardized coefficient −0.22; p = 0.04), cardiothoracic ratio (0.28; 0.02), and the ratio of early mitral valve inflow velocity to early diastolic annular velocity (E/e′) ratio (0.31; 0.004). Factors significantly associated with corrected VS diameter were diastolic blood pressure (−0.25; 0.02) and the E/e′ ratio (0.34; 0.001). Conclusions: In adults with repaired TOF, the rate of dilatation of the aortic diameter was associated with decreased diastolic blood pressure and left ventricular diastolic dysfunction, possibly reflecting decreased aortic wall elasticity.Graphic Abstract
Keywords
Tetralogy of Fallot (TOF) is the most common cyanotic heart disease and is characterized by the presence of sub-valvular pulmonary stenosis, a ventricular septal defect, overriding aorta, and right ventricular hypertrophy, including, broadly, pulmonary atresia with a ventricular septal defect. The results of intracardiac repair in childhood have been excellent, and the number of patients reaching adulthood has increased worldwide. In the long-term postoperative period, complications, such as pulmonary valve regurgitation, pulmonary artery stenosis, and arrhythmias, can appear, requiring reoperation to prevent sudden death. Furthermore, progressive aortic root dilatation due to medial degeneration, increased volume overload, genetic factors, or a combination of these factors may occur [1]. Aortic dilatation can induce an aortic aneurysm, rupture, or aortic regurgitation and provoke left ventricular hypertrophy, reduced coronary artery flow, and possibly left ventricular failure, leading to so-called “aortopathy” [1].
There are several studies of aortic dilatation in adults with surgically repaired TOF, with the reported prevalence differing by the criteria applied. In their historical article, Niwa et al. [2] reported that the diameter of the sino-tubular junction (STJ) of the ascending aorta was 1.5 times greater than the predicted value in 15% of their patients. Based on the Valsalva sinus (VS) diameter, Nagy et al. [3] reported that aortic dilatation with a Z score > 2.0 was present in 51% of their cohort and that 39% of their patients had an actual measurement >40 mm. Mongeon et al. [4] reported that 7% of their cohort had aortic dilatation >1.5 times the predicted value while 29% had an actual diameter >40 mm. While these studies were cross-sectional and used echocardiography, recent, longitudinal studies using magnetic resonance angiography [5] as well as echocardiography [6] have found various aortic dilatation rates. According to these studies [2–6], aortic dilatation was associated with several factors, including male sex, pulmonary atresia, right aortic arch, left ventricular enlargement, residual ventricular septal defect, and aortic regurgitation.
Because the studies cited above were monocentric and retrospective, limitations, such as selection bias, variable measurement methodology, and confounding factors, may have influenced their findings. To address these issues, the present, multicentric, prospective cohort study of aortopathy in adult patients with repaired TOF centrally analyzed echocardiographically measured aortic diameters; determined the annual dilatation rate and prevalence of aortic dilatation in relation to the actual diameter and the diameter corrected for the body surface area; assessed potentially associated factors to improve management strategies; and collected data on Japanese patients to redress the exclusive focus of previous studies on North American and European patients.
The inclusion criteria were patients aged 20 years or older with a diagnosis of TOF, including pulmonary atresia, who had undergone surgical repair for this condition and were followed up for three years. The exclusion criteria were the presence of any other congenital heart disease with aortic dilatation (bicuspid aortic valve, coarctation of aorta, or interruption of the aortic arch) or a connective tissue disease (Marfan syndrome or Loeys-Dietz syndrome). Between March 2015 and March 2018, we enrolled 124 patients from the seven participating centers: Chiba Cerebral and Cardiovascular Center, Chiba, Japan; University of Tsukuba, Tsukuba, Japan; St. Luke’s International Hospital, Tokyo, Japan; Tokyo Metropolitan Tama Medical Center, Tokyo, Japan; Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan; National Center for Child Health and Development, Tokyo, Japan; and Keio University School of Medicine, Tokyo, Japan. Of these, one patient withdrew consent, four were not followed up (one died), and 16 had no echocardiographic images available for central analysis. As a result, the number of subjects was 104 (Fig. 1).
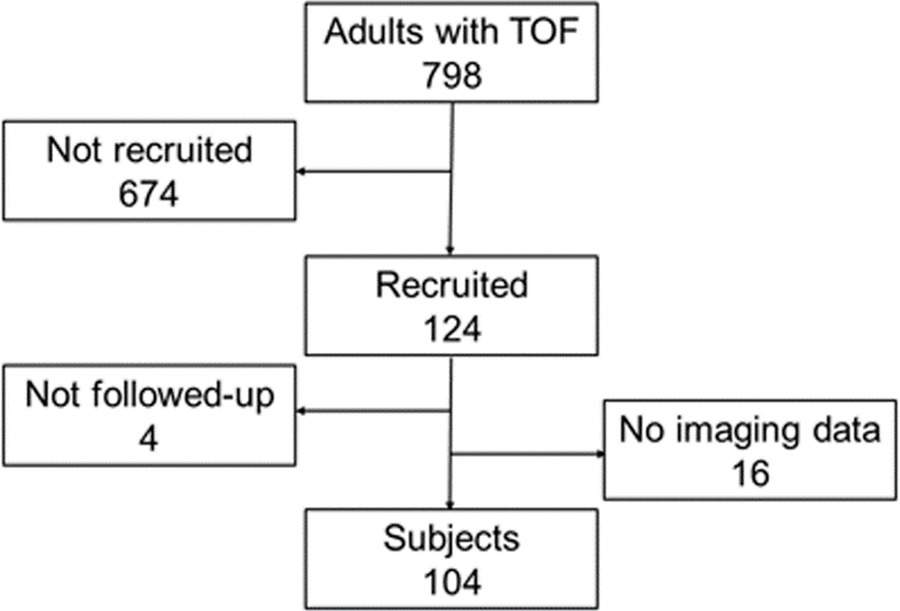
Figure 1: Patient flow chart. TOF, tetralogy of Fallot
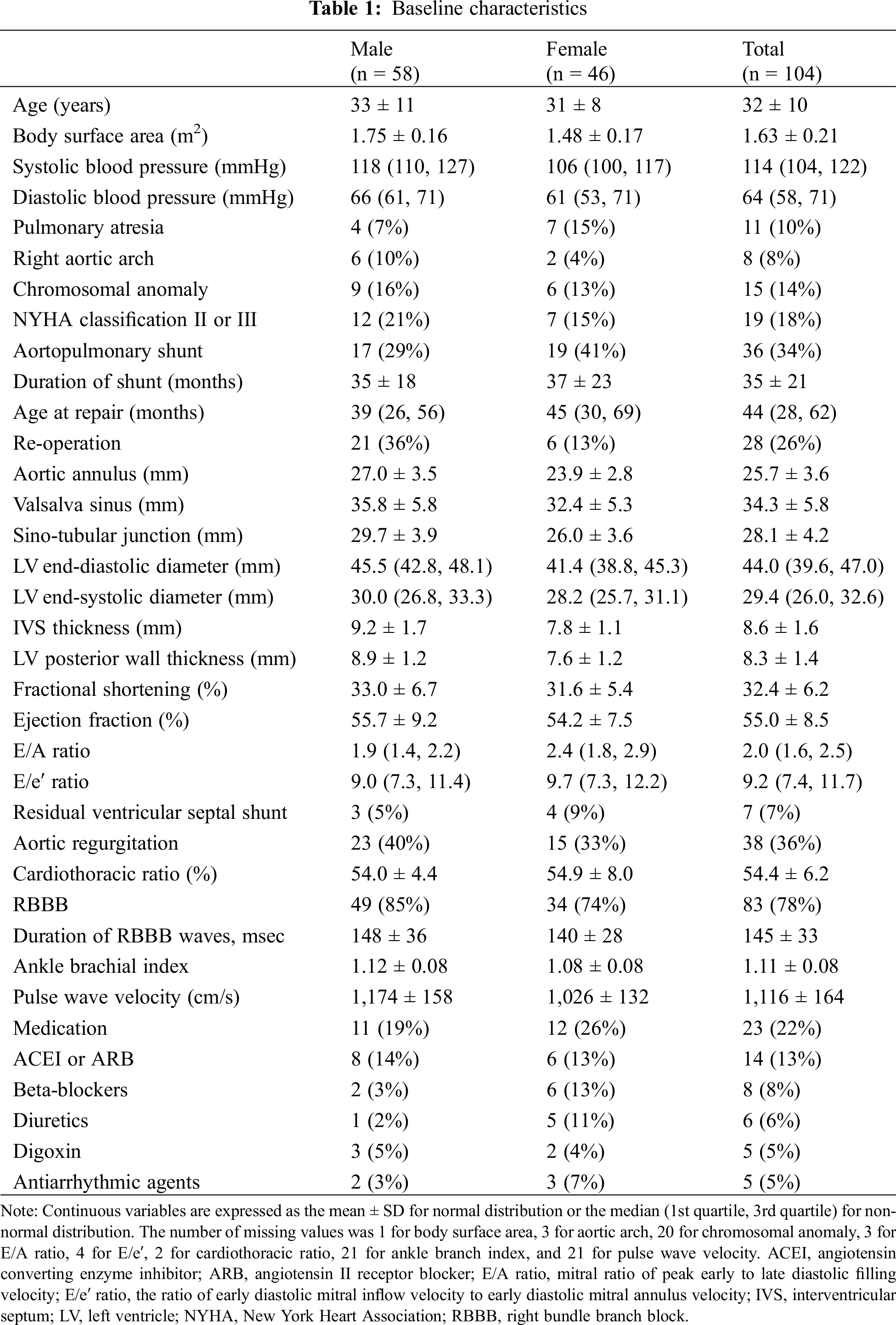
The following data were collected at enrollment: 1) Demographic data, including age and sex; 2) A diagnosis of TOF with pulmonary stenosis or atresia, the presence of major aortopulmonary collateral arteries, postoperative ventricular residual shunt, left or right aortic arch, and chromosomal abnormalities (Down syndrome, 22q11.2 deletion syndrome or others); 3) A history of treatment, including the timing and type of cardio-pulmonary shunt, unifocalization of major aortopulmonary collateral arteries, intracardiac surgical repair, re-operation, and catheter treatment; 4) Medication history, including use of angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), beta-blocker, other antiarrhythmic drugs, diuretics, and digoxin; and 5) Other data, including the New York Heart Association cardiac function classification and a history of pregnancy and childbirth in female patients.
This study was approved by the ethics committee of the main study center, Tokyo Metropolitan Children’s Medical Center (Approval No. H26-33), as well as those of the collaborating institutions. The study was also approved by the research committee of the Japanese Society for Adult Congenital Heart Disease and has been registered with University Hospital Medical Information Network Clinical Trials Registry (UMIN000017555) (https://www.umin.ac.jp/ctr/index-j.htm, accessed on 1 July 2024). The aim of the present study was explained to the patients or their guardians if the former was intellectually incompetent, and their oral or written consent (as per institutional protocol) was obtained.
2.2 Data Collection and Follow-Up
The date of an echocardiogram performed within one year of enrollment was used as the starting date, and the following data were collected: weight, height, blood pressure, echocardiographic findings, the cardiothoracic ratio on a plain chest radiogram, presence of right bundle branch block, and QRS wave width on electrocardiogram, and blood tests, including the complete blood count, blood chemistry, and the brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) value. The BNP value was converted into the NT-proBNP value using the formula published by Ishihara et al. [7]. The ankle brachial index and pulse wave velocity data were also collected only at institutions where they were available.
Echocardiographic measurements included the following: the inner diameter of the aortic valve annulus, VS, and STJ; end-diastolic and end-systolic diameters of the left ventricle; end-diastolic thickness of the ventricular septum and left ventricular posterior wall; E wave and A wave velocities of the mitral valve inflow waveform using the pulse wave method; e′ wave and a′ wave velocities of the mitral valve annulus using tissue Doppler at the ventricular septum; presence of aortic regurgitation; maximum flow velocity at the main pulmonary artery; maximum flow velocity of tricuspid regurgitation; and the presence of a residual ventricular septal shunt. Fractional shortening and the ejection fraction were calculated using the left ventricular diameter and the Teichholz formula as the systolic function and the E/A and E/e′ ratios as the diastolic function.
During follow-up, the patients’ clinical condition, including life or death (with the cause of death), cardiac complications, re-operation, catheter treatment, medication, activity level by the New York Heart Association classification, and pregnancy or childbirth, were monitored annually. The final examination was performed at 36 ± 3 months after the initial examination, and the similar findings were collected as above given in data collection. Regarding the echocardiographic measurements of the aortic root diameter, motion-mode images on the parasternal, left ventricular, and long axis view were recorded on compact discs, which were sent to a data center and evaluated by two pediatric cardiologists at a core laboratory. The diameter of the aortic valve annulus was measured at mid-systole, and the diameter of the VS and STJ was measured at end-diastole on a plane perpendicular to the long axis of the aorta using the inner edge-to-inner edge convention. Inter-observer and intra-observer reliability of the assessment of the VS diameter in a randomly selected cohort of 44 subjects was 0.91 for the former and 0.89 and 0.99 for the latter.
The primary outcome was the annual dilatation rate of the aortic diameter, defined as the VS diameter based on both the actual value and the value corrected by body surface area. The secondary outcomes were the prevalence of aortic dilatation at the initial and final examinations. Aortic dilatation was assessed on the basis of the diameter of the VS corrected by body surface area and was defined as exceeding the mean value + standard deviation (SD) × 2 of the normal value for Japanese adults [8]. For categorical variables, subjects were divided into two groups, and the annual dilatation rate of the VS diameter was compared using Student’s t-test or the Wilcoxon test. For continuous variables, the average of the initial and final examinations was calculated except for age at enrollment and surgical repair and shunt duration. Pearson’s or Spearman’s correlation analysis was then performed to assess for any correlation with the annual dilatation rate of the VS diameter. For multiple regression analysis, variables found to be significant in these analyses were used as explanatory variables in addition to those reportedly associated in previous studies with aortic dilatation [2–6], and the annual dilatation rate of the VS diameter was treated as an objective variable. Two-sided p < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS, version 27.0 (International Business Machines Corporation, Armonk, NY,USA).
The 104 patients included in the study comprised 58 males and 46 females ranging in age from 20 to 56 years, with the median age and the mean age being 29 years and 32 years, respectively. Table 1 shows the basic data and missing values. One patient had no weight or height data; thus, the corrected value for the aortic diameter was unable to be calculated for this patient.
The VS diameter at the initial and final examinations was compared, and the annual dilatation rate was calculated (Fig. 2). The actual VS diameter was significantly larger at the final examination than at the initial examination in the total cohort (dilatation rate: median [1st quartile, 3rd quartile], 0.64 [0.07,1.33] mm/year; mean ± SD, 0.58 ± 1.29 mm/year) as in the male patients but did not vary significantly among the female patients. The prevalence of aortic dilatation, as estimated using the normal values of Japanese adults [8], did not differ significantly between 51 patients (49%) at the first examination and 55 patients (53%) at the last examination (p = 0.58), and both male and female patients demonstrated similar findings. The corrected VS diameter increased significantly from the initial to the final examination in the total cohort (dilatation rate: 0.28 [−0.21, 0.76]; 0.29 ± 0.79 mm/m2/year) and among the male patients, but no significant difference was observed among the female patients. The prevalence of aortic dilatation did not differ significantly between 46 patients (44%) at the first examination and 54 patients (52%) at the final examination (p = 0.35), with both male and female patients demonstrating similar findings. During the follow-up period, two patients received surgery for pulmonary valve replacement, but none received surgery for aortic valve, aortic dilatation, or aortic dissection.
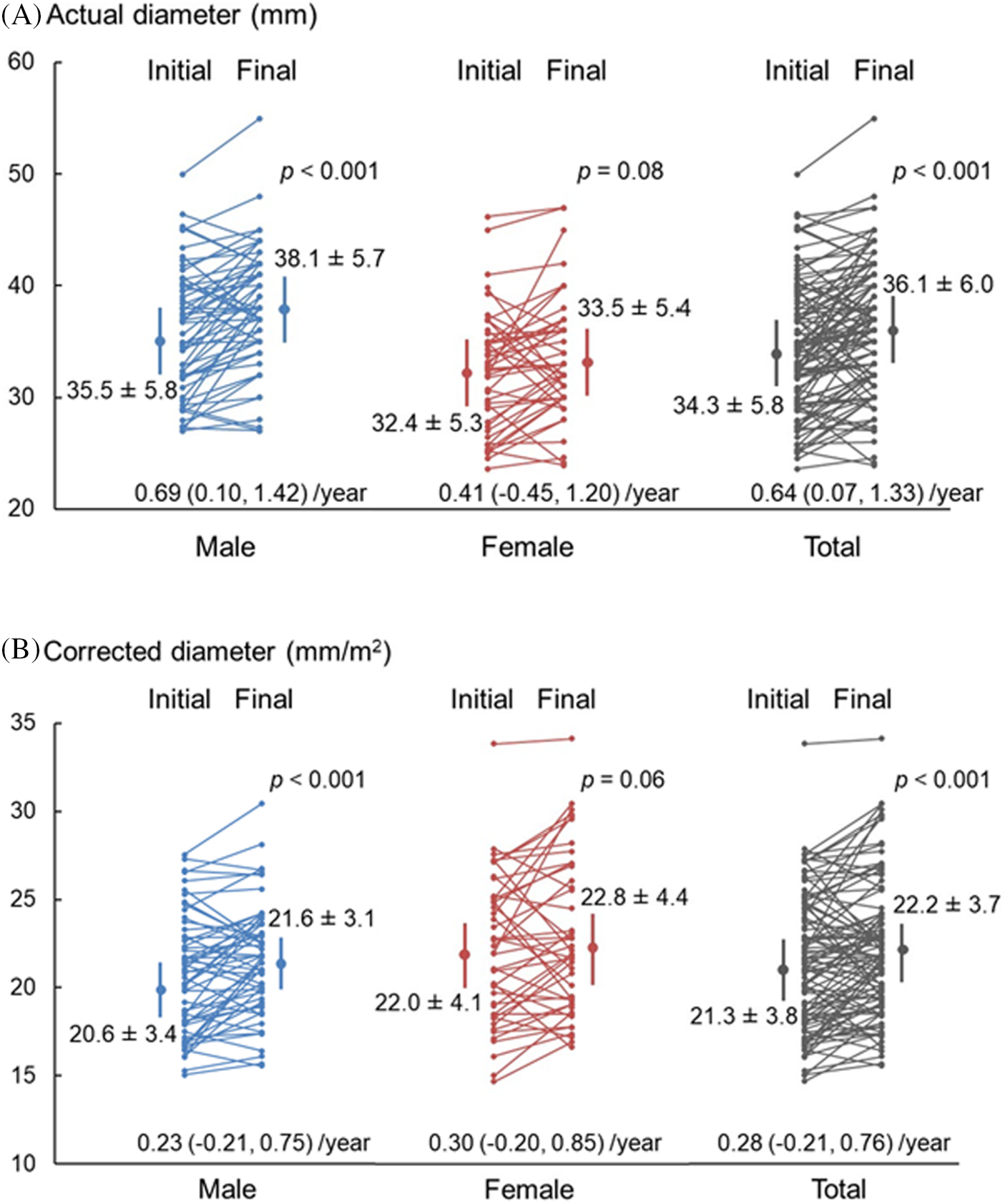
Figure 2: Change in Valsalva sinus diameter. Actual diameter (A) and corrected diameter (B) of the Valsalva sinus at the initial and final examinations and the annual dilatation rate for male, female, and total patients. The diameter is expressed as the mean ± SD, and the dilatation rate is expressed as the median (1st quartile, 3rd quartile) for dilatation rates
3.3 Factors Associated with Dilatation Rate of Aortic Diameter
Regarding the factors affecting the dilatation rate of the VS diameter, no significant difference was found in any of the categorical variables of the actual or corrected value including medications (Table 2). In terms of continuous variables, the cardiothoracic ratio, QRS width of the right bundle branch block, and mitral E/e′ ratio showed significant positive correlations, while the diastolic blood pressure showed a significant negative correlation with the actual values (Table 3). The cardiothoracic ratio, E/e′ ratio, and NT-proBNP showed a significant positive correlation, and the diastolic blood pressure showed a significant negative correlation with the corrected values. No significant correlation was found between the other blood test findings and the actual or corrected value.
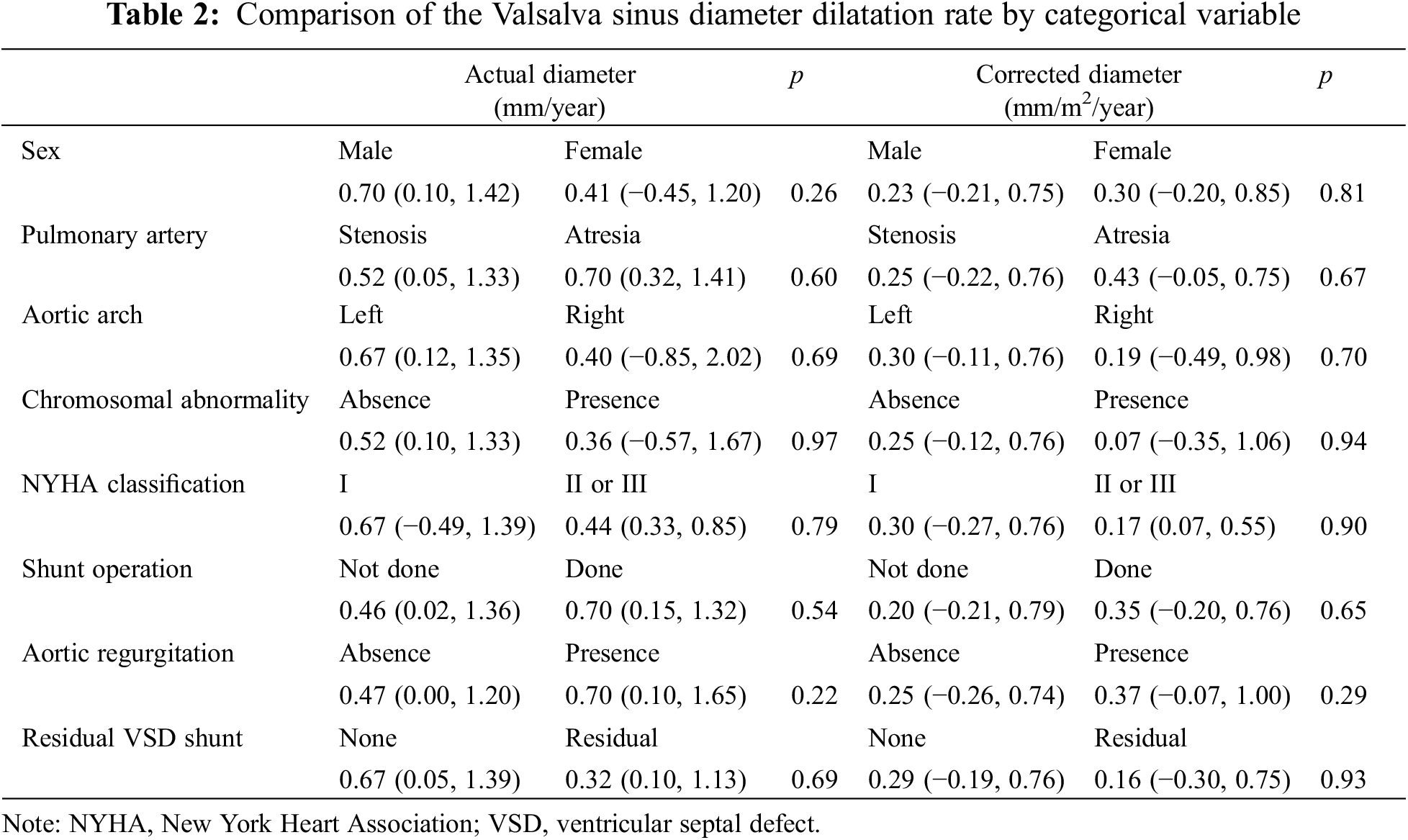
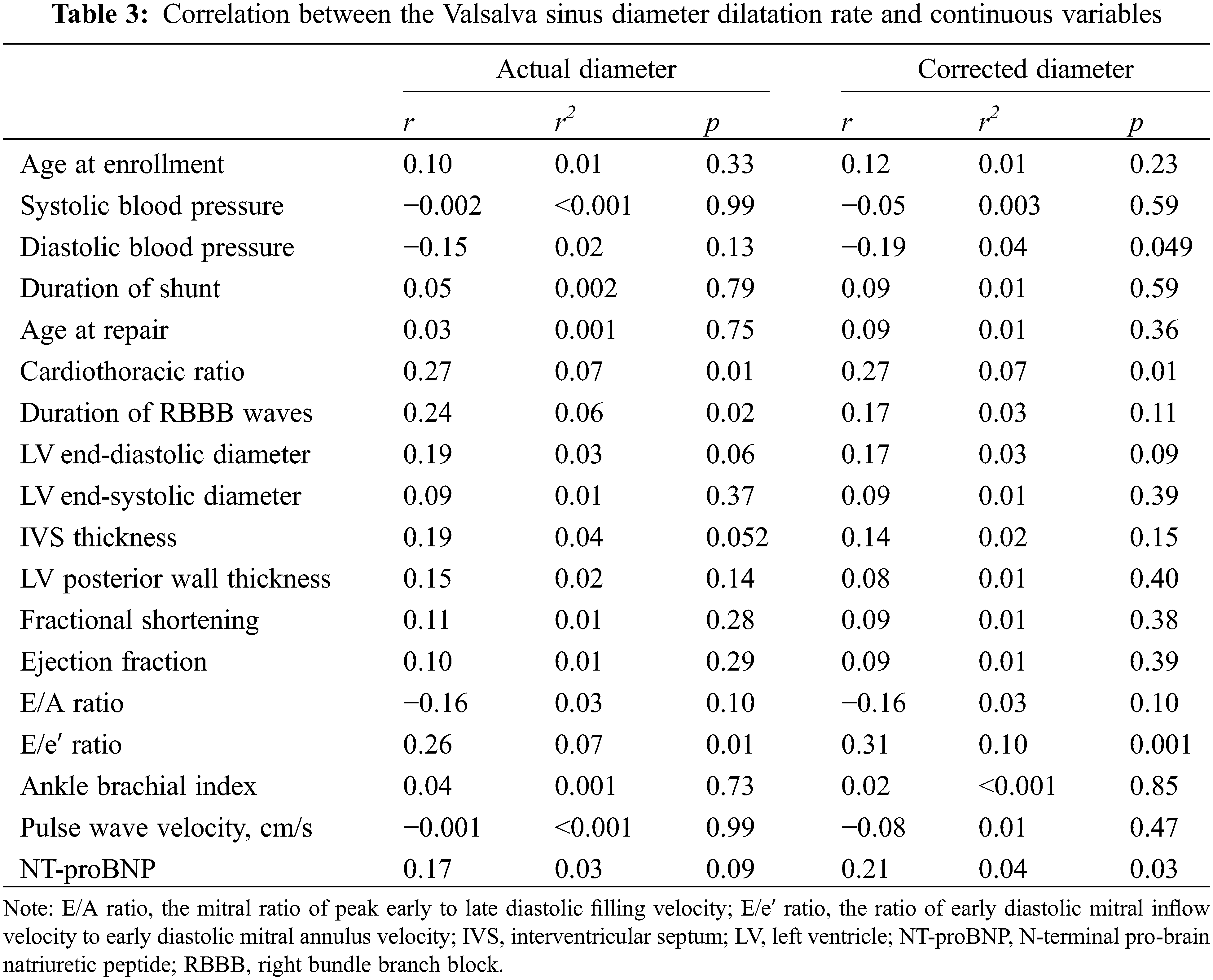
Multiple regression analysis was performed using the dilatation rate of the VS diameter as an objective variable (Table 4). The explanatory variables were male sex and pulmonary atresia, both of which have previously been reported as associated factors [2–6], and significantly correlated factors, including the diastolic blood pressure, cardiothoracic ratio, QRS width of right bundle branch block, mitral E/e′ ratio, and NT-proBNP value. The analysis found diastolic blood pressure, the cardiothoracic ratio, the E/e′ ratio in the actual values, and diastolic blood pressure and the E/e′ ratio in the corrected values to be significantly associated. Multiple correlation coefficient r and coefficient of determination r2 were 0.503 and 0.253 for the actual values and 0.502 and 0.252 for the corrected values, respectively.
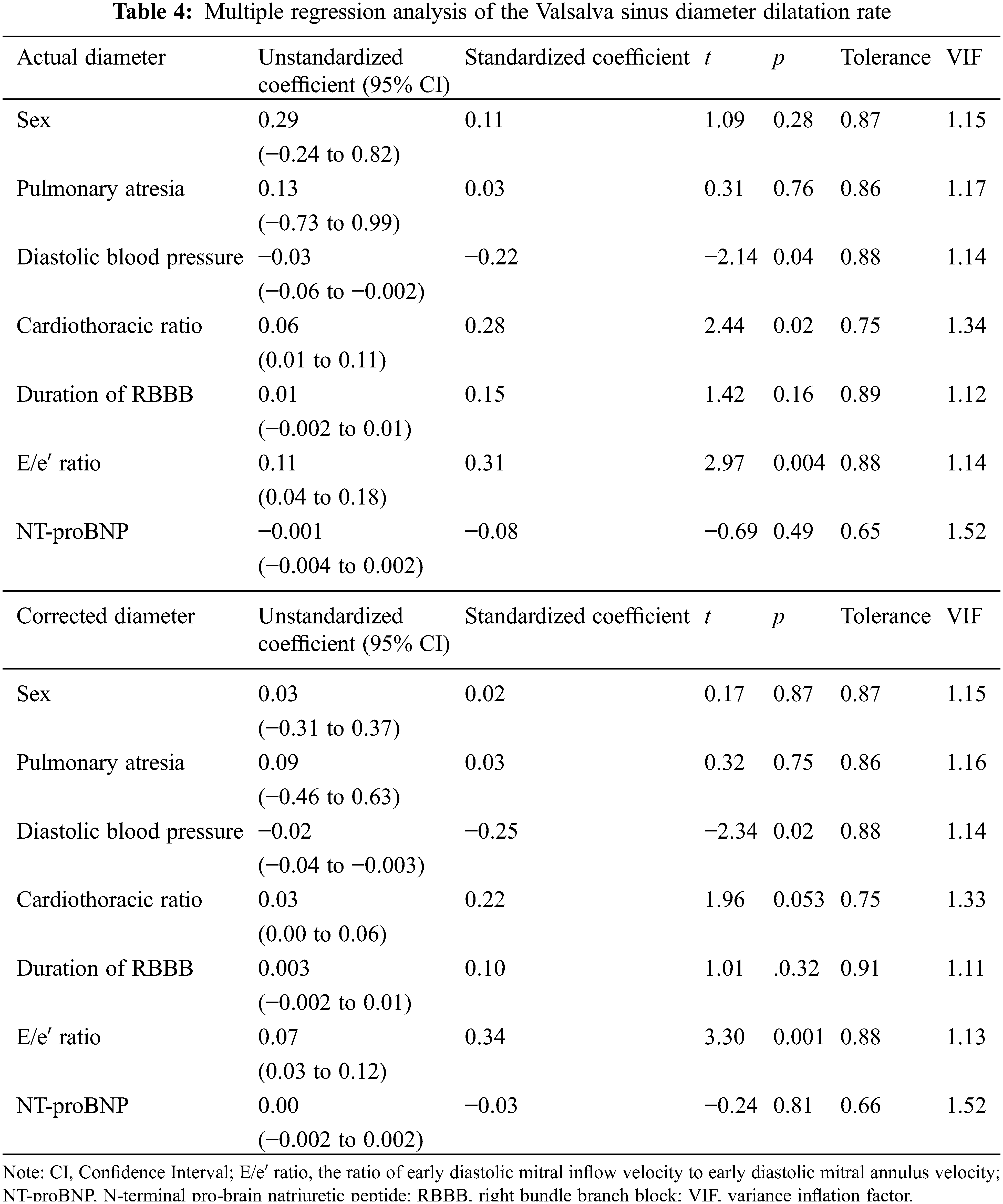
To the best of our knowledge, the present study is the first, multicentric, prospective study to date to examine aortic dilatation rate in adults with repaired TOF. On multiple regression analysis, the dilatation rate of both the actual and corrected VS diameters significantly correlated with a decrease in the diastolic blood pressure as well as with left ventricular diastolic dysfunction. Previous, cross-sectional studies [2–4] did not emphasize these factors, which in the present, longitudinal study were likely indices of aortopathy. The present study demonstrated that the median annual dilatation rate was 0.64 mm/year for the actual VS diameter and 0.28 mm/m2/year for the corrected VS diameter. Although there are few studies of the dilatation rate in TOF, the existing data of the actual VS diameter indicate a rate of 0.2–0.6 mm/year based on MRI [5] and echocardiographic findings [6]. Niwa et al. [2] reported that the dilatation rate of the actual STJ diameter was 1.7 mm/year in patients with aortic dilatation and 0.03 mm/year in those without aortic dilatation.
In the management of adults after TOF surgery, aortic enlargement is less well described than pulmonary artery stenosis and pulmonary valve regurgitation. U.S. guidelines [9] recommend transthoracic echocardiography every 24 months and cardiac computed tomography or cardiovascular magnetic resonance imaging every 36 months, even if the physiological stage is stable. They also recommend shortened intervals between examinations if the disease worsens. Changes in the aortic diameter, degree of aortic regurgitation, and left ventricular function should be evaluated whenever these imaging studies are performed. Based on the results of the present study, surgical intervention may be considered if aortic dilatation progresses rapidly.
In the present analysis, the diastolic blood pressure demonstrated a significant, negative correlation with the rate of dilation of both the actual and corrected VS diameters. Previous, pathophysiological studies [10,11] have demonstrated that the arterial wall stiffens and becomes more resistant to distension as arterial blood pooling becomes more limited during left ventricular ejection. The result is increased systolic pressure and decreased diastolic pressure. With aging, fractures in the elastin lamellae of the central arteries cause arterial stiffening and dilatation so that wave reflections return earlier to the heart; as a consequence, the aortic systolic pressure rises and the diastolic pressure falls [12]. Furthermore, if aortic dilatation induces aortic regurgitation, the diastolic pressure may decrease further. The lower diastolic blood pressure of adults with repaired TOF was associated with arterial stiffness and may predict aortic dilatation.
In the present study, the factor most relevant to the rate of dilation of both the actual and corrected VS diameters was left ventricular diastolic dysfunction, which was expressed as the mitral E/e′ ratio. Diastolic dysfunction is induced by aortic stiffening, which leads to ventricular hypertrophy and an increased myocardial oxygen requirement via left ventricular overload [10–12]. In the hypertrophied heart, the duration of the systole is accordingly lengthened, wave reflection causes augmentation of late systolic pressure, and the tension-time index and myocardial oxygen consumption needs are thereby boosted. On the other hand, coronary blood flow is impaired due to decreased diastolic pressure and a reduction in the duration of diastole. In their prospective study of 51 adults with repaired TOF using echocardiography and magnetic resonance imaging, Shiina et al. [13] reported that aortic dilatation was significantly related to aortic pressure wave reflection, which had a negative impact on the diastolic function but not on the systolic function of the left ventricle. Thus, it may be concluded that the diastolic rather than the systolic function of the left ventricle more strongly reflects aortopathy in adults with repaired TOF.
Unlike previous studies, the present study did not find male sex, pulmonary atresia, right aortic arch, left ventricular enlargement, residual ventricular septal defect, or aortic regurgitation to be associated with aortic dilatation [2–6]. However, a comparison of the initial and final examination findings demonstrated a significant increase in the VS diameter among the male patients but not in the female patients. On the other hand, multiple regression analysis found that the rate of dilatation rate of the actual VS diameter was significantly associated with a large cardiothoracic ratio. Furthermore, in simple correlation analysis, the QRS duration of the right bundle branch block significantly correlated with the dilatation rate of the actual VS diameter, and the NT-proBNP value significantly correlated with the corrected VS diameter, suggesting that these variables reflect dysfunction of both the left ventricle and right ventricles.
The present study found no association between the reduction in the dilatation rate of the VS diameter and any medications including ACEI, ARB, beta-blockers, diuretics, digoxin, and antiarrhythmic agents. ARB inhibits the activation of transforming growth factor-β; clinical trials of ARB administered alone or in combination with a beta-blocker to patients with Marfan syndrome found that the drug inhibited the progression of aortic dilatation [14]. Aortic dilatation in TOF involves tunica media necrosis like that found in Marfan syndrome [15]. Given the possible involvement of transforming growth factor-β [16], ARB may be effective, but further, detailed studies are needed.
The present study has several limitations. First, the number of eligible cases during the study period was large, and the recruitment criteria varied among the facilities and physicians, leading to a selection bias. Second, although the aortic diameter was measured echocardiographically at the central institution, the other participating centers may have used different recording methods with variable imaging accuracy. Third, the timing and method of surgical repair varied over time, making a comparison with the findings of previous reports difficult. Fourth, the subjects were a relatively small number of all ethnically Japanese. Fifth, because the indications for the drugs were not specified, their efficacy was difficult to assess owing to the confounding factor of illness severity.
To the best of our knowledge, the present study is the first, multicentric, prospective cohort study to analyze aortic dilatation in adults with repaired TOF. The dilatation rate of the aortic diameter was found to be associated with a decrease in diastolic blood pressure and left ventricular diastolic dysfunction, which are probably manifestations of aortopathy. These findings will hopefully inform future clinical research on the appropriate management of TOF.
Acknowledgement: We thank James R Valera, our in-house editor, for his assistance with editing and proofreading this manuscript. We also thank Yoko Saito, Masako Tomotsune, and Haruka Sasaki of the Clinical Research Support Center at Tokyo Metropolitan Children’s Medical Center for their assistance with data management. Cooperating institutions (with the number of patients contributed by each) were as follows: Chiba Cerebral and Cardiovascular Center (49), University of Tsukuba (28), St. Luke’s International Hospital (20), Tokyo Metropolitan Tama Medical Center (15), Tokyo Metropolitan Children’s Medical Center (7), National Center for Child Health and Development (3), and Keio University School of Medicine (2).
Funding Statement: This study was supported by grants from the Clinical Research Fund of Tokyo Metropolitan Government Hospitals.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Masaru Miura; data collection: Hiroki Nagamine, Jun Maeda, Fumie Takechi, Shigeru Tateno, Tomoko Ishizu, Yumi Shiina, Ken Kato, Hiroshi Ono, Hiroyuki Yamagishi; analysis and interpretation of results: Hiroki Nagamine, Masaru Miura; echocardiographic analysis: Hiroki Nagamine, Takumi Nishiki, Maasa Sato; draft manuscript preparation: Hiroki Nagamine; supervision: Koichiro Niwa. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: This study was approved by the ethics committee of the main study center, Tokyo Metropolitan Children’s Medical Center (Approval No. H26-33), as well as those of the collaborating institutions. The study was also approved by the research committee of the Japanese Society for Adult Congenital Heart Disease and has been registered with the UMIN-CTR clinical trials registry (UMIN000017555). The aim of the present study was explained to the patients or their guardians if the former was intellectually incompetent, and their oral or written consent (as per institutional protocol) was obtained.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Niwa K. Aortic dilatation in complex congenital heart disease. Cardiovasc Diagn Ther. 2018;8(6):725–38. doi:10.21037/cdt.2018.12.05. [Google Scholar] [PubMed] [CrossRef]
2. Niwa K, Siu SC, Webb GD, Gatzoulis MA. Progressive aortic root dilatation in adults late after repair of tetralogy of Fallot. Circulation. 2002;106(11):1374–8. doi:10.1161/01.CIR.0000028462.88597.AD. [Google Scholar] [PubMed] [CrossRef]
3. Nagy CD, Alejo DE, Corretti MC, Ravekes WJ, Crosson JE, Spevak PJ, et al. Tetralogy of Fallot and aortic root dilation: a long-term outlook. Pediatr Cardiol. 2013;34(4):809–86. doi:10.1007/s00246-012-0537-8. [Google Scholar] [PubMed] [CrossRef]
4. Mongeon FP, Gurvitz MZ, Broberg CS, Aboulhosn J, Opotowsky AR, Kay JD, et al. Aortic root dilatation in adults with surgically repaired tetralogy of Fallot: a multicenter cross-sectional study. Circulation. 2013;127(2):172–9. doi:10.1161/CIRCULATIONAHA.112.129585. [Google Scholar] [PubMed] [CrossRef]
5. Lyon SM, Ofner S, Cheng P, Powell S, Schloss D, Landis BJ, et al. Serial magnetic resonance imaging for aortic dilation in tetralogy of Fallot with pulmonary stenosis. Am J Cardiol. 2023;191:92–100. doi:10.1016/j.amjcard.2022.12.015. [Google Scholar] [PubMed] [CrossRef]
6. Sengupta A, Lee JM, Gauvreau K, Colan SD, del Nido PJ, Mayer JE, et al. Natural history of aortic root dilatation and pathologic aortic regurgitation in tetralogy of Fallot and its morphological variants. J Thorac Cardiovasc Surg. 2023;166(6):1718–28. doi:10.1016/j.jtcvs.2023.04.014. [Google Scholar] [PubMed] [CrossRef]
7. Ishihara S, Hiramitsu S, Kanaoka K, Taki M, Nakagawa H, Ueda T, et al. New conversion formula between B-type natriuretic peptide and N-terminal-pro-B-type Natriuretic peptide-analysis from a multicenter study. Circ J. 2022;86(12):2010–18. doi:10.1253/circj.CJ-22-0032. [Google Scholar] [PubMed] [CrossRef]
8. Daimon M, Watanabe H, Abe Y, Hirata K, Hozumi T, Ishii K, et al. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: the JAMP study. Circ J. 2008;72(11):1859–66. doi:10.1253/circj.CJ-08-0171. [Google Scholar] [PubMed] [CrossRef]
9. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(14):e698–800. [Google Scholar] [PubMed]
10. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82(4):388–400. doi:10.1038/ki.2012.131. [Google Scholar] [PubMed] [CrossRef]
11. Laurent S, Boutouyrie P. The structural factor of hypertension large and small artery alterations. Circ Res. 2015;116(6):1007–21. doi:10.1161/CIRCRESAHA.116.303596. [Google Scholar] [PubMed] [CrossRef]
12. O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50(1):1–13. doi:10.1016/j.jacc.2006.12.050. [Google Scholar] [PubMed] [CrossRef]
13. Shiina Y, Murakami T, Kawamatsu N, Niwa K. Aortopathy in adults with tetralogy of Fallot has a negative impact on the left ventricle. Int J Cardiol. 2017;228:380–4. doi:10.1016/j.ijcard.2016.11.252. [Google Scholar] [PubMed] [CrossRef]
14. Al-abcha A, Saleh Y, Mujer M, Boumegouas M, Herzallah K, Charles L, et al. Meta-analysis examining the usefulness of angiotensin receptor blockers for the prevention of aortic root dilation in patients with the Marfan syndrome. Am J Cardiol. 2020;128(7–8):101–6. doi:10.1016/j.amjcard.2020.04.034. [Google Scholar] [PubMed] [CrossRef]
15. Tan JL, Davlouros PA, McCarthy KP, Gatzoulis MA, Ho SY. Intrinsic histological abnormalities of aortic root and ascending aorta in tetralogy of Fallot: evidence of causative mechanism for aortic dilatation and aortopathy. Circulation. 2005;112(7):961–8. doi:10.1161/CIRCULATIONAHA.105.537928. [Google Scholar] [PubMed] [CrossRef]
16. Shiina Y, Niwa K. Cardio-ankle vascular index (CAVI) and plasma transforming growth factor-β1 (TGF-β1) level correlate with aortopathy in adults with repaired tetralogy of Fallot. Pediatr Cardiol. 2017;38(2):338–43. doi:10.1007/s00246-016-1519-z. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools