 Open Access
Open Access
CASE REPORT
Fate of Right Coronary Artery Occlusion after a Surgically Repaired Aorto-Ventricular Tunnel in a Neonate
1 Department of Pediatric Cardiac Surgery, Hôpital Timone enfant, Assistance Public-Hopitaux Marseille, Aix-Marseille Université, Marseille, France
2 Department of Cardiothoracic Surgery, Tanta University, Tanta, Egypt
3 Department of Pediatric Cardiology, Hôpital Timone enfant, Assistance Public-Hopitaux Marseille, Aix-Marseille Université, Marseille, France
4 Department of Neurology, Hôpital Timone enfant, Assistance Public-Hopitaux Marseille, Aix-Marseille Université, Marseille, France
* Corresponding Author: Marien Lenoir. Email:
Congenital Heart Disease 2024, 19(3), 267-273. https://doi.org/10.32604/chd.2024.051642
Received 11 March 2024; Accepted 17 May 2024; Issue published 26 July 2024
Abstract
The aorto-ventricular tunnel is a rare congenital cardiac anomaly. We present a case of aorto-ventricular tunnel diagnosed via fetal echocardiography. Emergency surgery was performed on the 2nd day of life to close the tunnel, located just in front of the right coronary ostium, due to the patient’s unstable health condition. The postoperative period revealed complete occlusion of the right coronary artery. Due to the patient’s stability, we opted not to reintervene on the right coronary artery. The patient fully recovered without the need for further coronary intervention. In cases of patients with an aorto-ventricular tunnel (AVT) and associated coronary lesions, it is crucial to exercise caution when intervening in the coronary arteries.Keywords
Nomenclature
| AVT | Aorto-ventricular tunnel |
| STJ | Sino-tubular junction |
| LVEF | Left ventricle ejection fraction |
| RCA | Right coronary artery |
| ECG | Electrocardiogram |
| TTE | Transthoracic echocardiography |
| CPAP | Continuous positive airway pressure |
| PDA | Patent ductus arteriosus |
| LCA | Left coronary artery |
| RCA | Right coronary artery |
Aorto-ventricular tunnel (AVT) is a highly uncommon congenital cardiac malformation among newborns, occurring in approximately 0.1% of congenital heart disease cases [1] and the incidence of congenital heart disease is estimated between 7 to 8 per 1000 births. Typically, the tunnel originates above the Sino-tubular junction (STJ), bypasses the hinge of the aortic valve, and terminates in the left ventricle, with rare instances of termination in the right ventricle. The right coronary sinus is the most frequently affected site of the AVT [2–5]. Patients with AVT typically present with symptoms of congestive heart failure during the neonatal period and within the first year of life, which is attributed to volume overload and stress on the myocardium transitioning from fetal to postnatal circulation. Prenatal echocardiography commonly reveals left ventricular dilatation [5]. Associated lesions have been reported [1], including bicuspid aortic valve, aortic valve dysplasia, ventricular septal defect, atrial septal defect, patent ductus arteriosus, pulmonary valve stenosis, and coronary anomalies.
We present a case of early surgical closure of AVT, with postoperative angiography revealing occlusion of the right coronary artery (RCA), which subsequently fully recovers without the need for coronary intervention. Informed consent was granted by the parents to publish this case report.
An antenatal follow-up (second trimester) by fetal echocardiography showed AVT with signs of global hypokinesia and dilatation of the left ventricle. The left ventricular function improved in the 3rd trimester with left ventricle ejection fraction (LVEF) 55%–66%. The mother had a normal delivery at 38 weeks with a neonatal birth weight of 3030 g.
The infant’s condition was unstable, requiring continuous positive airway pressure (CPAP), with oxygen saturation maintained at 99% while breathing room air. Chest X-ray was marked by cardiomegaly. On electrocardiogram (ECG), the rhythm was sinus with trouble of repolarization; ST elevation at L(I)-aVL, ST depression at V2: V5.
Transthoracic echocardiography (TTE) revealed several findings: a patent ductus arteriosus (PDA); an aorto-ventricular tunnel (AVT) located at the right coronary sinus, with an aortic end diameter measuring 7.4 mm and left ventricle (LV) end diameter measuring 4.7 mm (in and out); a bicuspid aortic valve without aortic valve regurgitation; a dilated left coronary ostium, with the diameter of the left main coronary artery (LCA) measuring 3.53 mm (Fig. 1); the right coronary artery (RCA) was not visualized; normal mitral valve morphology and annular diameter; a dilated LV, with a left ventricular end-diastolic diameter (LVEDd) measuring 26.8 mm (Z score 4.09), and an interventricular septum thickness of 4.65 mm (Z score 2.77). Additionally, there was left ventricular dysfunction with a left ventricular ejection fraction (LVEF) of 31%. Notably, there was flow reversal in the abdominal aorta pulsed wave Doppler, indicating diastolic runoff at 0.2 m/s.
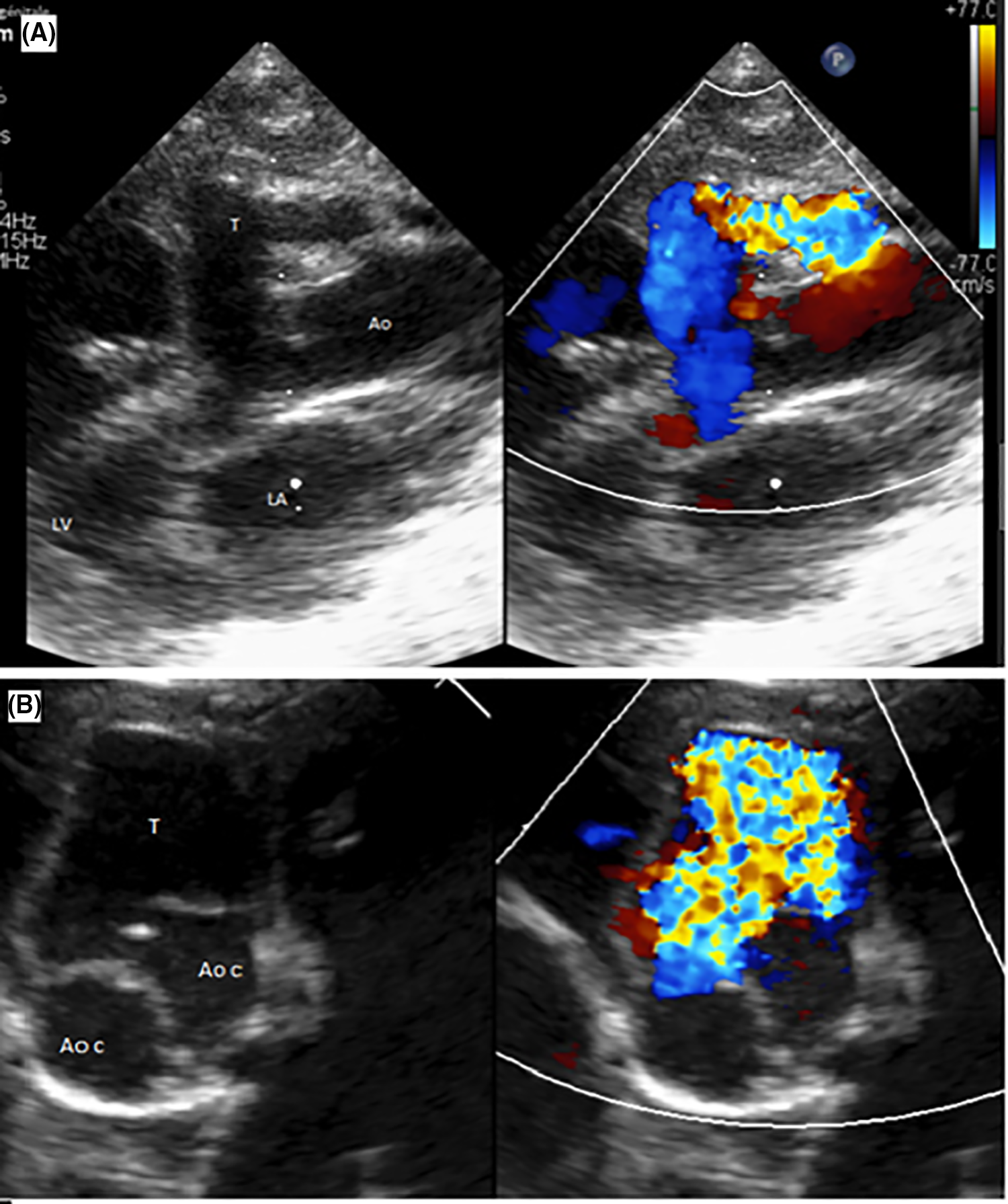
Figure 1: Transthoracic echocardiography: Aortic regurgitation through the tunnel. (A). Parasternal long-axis view showing the tunnel with its aortic and left ventricular orifices (T: tunnel; Ao: Aorta; LV: left ventricle; LA: left atrium). (B). Parasternal short axis view showing the relationship of the tunnel with the aortic valve (T: tunnel; Ao c: Aortic cusp)
Emergency surgery was done on the 2nd day of life for left ventricle dysfunction. After median sternotomy, we could see the aneurysmal dilatation at the site of the tunnel at the aortic root anteriorly, with small RCA (Fig. 2) and a PDA which we closed with a clip. After cardiopulmonary bypass, cross-clamping, and opening the aorta, we used selective cardioplegia first in the LCA then the RCA was very difficult to access as the ostium was very small, we could canulate it after multiple trials. We found a bicuspid aortic valve, AVT which we closed by bovine pericardial patch with 6.0 prolene below the level of the facing right coronary ostium. After the closure of the aorta and removal of the clamp, the heart started beating spontaneously in a sinus rhythm. Weaning off bypass was smooth under a small dose of adrenaline and milrinone. The transesophageal echocardiography revealed no dyskinesia, no gradient over the mitral valve, no aortic regurgitation, no aliasing across the left ventricular outflow tract, LCA well visible and dilated, and no residual shunt.
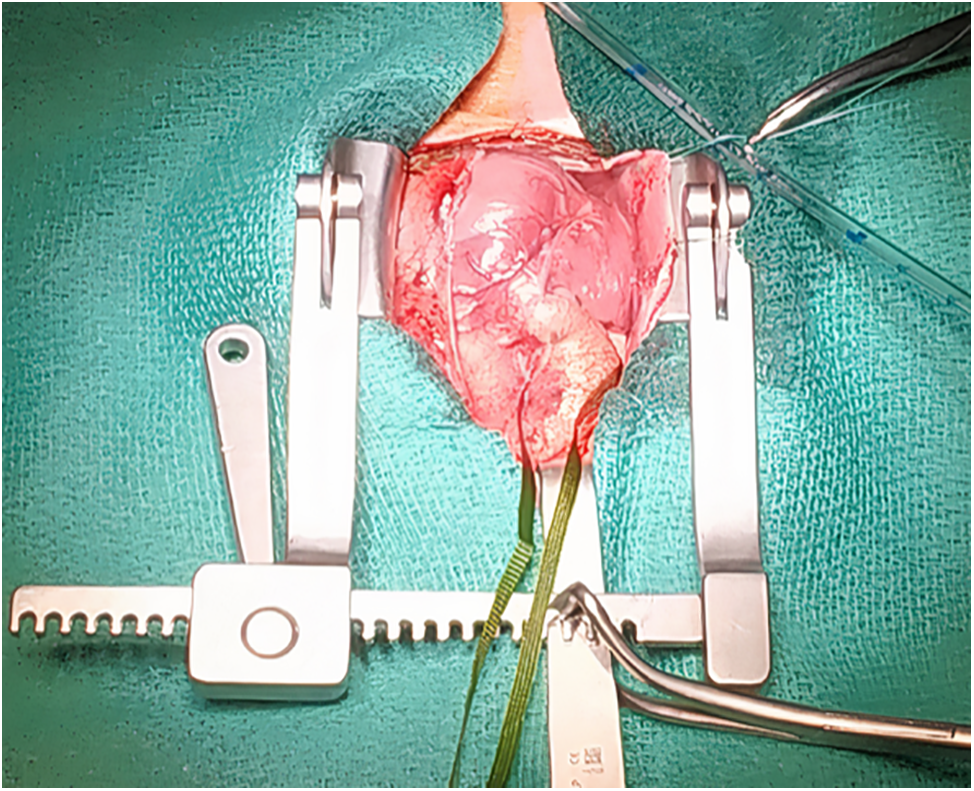
Figure 2: Intraoperative photo before cardiopulmonary bypass showed aneurysmal dilatation at the site of aorto-ventricular tunnel
On arrival to the intensive care unit, the echocardiography showed reduced LV function, septal dyskinesia, and apical hypokinesia with well-functioning anterolateral wall, LCA was well visible and well-perfused and right ventricle (RV) function was reduced with free wall hypokinesia. The troponin level was gradually rising and by postoperative day 2 it reached 3415 mg/l (Fig. 3A). We decided to do coronary angiography. We found a dilated left coronary ostium and trunk and occluded RCA with no anterograde flow (Fig. 3B). We decided not to intervene, as the clinical course of the patient was always stable and improving. RV function was improving slowly while troponin kept going up reaching 7100 mg/l at postoperative day 4. From day 5 the troponin level kept decreasing, with successful extubation under CPAP with a small dose of adrenaline and milrinone. At day 14, the global function of RV and LV was normal.
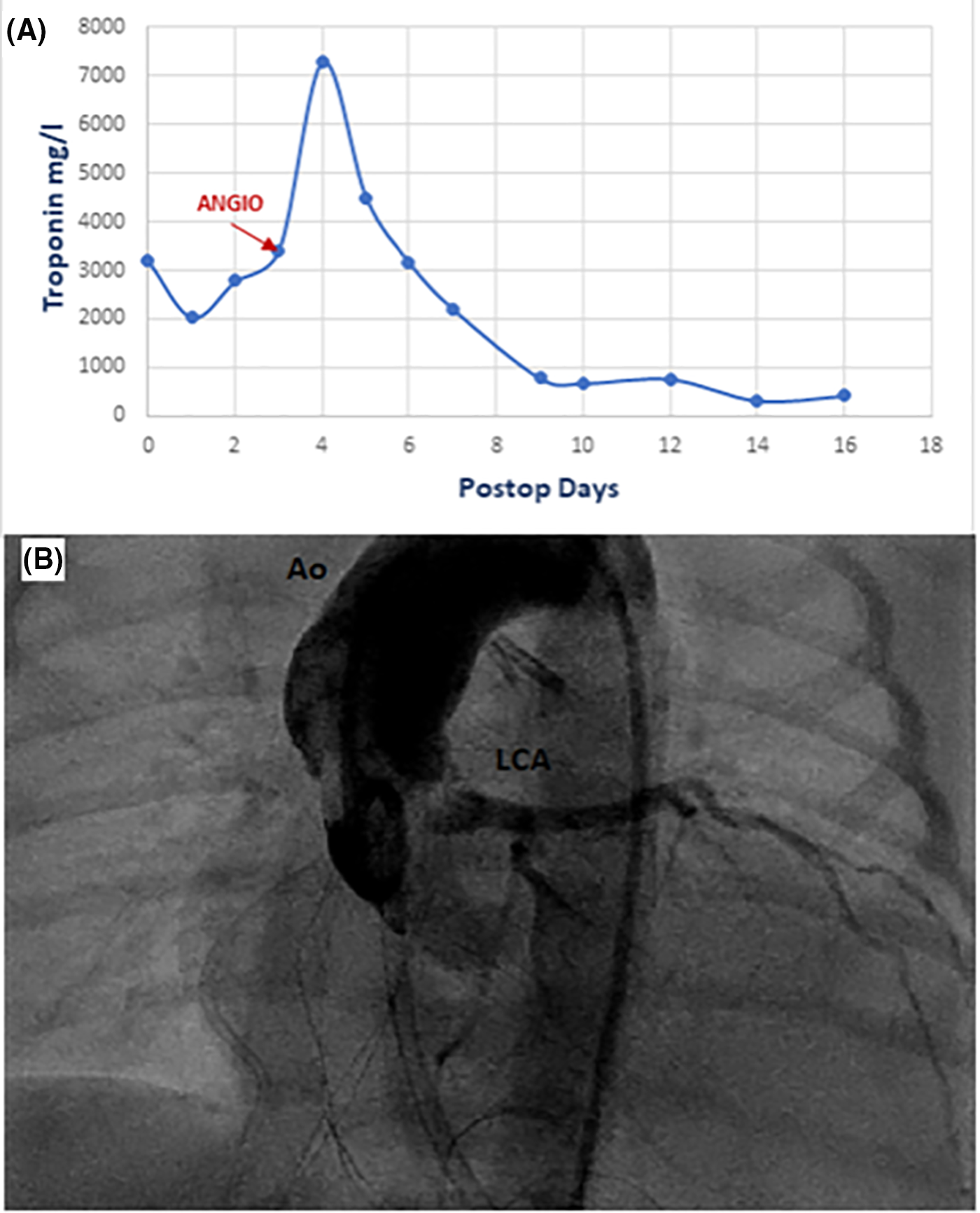
Figure 3: (A)-Graph for serum troponin T level postoperatively. (B)-Coronary angiography showed good perfusion of the left coronary artery and occlusion of the right coronary artery
The ECG during the postoperative period showed nonspecific signs of Q wave in V3,4,5,6 and negative T wave in V4,5,6.
We started antiplatelet treatment immediately after diagnosis of RCA occlusion, B blockers after weaning of all inotropes were introduced gradually to improve the cardiac function with angiotensin-converting enzyme inhibitors and diuretics. The patient was discharged on all 4 medications.
At 2 years follow-up the TTE showed normal function of both LV and RV and ECG showed sinus rhythm with isoelectric ST segment with only antiplatelet treatment and B blockers treatment. We did not observe aortic insufficiency and a large left ventricular outflow tract.
Congenital AVT is an extremely rare condition with an unknown etiology, but it is thought to result from the failure of cushion development responsible for separating the facing sinuses of the aorta and pulmonary artery [6–9]. Without surgical intervention, there is a high mortality risk within the first year of life.
While medical treatment is used solely to control heart failure, the main treatment modality is surgery, with most patients undergoing treatment before the age of six [8]. Patch closure is preferred by most surgeons over direct closure as it preserves the anatomical criteria of the aortic root [1,2]. Transcatheter closure has been reported in some cases [8,10,11], but it cannot be considered a primary treatment due to its common association with coronary anomalies and aortic valve dysplasia, which necessitate surgical intervention.
The long-term outcome varies. Valve replacement may be required later in some cases [12,13], and aortic root dilatation has been reported due to the lack of support in the area of the AVT and expected anomalies of the aortic valve or iatrogenic injury during surgical repair.
What is unique about our case is the early surgical intervention for severe left ventricle dysfunction. Then, surgery likely caused the stenosis of the right coronary artery. Hypotheses to explain this stenosis include either an injury during selective catheterization or folding of the coronary ostium due to the closure of the tunnel patch. But despite laboratory and radiological signs of myocardial ischemia, we chose not to intervene in the RCA because the patient’s clinical course was stable with LV and RV function improved and the RCA was particularly hypoplasic. We believe this scenario is explained by a significantly dominant LCA (and a small RCA) with collaterals developed during fetal life due to malperfusion of the RCA by AVT. Surgical angioplasty of a state of RCA atresia in an AVT patient was reported by Bonnet et al. [14]; it was repaired by an incision in the anterior wall of the aortic root extending through the right coronary sinus until the proper caliber of the artery, as it is a local lesion. However, this was not possible in our patient due to the small RCA ostium and caliber. In the future, we will conduct a coronary CT angiography around the age of 5 years, or earlier if there are any concerning signs on echocardiography. Based on the findings of the CT scan, we will adapt our approach as needed, whether through continued monitoring, intervention, or Cardiac catheterization.
Thus, coronary anomalies demonstrate a great capability of adaptation, as seen in the anomalous origin of the LCA from the pulmonary artery (ALCAPA), with a dilated RCA taking precedence and significant collaterals keeping some patients asymptomatic for up to 40 years [15]. There are also reported cases of a single RCA supplying both right and left coronary territories.
In cases of patients with an Aorto-Ventricular tunnel (AVT) and associated coronary lesions, it is crucial to exercise caution when intervening in the coronary arteries. For hypoplastic coronary arteries, choosing not to intervene on the coronary lesion can be a viable option, given the significant adaptability of coronary arteries. However, reintervention remains the standard practice for arteries of normal diameter.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Sherif Negm, Loic Mace, Beatrice Desnous, Virginie Fouilloux, Marien Lenoir. Data collection: Sherif Negm; analysis and interpretation of results: Fedoua El Louali, Philipe Aldebert, Marien Lenoir; draft manuscript preparation: Loic Mace, Beatrice Desnous, Virginie Fouilloux, Marien Lenoir. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All information related to the study’s data is available. Access to the data used in the study can be obtained through (marien.lenoir@ap-hm.fr).
Ethics Approval: Informed consent was granted by the parents to publish this case report.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Martins JD, Sherwood MC, Mayer JE, Keane JF. Aortico-left ventricular tunnel: 35-year experience. J Am Coll Cardiol. 2004;44(2):446–50. doi:10.1016/j.jacc.2004.04.032. [Google Scholar] [PubMed] [CrossRef]
2. Protopapas EM, Anderson RH, Backer CL, Fragata J, Hakim N, Vida VL, et al. Surgical management of aorto-ventricular tunnel. A multicenter study. Semin Thorac Cardiovasc Surg. 2020;32(2):271–9. doi:10.1053/j.semtcvs.2020.01.011. [Google Scholar] [PubMed] [CrossRef]
3. Wong AR, Amelia A, Mohd Zain MR, Sayuti KA. Left aorto-ventricular tunnel: a differential diagnosis to aortic regurgitation. Med J Malaysia. 2022 Jan;77(1):101–3. [Google Scholar] [PubMed]
4. Janeel M, Vaidyanathan S, Arvind A, Solomon NA. An interesting case of aorto-left ventricular tunnel. Ann Pediatr Cardiol. 2020 Jan–Mar;13(1):108–10. doi:10.4103/apc.APC_28_19. [Google Scholar] [PubMed] [CrossRef]
5. van Nisselrooij AEL, Moon-Grady AJ, Wacker-Gussmann A, Tomek V, Malčić I, Grzyb A, et al. The aorto-left ventricular tunnel from a fetal perspective: original case series and literature review. Prenat Diagn. 2022 Feb;42(2):267–77. doi:10.1002/pd.v42.2. [Google Scholar] [CrossRef]
6. Karacelik M, Onemli CS, Bilen MM, Sariosmanoglu ON, Yilmazer MM, Coskun M. Aorto-left ventricular tunnel: the necessity of early surgical intervention. Cardiol Young. 2024 Feb;34(2):442–4. doi:10.1017/S1047951123004183. [Google Scholar] [PubMed] [CrossRef]
7. Diraneyya OM, Alhabshan F, Alghamdi A, Moafa H, Alnasef M, Kabbani MS. Aorto-left ventricular tunnel: case series of a rare disease. Cardiol Young. 2020;31(1):47–51. [Google Scholar] [PubMed]
8. Kathare P, Subramanyam RG, Dash TK, Muthuswamy KS, Raghu K, Koneti NR. Diagnosis and management of aorto-left ventricular tunnel. Ann Pediatr Cardiol. 2015;8(2):103–7. doi:10.4103/0974-2069.157021. [Google Scholar] [PubMed] [CrossRef]
9. Liang Y, Wang J, Li D, Wan L, Gao Y, Liu R, et al. Mid-term outcome of surgical treatment in patients with aorto-left ventricular tunnel. Eur J Cardiothorac Surg. 2021 Jun 14;59(6):1312–9. doi:10.1093/ejcts/ezab110. [Google Scholar] [PubMed] [CrossRef]
10. Vida VL, Bottio T, Stellin G. An unusual case of aorto-left ventricular tunnel. Cardiol Young. 2004;14(2):203–5. doi:10.1017/S1047951104002161. [Google Scholar] [PubMed] [CrossRef]
11. Kalyanasundaram M, Kasha A, Gopalakrishnan S, Jothinath K. Transcatheter management of aorto-right ventricular tunnel: a surprise in the catheterization laboratory. Ann Pediatr Cardiol. 2022 Mar–Apr;15(2):199–202. doi:10.4103/apc.apc_108_21. [Google Scholar] [PubMed] [CrossRef]
12. Chowdhury UK, Anderson RH, George N, Singh S, Sankhyan LK, Pradeep D, et al. A review of the surgical management of aorto-ventricular tunnels. World J Pediatr Congenit Heart Surg. 2021 Jan;12(1):103–15. doi:10.1177/2150135120954809. [Google Scholar] [PubMed] [CrossRef]
13. Meldrum-Hanna W, Schroff R, Ross DN. Aortico-left ventricular tunnel: late follow-up. Ann Thorac Surg. 1986;42(3):304–6. doi:10.1016/S0003-4975(10)62740-3. [Google Scholar] [PubMed] [CrossRef]
14. Bonnet D, Bonhoeffer P, Sidi D, Kachaner J, Acar P, Villain E, et al. Surgical angioplasty of the main coronary arteries in children. J Thorac Cardiovasc Surg. 1999;117(2):352–7. doi:10.1016/S0022-5223(99)70433-2. [Google Scholar] [PubMed] [CrossRef]
15. Boutsikou M, Shore D, Li W, Rubens M, Pijuan A, Gatzoulis MA, et al. Anomalous left coronary artery from the pulmonary artery (ALCAPA) diagnosed in adulthood: varied clinical presentation, therapeutic approach and outcome. Int J Cardiol. 2018;261:49–53. doi:10.1016/j.ijcard.2018.02.082. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools