 Open Access
Open Access
ARTICLE
Reliability of Echocardiographic Pulmonary Vascular Resistance to Screen for the New Definition of Precapillary Pulmonary Hypertension in Uncorrected Secundum Atrial Septal Defect
1 Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, 57126, Indonesia
2 Department of Cardiology and Vascular Medicine, Universitas Sebelas Maret Hospital, Sukoharjo, 57161, Indonesia
3 Department of Cardiology and Vascular Medicine, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
4 Department of Cardiology and Vascular Medicine, Dr. Sardjito Hospital, Yogyakarta, 55284, Indonesia
* Corresponding Author: Risalina Myrtha. Email:
Congenital Heart Disease 2024, 19(3), 315-324. https://doi.org/10.32604/chd.2024.051587
Received 09 March 2024; Accepted 31 May 2024; Issue published 26 July 2024
Abstract
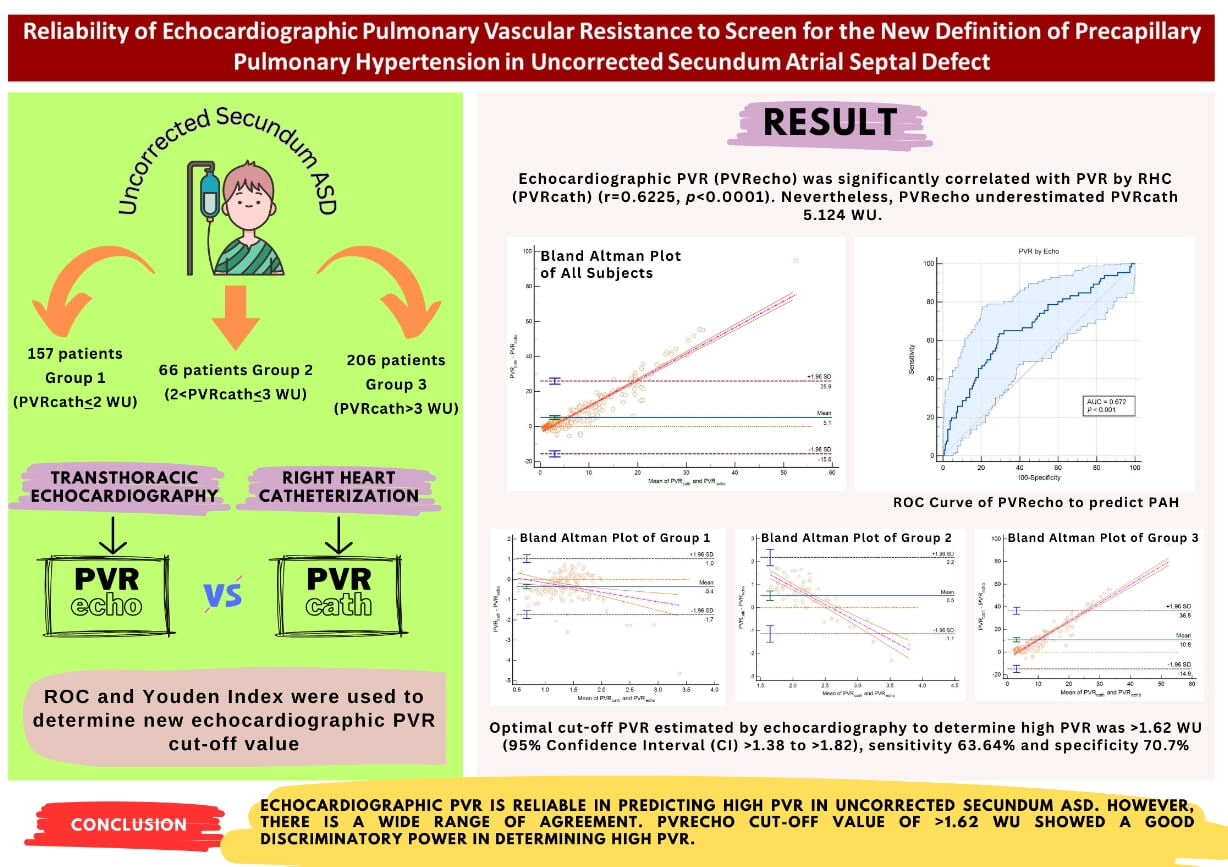
Background and Objective: The most feared complication of uncorrected secundum Atrial Septal Defect (ASD) is pulmonary arterial hypertension (PAH). Pulmonary vascular resistance (PVR) is crucial in detecting precapillary pulmonary hypertension (PH) to guide the need for PAH-specific therapy. There is a change in the cut-off value of PVR according to the recently updated PH guideline. How echocardiographic PVR (PVR) correlates to PVR by right heart catheterization (RHC) (PVR) according to the new guidelines has not been known. The aim of this study is to determine the reliability of PVR in detecting PAH in Uncorrected Ostium Secundum ASD based on the current updated guideline and to help screen the high PVR group. Methods: 429 ostium secundum ASD in the COngenital HeARt Disease in Adult and Pulmonary Hypertension (COHARD-PH) registry was divided into three groups according to the PVR. PVR was calculated using Abbas’ Formula and compared the its gold standard, the PVR. The correlation between the two methods was analyzed. The Bland-Altman plot was used to analyze the agreement between the two methods. Receiver operating characteristics (ROC) analysis was used to determine the PVR cut-off value for high PVR. Results: The majority of the population (63.5%) had high PVR. Female gender dominated the study population (84%). PVR was significantly correlated with PVR (r = 0.6225, p < 0.0001). Bland-Altman plot among all groups and in subgroups analysis showed a wide range of agreement. PVR underestimated PVR 5.124 WU. In subgroup analysis, PVR overestimated PVR 0.35 WU in those with PVR < 2 WU. In the second and third groups, PVR underestimated PVR 0.52 and 10.77 WU, respectively. Conclusion: PVR is reliable in predicting high PVR in uncorrected secundum ASD. However, there is a wide range of agreement. PVR cut-off value of >1.62 WU showed good discriminatory power in determining high PVR.Graphic Abstract
Keywords
Atrial Septal Defect (ASD) is the most common form of congenital heart disease (CHD) found in adults. The slow progression of the disease is the unique characteristic of ASD so it remains undiagnosed until young adulthood [1]. In the COngenital HeARt Disease in Adult and Pulmonary Hypertension (COHARD-PH) registry, secundum ASD constitutes the majority population in this hospital-based registry (73.4% of all population). The majority of patients (66.9%) in COHARD-PH suffered from pulmonary arterial hypertension (PAH) [2]. PAH may commonly develop in uncorrected ASD due to chronic volume overload to the pulmonary circulation. It can also cause pulmonary vascular remodeling and lead to irreversible pulmonary vascular disease, the most feared complication of uncorrected left-to-right shunt [3].
According to the European Society of Echocardiography (ESC) guideline on Pulmonary Hypertension 2015, pulmonary hypertension (PH) is diagnosed if the mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest in right heart catheterization (RHC). Pulmonary artery hypertension (PAH) is diagnosed if the pulmonary artery wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >3 Woods Unit (WU) without other causes of precapillary PH [4]. In the recent ESC guideline on PH, the lower limit of mPAP is lowered to >20 mmHg and PVR > 2 WU [5,6]. Echocardiography has not been studied in predicting PH based on the new criteria of mPAP >20 mmHg instead of ≥25 mmHg and PVR > 2 WU rather than >3 WU.
PVR is an important variable in the diagnosis of precapillary PH. PVR is significant in determining the diagnosis, management, and prognosis of adult congenital heart disease (ACHD) patients. PVR is crucial in the diagnosis of PH in the ACHD population, in order to differentiate group 2 PH associated with left heart disease from other groups. RHC remains the gold standard in the calculation of PVR in ACHD [5,7]. Nevertheless, in developing countries, the availability of cardiac catheterization laboratories is limited. Besides, echocardiography is widely available, easy to perform, and less costly. Doppler echocardiography using Abbas’ Formula has been known to be strongly correlated with invasively measured PVR. However, PVR using Abbas’ Formula has a mean difference of 2.07 WU and wide standard deviation in ASD with PVR < 3 WU. The sensitivity and specificity of the Abbas method in PVR > 3 WU are 100% and 31.5%, respectively [2,7,8].
PVR is an important parameter to obtain because it determines the need for PAH-specific drugs in ASD-associated PAH. The American Society of Echocardiography (ASE) recommendation suggests the use of the Abbas formula for assessing echocardiographic PVR. Non-invasive estimation of PVR should not be used to replace invasively measured PVR [9]. However, not all centers have the invasive modality. In addition, echocardiography is widely available and easier to perform in a bedside setting. We aimed to investigate whether echocardiographic PVR could help screen patients with high PVR who need PAH-targeted therapy in uncorrected secundum ASD.
Due to the new definition of PAH according to the 2022 ESC guideline on PH, we aimed to assess the reliability of echocardiographic PVR to diagnose PAH based on the recent guideline in uncorrected ostium secundum ASD and to help screen for the high PVR group.
COHARD-PH registry is a prospective, single-center, observational study enrolling adult patients with CHD and CHD-related PH at Dr. Sardjito Hospital in Jogjakarta, Indonesia. In this registry, patients who were suspected of having CHD and CHD-related PH performed a variety of diagnostic procedures to confirm their CHD and CHD-related PH. The subjects are recruited from outpatient clinics and inpatient wards in sequence. Enrollment and monitoring have been conducted since July 2012 to the present. Since July 2012, enrollment and follow-up have been conducted. This registry consisted of patients over 18 years of age. Uncorrected ostium secundum ASD with complete echocardiographic and catheterization data were included. ASD other than ostium secundum were excluded. If another shunt lesion or obstructive lesion were excluded. In this study, 429 ostium secundum ASD patients were included. The population was divided into three groups, i.e., group 1 (PVR ≤ 2 WU), group 2 (PVR > 2–3 WU), and group 3 (PVR > 3 WU). This research was approved by the ethical committee of the Faculty of Medicine, Public Health, and Nursing at Universitas Gadjah Mada with Approval No. KE/FK/0275/EC on 20 February, 2023. Informed consents were obtained from each patient before they were included in the registry.
Along with a physical exam, an electrocardiogram (ECG), and a chest X-ray, patients also conducted an interview. Patients suspected of having CHD underwent transthoracic echocardiography (TTE) as the main diagnostic test. Based on the most recent recommendations, TTE assessed the probability of PH. In certain cases, the agitated saline contrast echocardiography was carried out if the TTE study revealed any uncertainties concerning septal abnormalities or shunts. Transoesophageal echocardiography, also known as TOE, was carried out on patients whose ASD had been confirmed using TTE. The G.E. Vivid 7, G.E. Vivid S6, G.E. T8, G.E. E95 (G.E. Healthcare, Pittsburgh, PA, USA), Philips HD 15, Philips Epiq 7, and Philips Epiq CVx (Philips N.V., Amsterdam, Netherlands) were used for the TTE and TOE examination. Cardiologists serving as consultants carried out the TTE and TOE examinations’ validation and confirmation in this registry-specific center. Guidelines from the ASE and the European Association of Echocardiography (EAE) were followed in the acquisition, validation, and confirmation of the images. The interobserver variability coefficients of the cardiologist consultants were investigated, and the results revealed >80% agreement. At the time of the initial visit, a two-dimensional echocardiogram and a Doppler measurement were done. Using Abbas’ Formula, PVR by echocardiography was determined [10]:
After TTE and TOE confirmation of ASD and inclusion in the registry, all patients underwent RHC. Cardiologist consultants carried out the RHC on unsedated patients following standard protocols. In RHC, hemodynamics was calculated, PAH was identified, and the septal defect closure method was decided based on the RHC result. The indirect Fick method was employed to determine the cardiac output by hospital procedure. Using the equation: pulmonary blood flow (Qp)/systemic blood flow (Qs) = (aorta saturation − mixed vein (MV) saturation)/pulmonal vein (PV) saturation-pulmonary artery (PA) saturation, the flow ratio was obtained. A MV saturation was determined to be as follows:
The pulmonary vascular resistance index (PVRi) was determined using the following formula:
For statistical computation, MedCalc Version 20.014 was used. Patients with echocardiographically measurable tricuspid regurgitation (TR) were analyzed statistically (patients without TR were excluded). Initially, the correlation between echocardiographic PVR and invasively measured PVR was evaluated using Pearson’s correlation, with p < 0.05 indicating significance. Using the Bland-Altman test, the mean difference ± standard deviation (SD) between echocardiography and RHC was determined. We assessed the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of echocardiographic PVR for diagnosing precapillary PH. The receiver operating characteristics (ROC) curve and Youden index were utilized to establish the ideal new cut-off value of echocardiographic PVR as a screening tool for high PVR (>2 WU) according to the latest guideline. Continuous data were reported as either mean and standard deviation (SD) or median and interquartile range, depending on the normality of the data distribution. The category data were reported as numerical values and percentages.
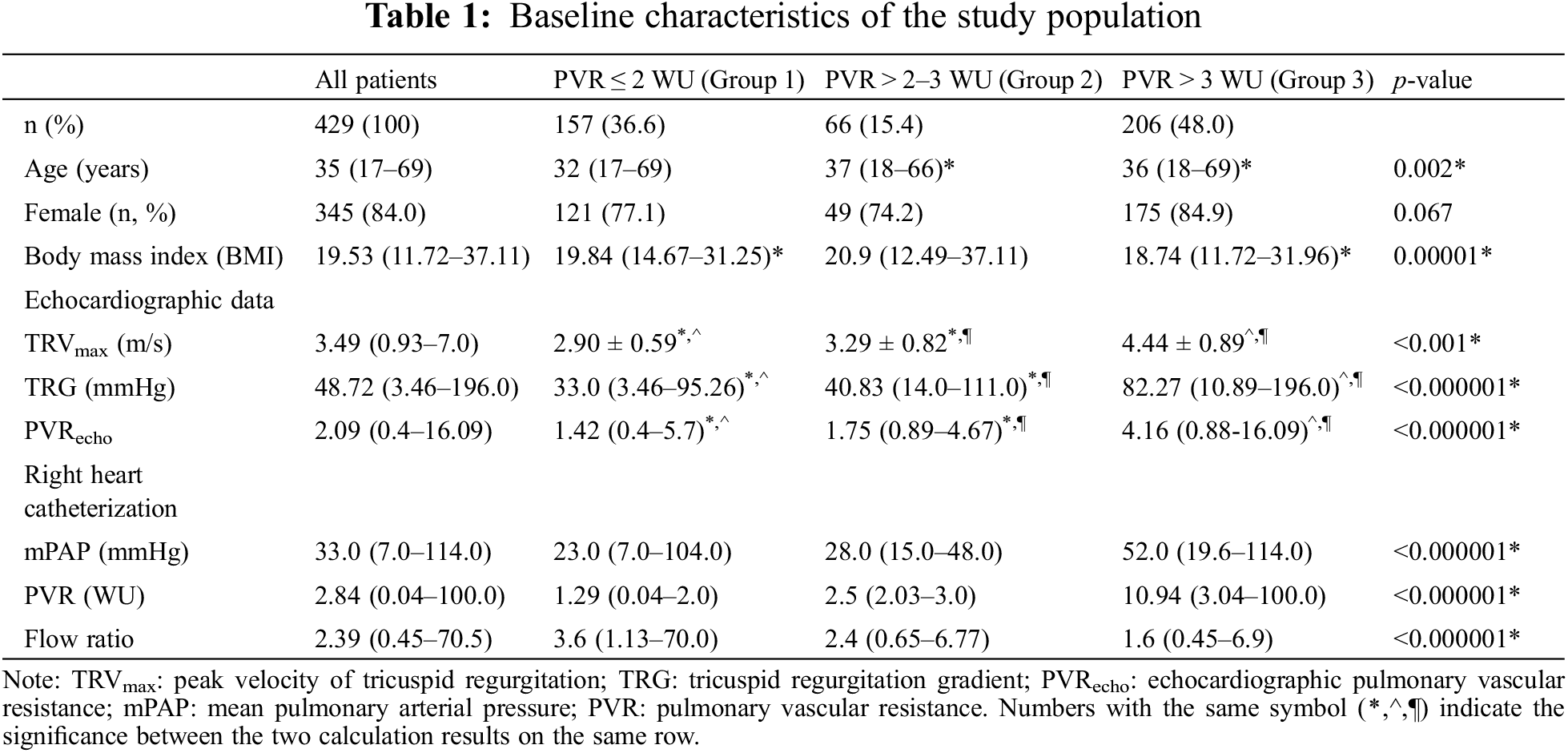
Between 2002 and 2022, 1272 ASD patients were included in the COHARD-PH registry. Of these patients, 1,021 patients (80.27%) were diagnosed with secundum ASD. Of these patients, 681 patients (66.70%) had complete RHC data, of whom 429 patients had echocardiographic PVR data (Fig. 1). The age of the study population ranges from 17 years old to 69 years old. Females dominated the study population (84.0%). Likewise, all the study groups were dominated by females. The median body mass index (BMI) was 19.53 kg/m2 (range from 11.72 to 37.11 kg/m2).
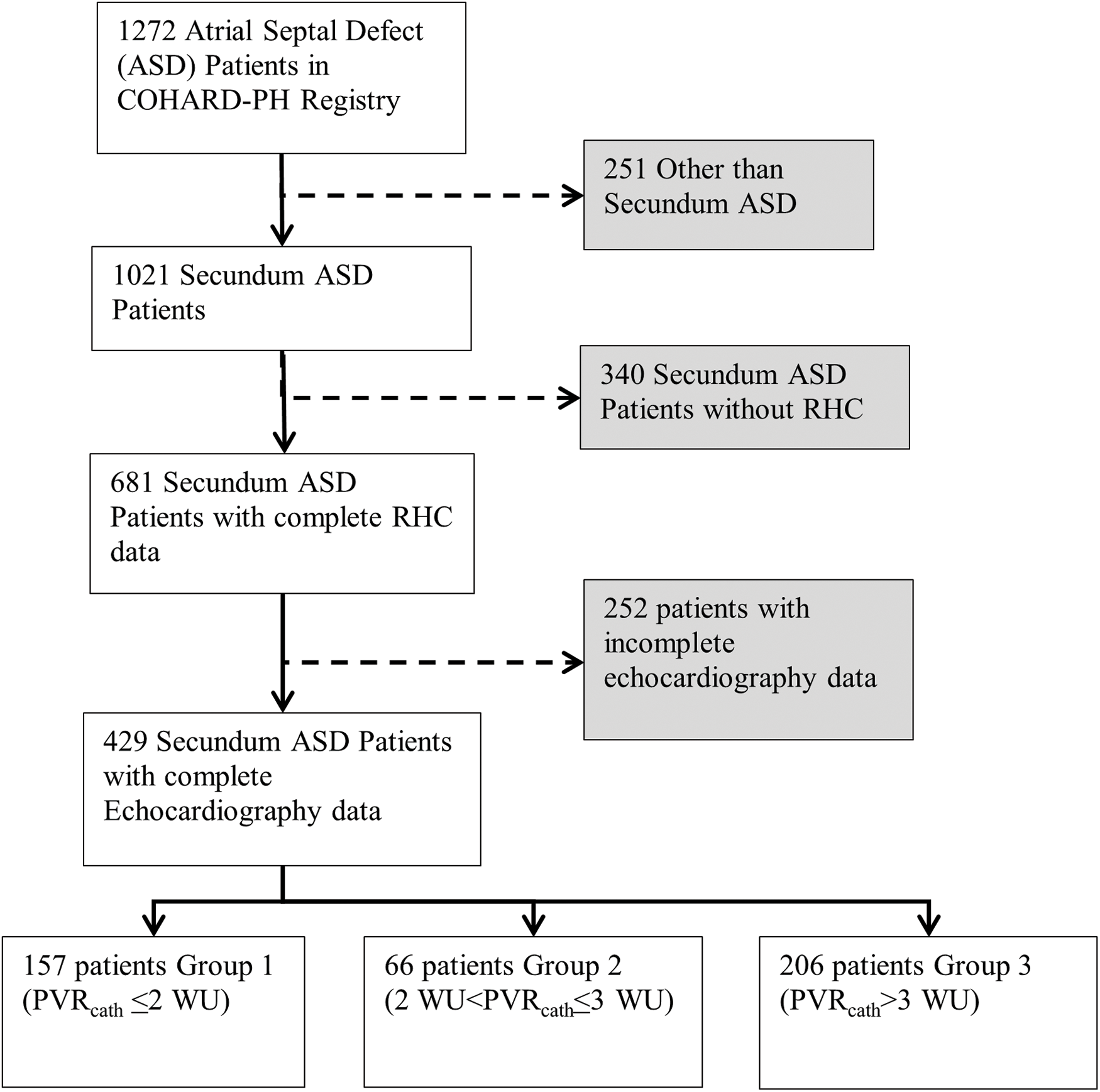
Figure 1: Study population flow chart
Among all the study population, 157 patients (36.60%) had normal PVR. As many as 66 patients (15.40%) were not diagnosed as PAH based on the former ESC PH guideline, but based on the recent ESC PH guideline, was moved to the high PVR group. The largest patient population (48%) had been already diagnosed as PAH based on both the former and recent ESC PH guidelines. The patients who are now moved to the PAH groups were slightly older than other groups (median age was 37 years old, youngest age was 18 years old, and oldest age was 66 years old). The BMI was slightly higher in the second group. The majority of the population (63.40%) had high PVR based on the recent ESC PH guideline (Table 1).
3.2 PVR Estimated by Echocardiography and PVR by Right Heart Catheterization
In the correlation test, echocardiographic PVR (PVRecho) was significantly correlated with PVR by RHC (PVRcath) (r = 0.6225, p < 0.0001). Nevertheless, PVRecho underestimated PVRcath 5.124 WU. In sub-group analysis, PVRecho overestimated PVRcath 0.35 WU in those with PVR ≤ 2 WU (group 1). However, in group 2 and group 3, PVRecho underestimated PVRcath 0.52 and 10.77 WU, respectively (Figs. 2–5).
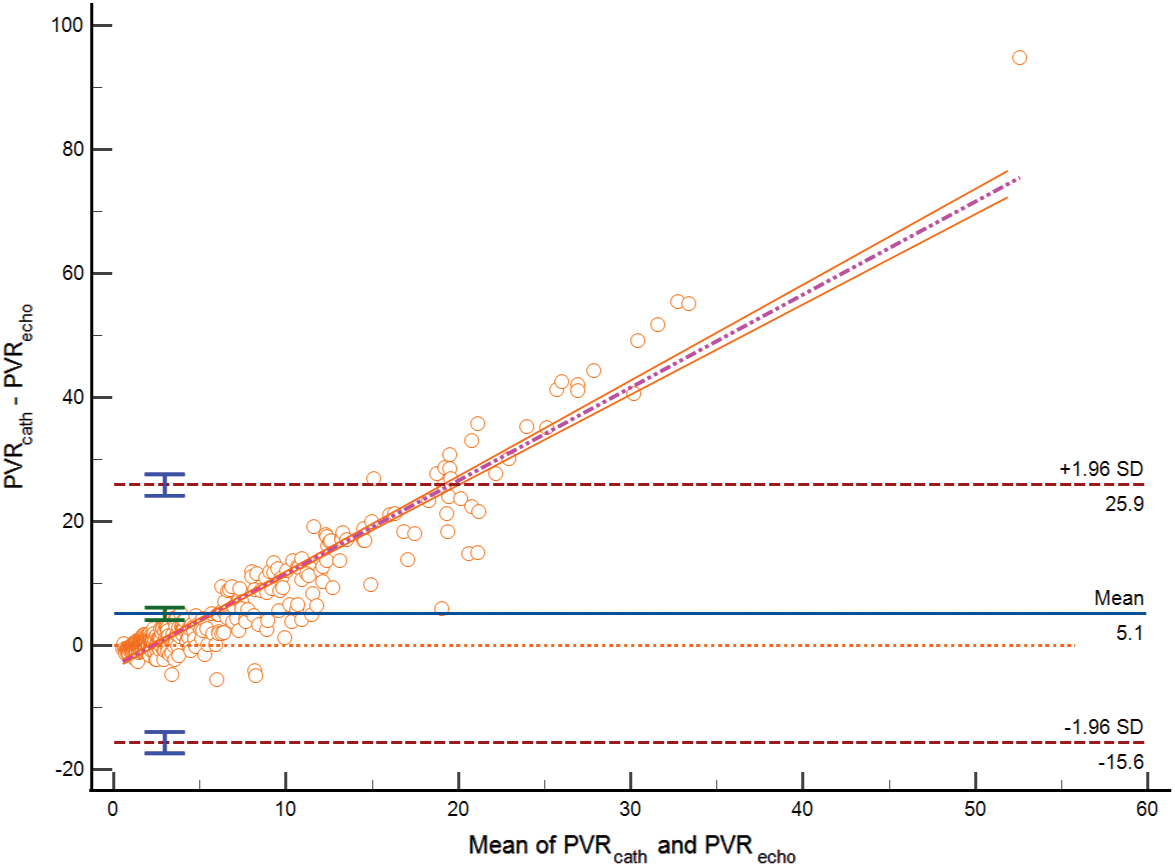
Figure 2: Bland-Altman plot PVR estimated by echocardiography vs. PVR by RHC among all study populations (Bias = 5.124, SD 10.59, lower limit of agreement −15.65, upper limit of agreement 25.897) (p < 0.0001)
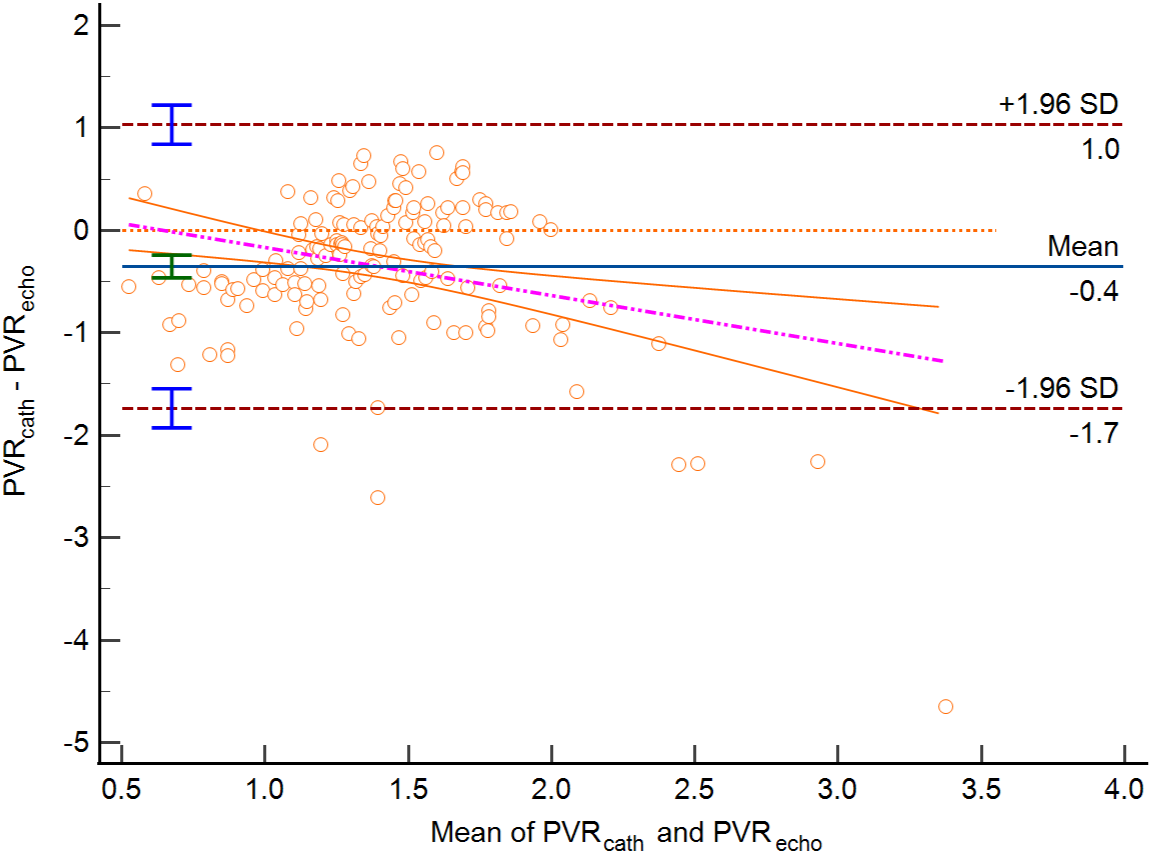
Figure 3: Bland-Altman plot PVR estimated by echocardiography vs. PVR by RHC in group 1 (Average difference/Bias −0.35 WU, lower limit of agreement −1.73, upper limit of agreement 1.03, p < 0.0001)
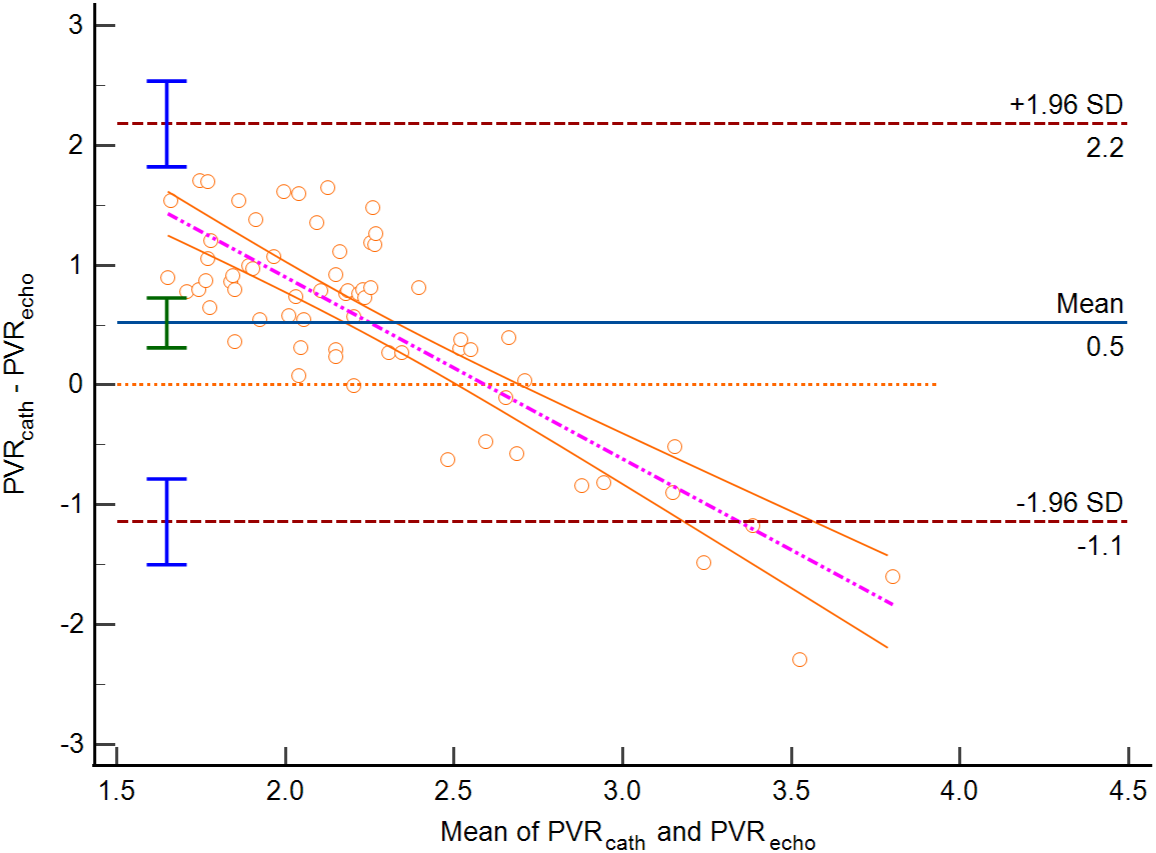
Figure 4: Bland-Altman plot PVR estimated by echocardiography vs. PVR by RHC in group 2 (Bias 0.52 WU, lower limit of agreement −1.14 WU, upper limit of agreement 2.18 WU (p < 0.0001))
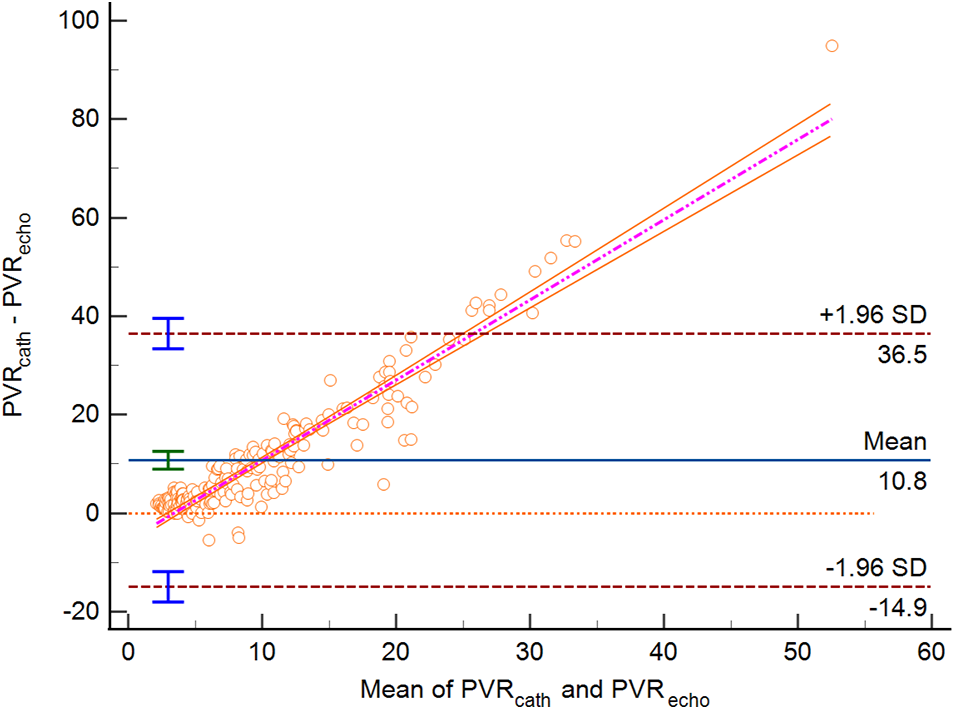
Figure 5: Bland-Altman Plot for PVR estimated by echocardiography vs. PVR by RHC in group 3 (Bias 10.77 WU (95% CI 8.97–12.57). Lower limit of agreement −14.94 WU, upper limit of agreement 36.48 WU, p < 0.0001)
When a high PVR is defined as >2 WU, the sensitivity of PVR estimated by echocardiography slightly increases (74.63% vs. 70.39%) compared to if a high PVR is defined as ≥3 WU. However, the sensitivity decreases if the PVR cut-off is lowered to 2 WU (85.99% vs. 94.61%). PPV is comparable in both cut-off values (90.22 vs. 92.36%). NPV is reduced than using the former cut-off PVR value (66.18% vs. 77.57%), and so is the accuracy (78.79% vs. 82.98%).
ROC analysis of echocardiographic PVR showed an area under the curve (AUC) of 0.672 (p < 0.0001) (Fig. 6). The optimal cut-off echocardiographic PVR to determine high PVR was >1.62 WU (95% Confidence Interval (CI) >1.38 to >1.82), sensitivity 63.64%, and specificity 70.7%.
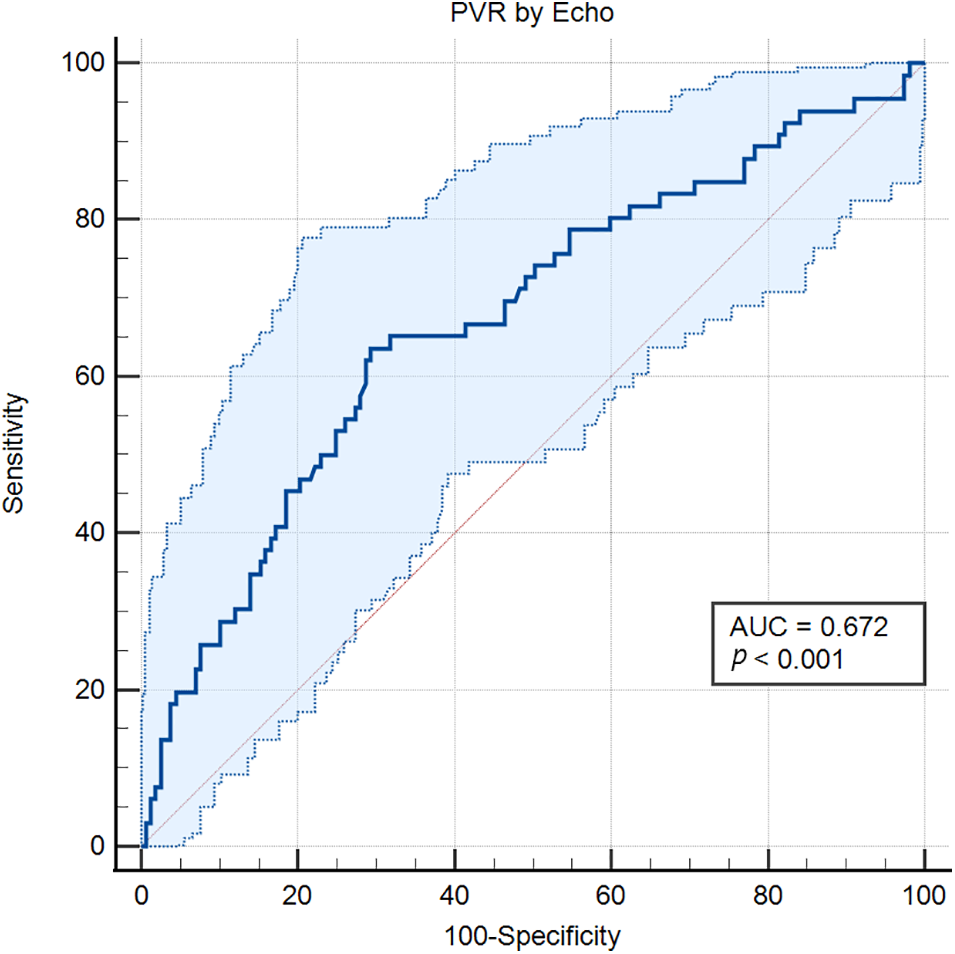
Figure 6: ROC curve of PVR estimated by echocardiography to predict PAH
PVR can serve as a dependable alternative for pulmonary vascular disease in individuals with CHD at a specific stage. The key factors influencing vascular resistance are transitional and muscular arterioles, which have smooth muscle cells in their walls and allow the vasoreactivity required to regulate pulmonary blood flow. Ohm’s law is applied hydraulically to calculate the PVR [11].
Although PVR by RHC is the gold standard, PVR can be assessed noninvasively by Doppler echocardiography. Invasive PVR is measured by ∆p and Qp. In echocardiography, ∆p is substituted by tricuspid regurgitation velocity (TRV), whereas Qp is quantified using TVIRVOT. As peak TRV increases, systolic pulmonary arterial pressure (SPAP) increases. It is widely acknowledged that, in the absence of a large pulmonary systolic gradient, the right ventricular (RV) systolic pressure measured from Doppler peak TRV corresponded well with invasive pulmonary arterial systolic pressure (PASP). Furthermore, it was previously understood that a non-invasive Doppler-derived metric that took into consideration the area of the aortic valve and the velocity time integral (VTI) of the aortic root had a significant association with cardiac output measured by catheterization [10,11]. Analysis of Qp is crucial for PVR estimation because increased PVR and hyperkinetic PAH both have the potential to raise PASP. TVIRVOT serves as a replacement for Qp. A conformational shift in TVIRVOT, which causes mid-systolic notching and early deceleration of pulmonary flow and results in a reduced right ventricular ejection time, reflects an increase in PVR. TVIRVOT declines while PVR rises. In numerous research, TRV/TVIRVOT has been verified as an estimation of PVRcath [12]. However, Doppler echocardiography as a non-invasive measurement has drawbacks due to the limitation of acoustic windows especially in evaluating pulmonary circulation, and is operator-dependent [11].
According to the underlying lesion, a variety of processes contribute to the gradual vascular remodeling that causes PAH in CHD. A common cause of this is a significant left-to-right shunt defect in CHD, resulting in elevated shear stress, endothelial dysfunction, proliferation, and gradual alteration of the pulmonary vessels, all contributing to the onset of PAH [13].
Pulmonary vascular disease is the most unwanted complication in ASD and other shunt lesions. Our study reported a surprisingly higher percentage. The bulk of patients belong to the high PVR group in our study (63.4%). A systematic review conducted in 2018 reported the prevalence of PAH before ASD closure varies from approximately 30%–70% [14]. PAH CHD associated with left-to-right shunt exhibits significant variation in outcome, depending on the severity and reversibility of vascular remodeling [15]. Although there seem to be more factors, the hemodynamic impact of increased pulmonary blood flow is the most widely known predisposing factor for developing PAH in ASD. Large ASD patients may experience long lifespans free of PAH. In middle age, some people experience arrhythmias or right heart failure without any indication of PAH. On the other hand, a tiny percentage of individuals with mild to moderate ASD, sometimes at a young age and without obvious risk factors, present with PAH. When and why specific patients gain PAH in ASD remains unanswered, despite findings indicating a potential genetic susceptibility [16].
In our study, PVRecho was significantly correlated with PVRcath. However, there is a wide limit of agreement between both methods. Using the new high PVR definition, the sensitivity of PVRecho slightly increases, but the sensitivity, NPV, and accuracy decrease. Abbas et al.’s simplified technique showed reduced accuracy while analyzing different causes of PH in a study [10]. PVR should be evaluated based on cardiac output, pulmonary artery mean pressure (PAMP), and pulmonary capillary wedge pressure (PCWP) instead of using Abbas’s formula. By substituting the ratio of the RVOT VTI to the peak velocity of TR for PA pressure and cardiac output. This method disregards the filling pressure on either the right or left heart [11,17,18]. Doppler method has inherent limitations linked to the echocardiography window and correct ultrasound beam alignment. For accurate measurement of TVIRVOT, the ultrasonic beam must be properly aligned. Accurate TVIRVOT measurements in patients with PH can occasionally be challenging due to anatomical differences in the right heart components, which carries a risk of introducing large inaccuracies in RV stroke volume estimation [18]. Furthermore, as the RHC and echocardiography measurements were not carried out at the same time, the results may be compromised by the inaccuracy of the separate hemodynamic measurements.
In low PVR situations, many studies found a strong association between Doppler-derived measures and invasive PVR. There is no concrete proof of the use of these Doppler surrogates in high PVR situations, despite the ROC curves showing good cut-off values for PVR. Abbas et al. demonstrated that 44 patients with high PVR, who had a pulmonary catheter in place, had a good correlation between their TRV/TVIRVOT ratio and postulated that these patients had higher TRV correlations when squared [10]. Abbas et al. concluded from their study that squaring the TRV in line with Bernoulli’s principle would lead to a robust correlation. However, only the ratio of the TRV and TVIRVOT is used in the non-invasive calculation of PVR [19]. As PVR rises, the TRV rises and TVIRVOT falls, while still preserving the linear correlation with PVR [12]. Most of the studies showed a good correlation between both methods. A study by Bech-Hensen et al. showed that PVR calculated using Abbas’ Formula showed a larger limit of agreement compared with PVRDoppler which was calculated using the stroke volume formula [17]. Studies that compare PVRecho and PVRcath showed varying results. However, previous studies used diverse populations, and there have been no studies examining the population of uncorrected secundum ASD.
Due to its wide limit of agreement, PVRecho cannot accurately calculate PVR values but can classify ASD patients into high PVR groups (>2 WU). Therefore, we proposed a new PVRecho cut-off value that could identify patients in the high PVR group. We proposed a cut-off value of PVRecho to determine high PVR. The cut-off value of PVRecho for high PVR is 1.62 WU, with a good discriminatory power. Given this finding, we hope that this new cut-off value can guide clinicians in starting anti-PAH therapy in ASD patients even though no catheterization has been performed. PAH-specific therapy could improve the hemodynamics and quality of life [15].
We conclude that PVRecho is reliable in predicting high PVR in uncorrected secundum ASD. PVR measured by echocardiography according to Abbas’ Formula had a significant correlation with its gold standard. However, there is a wide range of agreement between the two methods. We suggested using an PVRecho cut-off value of 1.62 WU to identify individuals in the high PVR group.
Acknowledgement: The authors expressed gratitude to Fika Humaeda Assilmi, Muhammad Reyhan Hadwiono, and Cindy Elica Cipta, research assistants who helped out in managing the COHARD-PH registry and provided friendly support during data collection. The authors also acknowledged the echo-lab sonographers and nurses who had assisted and supported the data collection. The authors also thank Rille Puspitoadhi Harjoko, FIHA for supporting the research.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Risalina Myrtha: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing–original draft, writing–review & editing. Hasanah Mumpuni: Conceptualization, formal analysis, investigation, resources, supervision, validation, writing–review & editing. Real Kusumanjaya Marsam: Formal analysis, supervision, resources, investigation, validation, writing–review & editing. Dyah Wulan Anggrahini: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, writing–review & editing. Anggoro Budi Hartopo: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, writing–review & editing. Lucia Kris Dinarti: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft, writing–review & editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The dataset produced and/or analyzed in the current study can be obtained from the COHARD-PH Registry Project Administrators upon a fair request.
Ethics Approval: This research was approved by the Ethical Committee of the Faculty of Medicine, Public Health, and Nursing at Universitas Gadjah Mada with Approval No. KE/FK/0275/EC on 20 February, 2023. Informed consents were obtained from each patient before they were included in the registry.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Brida M, Chessa M, Celermajer D, Li W, Geva T, Khairy P, et al. Atrial septal defect in adulthood: a new paradigm for congenital heart disease. Eur Heart J. 2022;43(28):2660–71. doi:10.1093/eurheartj/ehab646. [Google Scholar] [PubMed] [CrossRef]
2. Dinarti LK, Hartopo AB, Kusuma AD, Satwiko MG, Hadwiono MR, Pradana AD, et al. The COngenital HeARt Disease in adult and pulmonary hypertension (COHARD-PH) registry: a descriptive study from single-center hospital registry of adult congenital heart disease and pulmonary hypertension in Indonesia. BMC Cardiovasc Disord. 2020;20(1):1–11. doi:10.1186/s12872-020-01434-z. [Google Scholar] [PubMed] [CrossRef]
3. Le Gloan L, Legendre A, Iserin L, Ladouceur M. Pathophysiology and natural history of atrial septal defect. J Thorac Dis. 2018;10(S24):S2854–63. doi:10.21037/jtd.2018.02.80. [Google Scholar] [PubMed] [CrossRef]
4. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67–119. doi:10.1093/eurheartj/ehv317. [Google Scholar] [PubMed] [CrossRef]
5. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731. [Google Scholar] [PubMed]
6. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi:10.1183/13993003.01913-2018. [Google Scholar] [PubMed] [CrossRef]
7. Bhyravavajhala S, Velam V, Polapragada NV, Pallempati P, Iragavarapu TR, Patnaik AN, et al. Reliability of doppler-based measurement of pulmonary vascular resistance in congenital heart disease with left-to-right shunt lesions. Echocardiography. 2015;32(6):1009–14. doi:10.1111/echo.12779. [Google Scholar] [PubMed] [CrossRef]
8. Tarigan LB, Dinarti LK. Korelasi Pengukuran Resistensi Vaskular Paru Antara Ekokardiografi dengan Invasif Pada Pasien Defek Septum Atrium Dewasa 2013. Available from: http://etd.repository.ugm.ac.id/penelitian/detail/66214. [Accessed 2022]. [Google Scholar]
9. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography J Am Soc Echocardiogr. 2010;23(7):685–713. doi:10.1016/j.echo.2010.05.010. [Google Scholar] [PubMed] [CrossRef]
10. Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41(6):1021–7. doi:10.1016/S0735-1097(02)02973-X. [Google Scholar] [PubMed] [CrossRef]
11. Tamimi O, Mohammed MHA. Pulmonary vascular resistance measurement remains keystone in congenital heart disease management. Front Cardiovasc Med. 2021;8:1–5. doi:10.3389/fcvm.2021.607104. [Google Scholar] [PubMed] [CrossRef]
12. Bhyravavajhala S, Yerram S, Galla R, Kotapati VSK. Reliability of Doppler echocardiography in the assessment of high pulmonary vascular resistance in patients with severe pulmonary arterial hypertension. Indian Heart J. 2018;70(8):S241–4. doi:10.1016/j.ihj.2018.10.031. [Google Scholar] [PubMed] [CrossRef]
13. Nashat H, Montanaro C, Li W, Kempny A, Wort SJ, Dimopoulos K, et al. Atrial septal defects and pulmonary arterial hypertension. J Thorac Dis. 2018;10(S24):S2953–65. doi:10.21037/jtd.2018.08.92. [Google Scholar] [PubMed] [CrossRef]
14. Zwijnenburg RD, Baggen VJM, Geenen LW, Voigt KR, Roos-Hesselink JW, van den Bosch AE. The prevalence of pulmonary arterial hypertension before and after atrial septal defect closure at adult age: a systematic review. Am Heart J. 2018;201(21):63–71. doi:10.1016/j.ahj.2018.03.020. [Google Scholar] [PubMed] [CrossRef]
15. Jone PN, Ivy DD, Hauck A, Karamlou T, Truong U, Coleman RD, et al. Pulmonary hypertension in congenital heart disease: a scientific statement from the American Heart Association. Circ Heart Fail. 2023;16(7). doi:10.1161/HHF.0000000000000080. [Google Scholar] [PubMed] [CrossRef]
16. Opotowsky AR, Cedars A, Kutty S. Atrial septal defects and pulmonary hemodynamics: a time for holey reflection. Am J Physiol Heart Circ Physiol. 2020;318(5):H1159–61. doi:10.1152/AJPHEART.00226.2020. [Google Scholar] [PubMed] [CrossRef]
17. Bech-Hanssen O, Karason K, Rundqvist B, Bollano E, Lindgren F, Selimovic N. Can pulmonary hypertension and increased pulmonary vascular resistance be ruled in and ruled out by echocardiography? J Am Soc Echocardiogr. 2013;26(5):469–78. doi:10.1016/j.echo.2013.02.011. [Google Scholar] [PubMed] [CrossRef]
18. Roule V, Labombarda F, Pellissier A, Sabatier R, Lognoné T, Gomes S, et al. Echocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8(1):21. doi:10.1186/1476-7120-8-21. [Google Scholar] [PubMed] [CrossRef]
19. Abbas AE, Franey LM, Marwick T, Maeder MT, Kaye DM, Vlahos AP, et al. Noninvasive Assessment of Pulmonary Vascular Resistance by Doppler Echocardiography. J Am Soc Echocardiogr. 2013;26:1170–7. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools