 Open Access
Open Access
ARTICLE
The Impact of a Prior Norwood Procedure on Cardiac Transplantation in Failed Fontan Physiology
1 Medical College of Wisconsin, Herma Heart Institute at Children’s Wisconsin, Milwaukee, USA
2 Division of Pediatric Cardiology, Medical College of Wisconsin, Herma Heart Institute at Children’s Wisconsin, Milwaukee, USA
3 Division of Pediatric Cardiothoracic Surgery, Medical College of Wisconsin, Herma Heart Institute at Children’s Wisconsin, Milwaukee, USA
* Corresponding Author: Ronald K. Woods. Email:
Congenital Heart Disease 2024, 19(3), 257-266. https://doi.org/10.32604/chd.2024.052108
Received 23 March 2024; Accepted 28 April 2024; Issue published 26 July 2024
Abstract
Objective: The objective of this study was to compare cardiac transplant operative and postoperative courses of patients with failed Fontan physiology who were initially palliated with a Norwood (FFN) to those without a prior Norwood (FF). Methods: A single-institution retrospective review of all patients with Fontan failure who underwent cardiac transplantation from 2003–2021 was completed—22 underwent prior Norwood (FFN) and 11 did not (FF). Descriptive and inferential statistics were calculated for operative course and patient outcomes. Results: The operative course of the FFN cohort appeared to be more complex (not statistically significant, but clinically relevant)—this group exclusively experienced sternal re-entry events (3 of 22 patients) and concomitant neo-aortic reconstruction (6 patients), had a longer duration of surgery (median of 682 min vs. 575.5 min), more time on circulatory arrest (median of 25.5 min vs. 12.5 min), and more frequent use of open sternal management [50% of patients (11/22) vs. 27.3% of patients (3/11)]. Postoperatively, these patients underwent more mediastinal explorations [other than sternal closure; 40.9% of patients (9/22) vs. 18.2% of patients (2/11)], spent more time on mechanical ventilation (median of 5 days vs. 2 days), had a longer length of stay (median of 30 days vs. 19 days), and required more catheter-based re-interventions [22.7% of patients (5/22) vs. 9.1% of patients (1/11)]. Conclusion: Although underpowered, our results suggest that the operative course of FFN patients is more challenging, based mostly on neo-aortic arch issues. In turn, this likely leads to a more complex postoperative course. We are currently collaborating with other institutions to increase the cohort size and power of the study.Keywords
Several authors have reported the challenges associated with cardiac transplantation of patients with failed Fontan physiology [1–4]. Some of the aforementioned challenges include the re-do nature of the surgery, the frequent need for complex reconstruction, and the unique anatomy of Fontan patients [1,2]. Two pathophysiological mechanisms have been described as underlying failed Fontan physiology—elevated pulmonary vascular resistance in the setting of preserved ventricular function and elevated pressures in the setting of ventricular dysfunction—ultimately leading to chronic right-sided heart failure and subsequent multi-organ system failure [3]. Despite the abundance of studies exploring the transplantation of Fontan patients, studies accounting for the differences in prior palliative surgeries are sparse.
It has been our observation that patients with failed Fontan physiology who originally underwent Norwood palliation pose even greater challenges than those who did not, mostly due to the nature of the post-Norwood ascending aorta (short, dilated, friable) and stents in the pulmonary arteries (PA). In light of these observations, we investigated whether this is indeed true based on objective perioperative data.
All patients with Fontan failure who underwent cardiac transplantation at Children’s Wisconsin between 2003 and 2021 were included in this study. Patients were divided into two groups, those who underwent a prior Norwood (FFN, n = 22) and those who did not undergo a prior Norwood (FF, n = 11). The Children’s Wisconsin Institutional Review Board determined this study to meet exempt criteria (ID: 1883417-1) and waiver of the Health Insurance Portability and Accountability Act was granted on May 13, 2022.
Patient information was obtained from the general medical and cardiology records, as well as our transplantation records and data submitted to the United Network for Organ Sharing (UNOS). De-identified patient information was collected utilizing a password-protected web-based server with Excel capability.
Patient charts were systematically reviewed for predetermined variables and outcomes. Donor demographics and the ejection fraction of donor hearts were taken from UNOS. Prior sternotomies were considered any entry into a closed sternum regardless of the time between procedures. Ventricular dysfunction was determined by the most immediate echocardiogram preceding cardiac transplantation, with mildly diminished or worse considered dysfunctional. Atrioventricular valve insufficiency was met if graded moderate or worse on echocardiogram. Pre-transplant circulatory support included patients who were taken to the operating room on ventricular assist devices. We intended to gather all panel reactive antibody (PRA) results immediately pre-transplant, however, for several patients, this was not possible and we only had access to PRAs that were taken months in advance. Moreover, we could not obtain PRA data for some patients in the earlier period of this cohort.
Intraoperative variables and outcomes were taken from the operative report, cardiopulmonary perfusion record, and anesthesia record. Any significant injury or event that occurred during re-entry into the sternum was considered a sternal re-entry event.
Post-operative variables and outcomes were attained from progress reports, post-operative procedure notes, and biopsy results. For this study, acute rejection was considered any grade of rejection within 1 year of transplant. Primary graft non-function (PGNF) was defined as the use of extra-corporeal membrane oxygenation (ECMO) within 48 h of transplant. Reoperations were defined as any operation that occurred post-transplant during transplant admission. Appropriate management (mediastinal exploration for simple removal of packing or vents), vacuum dressing changes, routine catheterizations, and sternal closure were not counted as a reoperation. Catheterizations were only counted if they included ballooning, stenting, or coiling of collaterals. Similarly, other procedures that did not involve the mediastinum were not counted with the exception of diaphragm plication and vocal fold injections. All patients who met the 1-year survival outcome had a last known alive date beyond 1-year post-transplant.
Medians and ranges were used to summarize continuous variables while counts and percentages were used for categorical variables. Wilcoxon rank-sum test and Fisher’s exact test were used to compare continuous variables and categorical variables, respectively, between different groups of patients. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). A threshold p-value of 0.05 was used to determine statistical significance.
3.1 Preoperative Characteristics
Table 1 outlines preoperative characteristics and demographics between the two groups. The only significant differences between the groups were their pre-operative cardiac diagnoses (p < 0.0001), as well as median age and height at transplant. Notably, the vast majority of the FFN cohort was diagnosed with hypoplastic left heart syndrome (95.5%), while the majority of FF patients were diagnosed with double inlet left ventricle (Fig. 1). Furthermore, 6 FFN patients (27.3%) had a PA stent pre-transplant, while only 1 FF patient (9.1%) had a PA stent pre-transplant (p = 0.378). The groups were similar with respect to sensitization, ventricular function, creatinine, and bilirubin.
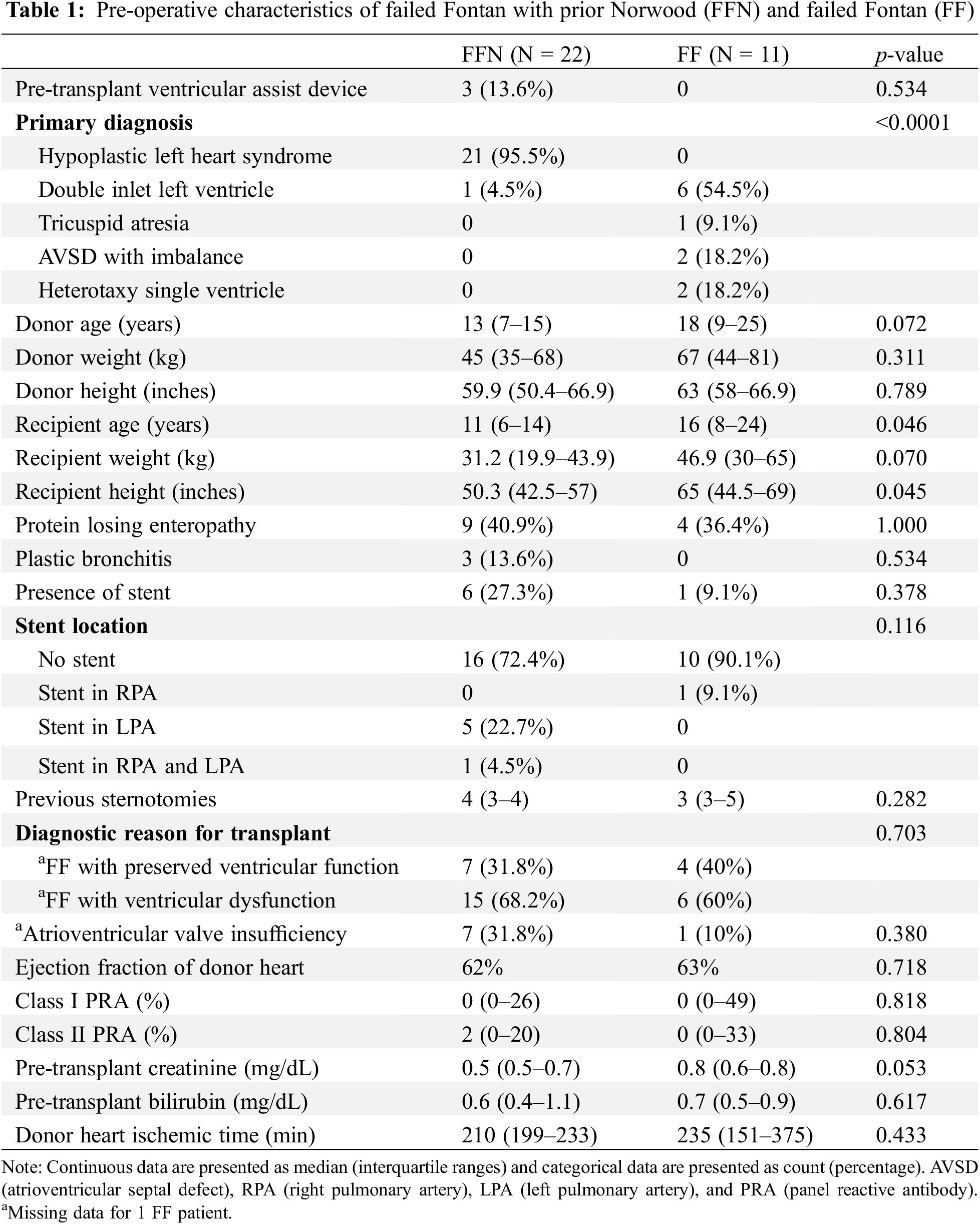
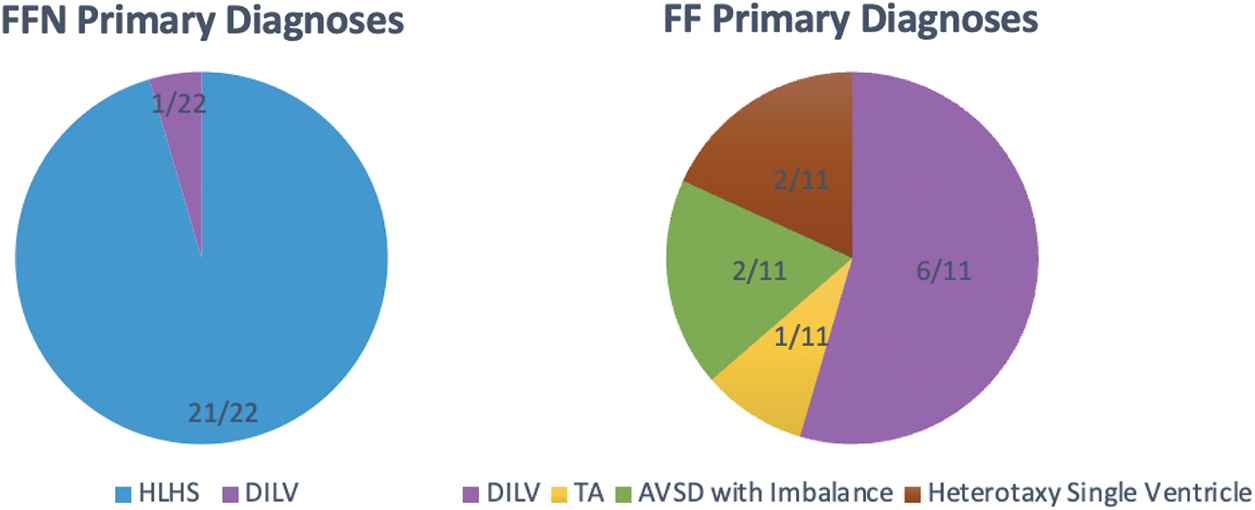
Figure 1: Primary diagnoses of FFN and FF patients. HLHS (hypoplastic left heart syndrome), DILV (double inlet left ventricle), TA (tricuspid atresia), AVSD (atrioventricular septal defect)
Table 2 demonstrates intraoperative data between the two groups. Compared to the FF group, the FFN group had a longer duration of surgery (median of 682 min vs. 575.5 min, p = 0.092) and required longer circulatory arrest times (median of 25.5 min vs. 12.5 min, p = 0.123). Moreover, the FFN cohort exclusively experienced sternal re-entry events [3 patients (13.6%) vs. 0 patients, p = 0.534] and concomitant neo-aortic reconstruction [6 patients (27.3%) vs. 0 patients, p = 0.077]. The sternal re-entry events consisted of a problem with the pacemaker that caused a drop in blood pressure, a drop in blood pressure that required cardiac massage, and direct aortic injury. Fig. 2 demonstrates the cannulation strategy for the two groups. The majority of patients were managed with central canulation (90.9% of FF patients and 81.8% of FFN patients), while a larger portion of FFN patients required initial peripheral cannulation or a combination of peripheral and central cannulation (18.1% vs. 9%, overall p = 0.583). It should be noted the above-mentioned results did not reach our threshold for statistical significance.
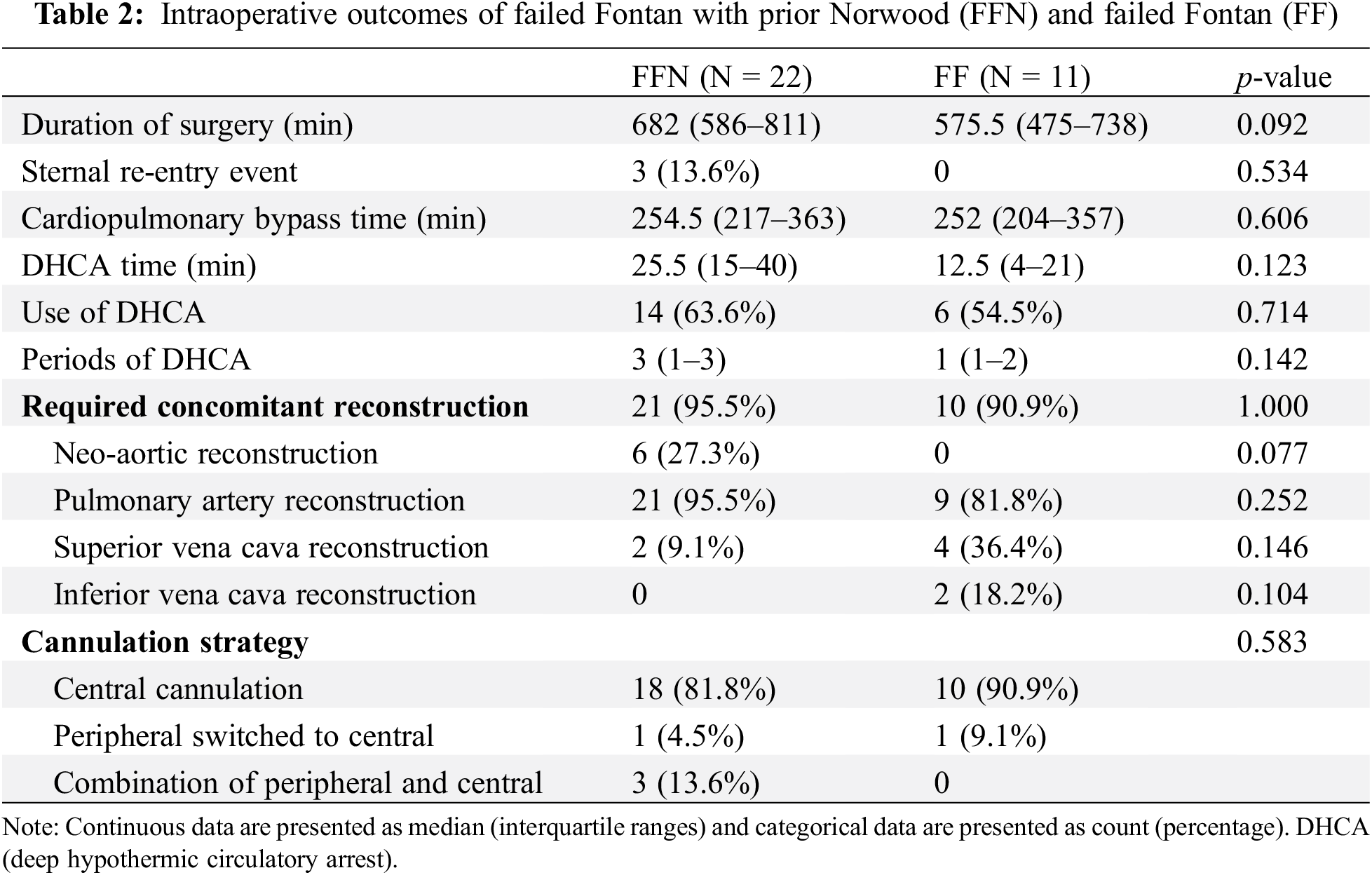
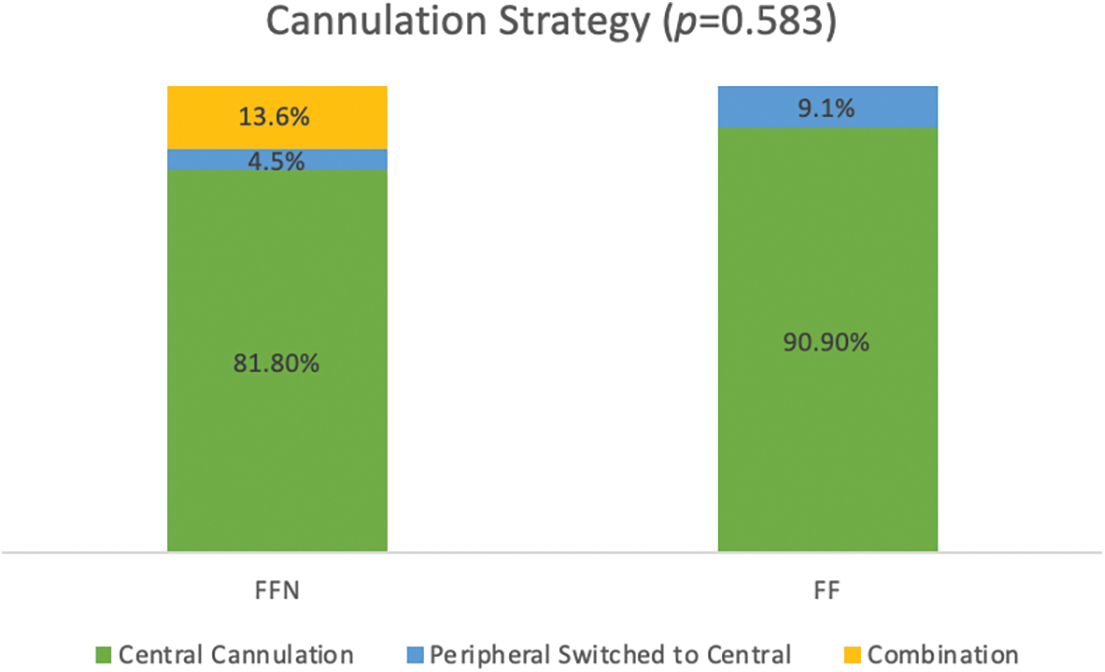
Figure 2: Distribution of intra-operative cannulation strategies between FFN and FF patients
Table 3 demonstrates similar discharge survival between groups. The causes of death for the three FFN patients were multi-organ failure and global hypoxic ischemic injury two weeks post-transplant, multi-organ failure and diffuse watershed injury 3 months post-transplant, and fungemia progressing to cardiogenic and septic shock 3.5 months post-transplant. In the FF group, one patient died due to severe end-organ dysfunction despite adequate flows on ECMO 2 weeks post-transplant and the other patient died of multi-organ failure in the context of septic shock 3.5 months following transplant.
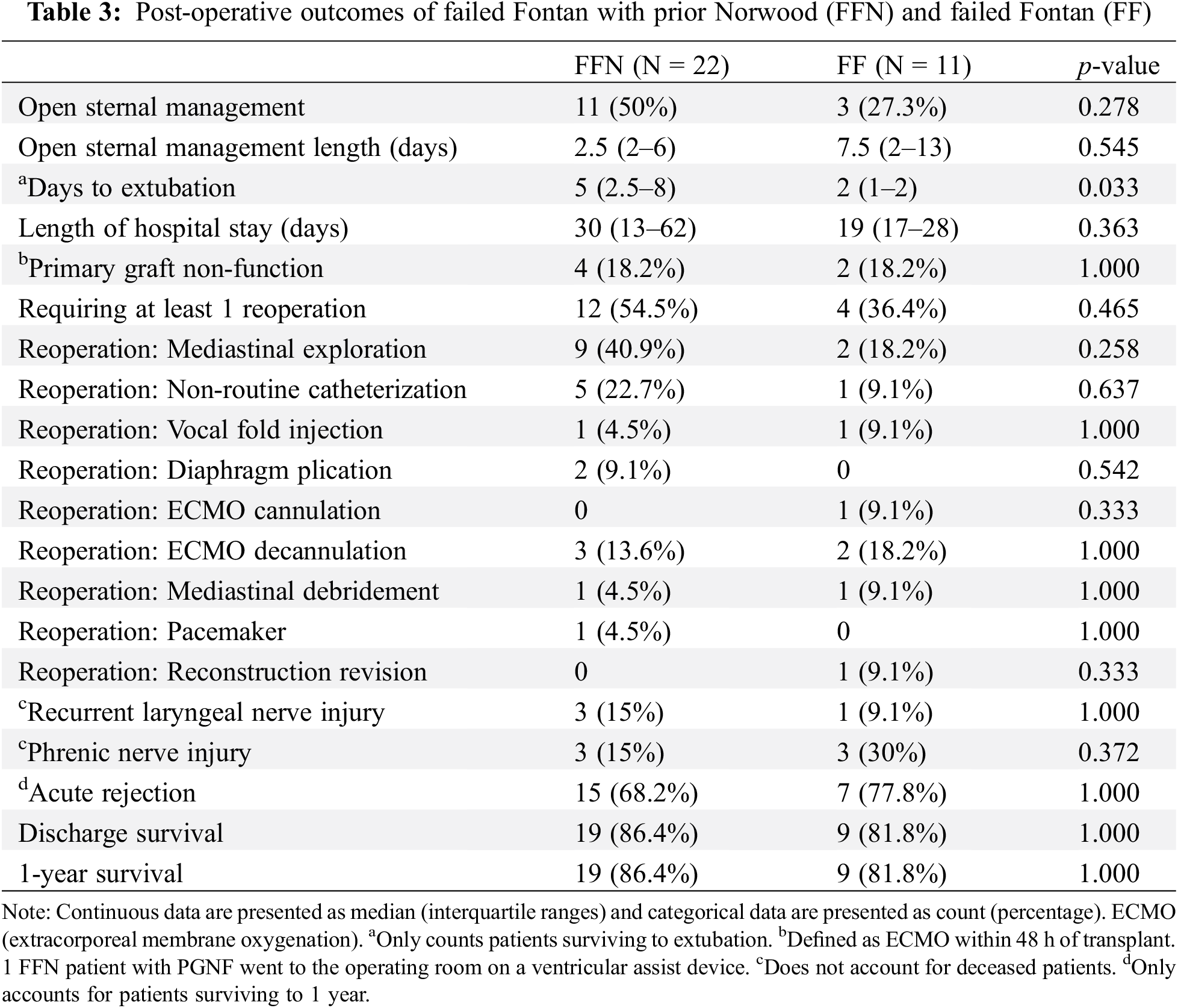
Figs. 3 and 4 show a graphical representation of post-operative management and morbidity trends. The FFN cohort experienced a longer duration of mechanical ventilation post-operatively (median of 5 days vs. 2 days, p = 0.033). While not statistically significant the FFN cohort demonstrated more use of open sternal management [11 patients (50%) vs. 3 patients (27.3%), p = 0.278] and longer length of stay (median of 30 days vs. 19 days, p = 0.363). Additionally, more patients in the FFN cohort required at least 1 reoperation [12 patients (54.5%) vs. 4 patients (36.4%), p = 0.465]—a larger number of FFN patients specifically required mediastinal exploration [9 patients (40.9%) vs. 2 patients (18.2%), p = 0.258] and non-routine catheterization [5 patients (22.7%) vs. 1 patient (9.1%), p = 0.637].
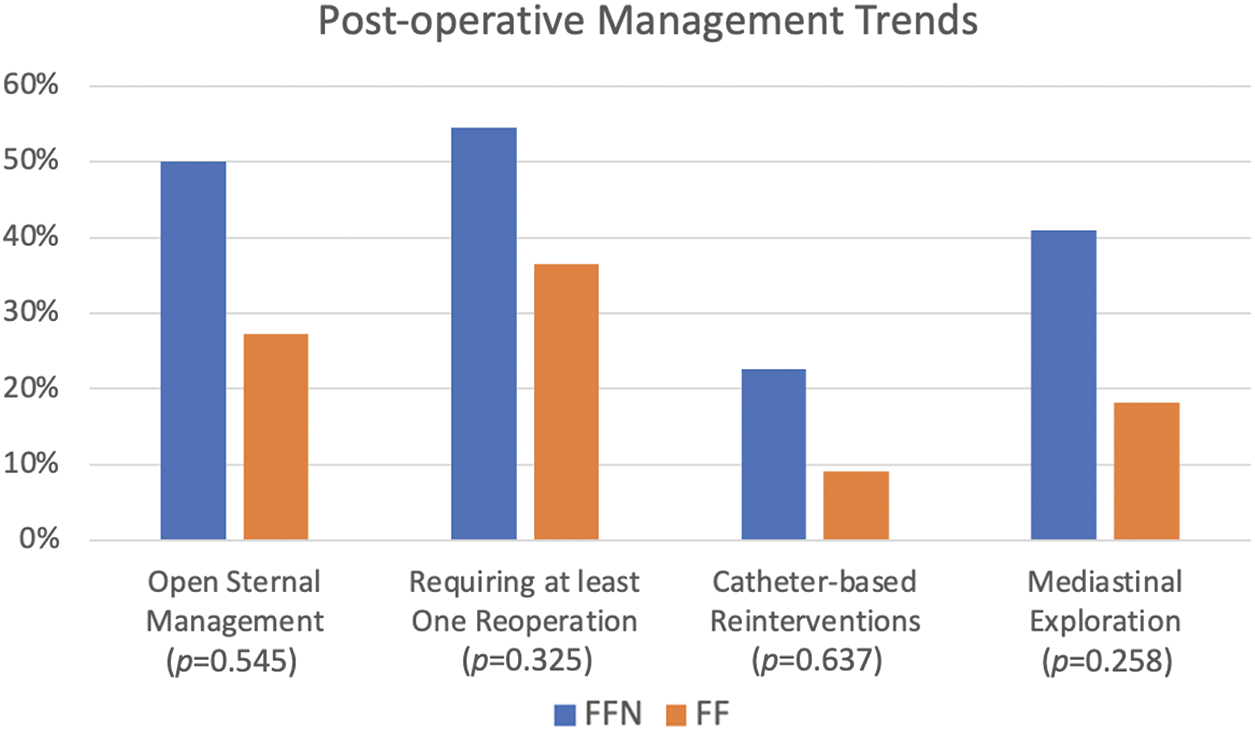
Figure 3: Comparison of post-operative management between FFN and FF patients
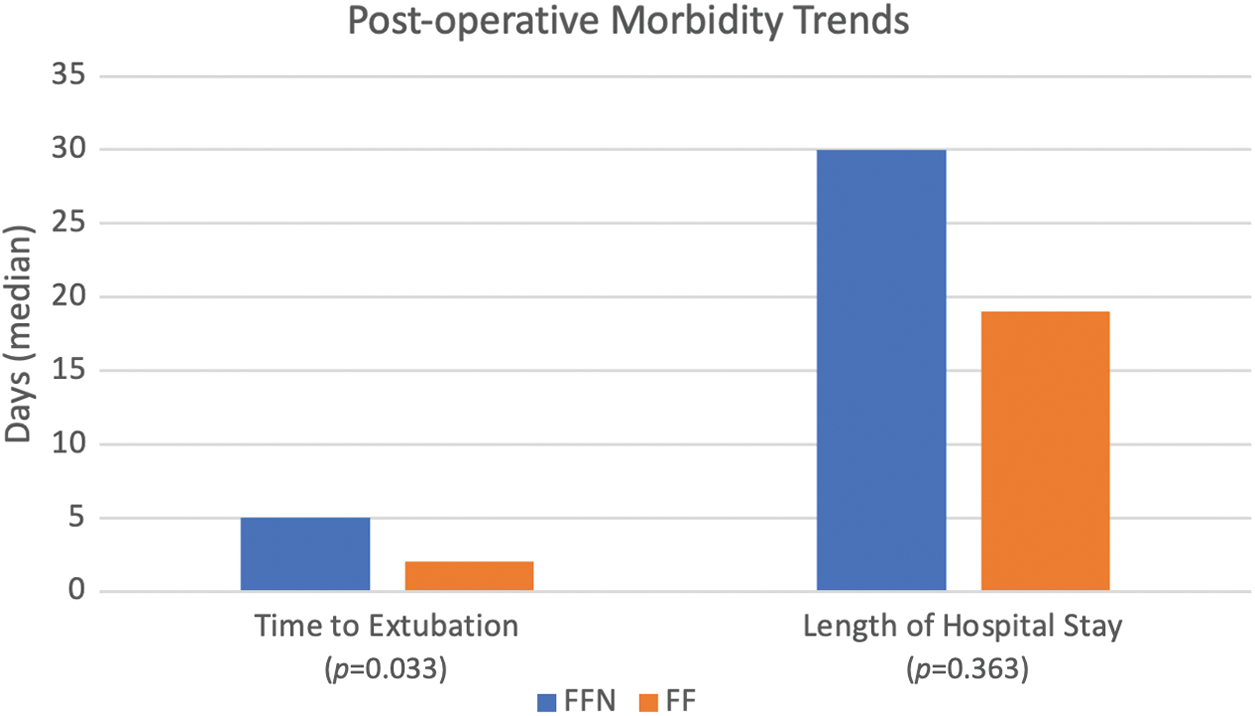
Figure 4: Comparison of post-operative morbidity trends between FFN and FF patients
Many studies have categorized the evolution and impact of Fontan physiology over the last several decades [5–8]. In the recent era, the number of Fontan cardiac transplants has been increasing, primarily due to an increase in HLHS patients [9]. Despite known challenges to transplantation in Fontan patients, one-year post transplant survival has been approaching rates similar to non-Fontan congenital heart disease transplant patients (89% vs. 91%, respectively) [10]. Additionally, waitlist survival for congenital heart disease patients has increased over recent decades to 84% at six months and 80% at one year [11]. While these trends are promising, it is been suggested that operative mortality during cardiac transplantation of patients with failed Fontan physiology may be as much as 30% higher than other congenital heart disease transplant patients [12].
Experts have advocated for a more granular approach to listing pediatric patients for cardiac transplantation [13]. Moreover, contemporary risk stratification of pediatric cardiac transplantation patients does not account for several factors that most surgeons deem clinically relevant [14]. We also believe that even within the specific subset of congenital heart disease patients, namely Fontan patients, there are important differences and risks, particularly regarding the types of prior palliation. Therefore, we undertook this study to explore this topic in more detail, focusing on the issue of a prior Norwood (FFN).
We found that FFN patients had longer operative times; longer circulatory arrest times; and exclusively experienced sternal reentry events and concomitant neo-aortic/aortic arch reconstruction. This patient group was also associated with more frequent open sternal management, the need for reoperation, longer times on mechanical ventilation, and longer lengths of stay.
The explanation for these findings is likely multifactorial, but we believe the dominant issue is the post-Norwood neo-aorta. In general, these aortas tend to be dilated, sometimes quite aneurysmal, and have a very short ascending segment which limits room for cannulation and clamping. Tissue quality can also be an issue–friability as well as calcification, both of which can preclude establishing a safe, reliable suture line. The need to resect most if not all of the ascending aorta in some of these patients explains the higher percentage of concomitant ascending aorta/arch reconstruction. Finally, dense adherence of a dilated, thin-walled aorta to the posterior sternal table can pose challenges to re-entry.
While similar in both groups, PGNF (defined as being placed on ECMO within 48 h of transplant) was much higher compared to non-Fontan transplant patients at our institution (18% vs. approximately 7%–8%). In the non-Fontan group, we have not seen PGNF with donor ischemic times under four hours. However, the median times for the entire Fontan cohort were less than four hours. As this was not the focus of the current study, we can only speculate on contributory factors–systemic to pulmonary artery collateral burden; blood and product transfusion. We do believe it is an important finding, though, and merits further investigation.
Based solely on clinical impression (which prompted the initiation of this study), three years ago we modified our operative strategy in an effort to mitigate many of the issues described in this study. We have been more liberal in our use of peripheral cannulation strategies (arterial chimney grafts to the carotid or femoral arteries), as well as our use of circulatory arrest or low flow CPB, especially when dissecting out the neo-aorta. However, we are very careful in the manner in which we conduct circulatory arrest. We limit it to periods of approximately 10 min with 2–3 min of interposed full reperfusion, and utilize oximetry (near-infrared spectroscopy) to avoid the anaerobic threshold. We also chose to cut across and filet the PA stents during hilum-to-hilum reconstruction, where in the past we would attempt to carefully remove stents that left friable tissue prone to bleed after the reconstruction [15]. Although it is our informal impression that this approach is working better than our traditional approach, we do not yet have sufficient data to permit such an analysis. It is conceivable, however, that some of the results of this study (cannulation strategy, use of DHCA, CPB times, etc.) may be influenced by these changes.
This study was underpowered, hence descriptive univariable statistics only, and failed to meet statistical significance for many outcomes. It must be acknowledged that due to the lack of statistical significance, our findings may be related to chance. However, we still regard many of the results as clinically relevant and believe further investigation is warranted. Surgeon bias in specific case strategy likely existed in some form and the potential impact of patient selection bias in cardiac transplantation remains unclear. It is also worth mentioning the lack of data regarding blood product administration and collateral flow. The cohort spans a long period of time during which clinical practice likely changed. A low number of patients precluded a rigorous analysis of this issue. We will attempt to overcome some of these limitations in the future by collaborating with other institutions to expand the cohort size.
Our preliminary findings suggest that cardiac transplantation of Fontan patients with a prior Norwood is associated with notable differences in operative and postoperative morbidity as well as resource utilization. A more rigorous analysis of a much larger cohort is needed to confirm or refute these findings.
Acknowledgement: We would like to thank the patients included in this study for making this research possible and the organ donors, for their selfless gift of life.
Funding Statement: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T35HL072483. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ryan G. McQueen, Ronald K. Woods data collection: Ryan G. McQueen, Nikki M. Singh analysis and interpretation of results: Ryan G. McQueen, Ronald K. Woods draft manuscript preparation: Ryan G. McQueen, Nikki M. Singh, Ronald K. Woods. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request with appropriate permissions.
Ethics Approval: The Children’s Wisconsin Institutional Review Board determined this study to meet exempt criteria (ID: 1883417-1) and waiver of the Health Insurance Portability and Accountability Act was granted on May 13, 2022.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Mauchley DC, Mitchell MB. Transplantation in the Fontan patient. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18:7–16. doi:10.1053/j.pcsu.2015.01.001. [Google Scholar] [CrossRef]
2. McCormick AD, Schumacher KR. Transplantation of the failing Fontan. Transl Pediatr. 2019;8:290–301. doi:10.21037/tp.2019.06.03. [Google Scholar] [PubMed] [CrossRef]
3. Kirklin JK, Pearce FB, Dabal RJ, Carlo WF Jr, Mauchley DC. Challenges of cardiac transplantation following the Fontan procedure. World J Pediatr Congenit Heart Surg. 2017;8:480–6. doi:10.1177/2150135117714. [Google Scholar] [PubMed] [CrossRef]
4. Gil-Jaurena JM, Pardo C, Pita A, Pérez-Caballero R, Zamorano J, Camino M, et al. Technical modifications for transplant in the failing Fontan. Cardiol Young. 2021;31(3):400–5. doi:10.1017/S104795112000414X. [Google Scholar] [PubMed] [CrossRef]
5. Kutty S, Jacobs ML, Thompson WR, Danford DA. Fontan circulation of the next generation: why it’s necessary, what it might look like. J Am Heart Assoc. 2020;9(1):e013691. doi:10.1161/JAHA.119.013691. [Google Scholar] [PubMed] [CrossRef]
6. Poh CL, d’Udekem Y. Life after surviving Fontan surgery: a meta-analysis of the incidence and predictors of late death. Heart Lung Circ. 2018;27(5):552–9. doi:10.1016/j.hlc.2017.11.007. [Google Scholar] [PubMed] [CrossRef]
7. Mazza GA, Gribaudo E, Agnoletti G. The pathophysiology and complications of Fontan circulation. Acta Biomed. 2021;92(5):e2021260. [Google Scholar] [PubMed]
8. Kverneland LS, Kramer P, Ovroutski S. Five decades of the Fontan operation: a systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. 2018;13(2):181–93. doi:10.1111/chd.2018.13.issue-2. [Google Scholar] [CrossRef]
9. Serfas JD, Thibault D, Andersen ND, Chiswell K, Jacobs JP, Jacobs ML, et al. The evolving surgical burden of Fontan failure: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. 2021;112(1):179–87. doi:10.1016/j.athoracsur.2020.05.174. [Google Scholar] [PubMed] [CrossRef]
10. Simpson KE, Pruitt E, Kirklin JK, Naftel DC, Singh RK, Edens RE, et al. Fontan patient survival after pediatric heart transplantation has improved in the current era. Ann Thorac Surg. 2017;103(4):1315–20. doi:10.1016/j.athoracsur.2016.08.110. [Google Scholar] [PubMed] [CrossRef]
11. Richmond ME, Conway J, Kirklin JK, Cantor RS, Koehl DA, Lal AK, et al. Three decades of collaboration through the pediatric heart transplant society registry: a journey through registry data with a highlight on children with single ventricle anatomy. Pediatr Transplant. 2024;28(1):e14615. doi:10.1111/petr.v28.1. [Google Scholar] [CrossRef]
12. Bearl DW. Can the pediatric heart transplant waitlist please get more granular? Pediatr Transplant. 2023;27(6):e14568. doi:10.1111/petr.v27.6. [Google Scholar] [CrossRef]
13. Chowdhury UK, George N, Sankhyan LK, Pradeep D, Chittimuri C, Chauhan A, et al. Fontan failure: phenotypes, evaluation, management, and future directions. Cardiol Young. 2022;32(10):1554–63. doi:10.1017/S1047951122001433. [Google Scholar] [PubMed] [CrossRef]
14. Woods RK, Kirklin JK, Maeda K, Adachi I. We need better pediatric cardiac transplantation risk modeling. J Thorac Cardiovasc Surg. 2022;164:2036–9. doi:10.1016/j.jtcvs.2021.12.046. [Google Scholar] [PubMed] [CrossRef]
15. Woods RK, Mitchell ME. Cardiac transplantation of the Fontan patient with a prior Norwood procedure. Oper Tech Thorac Cardiovasc Surg. 2023;28:35–46. doi:10.1053/j.optechstcvs.2022.07.004. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools