 Open Access
Open Access
ARTICLE
Transcatheter Closure of Postoperative Residual Atrial or Ventricular Septal Shunts in Patients with Congenital Heart Disease
Department of Congenital Heart Disease, General Hospital of Northern Theater Command, Shenyang, 110016, China
* Corresponding Author: Qiguang Wang. Email:
Congenital Heart Disease 2024, 19(3), 293-303. https://doi.org/10.32604/chd.2024.051427
Received 05 March 2024; Accepted 23 April 2024; Issue published 26 July 2024
Abstract
Background: Transcatheter closure (TCC) has emerged as the preferred treatment for selected congenital heart disease (CHD). While TCC offers benefits for patients with postoperative residual shunts, understanding its mid- and long-term efficacy and safety remains crucial. Objective: This study aims to assess the mid- and long-term safety and efficacy of TCC for patients with residual atrial or ventricular septal shunts following CHD correction. Methods: In this consecutive retrospective study, we enrolled 35 patients with residual shunt who underwent TCC or surgical repair of CHD between June 2011 to October 2022. TCC candidacy was determined based on established criteria. Echocardiography and electrocardiogram were conducted during the perioperative period and continued as part of long-term follow-up. Results: Among the patients, 5 (14.3%) exhibited interatrial shunting, while 30 (85.7%) had interventricular shunting. TCC was successfully implemented in 33 of 35 patients, with exceptions in two cases of post-ventricular septal defect repair due to anatomical challenges involving the shape and aortic angulation. This resulted in a TCC success rate of 94.3%. Trace residual shunt was detected in two interventricular shunting cases and a mild residual shunt in one interventricular shunting case; all resolved by the three-month follow-up after TCC. Minor complications included one hematoma at the puncture site and one transient junctional rhythm during the perioperative period. During a median follow-up of 73 months, there were no instances of residual shunt, device embolization, occluder displacement, valve insufficiency, malignant arrhythmia, infective endocarditis, death, or other serious complications. Conclusion: TCC is an effective and safe therapy for patients with residual atrial or ventricular septal shunts following CHD correction. These findings support the consideration of TCC as the preferred treatment option for appropriate patient populations.Keywords
Abbreviations
| CHD | Congenital Heart Disease |
| TCC | Transcatheter Closure |
| IAS | Interatrial Shunting |
| IVS | Interventricular Shunting |
Congenital heart disease (CHD) stands as the preeminent congenital malformation, affecting approximately 1% of all live births. In China alone, the annual birth of infants with CHD is estimated to range between 150,000 and 200,000 [1]. The majority of CHD patients have the potential for cure through either surgical intervention or transcatheter closure (TCC). Statistics reveal that over 100,000 CHD-related operations are performed annually in China, which include approximately 30,000 interventional procedures and 4,000 complex surgical operations. Intriguingly, about 70% of these new diagnoses each year undergo some form of CHD correction [2,3]. Despite these figures, a subset of patients continues to grapple with residual lesions post-procedure. Among these, various residual shunts, such as interatrial shunting (IAS) and interventricular shunting (IVS), are most frequently encountered. The challenge with these residual shunts lies in the local scar tissue adhesion, which renders a second surgical intervention not only exceedingly complex and invasive but also potentially ineffective, as the risk of persistent shunting remains. For most patients, the prospect of re-thoracotomy poses significant risks [4]. The introduction of the Amplatzer septal occluder in the 1990s marked a pivotal turning point, propelling the development of TCC for CHD and offering an innovative therapeutic approach for treating residual atrial or ventricular septal shunts after CHD correction. This study is designed to assess the safety and efficacy of TCC in patients presenting with residual atrial or ventricular septal shunts following CHD correction, with a focus on examining the mid-and long-term follow-up outcomes.
In this retrospective study, we enrolled a cohort of thirty-five patients who underwent TCC for residual shunts following CHD correction at our institution from June 2011 and October 2022. The study population comprised all patients who met the following criterion: (1) Individuals with residual shunt following TCC or surgical repair of CHD, including IAS, IVS; (2) As per the Chinese experts’ consensus on the interventional treatment of common congenital heart diseases [5], candidates were deemed eligible for TCC if they presented with: a. IAS with right heart volume overload without significant pulmonary arterial hypertension; b. IVS with symptoms of heart failure symptoms, dilatation of left heart chambers, or the pulmonary to systemic flow (QP/QS) ratio of ≥1.5 during catheterization; (3) Absence of other cardiac conditions necessitating surgical intervention. This investigation was approved by the Ethics Committee of General Hospital of Northern Theater Command Ethics [Y (2024) 055] and conformed to the principles of the Declaration of Helsinki and its later amendments. The requirement for informed consent was waived due to the retrospective nature of the study.
In this study, the TCC procedures were conducted by two skilled doctors with over 10 years of experience in CHD interventional treatment. We used the Amplatzer Septal Occluder (ASO, Abbott Medical, MN, USA), the Second-generation Amplatzer Ductus Occluder (ADOII, Abbott Medical, MN, USA), and a domestically manufactured atrial/ventricular septal occluder (Shanghai Shape Memory Alloy Co., Ltd., Shanghai, China). The choice of occluder was informed by transthoracic echocardiography (TTE), and left ventricular angiography, with the occluder’s positioning confirmed through fluoroscopy and TTE. TCC procedures were performed under local or general anesthesia, guided by both fluoroscopy and TTE. Access was obtained via puncture of the right femoral vein and/or artery, with a vascular sheath placed in the inguinal region. Patients received routine intravenous heparin administration at a dosage of 80–100 IU/kg. Standard right heart catheterization was conducted on all patients, followed by the TCC procedure as detailed in previous literature [5]. An appropriately selected occluder, attached to its delivery cable, was introduced through the femoral venous route using a delivery sheath and was precisely deployed at the designated site. Simplified illustrations of the TCC procedures for IAS (Fig. 1) and IVS (Fig. 2) are provided to offer visual clarity on the techniques employed.
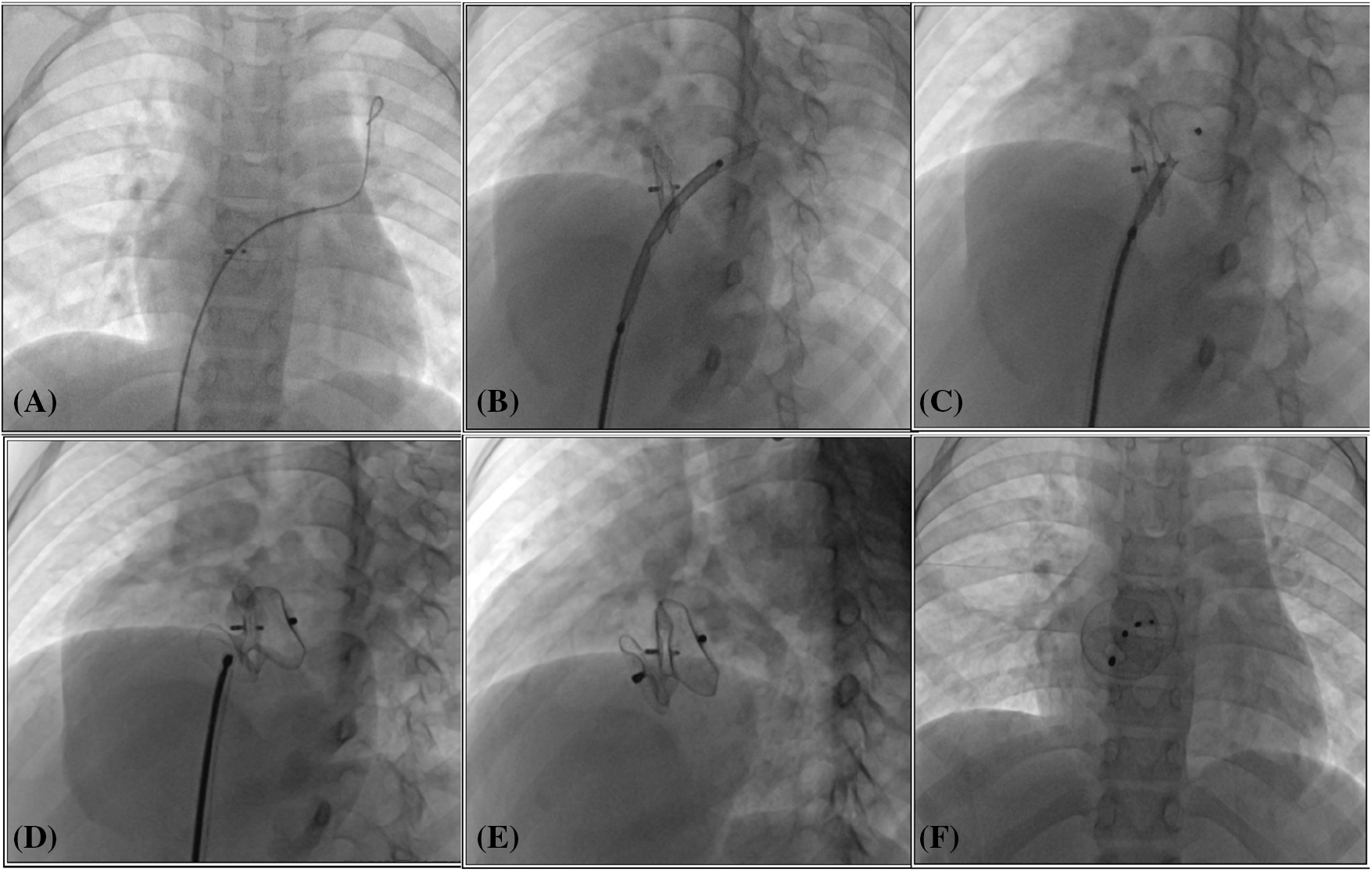
Figure 1: Transcatheter closure of residual shunt following atrial septal defect occlusion. (A) The catheter and guide wire were adeptly navigated through the previously implanted occluder, successfully reaching the left upper pulmonary vein; (B) The occluder, attached to its delivery cable, was meticulously advanced through the delivery sheath, ultimately arriving at the left atrium; (C, D) Sequentially, the left and right discs of the occluder were deployed, ensuring a secure fit; (E, F) Following the occlusion, the occluder’s form and placement were carefully assessed using fluoroscopic imaging in both left anterior oblique and lateral views to confirm optimal positioning and functionality
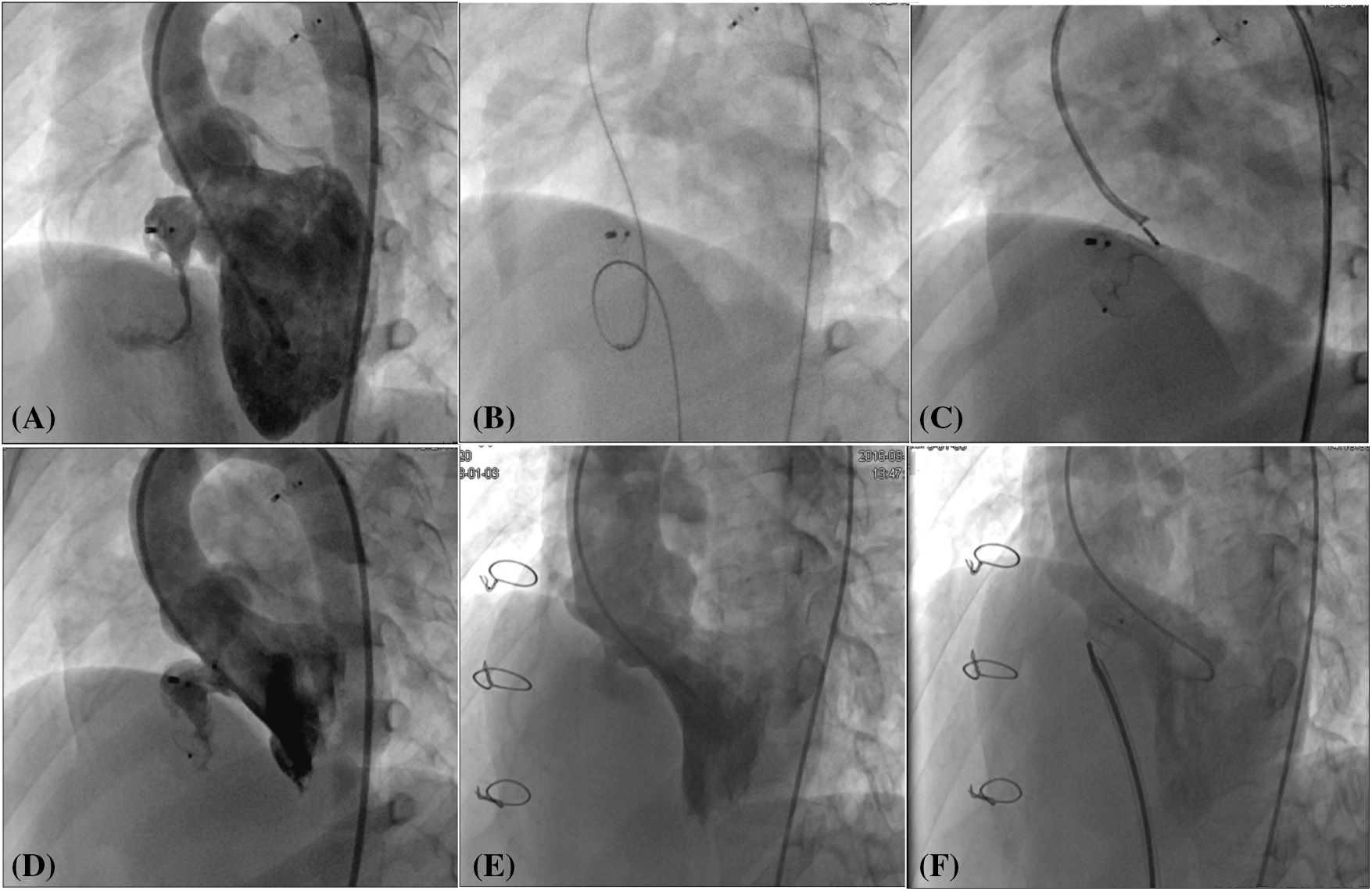
Figure 2: Transcatheter closure of residual shunt following ventricular septal defect occlusion and surgical repair. (A) Angiography of the left ventricle revealed a protracted residual shunt at the periphery of the previously placed occluder. (B) An arteriovenous circuit was skillfully established. (C) A second-generation Amplatzer Duct Occluder was expertly implanted via the transfemoral artery route. (D) Subsequent angiography of the left ventricle post-occlusion confirmed the complete disappearance of the residual shunt. (E) In a patient with a residual shunt post-surgical repair of the ventricular septal defect, left ventricular angiography was performed to assess the situation. (F) Following the occlusion, a repeat angiography of the left ventricle demonstrated the residual shunt had vanished
2.3 Follow-Up and Outcome Assessment
Prophylactic antibiotics were routinely administered to all patients after the procedure for a duration of two days as a standard precaution. Additionally, patients were prescribed oral aspirin at a dosage of 3–5 mg/kg daily, which was continued for six months following the occlusion. To monitor the patients’ progress, a comprehensive TTE and electrocardiogram evaluation was conducted at multiple time points: at discharge, and then at 1-, 3-, and 6-month intervals, and annual assessments thereafter. The echocardiographic assessment was meticulous, encompassing the evaluation of the device’s position and structural integrity, its contact with valve structures, the presence and severity of any residual flow, and the internal diameters of the heart chambers. The electrocardiographic assessment was equally thorough, with particular attention to heart rate and the emergence of any new arrhythmia, paying special heed to the occurrence of atrioventricular heart block.
The data were analyzed with the statistic software SPSS 25.0 (SPSS, Inc. Chicago, Illinois, USA). All continuous variables are expressed as mean values and standard deviation, which was performed by paired sample student t-test for comparison before and after TCC. Discrete variables are presented as percentages, which was performed by the Chi-square test or Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
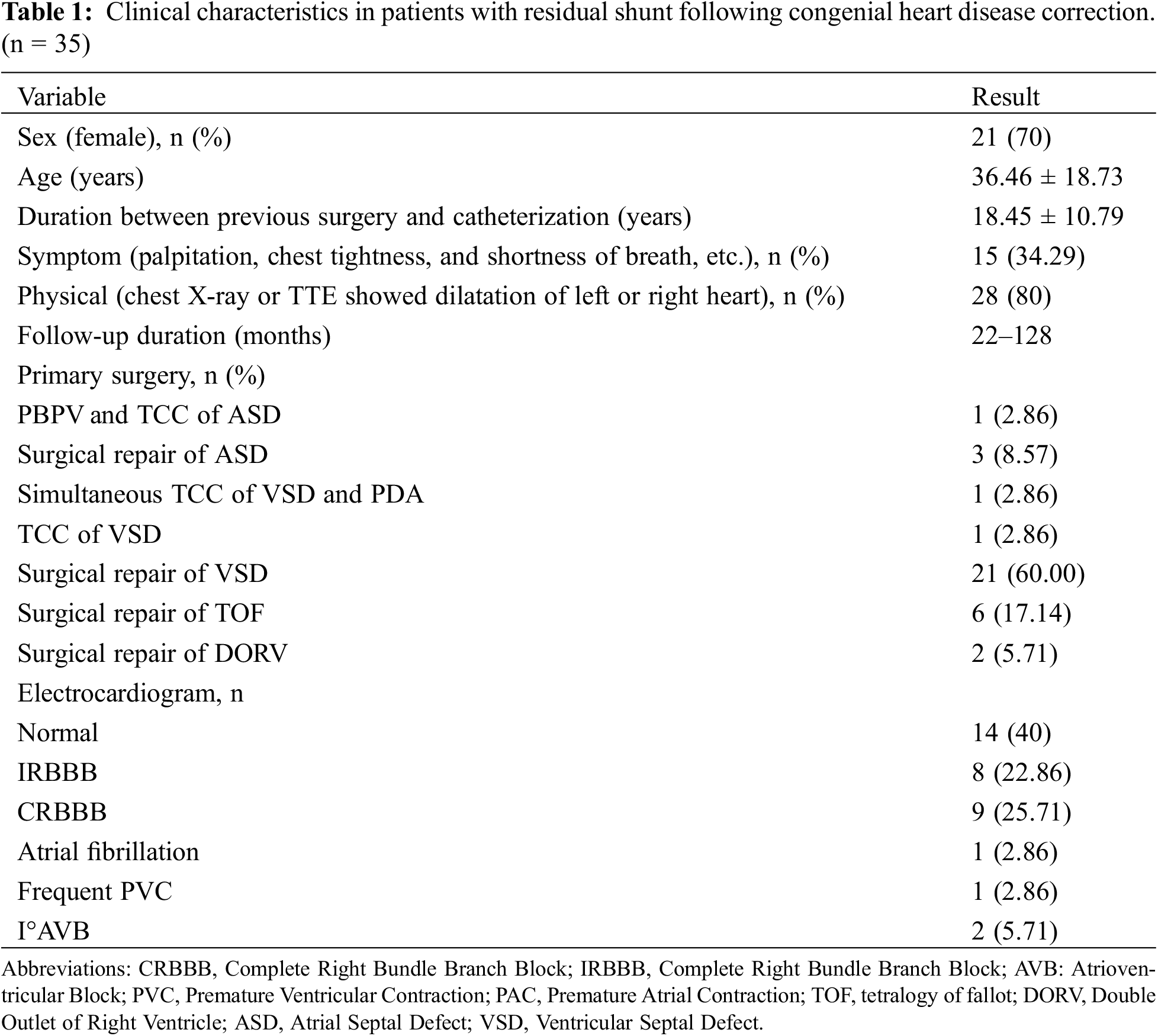
In this study, we enrolled a cohort of thirty-five patients (14 male and 21 female) who had undergone correction for CHD and subsequently presented with a residual shunt. The mean age of the participants was 36.46 years, with a standard deviation of 18.73 years. The interval between the initial CHD correction and the subsequent intervention averaged 18.45 ± 10.79 years. Within the group of thirty-five patients, 5 (14.3%) were diagnosed with IAS, while the majority, 30 (85.7%), had IVS. Among the study participants, 15 (34.29%) presented with a range of clinical symptoms, including palpitations, chest discomfort, and dyspnea. Furthermore, a substantial proportion of 28 (80%) patients demonstrated signs of heart enlargement, either on their chest X-ray imaging or through TTE assessments. The precise details of these diagnoses and patient characteristics are delineated in Table 1.
The TCC procedures for residual shunts were successfully completed in 33 out of 35 patients. The two exceptions were patients with residual shunts following ventricular septal defect (VSD) repair, where the unique anatomical shape and orientation presented challenges. Consequently, the overall success rate of TCC in this study reached 94.3%. Complications during the perioperative period were minimal, with one instance of a hematoma at the puncture site and one instance of transient junctional rhythm disturbance.
Among the cohort, five patients were identified with IAS: one had a post-TCC atrial septal defect (ASD) and four had post-surgical repair ASD. The former was an 8-year-old girl with ASD and pulmonary valve stenosis. Five years after balloon pulmonary angioplasty, and transcatheter ASD closure, an 8 mm interatrial shunt was discovered via TTE. To address this, an additional 16 mm ASD occluder was effectively deployed, resulting in a complete obstruction of the shunt with no residual blood flow (Fig. 1).
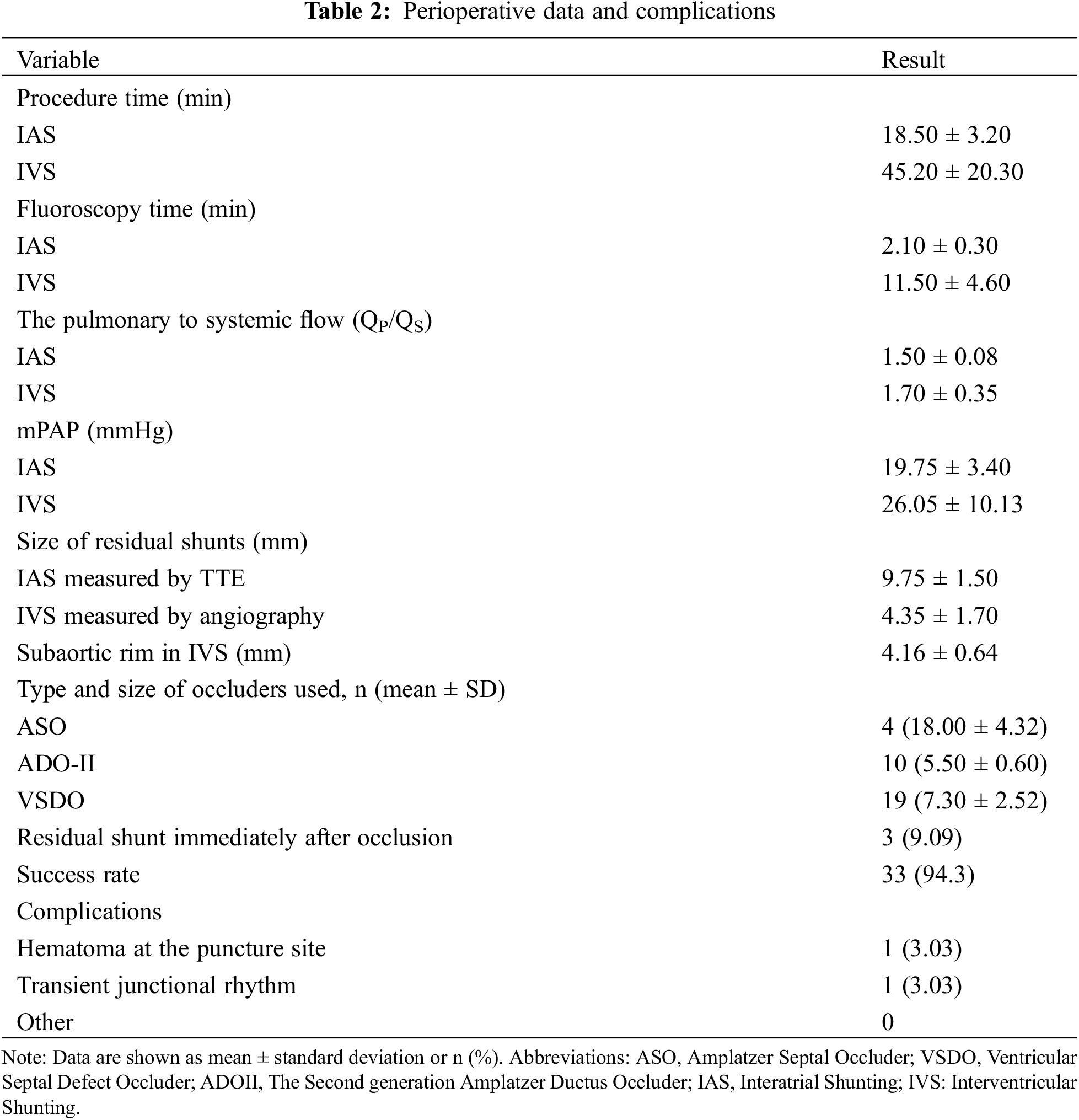
As is shown in Table 2, 30 patients (11 males) had IVS: 2 patients following transcatheter VSD closure and 28 patients following surgical repair. The mean pulmonary arterial pressure, as determined by right heart catheterization, was 26.05 ± 10.13 mmHg with a QP/QS of 1.70 ± 0.35. The IVS diameter, assessed via left ventricular angiography, was on average 4.35 ± 1.70 mm, and the subaortic rim measured 4.16 ± 0.64 mm. TCC of IVS was successfully performed in 28 patients, while in two cases post-ventricular septal defect repair failed due to anatomical challenges involving the shape and aortic angulation. The average procedure time was 45.20 ± 20.30 min, and the fluoroscopy time was 11.50 ± 4.60 min. In the IVS cohort, three distinct device types were used: small-waist double-disk VSD occluders were employed in 14 cases, Symmetric double-disk VSD occluders in 5 cases, and ADO II devices in 10 cases.
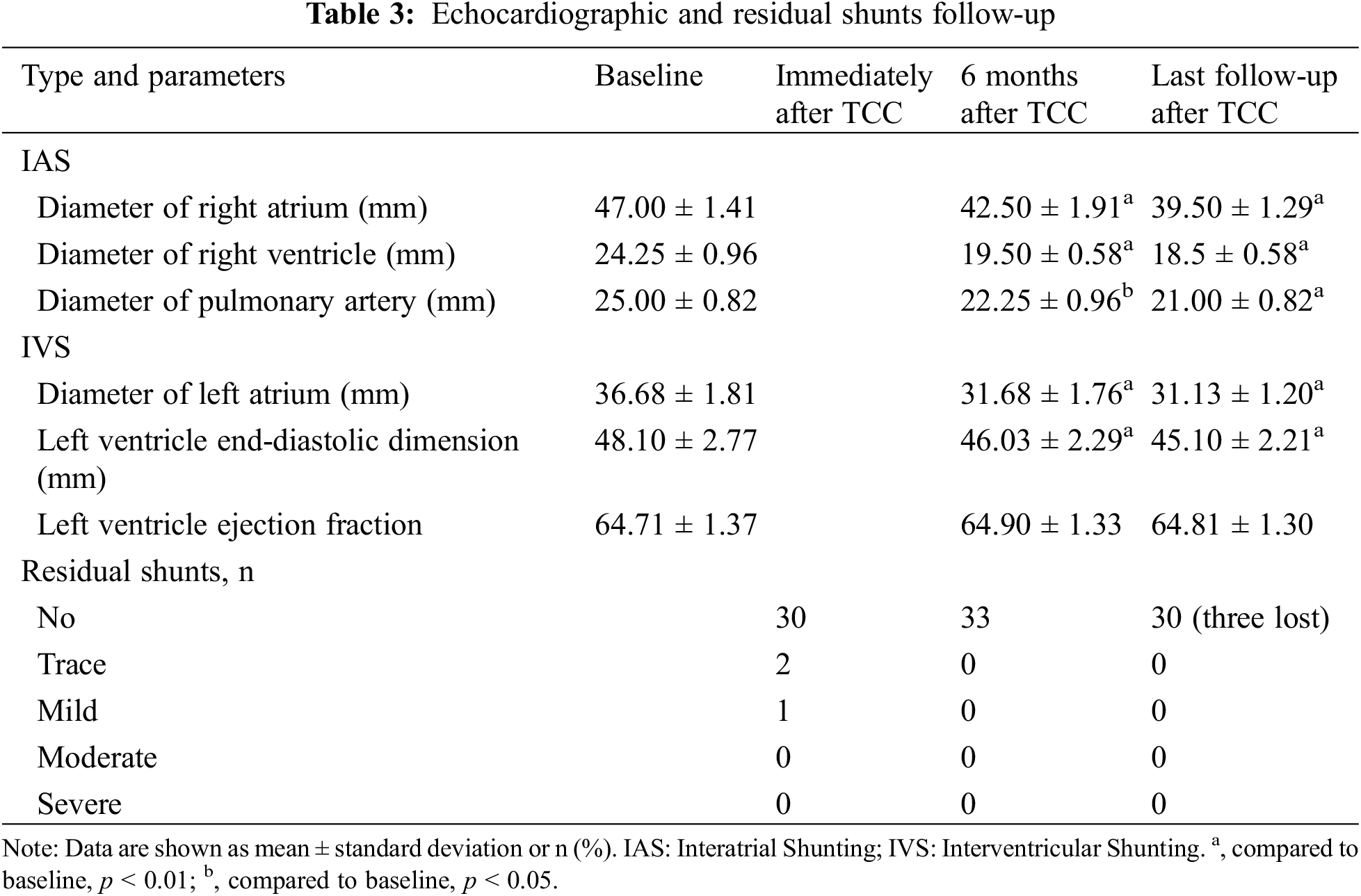
Among the 33 patients who successfully underwent TCC for residual shunts, a trace residual shunt was detected in two IVS cases, and a mild residual shunt in one IVS case; These residual shunts had resolved by the three-month follow-up as assessed by TTE. Unfortunately, follow-up was lost for three patients after their hospital discharge. A total of thirty patients (90.9%) adhered to the annual routine follow-up, which included TTE and electrocardiogram evaluations. During a median follow-up of 73 months, ranging from 22 to 128 months, there were no instances of residual shunts, device embolization, occluder displacement, valve insufficiency, new-onset arrhythmia, infective endocarditis, death, or other serious complications. As shown in Table 3, compared with the preoperative state, TTE results at 6 months postoperatively and at the last follow-up showed that both the right atrium and right ventricular diameters in patients with IAS were significantly reduced (all p-values < 0.05), the left ventricular end-diastolic diameter in patients with IVS was also significantly reduced (p-value < 0.05), while the left ventricular ejection fraction showed no significant change.
Since the initial report of patent ductus arteriosus (PDA) ligation in 1938, traditional surgery has evolved, amassing extensive experience in surgical techniques, anesthesia, extracorporeal circulation, perioperative monitoring, and other aspects, remaining a primary modality for the treatment of CHD. In 1966, Rashkind’s [6] pioneering use of balloon atrial septostomy to treat infants with complete transposition of the great arteries marked the dawn of the interventional treatment era for CHD. Today, TCC has emerged as a vital treatment approach within this realm. Despite the advancements, the scope of interventional therapy remains narrow, primarily addressing simpler malformations such as secundum ASD, VSD, PDA, and pulmonary valve stenosis. For the majority of complex CHD, including tetralogy of Fallot, double outlet right ventricular, partial atrioventricular septal defect, and specialized forms of ASD and VSD, surgical repair continues to be the gold standard. Nevertheless, regardless of the treatment approach, complications such as arrhythmia, heart failure, residual shunts, and residual obstructions are not uncommon, with residual shunts being of particular concern. In our study, among thirty-five patients, 5 (14.3%) exhibited IAS, and 30 (85.7%) had IVS. Residual shunts were observed in 32 patients post-surgical repair and 3 patients following TCC, underscoring the need for vigilant post-intervention monitoring and management.
For patients who have undergone initial surgery, subsequent surgical repairs often present increased challenges due to significant adhesions within the mediastinum and chest cavity, which can complicate the surgical field. In contrast, TCC offers a less invasive alternative with a lower risk of complications and a higher success rate, making it a more appealing option in terms of both physical and psychological trauma, avoiding the need for myocardial scarring, extracorporeal circulation, and the risks associated with bleeding. Consequently, TCC for residual shunts post-surgical correction of CHD is increasingly becoming the treatment of choice [7,8]. In this study, we present outcomes from 35 patients, who underwent attempted TCC of residual shunts following CHD correction. Devices were implanted successfully in 33 of 35 patients in our study, yielding a success rate of 94.3%. The efficacy of TCC of residual shunts following CHD correction was demonstrated by the stable positioning of devices and a marked reduction in residual flow as monitored by TTE throughout the follow-up period. Minor residual shunts are frequently seen after VSD device closure; these tiny residuals usually close over time. In our study, minor residual shunts in 3 cases were detected immediately after TCC, and residual shunts all disappeared during follow-up. The results of our study are consistent with the literature [9]. The efficacy of TCC for residual shunts was demonstrated by the stable positioning of devices and a marked reduction in residual flow as monitored by TTE throughout the follow-up period.
Residual shunts following surgical repair of CHD represent a significant complication in cardiac surgery, with reported incidence rates ranging from 1% to 10%, and in some cases, as high as 25%. Specifically, the occurrence of residual shunts after VSD repair is estimated to be around 3.8% to 10% [10]. The emergence of residual VSDs can be attributed to various factors, including the nature and severity of CHD, the patch material used, and the surgical suturing techniques and expertise [11–13]. In our study, we encountered 28 cases with residual IVS after CHD surgical repair, which included 20 instances post-VSD repair, 6 post-Tetralogy of Fallot repair, and 2 post-double outlet right ventricular repair. The surgical correction of ASD typically results in a clear and accessible visual field with a minimal pressure gradient between the left and right atrium, making residual shunts after ASD repair a rare clinical occurrence. The occurrence of such residual shunts is primarily linked to the surgical approach, the characteristics of the defects, and the suturing methodology. Residual shunts of less than 5 mm do not significantly impact hemodynamics and thus, a conservative approach is often warranted. However, for residual shunts exceeding 5 mm, TCC emerges as the preferred therapeutic strategy. In our cohort, only four patients exhibited residual shunts following ASD repair, underscoring the efficacy of the surgical intervention and the selective application of TCC for larger defects.
While residual shunts are infrequent following the TCC of CHD, they can occur in specific scenarios, such as when there is an improper choice of occluder type or size, postoperative displacement of the occluder, or in cases with multiple defects. Only in a few special cases, such as improper type and specification of occluders, postoperative displacement of occluders, and multiple defects. Long-term follow-up has revealed an increasing detection of residual shunts in patients after TCC of CHD. In our study, 3 cases exhibited residual shunts post-TCC, which included IAS in one patient, and IVS in two patients. The primary reasons for residual shunts after TCC in our observation are as follows: (1) In the early stages, The technology of domestic occluders designed to block fluid-filled materials is not yet fully developed. As the substances within these occluders are gradually absorbed or become damaged, there is a potential for an increased incidence of residual shunts over time. Furthermore, if the chosen device is oversized, it may fail to fully conform to the in vivo anatomy, leading to incomplete endothelialization. This could result in the gradual development of residual shunts during long-term follow-up. (2) An oversized device leading to fully conform to the in vivo anatomy, leading to incomplete endothelialization, may result in the gradual development of residual shunts during long-term follow-up. (3) Improper device selection, such as the use of an ASD occluder for large PDA in the absence of appropriately sized devices in the early stages. (4) The presence of multiple defects complicates the closure process. (5) An undersized device may lead to displacement post-implantation. Additionally, the atrial septum’s non-planar and angled anatomy may contribute to device abrasion and subsequent residual shunts after ASD occlusion, as seen in one patient in our study. In an early review using TTE after the initial percutaneous balloon pulmonary valvuloplasty and ASD occlusion, no residual shunt was detected. However, an 8 mm residual shunt was identified by TTE five years later, likely due to device abrasion. Fortunately, we were able to successfully implant an additional occluder adjacent to the original device, addressing the issue effectively.
During the process of ASD and VSD closure, we routinely use TTE for intraoperative monitoring and guidance. The preference for TTE over Transesophageal Echocardiography (TEE) in closure procedures is due to TTE’s non-invasive nature, which ensures patient comfort without the need for sedation, and its lower risk of complications. The simplicity of TTE allows for ease of performance in routine clinical practice, and it facilitates straightforward post-procedure monitoring for immediate results and potential complications. While TEE offers higher-resolution imaging, TTE is generally more suitable for the task due to these various advantages.
The indications, technical details, and procedure steps for interventional therapy of residual shunts following CHD correction are fundamentally aligned with CHD without prior surgery. The crux of TCC for these residual shunts following CHD correction lies in the establishment of a vascular circuit and the deployment of a long sheath. For postoperative ASD occlusion, the procedure is relatively straightforward, as it is not challenging to create the circuit and guide the sheath. However, the occluder size we often select is larger than what would be used for ASD without surgery. Postoperative residual VSDs typically result from partial avulsion at the patch-margin interface, necessitating the selection of a larger occluder size compared to VSDs without surgery to facilitate device anchoring and enhance the success rate. Moreover, the morphology of residual shunts is often irregular, presenting challenges in establishing the circuit and sheath delivery, especially with postoperative residual VSDs. In this study, TCC was unsuccessful in two out of 30 patients with postoperative residual VSDs, primarily due to the inability to establish an arteriovenous circuit because of the defect’s unique shape and its angulation with the aorta. Furthermore, the entire process—from establishing the circuit and delivering the long sheath to deploying the occluder—must be executed with gentleness to prevent additional damage to the patch. Careful technique is paramount to avoid exacerbating the existing defect and to ensure the best possible outcome for the patient.
The ADO II occluder is a soft, flexible, and self-expanding nitinol mesh device designed for minimally invasive implantation from either the arterial or venous side. This device features two retention discs on either side of the defect, connected by a central waist. The distinct advantage of the ADO II is its low profile requirement, necessitating only four or five French sheaths for deployment, which simplifies the procedure. After a thorough evaluation of our single-centre experience, we can confidently affirm that the ADO II is both safe and highly effective for TCC of residual shunts following CHD correction. It is particularly well-suited for elongated residual ducts and small residual VSDs [14]. In scenarios where residual ducts and VSDs are elongated and angulated, making it challenging to establish a circuit from the venous side, we opt for an arterial approach. Utilizing a right coronary artery catheter, we can delicately manipulate the guide wire, either withdrawing or advancing it as needed to traverse the residual shunt into the pulmonary artery or superior vena cava. The wire is subsequently snared to establish an arteriovenous circuit. Leveraging the unique structure of the ADO II, we can release the device through either arterial or venous access, providing flexibility in our interventional strategy. In the context of this study, the ADO II was deployed in 10 patients with residual VSDs, and in each case, it successfully achieved complete closure of residual shunts.
Our study has several limitations that warrant consideration. Firstly, the retrospective design may introduce potential biases, associated with a limited sample size. Secondly, the criteria for patient selection were rigorous, and primarily formulated based on the expertise and judgment of experienced clinicians. Additionally, the use of the ADO II occluder for VSD closure is still considered an “off-label” application, utilized judiciously only when dedicated occluders are not suitable. Given the varied surgical histories among patients, it is essential to validate the treatment’s safety and efficacy through larger-scale, prospectively designed studies across diverse patient populations and healthcare centers.
Transcatheter device closure stands out as an efficacious and secure strategy for managing patients with residual shunts following either TCC or surgical repair of CHD. This approach has yielded commendable long-term follow-up outcomes and is poised to emerge as the favored treatment for patients who meet the appropriate criteria. The vast majority of patients achieved successful and enduring closure of residual shunts, characterized by a complete cessation of shunting.
Acknowledgement: We would like to thank all the other staff from the Department of Congenital Heart Disease, General Hospital of Northern Theater Command for their great help with the arrangement and collection of paper and electronic materials.
Funding Statement: None.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Qiguang Wang, Jiawang Xiao; data collection: Jiawang Xiao, Jianming Wang; analysis and interpretation of results: all authors; draft manuscript preparation: Jiawang Xiao. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Readers can access the data used in the study by contacting the corresponding author.
Ethics Approval: This investigation was approved by the Ethics Committee of General Hospital of Northern Theater Command Ethics [Y (2024) 055] and conformed to the principles of the Declaration of Helsinki and its later amendments. The requirement for informed consent was waived due to the retrospective nature of the study.
Conflicts of Interest: The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Tang CZ, Zhao H, Gao WG, Hu DY. Current status of surgical treatment of congenital heart disease in China. Chin J Evid Based Cardiovasc Med. 2009;1(1):68–9 (In Chinese). doi:10.3969/j.issn.1674-4055.2009.01.003. [Google Scholar] [CrossRef]
2. National Health Commission, National Center for Interventional Quality Control, National Center for Cardiovascular Disease, Interventional Quality Control Center for Structural Heart Disease, Working Group for Percutaneous Intervention Treatment of Primary Heart Disease, Cardiovascular Disease Society of Chinese Medical Association, et al. Guidelines for percutaneous interventional treatment of common congenital heart disease (2021 edition). Natl Med J China. 2021;101(38):3054–76 (In Chinese). [Google Scholar]
3. Chinese Society of Extracorporeal Circulation. White book of Chinese cardiovascular Surgery and extracorporeal circulation in 2019. Chin J ECC. 2020;18(4):193–6 (In Chinese). [Google Scholar]
4. Knauth AL, Lock JE, Perry SB, McElhinney DB, Gauvreau K, Landzberg MJ, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004;110(5):501–7. doi:10.1161/01.CIR.0000137116.12176.A6. [Google Scholar] [PubMed] [CrossRef]
5. Congenital Heart Disease Expert Committee of Pediatricians Branch of Chinese Medical Doctor Association, Cardiovascular Group of Pediatrics Branch of Chinese Medical Society, Editorial Committee of Chinese Journal of Pediatrics. Expert consensus on interventional treatment of common congenital heart disease in children. Chin J Pediatr. 2015;53(1):17–24. [Google Scholar]
6. Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA. 1966;196(11):991–2. doi:10.1001/jama.1966.03100240125026. [Google Scholar] [CrossRef]
7. Gao W, Yu ZQ, Li F, Huang MR, Li J, Zhong YM. Interventional management for the post-operative residual lesions in children with congenital heart disease. J Clin Pediatr. 2011;29(7):613–6. [Google Scholar]
8. Zhou W, Li F, Fu L, Gao W, Guo Y, Liu T, et al. Clinical experience of transcatheter closure for residual ventricular septal defect in pediatric patients. Congeni Heart Dis. 2016;11(4):323–31. doi:10.1111/chd.12357. [Google Scholar] [PubMed] [CrossRef]
9. Abdelmohsen GA, Gabel HA, Al-Ata JA, Bahaidarah SA, Alkhushi NA, Abdelsalam MH, et al. Percutaneous closure of postoperative residual ventricular septal defects, including dehiscence of surgical patches. Cardiovasc Diagn Ther. 2023;13(4):710–27. doi:10.21037/cdt-22-624. [Google Scholar] [PubMed] [CrossRef]
10. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, Van Rijen EH, Utens EM, et al. Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22–34 years. Eur Heart J. 2004;25(12):1057–62. doi:10.1016/j.ehj.2004.04.012. [Google Scholar] [PubMed] [CrossRef]
11. Bibevski S, Ruzmetov M, Mendoza L, Decker J, Vandale B, Jayakumar KA, et al. The destiny of postoperative residual ventricular septal defects after surgical repair in infants and children. World J Pediatr Congenital Heart Surg. 2020;11(4):438–43. doi:10.1177/2150135120918537. [Google Scholar] [PubMed] [CrossRef]
12. Deng CX, Huang P, Luo JW, Chen RW, Yang GX, Chen WJ, et al. Residual shunts following isolated surgical ventricular septal defect closure: risk factors and spontaneous closure. Pediatr cardiol. 2020;41(1):38–45. doi:10.1007/s00246-019-02218-9. [Google Scholar] [PubMed] [CrossRef]
13. Schipper M, Slieker MG, Schoof PH, Breur JM. Surgical repair of ventricular septal defect; contemporary results and risk factors for a complicated course. Pediatr Cardiol. 2017;38(2):264–70. doi:10.1007/s00246-016-1508-2. [Google Scholar] [PubMed] [CrossRef]
14. Venczelova Z, Tittel P, Masura J. The new Amplatzer duct occluder II: when is its use advantageous? Cardiol Young. 2011;21(5):495–504. doi:10.1017/S1047951111000230. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools