 Open Access
Open Access
REVIEW
Z-Score in Fetal Echocardiography–Is there Still Room for New Studies?
1 Department of Obstetrics, Paulista School of Medicine, Federal University of São Paulo (EPM-UNIFESP), São Paulo-SP, 04023-062, Brazil
2 Department of Obstetrics, Federal University of Ceará (UFC), Sobral-CE, 60020-181, Brazil
3 Department of Pediatrics, Pediatric Cardiology, School of Medicine, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro-RJ, 21941-853, Brazil
* Corresponding Author: Edward Araujo Júnior. Email:
Congenital Heart Disease 2024, 19(3), 305-314. https://doi.org/10.32604/chd.2024.053484
Received 01 May 2024; Accepted 26 June 2024; Issue published 26 July 2024
Abstract
Congenital heart disease (CHD) is the most common type of birth defect, representing a significant cause of perinatal morbidity and mortality. Early diagnosis of such anomalies is crucial for improving outcomes. Current protocols recommend a qualitative assessment of cardiac structures using two-dimensional ultrasound (2DUS) and color Doppler imaging. In cases of suspected abnormalities, quantitative assessments through cardiac structure measurements and reference curves can aid in accurate diagnosis. Similar to centiles widely employed in obstetrics, Z-scores provide more precise quantification of various cardiac structures, particularly at the extremes of the curve. While the development of reference curves and Z-scores has progressed over the past two decades, a lack of standardization in measurements and statistical methodology for their determination is evident. Establishing reference curves requires adherence to specific recommendations to improve their accuracy. The purpose of this study is to provide a narrative review of the major studies that have generated reference values for cardiac structures using 2DUS and Z-scores, to evaluate their methodology, and to provide a summary of the results.Keywords
Congenital heart disease (CHD) is the most common type of malformation at birth, having shown an increase in prevalence over the last few decades. Liu et al. [1] determined a prevalence of approximately 8.2 cases per 1000 births after conducting a systematic review covering more than 130 million births. CHD is still one of the main causes of perinatal morbidity and mortality. Prenatal diagnosis can improve the prognosis of CHD, enabling better birth planning in tertiary care centers including a multidisciplinary team. Unfortunately, this early diagnosis still occurs only in less than half of all cases [2].
In an attempt to improve prenatal diagnosis of CHD, several protocols have been published to guide and standardize appropriate fetal cardiac assessment [3–5]. These protocols recommend a qualitative assessment of cardiac structures using two-dimensional ultrasound (2DUS) with the aid of color Doppler. If an abnormality is suspected, quantitative assessments using measurements of cardiac structures, reference values and Z-scores can assist in the correct diagnosis.
Reference values are determined using regression analysis considering the studied cardiac structure as the dependent variable and a fetal biometric parameter or gestational age (GA) as independent variables. After finding the equation with the best correlation between these variables, reference tables and charts are created using centiles.
Z-scores are an alternative to using centiles. The Z-score is an index that represents how much a value is above or below the mean for a given population. The formula integrates the measurement, the predicted mean, and the standard deviation (SD) into a single number (Fig. 1). The Z-score has the advantage of accurate quantification, especially at the extremes of the distribution curve. It is also useful in research, as it allows more accurate comparisons between studies [6].

Figure 1: Formula of Z-score. The predicted mean is obtained from regression analysis, as is the standard deviation (SD). *Some authors use the root mean square error (RMSE) instead of the SD
This study aimed to provide a clear and objective narrative review of the main studies that determined Z-scores of various cardiac structures assessed by 2DUS. In addition to the results, an attempt was made to evaluate the methodology of the studies, highlighting their strengths and weaknesses.
2 Calculating Reference Curves and Z-Scores
According to the precepts proposed by Royston et al. [7], some recommendations and assumptions must be followed to determine centiles and Z-scores using regression analysis. A well-designed study should be cross-sectional, with each patient being examined once, with an equal number of patients in each gestational age range, and for each gestational age interval, the population must have a normal distribution.
The most used equations are derived from linear, polynomial (quadratic or cubic), or fractional polynomial regression. When the residuals do not present a normal distribution, or heteroscedasticity is present, the dependent variables may be subjected to a transformation before analysis-usually logarithmic. The regression model must account for changes in SD as GA increases. Data must be tested for normality and homoscedasticity of residuals through statistical tests (Shapiro-Wilk, e.g.), scatterplots, or histograms (Fig. 2) [8].
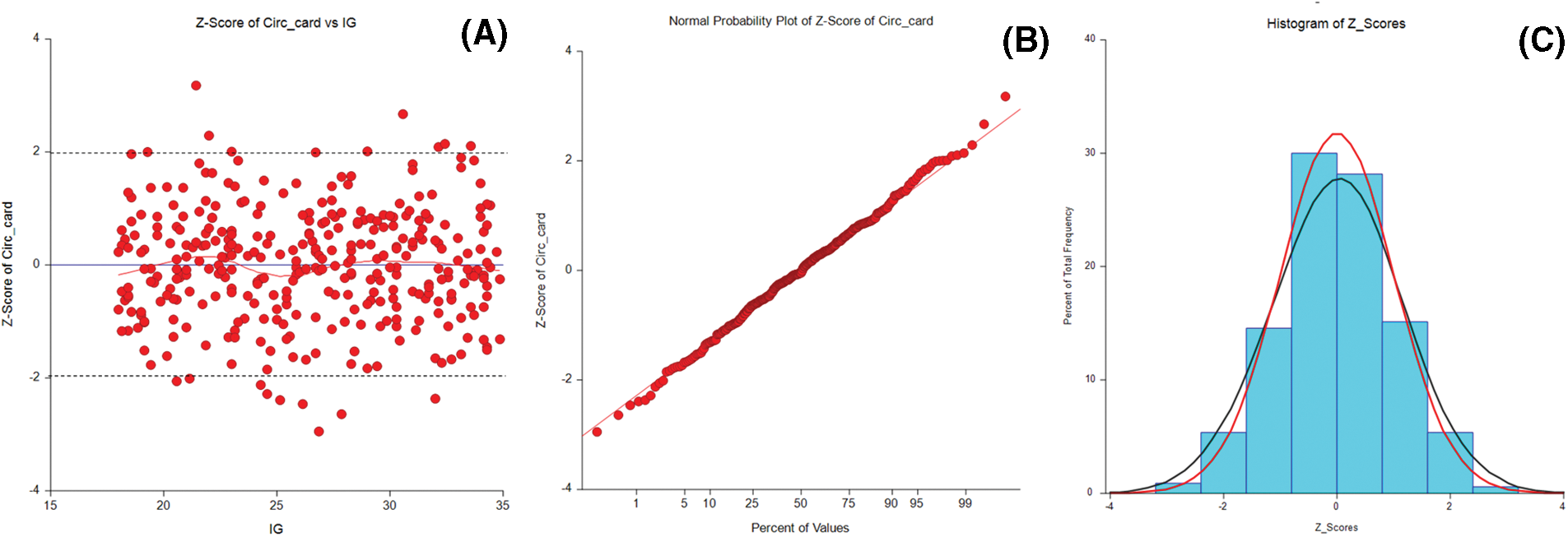
Figure 2: Graphs for qualitative assessment of data normality. (A) A scatterplot of the Z-score for gestational age against the cardiac circumference with tramlines at 1.96, 0, and –1.96. (B) Normal plot of Z-score from the fitted model for cardiac circumference. (C) The frequency of the Z-scores with an approximately normal distribution
3 Z-Score–A New Tool in Fetal Cardiac Assessment
Since 2005, several articles have been published determining the Z-scores of various cardiac parameters in different populations. Schneider et al. [6] conducted the first study to correlate Z-scores of cardiac structures with fetal biometric parameters. They evaluated 17 cardiac measurements as a function of GA, femur length (FL) and biparietal diameter (BPD), in 130 normal fetuses between 15 and 39 weeks of pregnancy. The fetal parameter that most correlated with cardiac measurements was FL, followed by BDP and GA. In this study, the best equation for this correlation was linear with logarithmic transformation (Ln). The authors considered that the use of Z-scores would provide a better assessment of the growth of structures in normal fetal hearts. This would allow for better interpretation in cases of congenital anomalies where the quantitative assessment of cardiac structures is important for diagnosing and planning treatment. The small sample size and the heterogeneous distribution of cases by gestational age interval were limitations of the study.
In 2007, aiming at specifically improve coarctation of the aorta (CoA) diagnosis, Pasquini et al. [9] created reference values with Z-scores for the aortic isthmus (AoI) and ductus arteriosus (DA) in the sagittal and three-vessel and trachea (3VT) planes, in pregnant women between 18 and 37 weeks. These values were correlated with FL and GA. After normalization of the data by logarithmic transformation, the regression equations had a coefficient of determination (R2) of 0.69 (AoI-3VT), 0.71 (DA) and 0.60 (AoI-sag) based on GA, and very close values based on FL. In addition to the measurements, a ratio between AoI and DA in the 3VT plane was calculated and found to be close to 1–0.99 (95% Confidence Interval 0.97–1.01)–regardless of the FL or GA value, with 95% of all values between 0.74 and 1.23. The authors argued that the use of this ratio could improve specificity in the diagnosis of CoA requiring postnatal surgery. The interobserver variability was tested using Bland-Altmann plots and found little variation between measurements.
A large retrospective study was carried out by Lee et al. [10] to determine cardiac Z-scores. The study involved 2735 fetuses between 17 and 41 weeks of pregnancy and evaluated cardiac measurements with the biometric parameters of FL, BPD, and GA. Simple linear regression was the best model found to relate cardiac measurements to fetal biometric variables. This study was the first to not assume a fixed SD, using regression analysis to determine its variation over GA. As a limitation of the study, the authors stated that echocardiography was not performed on newborns to prove the absence of CHD. In addition, a database was used with cases from the early 1990s, when the quality of the equipment was much lower than today; only 5 measurements were tested; no variability tests were performed; and the R2 of the equations was not reported.
The same group of researchers tried to determine the best screening parameter for hypoplastic cardiac lesions using the Z-score of the diameter of the left ventricle (LV), right ventricle (RV), aorta (Ao), pulmonary artery (PA); and the RV:LV and PA:Ao ratios [11]. A total of 90 fetuses with CHD were retrospectively studied and divided into two groups: the first with obstructive lesions on the right (n = 35) and the second with obstructive lesions on the left (n = 55). The Z-scores were compared with the Z-scores of the control group (2735 fetuses) determined in a previous study [10]. In group 1, the RV:LV ratio, RV Z-score, and PA:Ao ratio were the best parameters. In group 2, the Ao Z-score, PA:Ao ratio, and RV:LV were the best tests. The authors concluded that none of the evaluated parameters have high accuracy when analyzed alone; however, they could improve detection sensitivity when combined.
Li et al. [12] published a methodologically well-designed article constructing Z-score reference ranges of the fetal transverse heart diameter, heart length, cardiac circumference and area. They prospectively evaluated 809 Chinese pregnant women, homogeneously distributed from 11 to 40 weeks of gestation. Linear and third-degree polynomial regression were used to determine equations for mean and SD. They found high correlation coefficients between fetal heart measurements and fetal variables (BPD, FL, and GA). A group of 47 fetuses with CHD was used to validate the equations. They observed that in cases of Ebstein’s disease and fetuses with α-thalassemia, the Z-scores of the cardiac dimensions were increased. Reproducibility tests were not performed.
DeVore et al. [13] compared measurements of cardiac circumference and area obtained by using an ellipse or manual tracing in 200 fetuses between 20 and 40 weeks of pregnancy. The correlation coefficients between cardiac circumference and area and 7 independent variables ranged from 0.885 to 0.965 with no significant difference between manual tracing and ellipse. Based on these findings, the authors developed a calculator to evaluate cardiac circumference and area based on Z-scores; cardiomegaly was suspected when the Z-score was >+1.65 (which would correspond to the 95th centile).
In the same year, Krishnan et al. [14] published a study in which they determined Z-score reference values for 13 fetal cardiac structures between 12 and 39 weeks of pregnancy. They used a heterogeneous population (such as twin pregnancies, for example), retrospectively evaluating data stored in digital files. The GA showed the best correlation coefficients. The other parameters (BPD, FL, and estimated fetal weight-EFW) also showed good accuracy. Although GA ranged from 12 to 39 weeks, the distribution of cases was not uniform, with 90% of them between 18 and 34 weeks. During the regression analysis, no equations were determined for the SD; and the authors used the root mean square error (RMSE) to calculate the Z-score. Data transformation was required for most of the equations. The GA-based models had the highest R2, ranging from 0.893 (LV length) to 0.722 (right PA). As with most studies, the authors did not test the reproducibility of their measurements.
Mao et al. [15] studied the use of Z-scores for the dimensions of the left atrium (LA) and the distance between the LA and the descending Ao (DAo) in the diagnosis of fetuses with total anomalous pulmonary vein drainage (TAPVD). They evaluated 333 normal fetuses between 20 and 40 weeks of pregnancy by taking 6 measurements obtained by 2DUS-the reference values of Z-scores for these measures were determined using regression analysis. GA and biometric parameters served as independent variables. The authors subsequently validated these data in 10 fetuses diagnosed with TAPVD. The best formula for most measurements was simple linear regression, except for measuring the distance between the LA and DAo, which used quadratic regression. Eight of the 10 fetuses had a Z-score lower than –2 for the LA dimensions. All 10 fetuses had a Z-score greater than 2 for the distance between LA and DAo. These findings corroborated the use of these measurements’ Z-scores in the diagnosis of TAPVD.
A prospective study published in 2018 established reference ranges for the heart’s outflow tracts using the largest series to date. Vigneswaran et al. [16] evaluated the aortic valve (AoV), pulmonary valve (PV), distal transverse aortic arch (TAo), and DA in 7945 fetuses between 13 and 36 weeks of pregnancy. In this study, AoV and PV were measured during diastole while they were closed. All data had a normal distribution. To calculate Z-scores, the authors created regression formulas for mean and SD. Tables were created with reference values for the measurements and for TAo:DA ratio. No data on R2 or reproducibility assessment are available. As a limitation of the study, the authors reported the fact that the examined fetuses were referred for echocardiography for some reason (mostly due to increased nuchal translucency) and that the possibility that some of them could have a genetic disease cannot be ruled out.
Lussier et al. [17] established reference curves for 13 cardiac measurements and Z-scores in an Asian population. They assessed 575 Taiwanese pregnant women with GA between 14 and 38 weeks. GA, FL, BPD, abdominal circumference (AC), and head circumference (HC) were used as independent variables. The authors highlight the uniform distribution of cases across gestational age as a differential. Of the 65 equations, 63 were linear and 3 were quadratic. Data transformation was required for 10 equations. GA was the best predictor variable overall. Based on this data, centile graphs and nomograms were created for heart circumference based on the independent variables. The authors reinforce this as the first study to determine reference curves and nomograms for fetal echocardiographic parameters in a Taiwanese population. Comparing their results with other studies, they observed that the rate of growth of the structures is slower than in Caucasian populations.
Rocha et al. [18] created reference curves of percentiles and Z-scores of ventricular wall thickness and interventricular septum thickness in a Brazilian population. They assessed 873 cases with GA between 24 and 34 weeks. Only GA was used as an independent variable. Simple linear regression equations were determined. The authors found that all measurements increased slightly during pregnancy, probably due to the architectural development of myocardial structures. Table 1 provides a summary of the statistical methodology used in the cited studies.
4 Determination of Z-Scores of Doppler Velocimetric Parameters and Cardiac Function
A group of Canadian researchers carried out a study to determine reference values for measurements obtained using Doppler and M-mode. Gagnon et al. [20] evaluated 104 fetuses from singleton pregnancies taking 57 measurements including pulsed-wave Doppler (PWD) and M-mode measurements; and the calculation of systolic, diastolic, and global functions. They tested several regression formulas with GA as the independent variable. For most measures, they observed a non-linear relationship. Parametric normalization was successful for most parameters, making it possible to create Z-score equations with minimal residuals and a normal distribution. They concluded that some parameters did not yet have published formulas and that these equations could help echocardiographers identify early potential changes in cardiac function.
Mao et al. [21] determined Z-scores and reference values of PWD indices of the heart outflow tracts (AoV and PV) in normal fetuses. They evaluated 506 fetuses between 18 and 40 weeks of pregnancy, analyzing 12 PWD parameters. GA and EFW were considered independent variables for regression analysis. A strong correlation was observed between cardiac measurements and GA and EFW. A linear-quadratic regression model showed the best results for 11 out of the 12 parameters studied. The authors concluded that these data could help in the evaluation of fetuses with growth restriction and those with outflow tract malformations, although prospective studies would be necessary to validate these data.
Several studies evaluating Z-scores of fetal cardiac structures have already been published; however, few studies have evaluated cardiac function. Rocha et al. [22] carried out the first study on Brazilian pregnant women that determined reference values for cardiac function measurements and generated Z-score equations for all parameters. A total of 612 fetuses between 24 and 34 weeks of pregnancy were prospectively and cross-sectionally evaluated. Right (RCO), left (LCO), and combined cardiac output (CCO), mitral and tricuspid flow, inferior vena cava (IVC), and pulmonary vein flow were calculated. The study showed that cardiac output increased as GA progressed, with a predominance of RCO. No statistically significant difference was observed in the analysis of mitral and tricuspid flow, nor of the IVC and pulmonary veins considering GA. The Z-scores of cardiac function parameters could help monitor fetuses exposed to possible factors that could compromise cardiac function, such as growth restriction or gestational diabetes.
Luewan et al. [23] determined reference curves, centiles, and formulas for calculating cardiac output Z-scores in 700 fetuses of Thai pregnant women between 12 and 40 weeks of pregnancy. RCO, LCO, and CCO were evaluated as a function of GA and BPD. The authors noticed an exponential increase in variables as GA advanced, especially RCO. They evaluated fetuses with Bart’s hemoglobin disease, demonstrating an increase in cardiac output in the compensatory phase of the disease. Fetuses with growth restriction and Doppler changes tended to decrease cardiac output. They emphasize that the results from this study can help detect changes in cardiac function in fetuses with cardiomyopathy, cardiac anomalies, fetal anemia, and fetal growth restriction.
A study with a large sample (n = 7885) evaluated the inflow and outflow of the ventricles using PWD [24]. Flow measurements were taken through the mitral valve (MV), tricuspid valves (TV), AoV and PV in fetuses from 13 to 36 weeks of pregnancy. The regression analysis was performed with GA as the independent variable. Formulas were developed for aortic and pulmonary artery flow velocities, E wave and A wave velocities, mitral and tricuspid E/A ratio, and fetal heart rate. An R2 < 0.10 was obtained for all formulas. The distribution of cases by GA was a limitation of the study because >90% of cases were between 19 and 24 weeks of pregnancy. Furthermore, this was a retrospective study with cases spanning over 13 years.
5 Perspectives and Recommendations
Studies have shown that different populations and ethnicities can influence fetal growth [25,26]. Therefore, fetuses with the same GA may have different sizes within the same population and between different populations. This is one of the limitations of most of the studies mentioned above, as each study evaluated specific populations. Therefore, when analyzing the reference curves determined by Z-scores, it is essential to consider ethnic differences to ensure that these parameters are accurate and relevant for each population.
Another major limitation of the studies is the lack of standardization in their methodology: sample size; range of GA included in the study and distribution of the population over this range; structures to be measured and measurement technique; determination of independent variables; methodology of regression analysis; assessment of reliability of measurements by reproducibility test.
Moon-Grady et al. [27] conducted a feasibility study that could provide elements to guide a large multicenter study to develop reference values based on Z-scores of cardiac structures. Six experienced fetal cardiologists measured 53 structures in 72 sets of images. All the professionals received specific training to take part in the study. They were all instructed on which structures should be assessed and how to measure them. Some structures could not be assessed in up to 29% of cases. In addition, the interobserver intraclass correlation coefficient (ICC) for 2D measurements showed poor correlation (ICC < 0.50), especially in more advanced GA. This study provides a real insight into the difficulties that can be encountered when developing a multicenter study.
Despite these difficulties, a large multicenter study involving different populations and following strict standardization in the acquisition and performance of measurements, combined with a well-defined methodology and statistics, could generate a large database. Derived equations and graphs could be reliably used by a larger number of professionals and researchers.
Until these studies are available, professionals should use reference values derived from their population or use existing studies after validation.
The use of the Z-score is an important tool in the accurate and objective assessment of fetal cardiac measurements, which can help in the early detection of cardiac anomalies consequently improving clinical management. As with the use of centiles to evaluate fetal growth, Z-scores will likely be increasingly used as a way of evaluating cardiac structures and function. Its systematic use can significantly contribute to improving perinatal outcomes and the quality of care provided to patients and their families. Despite the many studies already published within this field, further and new research is needed. Future studies should respect statistical precepts and standardized measurement techniques to further improve the accuracy of regression equations, especially in cases of fetuses with different demographic characteristics or in specific clinical situations.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: Marcio Fragoso Vieira, Nathalie Jeanne Bravo-Valenzuela, Edward Araujo Júnior; data collection: Marcio Fragoso Vieira; manuscript preparation: Marcio Fragoso Vieira; proofreading: Nathalie Jeanne Bravo-Valenzuela, Edward Araujo Júnior. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data availability is not applicable to this article as no new data were created in this study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–63. doi:10.1093/ije/dyz009. [Google Scholar] [PubMed] [CrossRef]
2. Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, et al. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136(2):e378–85. doi:10.1542/peds.2014-3783. [Google Scholar] [PubMed] [CrossRef]
3. Carvalho JS, Axt-Fliedner R, Chaoui R, Copel JA, Cuneo BF, Goff D, et al. ISUOG practice guidelines (updatedfetal cardiac screening. Ultrasound Obstet Gynecol. 2023;61(6):788–803. doi:10.1002/uog.v61.6. [Google Scholar] [CrossRef]
4. AIUM practice parameter for the performance of fetal echocardiography. J Ultrasound Med. 2020;39(1):E5–16. [Google Scholar]
5. Moon-Grady AJ, Donofrio MT, Gelehrter S, Hornberger L, Kreeger J, Lee W, et al. Guidelines and recommendations for performance of the fetal echocardiogram: an update from the American society of echocardiography. J Am Soc Echocardiogr. 2023;36(7):679–723. doi:10.1016/j.echo.2023.04.014. [Google Scholar] [PubMed] [CrossRef]
6. Schneider C, McCrindle BW, Carvalho JS, Hornberger LK, McCarthy KP, Daubeney PEF. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26(6):599–605. doi:10.1002/uog.v26:6. [Google Scholar] [CrossRef]
7. Royston P, Wright EM. How to construct “normal ranges” for fetal variables. Ultrasound Obstet Gynecol. 1998;11(1):30–8. doi:10.1046/j.1469-0705.1998.11010030.x. [Google Scholar] [PubMed] [CrossRef]
8. DeVore GR. Computing the Z score and centiles for cross-sectional analysis: a practical approach. J Ultrasound Med. 2017;36(3):459–73. doi:10.7863/ultra.16.03025. [Google Scholar] [PubMed] [CrossRef]
9. Pasquini L, Mellander M, Seale A, Matsui H, Roughton M, Ho SY, et al. Z-scores of the fetal aortic isthmus and duct: an aid to assessing arch hypoplasia. Ultrasound Obstet Gynecol. 2007;29(6):628–33. doi:10.1002/uog.v29:6. [Google Scholar] [CrossRef]
10. Lee W, Riggs T, Amula V, Tsimis M, Cutler N, Bronsteen R, et al. Fetal echocardiography: z-score reference ranges for a large patient population. Ultrasound Obstet Gynecol. 2010;35(1):28–34. doi:10.1002/uog.7483. [Google Scholar] [PubMed] [CrossRef]
11. Riggs T, Saini AP, Comstock CH, Lee W. Comparison of cardiac Z-score with cardiac asymmetry for prenatal screening of congenital heart disease. Ultrasound Obstet Gynecol. 2011;38(3):332–6. doi:10.1002/uog.8989. [Google Scholar] [PubMed] [CrossRef]
12. Li X, Zhou Q, Huang H, Tian X, Peng Q. Z-score reference ranges for normal fetal heart sizes throughout pregnancy derived from fetal echocardiography. Prenat Diagn. 2015;35(2):117–24. doi:10.1002/pd.4498. [Google Scholar] [PubMed] [CrossRef]
13. DeVore GR, Tabsh K, Polanco B, Satou G, Sklansky M. A comparison between the point-to-point trace and automated ellipse methods between 20 and 40 weeks’ gestation. J Ultrasound Med. 2016;35(12):2543–62. doi:10.7863/ultra.16.02019. [Google Scholar] [PubMed] [CrossRef]
14. Krishnan A, Pike JI, McCarter R, Fulgium AL, Wilson E, Donofrio MT, et al. Predictive models for normal fetal cardiac structures. J Am Soc Echocardiogr. 2016;29(12):1197–206. doi:10.1016/j.echo.2016.08.019. [Google Scholar] [PubMed] [CrossRef]
15. Mao YK, Zhao BW, Zheng FH, Wang B, Peng XH, Chen R, et al. Z-scores for fetal left atrial size and left atrium-descending aorta distance in fetuses with isolated total anomalous pulmonary venous connection. Prenat Diagn. 2017;37(10):992–1000. doi:10.1002/pd.5119. [Google Scholar] [PubMed] [CrossRef]
16. Vigneswaran TV, Akolekar R, Syngelaki A, Charakida M, Allan LD, Nicolaides KH, et al. Reference ranges for the size of the fetal cardiac outflow tracts from 13 to 36 weeks gestation: a single-center study of over 7000 cases. Circ Cardiovasc Imaging. 2018;11(7):e007575. doi:10.1161/CIRCIMAGING.118.007575. [Google Scholar] [PubMed] [CrossRef]
17. Lussier EC, Yeh SJ, Chih WL, Lin SM, Chou YC, Huang SP, et al. Reference ranges and Z-scores for fetal cardiac measurements from two-dimensional echocardiography in Asian population. PLoS One. 2020;15(6):e0233179. doi:10.1371/journal.pone.0233179. [Google Scholar] [PubMed] [CrossRef]
18. Rocha LA, Zielinsky P, Nicoloso LHS, Araujo Junior E. Development of the Z-score for the measurement of myocardial thickness by two-dimensional echocardiography in normal fetuses. Echocardiography. 2021;38(1):97–102. doi:10.1111/echo.v38.1. [Google Scholar] [CrossRef]
19. Wu LH, Wang N, Xie HN, Du L, Peng R. Cardiovascular z-scores in fetuses with tetralogy of Fallot. Ultrasound Obstet Gynecol. 2014;44(6):674–81. doi:10.1002/uog.13419. [Google Scholar] [PubMed] [CrossRef]
20. Gagnon C, Bigras JL, Fouron JC, Dallaire F. Reference values and Z scores for pulsed-wave doppler and M-mode measurements in fetal echocardiography. J Am Soc Echocardiogr. 2016;29(5):448–60.e9. doi:10.1016/j.echo.2016.01.002. [Google Scholar] [PubMed] [CrossRef]
21. Mao YK, Zhao BW, Zhou L, Wang B, Chen R, Wang SS. Z-score reference ranges for pulsed-wave Doppler indices of the cardiac outflow tracts in normal fetuses. Int J Cardiovasc Imaging. 2019;35(5):811–25. doi:10.1007/s10554-018-01517-1. [Google Scholar] [PubMed] [CrossRef]
22. Rocha LA, Rolo LC, Nardozza LMM, Tonni G, Araujo Júnior E. Z-score reference ranges for fetal heart functional measurements in a large Brazilian pregnant women sample. Pediatr Cardiol. 2019;40(3):554–62. doi:10.1007/s00246-018-2026-1. [Google Scholar] [PubMed] [CrossRef]
23. Luewan S, Srisupundit K, Tongprasert F, Traisrisilp K, Jatavan P, Tongsong T. Z score reference ranges of fetal cardiac output from 12 to 40 weeks of pregnancy. J Ultrasound Med. 2020;39(3):515–27. doi:10.1002/jum.15128. [Google Scholar] [PubMed] [CrossRef]
24. Zidere V, Vigneswaran TV, Syngelaki A, Charakida M, Allan LD, Nicolaides KH, et al. Reference ranges for pulsed-wave doppler of the fetal cardiac inflow and outflow tracts from 13 to 36 weeks’ gestation. J Am Soc Echocardiogr. 2021;34(9):1007–16.e10. doi:10.1016/j.echo.2021.04.017. [Google Scholar] [PubMed] [CrossRef]
25. Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/Ethnic standards for fetal growth, the NICHD fetal growth studies. Am J Obstet Gynecol. 2015;213(4):449.e1–41. doi:10.1016/j.ajog.2015.08.032. [Google Scholar] [PubMed] [CrossRef]
26. Grantz KL, Hediger ML, Liu D, Buck Louis GM. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the world health organization multicentre growth reference study. Am J Obstet Gynecol. 2018;218(2):S641–55.e28. [Google Scholar]
27. Moon-Grady AJ, Lee H, Lopez L, Fatusin O, Freud LR, Hogan W, et al. Fetal echocardiographic Z score pilot project: study design and impact of gestational age and variable type on reproducibility of measurements within and across investigators. J Am Soc Echocardiogr. 2023;36(9):978–97. doi:10.1016/j.echo.2023.05.010. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF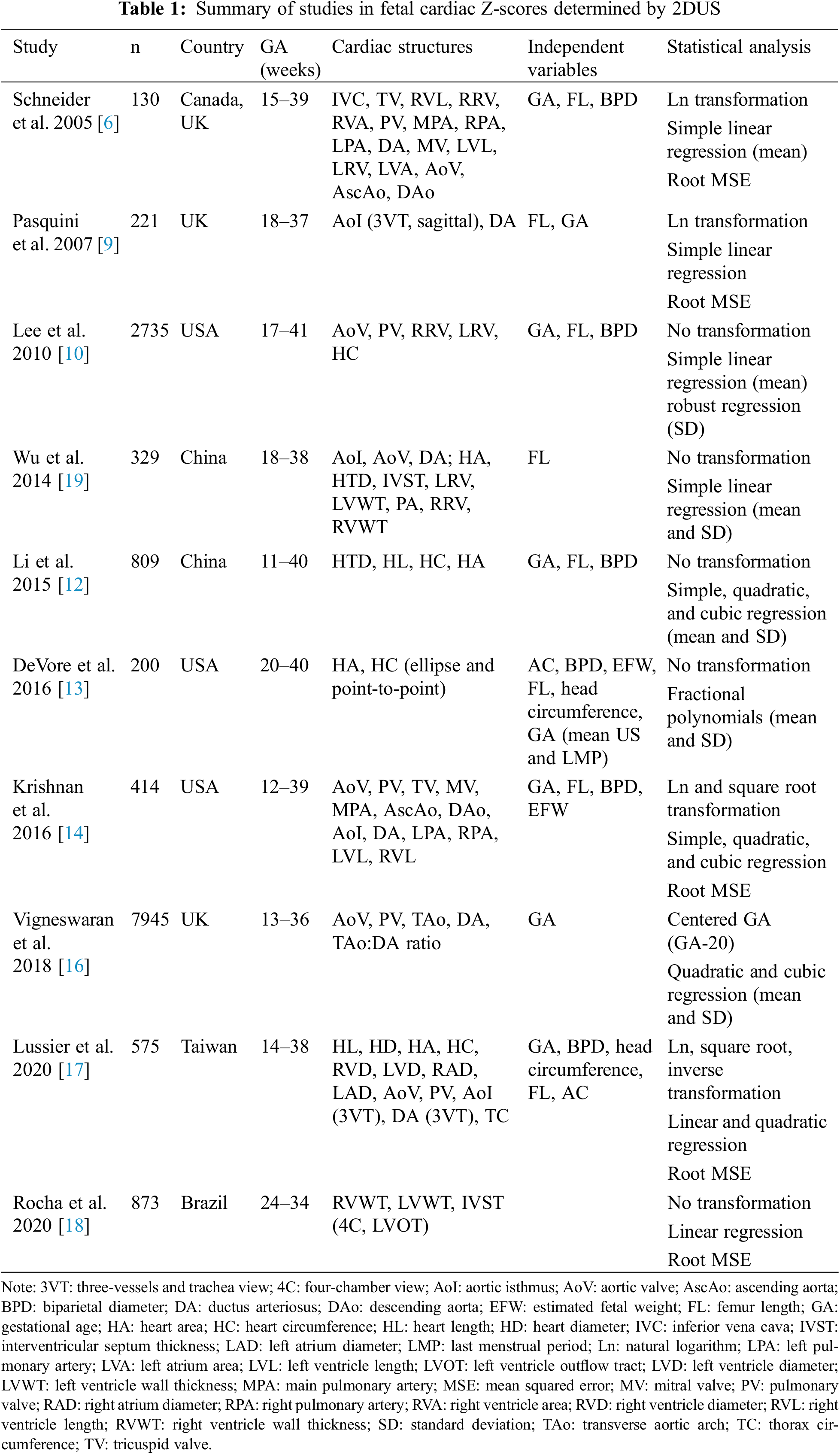
 Downloads
Downloads
 Citation Tools
Citation Tools