 Open Access
Open Access
REVIEW
Arterial Duct Stenting Versus Modified Blalock-Taussig Shunt in Patient with Ductal-Dependent Pulmonary Circulation: Systematic Review & Meta-Analysis
1 Department of Cardiothoracic & Vascular Surgery, Prof. Dr. I.G.N.G Ngoerah General Hospital, Denpasar, 80113, Indonesia
2 Faculty of Medicine, Udayana University, Denpasar, 80232, Indonesia
* Corresponding Author: Ketut Putu Yasa. Email:
Congenital Heart Disease 2024, 19(2), 139-156. https://doi.org/10.32604/chd.2024.050348
Received 03 February 2024; Accepted 08 April 2024; Issue published 16 May 2024
Abstract
Objective: Patients with ductal-dependent pulmonary circulation require alternative blood flow to provide and maintain adequate oxygenation. Modified Blalock-Taussig Shunt (MBTS) has been the standard for providing such a result. Currently, less invasive methods such as Arterial Duct (AD) stenting have been performed as alternatives. This study aims to compare the outcome of AD stenting and MBTS. Method: Systematic research was performed in online databases using the PRISMA protocol. The outcomes measured were 30-day mortality, complication, unplanned intervention, oxygen saturation, duration of hospital, and ICU length of stay. Any comparative study provided with full text is included. The outcome of each study was analyzed using a trandom effects model with relative risk and mean difference as the effect size. Bias risk assessment was conducted using the Newcastle-Ottawa Scale. All analyses were performed using Review Manager 5.4.1. Result: A total of 11 studies with 3154 samples included in this study. There is no significant difference in 30-day mortality between the two groups (p-value = 0.10). However, there is significantly less complication (RR 0.53 [0.35, 0.82]; p-value = 0.004) and unplanned intervention (RR 0.59 [0.38, 0.92]; p-value = 0.02) in the AD stent group. Comparison of the Nakata index showed no significant difference (p-value = 0.88). Post-operative oxygen saturation was measured significantly higher in the AD stenting (MD 1.80 [0.85, 2.74]; p-value = 0.0002). However, AD stent group shows significantly lower long-term oxygen saturation (MD −8.43 [−14.38, −2.48]; p-value = 0.005). Both hospital and ICU length of stay was significantly shorter in the AD stent group (MD −8.30 [−11.13, −5.48]; p-value < 0.00001; MD −5.09 [−7.79, −2.38]; p-value = 0.0002). Conclusion: AD stenting provides comparable outcomes relative to MBTS as it provides less complication and unplanned intervention and higher post-procedural O saturation. However, MBTS proved its superiority in maintaining higher long-term oxygen saturation and still became the preferred option to manage complex cases where stenting is either challenging or unsuccessful.Keywords
Nomenclature
| DDPC | Duct-dependent Pulmonary Circulation |
| AD stent | Arterial Duct Stent |
| MBTS | Modified Blalock-Taussig Shunt |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PA | Pulmonary Artery |
| ICU | Intensive Care Unit |
| IQR | Inter Quartile Range |
| RR | Relative Risk/Risk Ratio |
| MD | Mean Difference |
| CI | Confidence Interval |
Neonates and infants with duct-dependent cardiac lesions to the pulmonary circulation may require initial palliative treatment to survive critical conditions. The goal is simply to provide adequate flow to the pulmonary circulation so that adequate oxygenation can be achieved and maintained until the next palliative stage commences. The creation of surgical shunt has long been the standard to treat such cases. Since the discovery of systemic to pulmonary shunt by Alfred Blalock and Hellen Taussig in 1944 many methods have been tried with various outcomes. Among all variations of shunts, the BT Shunt and Modified Blalock-Taussig Shunt (MBTS) have been widely accepted as the initial palliative stage to provide and maintain adequate flow to the pulmonary circulation. The fundamental difference between these two is the use of prosthetic graft which provides more benefits such as less tendency to deform hypoplastic PAs, less need for mediastinal dissection, preservation of upper extremity blood flow, consistent shunt flow (regulated by the internal diameter of the ostia of the innominate or subclavian arteries), and adequate and flexible length. With these benefits, MBTS remains the preferred choice for surgical systemic-to-pulmonary shunt [1,2].
However, MBTS still requires invasive open surgery to perform and comes with its risks and complications. Rather than creating new shunts, alternatives were then developed to make use of available physiological shunts simply by preventing them from closing. The arterial duct which is part of fetal circulation provides a shunt from pulmonary to systemic circulation. When this duct is preserved either with drugs or stenting, in neonates and infants the shunt will be reversed by the pressure gradient and may provide alternative blood flow to pulmonary circulation. Hence, Arterial Duct (AD) stenting starts to become the alternative to provide adequate flow to the pulmonary circulation therefore providing and maintaining adequate oxygenation and increasing the survival of patients in such cases. AD stenting also has its risks and possible complications, such as stent stenosis, thrombosis, dislodgement, cardiac perforation, and other risks that can result in mortality and morbidity that need unplanned reintervention or even conversion to surgical shunt. However, the pre-operative protocol may be placed in the patient selection stage, such as assessing procedural risk, considering comorbid and cardiac anomaly, and assessing Patent Ductus Arteriosus (PDA) size and tortuosity to ensure successful stenting, thus improving clinical outcomes and preventing dire complications.
Individual comparative studies between both interventions showed that AD stenting may become a comparable alternative, some study claims AD stenting provides better outcomes. However, some of these studies are limited to small sample sizes, and their single-center study design. This study aims to fill this gap by performing a systematic review and meta-analysis to evaluate any significant difference in outcomes between AD stenting and MBTS.
Systematic searching was conducted in online databases (PubMed, ScienceDirect, and Google Scholar) in December 2023 following PRISMA guidelines [3]. Keyword used in this study was aimed at capturing population (infants or neonates with DDPC: ductal [All Fields] AND dependent [All Fields] AND (“pulmonary circulation” [MeSH Terms] OR (“pulmonary” [All Fields] AND “circulation” [All Fields]) OR “pulmonary circulation” [All Fields])), intervention (AD stenting: (PDA [All Fields] OR “Ductus Arteriosus, Patent” [Mesh]) AND (“stents” [MeSH Terms] OR “stents” [All Fields] OR “stenting” [All Fields])), and comparator (MBTS: (“Blalock-Taussig Procedure” [Mesh] OR (“surgical procedures, operative” [MeSH Terms] OR surgical [Text Word]) AND shunt [All Fields])). The study included was full text of a comparative study. Any non-comparative study, case series/report, or letter to the editor was excluded.
2.2 Outcomes & Quality and Bias Risk Assessment
The outcome measured was 30-day mortality, complication (any complication post-procedure), unplanned intervention (any intervention to treat cyanosis), oxygen saturation (post-procedure measured within 24-h and long term measured near the next stage commence), duration of hospital and ICU length of stay. Two authors independently screened, extract the data, and asses each study’s quality and bias using Newcastle-Ottawa Scale (NOS) and categorized them as follows: (1) Good; (2) Fair; (3) Poor [4]. We only include good and fair studies and exclude poor-quality studies. Independent screening and data extraction were conducted to prevent misinterpretation and error in data collection. Any disagreements were resolved by discussion and consensus.
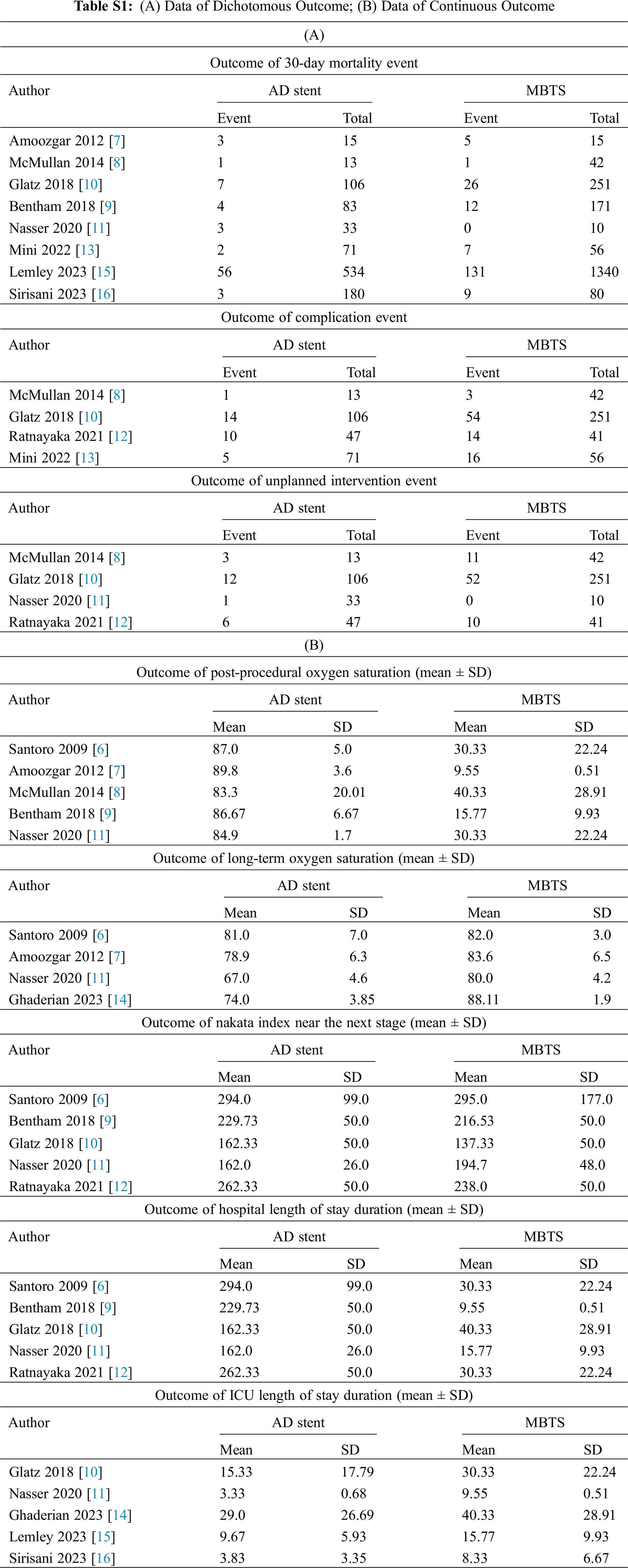
Meta-analysis was performed for similar outcomes reported by a minimum of two studies. Data were classified as dichotomous and continuous outcomes. Some continuous outcomes (Nakata index, post-procedure oxygen saturation, length of stay) were reported using mean and standard deviation, as well as median and interquartile range. Values that were provided as median and IQR were converted to mean and standard deviation using the method described by Hozo et al. [5] Dichotomous data were analyzed using relative risk (RR) as effect size with 95% CI. Meanwhile, continuous data were analyzed using mean difference (MD) as effect size with 95% CI. Both sets of data were analyzed using a random-effects model. All analyses were performed using Review Manager 5.4.1. Statistical heterogeneity to was calculated using I2 which is classified as follows: (1) I2 < 25%: low heterogeneity; (2) I2 = 25%–75%: moderate heterogeneity; (3) I2 > 75%: high heterogeneity. p-value < 0.05 was defined as a statistically significant result. All data were tabulated in the Appendix Files, and all analyses were presented using a forest plot.
3.1 Study Selection & Characteristics
Keyword search initially identified 903 individual studies, which were then screened using title and abstract alone. Of these, 892 and 21 studies were excluded for not being related to the topics or fulfilling the aim of the meta-analysis and for lacking accessible abstracts. Out of all included studies, 22 were retrieved, but 11 studies were subsequently excluded due to the wrong type of study design and the same sample population. A total of 11 studies comprising 3154 samples were included in this paper. The PRISMA flow diagram of the study selection process is depicted in Appendix A. Three papers were multicenter studies, one study included two centers as a population, and seven studies were single-center studies. One paper by Ghaderian in 2023 was cross-sectional, two studies by Bentham in 2017 and Nasser in 2020 were cohort prospective, and the rest were cohort retrospectives. The general characteristics of the studies are described in Table 1. Details of clinical characteristics are depicted in Appendix B.
3.2 Quality and Bias Risk Assessment
The assessment was performed by two independent reviewers using the Newcastle-Ottawa Scale (NOS) for all 11 included studies. Ten studies were classified as good quality, while one study was classified as fair (Appendix C). Any disagreements were resolved through discussion and consensus.
The detailed data for each study are depicted in Appendix D, Table S1. The analysis of 30-day mortality was conducted using data from seven studies. The pooled mortality rate in the AD stent group was 7.88%, whereas in the MBTS group was 9.98%. Although the forest plot appears to favor the AD stent group, there is no significant difference between AD stenting and MBTS (RR 0.63 [0.36, 1.10]; p-value = 0.10; I2 = 53%, Fig. 1) [7–11,13,15,16].
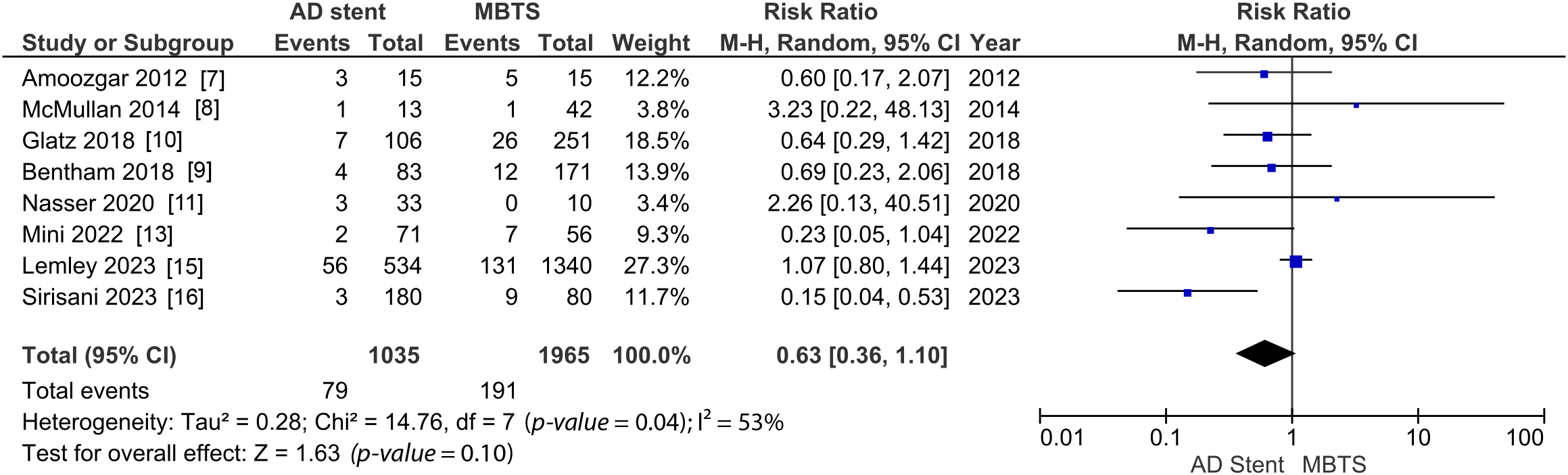
Figure 1: Pooled estimate of 30-day mortality for AD stent vs. MBTS
However, there are significantly fewer complications (RR 0.53 [0.35, 0.82]; p-value = 0.004; I2 = 13%, Fig. 2) and unplanned intervention to treat cyanosis (RR 0.59 [0.38, 0.92]; p-value = 0.02; I2 = 0%, Fig. 3) observed in the AD stent group [8,10–13].
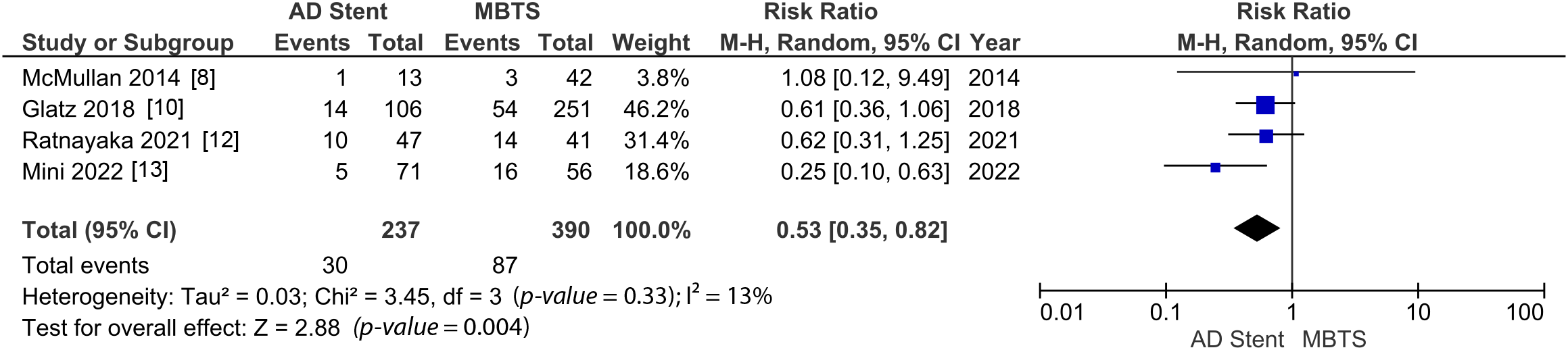
Figure 2: Pooled estimate of post-procedural complication for AD stent vs. MBTS
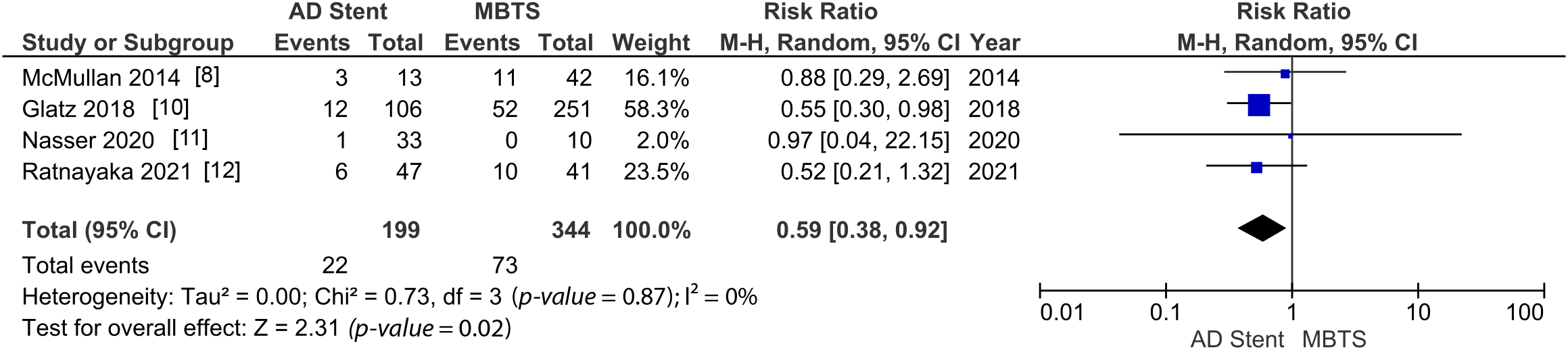
Figure 3: A pooled estimate of unplanned intervention for AD stent vs. MBTS
The comparison of the Nakata index revealed no significant difference between the two groups (MD 1.87 [−23.20, 26.93]; p-value = 0.88; I2 = 93%, Fig. 4) [6,9–13,14].
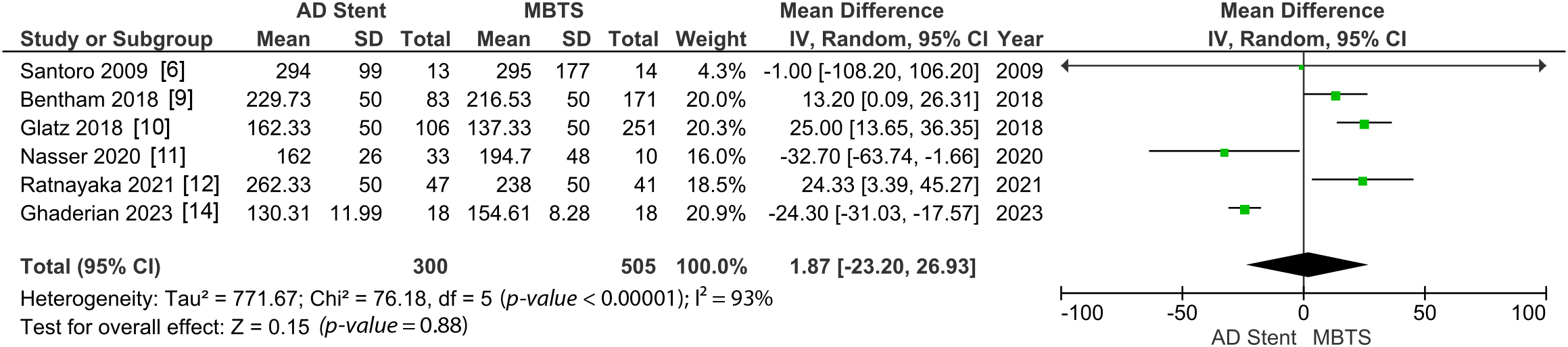
Figure 4: Pooled estimate of Nakata index for AD stent vs. MBTS
Postoperative oxygen saturation was measured significantly higher in the AD stenting (MD 1.80 [0.85, 2.74]; p-value = 0.0002; I2 = 0%, Fig. 5). However, in the long term, the AD stent group shows significantly lower oxygen saturation (MD −8.43 [−14.38, −2.48]; p-value = 0.005; I2 = 93%, Fig. 6) [6–9,11,14].
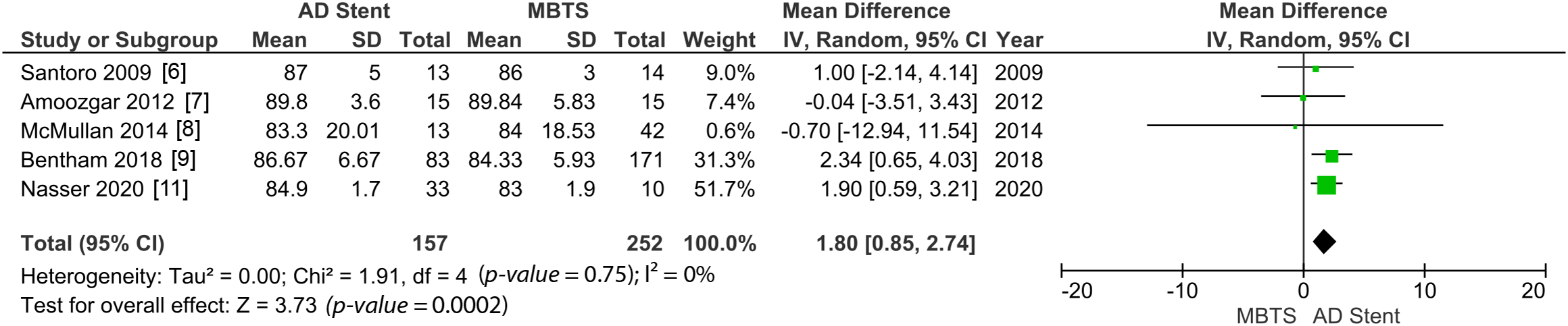
Figure 5: A pooled estimate of post-procedural oxygen saturation for AD stent vs. MBTS
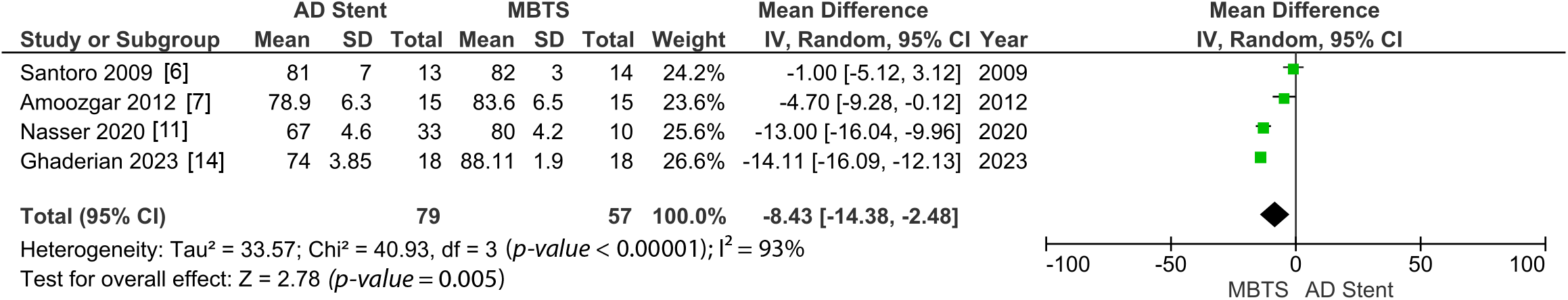
Figure 6: A pooled estimate of long-term oxygen saturation for AD stent vs. MBTS
Both hospital and ICU length of stay were significantly shorter in the AD stent group (MD −8.30 [−11.13, −5.48]; p-value < 0.00001; I2 = 82%, Fig. 7; MD −5.09 [−7.79, −2.38]; p-value = 0.0002; I2 = 94%, Fig. 8) [10–12,14–16].
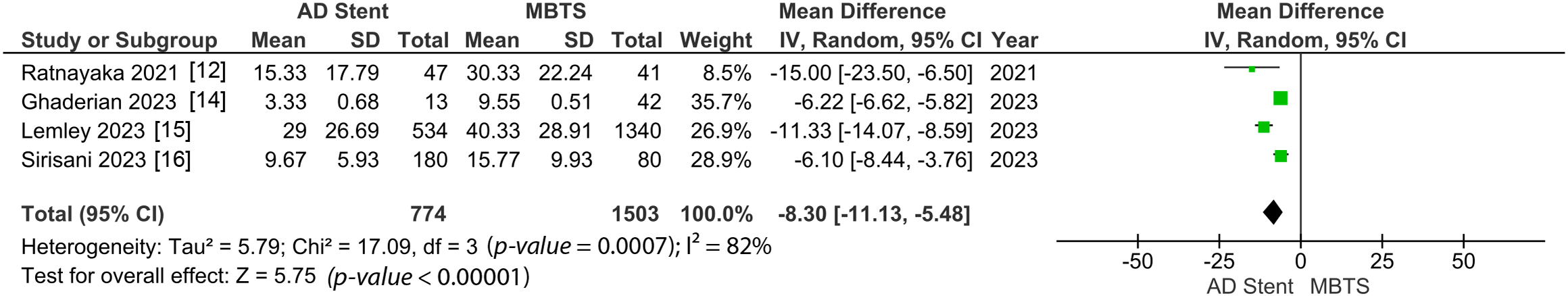
Figure 7: Pooled estimate of hospital length of stay for AD stent vs. MBTS
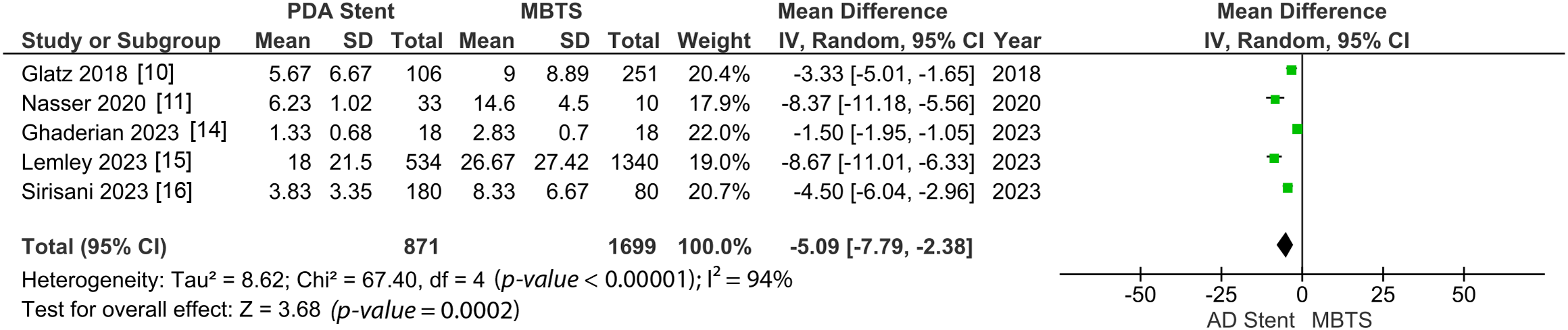
Figure 8: Pooled estimate of ICU length of stay for AD stent vs. MBTS
The goal of palliative treatment in critical congenital heart disease is generally to provide adequate circulation and prepare the patient for the next stage. In cases of ductal-dependent pulmonary circulation (DDPC), the palliative procedure involves redirecting blood flow to the pulmonary artery from another source, thereby ensuring sufficient blood flow to the pulmonary circulation and, consequently, adequate oxygenation to ensure overall survival. For a long time, MBTS has been the accepted method to address this problem. However, in this era, less invasive methods have been developed to achieve comparable or even superior outcomes to invasive surgical procedures. Several individual studies have been conducted, mostly retrospectively, aiming to compare outcomes between the two groups. The limitation of small sample size and single-center study designs need to be addressed before applying the findings of those studies to other centers. This study aims to systematically review these studies and conduct a meta-analysis of the same outcome measured thereby generating a more precise estimation that can be applied considerably in the future.
4.1 The Decision of AD Stenting & MBTS
Different centers follow distinct protocols regarding the selection of palliative treatment in DDPC cases. Two predominant patterns have been observed: universal and selective AD stenting. Notably, only one study, conducted by Nasser et al. implemented universal AD stenting from the outset, resulting in 21.4% (9/42) cases requiring immediate conversion to surgical shunt. Another study by Ratnayaka et al. initially employed selective AD stenting for the first five years of their study, before transitioning to universal AD stenting in 2018–2020 [12]. Although this change led to an imbalance increase in AD stenting procedures in their center, their study compared outcomes between the selective stenting and the universal stenting eras, revealing no significant difference in interstage and overall mortality, complications, and unplanned interventions. The remaining study adopted selective AD stenting as a protocol. In the study by Santoro et al., AD stenting was attempted in patients with high surgical risk or when short-term support to pulmonary circulation was anticipated [6]. McMullan et al. made case-by-case decisions between AD stenting and MBTS based on procedural risk, appropriateness of PA size, AD anatomy, and patient comorbidities [8]. Ghaderian et al. strategy relied on age; all infants aged under two months old were offered AD stent placement, while MBTS was performed in patients over 2 months old [14]. These considerations were aimed at anticipating the outcomes in order to enhance survival and mitigate complications.
In other related studies by Qureshi et al., a classification scheme has been proposed to assess ductal morphology in patients undergoing AD stenting. This classification is based on the tortuosity index (TI) using Ductal Curvature Index (DCI), with three types identified: (1) Type I (straight–DCI 0–0.16); (2) Type II (one turn–DCI 0.12–0.38); (3) Type III (multiple turns–DCI 0.17–0.45), where a greater TI is associated with greater risk of unplanned intervention. This study demonstrates that AD stenting can be performed in high tortuosity Patent Ductus Arteriosus (PDA), with the classification scheme aiding in vascular approach decisions. Subsequent studies by Mini et al. further examine ductal stenting outcomes in patients with high tortuosity PDA (DCI ≥ 0.45). In their initial study, 80% of patients with DCI ≥ 0.45 failed to achieve the primary outcome. Further analysis revealed that this particular group is at elevated risk for early surgery or unplanned reintervention. In a later study by the same author, 15 patients with DCI ≥ 0.45 underwent PDA stenting, of whom 8 required later conversion to surgical shunt due to desaturation or acute stent occlusion. This scenario poses a challenge in the perioperative stage due to pre-operative desaturation and its negative changes to pulmonary arteries and the lungs. Consequently, Mini et al. suggest that: (1) the DCI classification can serve as a useful guide in clinical decision-making for patients with challenging anatomy; (2) although PDA stenting is feasible in cases of high tortuosity, and while surgical shunts carry their own risk, MBTS could be preferred as alternative or prepared as emergency measure. This underscores the importance of considering procedural risk (based on age, clinical condition, cardiac anomaly), pulmonary artery size, and ductal anatomy in choosing appropriate treatment to achieve optimal outcomes [13,17,18].
Each study demonstrates the growing interest in utilizing AD stenting as the initial choice for palliative treatments, as it offers similar or superior overall outcomes. In a study by Glatz et al., 43.3% of the AD stenting group had pulmonary atresia with the intact interventricular septum (PA/IVS), a condition conducive to stent placement due to favorable anatomical characteristic (normal origin from proximal descending aorta, short straight course, and insert right to main PA) [10]. While certain conditions such as PA/IVS may facilitate stent placement, several morphological features may pose challenges for stent placement, making MBTS a safer option for palliative treatment in these cases. Hence, it is evident that selection bias exists in the studies we examined.
4.2 Mortality, Complication, and Unplanned Intervention
The primary cause of death in both AD stenting and MBTS is primarily related to complications associated with the interventions. The overall mortality rates for modified BT shunt vary across different centers and are attributed to factors such as shunt thrombosis, over-shunting, atrioventricular valve regurgitation, myocardial ischemia, necrotizing enterocolitis, and the need for extracorporeal membrane oxygenation support. For instance, in one study by Dave, the overall mortality rate of MBTS ranged from 7% to 9%. In another cohort study by Oofuvong et al., the mortality rate prior to Glenn/total repair could be as high as 31.3% [2,19]. Thrombotic occlusion of an MBTS can result in life-threatening hypoxemia, necessitating surgical or catheter-based reintervention as a rescue procedure. Conversely, in the case of AD stenting, there is a progressive decline in oxygen levels due to intimal ingrowth within the stent, fibro intimal peel formation, and thrombus formation within the shunt, leading to sudden death in 5% to 20% of patients during follow-up [10]. However, the overall mortality rate between patients undergoing AD stenting and MBTS did not show a significant difference [20].
In this systematic review, each included study indicates no significant difference in 30-day mortality, consistent with two recent meta-analyses [21,22]. Additionally, McMullan et al. demonstrated no significant difference in survival to the next stage (second-stage palliation or definitive repair) [8]. Conversely, Sirisani et al. reported a significantly lower overall mortality rate in the AD stent group compared to the MBTS group. (11.25% vs. 1.64%; p = 0.001) [16]. Bentham et al. conducted a survival analysis of longer-term outcomes revealing a significant reduction in mortality associated with AD stenting before repair (HR of 0.25 [0.07–0.85], p = 0.026) [9]. However, Glatz et al. analyzed the adjusted effect of treatment strategy on time-dependent outcomes with survival models, showing no significant difference in terms of death between the two groups [10].
Significant differences were observed in complications and unplanned interventions. Our study found that there is a 51% reduced risk of complication in the AD stenting group compared to the MBTS group, consistent with findings from three recent meta-analyses [21,23]. This result pooled various complications including acute kidney injury, necrotizing enterocolitis, diaphragm paralysis, vocal cord palsy, thrombosis, stroke, bleeding, post-operative infection, cardiac arrest, and arrhythmia [8,10,12,13].
Threatening hypoxemia can result from occlusion in the stent/shunt, significant leakage, and stent migration, necessitating unplanned interventions to restore blood flow and alleviate cyanosis. Our study revealed a 59% less risk of unplanned intervention in the AD stent compared to MBTS. Unplanned intervention was defined as any surgical or transcatheter intervention intended to treat cyanosis of any cause after the procedure. Stenting remains a challenging procedure to perform due to various conditions and anatomical variation, often requiring immediate intervention even emergency surgical shunt creation. Santoro et al. report four failed stenting attempts which were excluded from their study without clear information on the reason for subsequent treatment done in these patients [6]. Amoozgar et al. reported three failed stent attempts, with one of them due to difficulty achieving vascular access, and in the two remaining patients the wire cannot cross even with axillary access, all of which require immediate referral for surgery [7]. McMullan et al. included 13 successfully stented patients with no procedure-related death. This study also demonstrated that all MBTS patients needing unplanned intervention required multiple reintervention (surgical and transcatheter), while no case in the AD stent group required more than one reintervention [8]. Bentham et al. reported 13 failed attempts, with various reasons including four cases due to failure to cross tortuous duct; two cases failed due to inability to cover proximal duct; one failure due to RV perforation; one case not achieving adequate flow, one failure due to duct dissection; and four early stent failure (inadequate flow, thrombosis, in-stent stenosis), all of which required surgical referral [9]. Glatz et al. reported 12 cases of AD stent requiring reintervention, with five cases converted to surgical shunts. Their multicenter study in 2018 presented the most detailed data and analysis, recording unplanned interventions including stenting of the pulmonary or systemic artery, angioplasty of stent/shunt or pulmonary artery (with or without stent), RVOT stent, balloon atrial septostomy, surgical revision of both groups, drainage of hemopericardium, and early superior cavopulmonary connection [10]. Nasser et al. encountered similar cases where nine out of 42 patients initially offered AD stenting later failed to be performed and were immediately referred to surgical shunt, which later classified and followed up as MBTS group. In one case, a second stent placement was needed to cover the entire ductus after the first stent proved inadequate [11]. Ratnayaka et al. specifically reported conversion to surgical shunt in eight of 15 patients who have tortuous PDA anatomy. Ratnayaka et al. showed two general intervention post-procedures: surgical revision and transcatheter approach. Their study showed there is significantly more surgical revision in the MBTS group compared to the AD stent. Although more transcatheter approach re-intervention (angioplasty with or without stent) were seen in the AD stent group, group analysis showed no statistical significance [12]. All this information further highlights the importance of pre-operative consideration in terms of patient selection and consideration to improve clinical outcomes in each group.
Several individual cases have demonstrated that successful stenting or the use of drug-eluting stent in occluded MBTS may prolong survival, improve saturation, improve exercise tolerance, and lower hematocrit levels [24–27]. In studies by Bonnet et al., percutaneous interventions have been shown to effectively restore MBTS patency albeit with potential major complications [27]. These findings suggest that, despite the higher prevalence of complications and unplanned interventions in MBTS groups, reliable rescue measures are available to prolong survival and improve outcomes. However, further research is needed to investigate this issue more specifically.
4.3 Nakata Index and Oxygen Saturation
Pulmonary artery size is measured near the next stage of palliation. with the distance to the next stages varying between studies based on each center’s protocol and the patient’s general condition. Individual studies in our analysis showed no significant difference in the Nakata index in both groups, and so does the meta-analysis of the pooled result. This finding is consistent with one recent meta-analysis by Tseng et al. in 2022, but not consistent with one study by Abdelmassih et al. in 2020. The difference between our study and the study by Abdelmassih is that they use a fixed effect model for analysis [21,23]. However, even Abdelmassih’s random effect analysis showed no significant difference in this outcome. Two individual studies indicated that AD stents showed more symmetrical growth of the pulmonary artery [6,10].
As a general rule, an average Nakata index of 330 mm2/m2 is considered normal. However, the lowest Nakata index to preclude a patient from surgery has not yet been established. One study suggested that a preoperative Nakata index <250 mm2/m2 does not restrict the functional efficacy of the later Fontal circulation [28]. Another study by Itatani et al. showed that only those with a Nakata index below 110 mm2/m2 would experience exercise intolerance and increased pressure in later Fontan circulation [29]. Overall, the accepted Nakata index for the next palliation procedure may depend on various factors and should be determined on a case-by-case basis. Another method can be used to measure the PA index such as the McGoon ratio and Lower Lobe Index (LLI). Further analysis is required to determine which method is better for estimating the prognosis of the later stage.
In terms of oxygen saturation, both AD stenting and MBTS showed significant improvement. Our study indicated significantly higher post-procedural saturation in the AD stent groups, consistent with two recent meta-analyses [22,23]. However, in the longer term, saturation was significantly higher in the MBTS groups. This difference may be attributed to intimal ingrowth within the stent, fibro intimal peel formation and thrombus within the shunt, affecting the patency and durability of the stent, causing a progressive decline of saturation in AD stent in the long run [10,20]. Nonetheless, reintervention such as stent re-dilatation after AD stenting is a feasible and safe treatment option that can improve pulmonary blood flow. Strut dilation of branch pulmonary artery stenosis after arterial duct stenting has been shown to augment pulmonary blood flow and improve oxygen saturation levels [30].
In this study, the hospital and ICU length of stay (LOS) were significantly shorter in the AD stent group, a result consistent with three recent meta-analyses [21–23]. Post-procedural hospital LOS and ICU LOS depended on postoperative complications and reintervention. In one multi-center study by Valencia et al., longer hospital and ICU LOS in MBTS were associated with higher hospital charges and costs. However, this analysis also needs to consider the use of other services such as imaging, lab, drugs, and all the costs contributed to the overall cost which will be different in each case in each center [31]. In this meta-analysis, we were unable to calculate the cost across studies due to a lack of data measuring that particular outcome.
The nature of the observational study remains a limitation in this study. All studies are prone to confounders and covariates that may not have been considered in each analysis. The variability in the selection protocol of treatment in DDPC cases across different centers introduces selection bias in the included studies. Furthermore, the imbalance in sample size, difference in follow-up time, and variation in baseline characteristics across studies contribute to high heterogeneity in this study. To determine the superiority of both treatments, a randomized trial with a detailed protocol is necessary to provide results with greater confidence.
This meta-analysis demonstrates that AD stenting provides comparable outcomes relative to MBTS, with fewer complications and unplanned interventions, higher short-term oxygen saturation, and shorter lengths of stay. However, MBTS has shown superiority in maintaining higher long-term oxygen saturation. Additionally, MBTS remains the preferred option for managing complex cases where stenting is either challenging or unsuccessful. Therefore, to achieve the most optimal outcomes, the pre-operative protocol should be placed regarding patient selection and careful consideration when choosing the appropriate intervention.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: KPY, NSSB; data collection: NSSB, PFKP; analysis and interpretation of results: KPY, NSSB; draft manuscript preparation: KPY, NSSB, PFKP. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data set used for this meta-analysis will be shared upon request from the study authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Yuh DD, Vricella LA, Yang S, Doty JR. Johns Hopkins textbook of cardiothoracic surgery. 2ndedition. New York City, New York, US: McGraw Hill Education; 2014. [Google Scholar]
2. Dave HH. Modified Blalock-Taussig shunt: simple but unpredictable. Eur J Carido-Thoracic Surg. 2016;50:178–9. doi:10.1093/ejcts/ezw115. [Google Scholar] [PubMed] [CrossRef]
3. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA, 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:1–7. doi:10.1136/bmj.n71. [Google Scholar] [PubMed] [CrossRef]
4. O’Connell GWD, Peterson J, Welch V, Losos M, Tugwell PBS. Newcastle-Ottawa quality assessment scale. Ottawa Hosp Res Inst. 2014;7:2–4. [Google Scholar]
5. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10. [Google Scholar]
6. Santoro G, Capozzi G, Caianiello G, Palladino MT, Marrone C, Farina G, et al. Pulmonary artery growth after palliation of congenital heart disease with duct-dependent pulmonary circulation. J Am Coll Cardiol. 2009;54:2180–6. doi:10.1016/j.jacc.2009.07.043. [Google Scholar] [PubMed] [CrossRef]
7. Amoozgar H, Cheriki S, Borzoee M. Short-term result of ductus arteriosus stent implantation compared with surgically created shunts. Pediatr Cardiol. 2012;33(8):1288–94. doi:10.1007/s00246-012-0304-x. [Google Scholar] [PubMed] [CrossRef]
8. Mcmullan DM, Permut C, Jones K, Johnston TA, Rubio AE. Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. J Thorac Cardiovasc Surg. 2014;147:397–403. doi:10.1016/j.jtcvs.2013.07.052. [Google Scholar] [PubMed] [CrossRef]
9. Bentham JR, Zava NK, Harrison WJ, Shauq A, Kalantre A, Derrick G, et al. Duct stenting versus modified blalock-taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018;137(6):581–8. [Google Scholar] [PubMed]
10. Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, McDonnell A, et al. Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow. Am Hear Assoc. 2018;137(6):589–601. [Google Scholar]
11. Nasser BA, Abdulrahman M, Qwaee AAL, Alakfash A, Mohamad T, Kabbani MS. Impact of stent of ductus arteriosus and modified Blalock–Taussig shunt on pulmonary arteries growth and second-stage surgery in infants with ductus-dependent pulmonary circulation impact of stent of ductus arteriosus and modified Blalocke Taussigs. J Saudi Hear Assoc. 2020;32:86–92. doi:10.37616/2212-5043.1014. [Google Scholar] [PubMed] [CrossRef]
12. Ratnayaka K, Nageotte SJ, Moore JW, Guyon PW, Bhandari K, Weber RL, et al. Patent ductus arteriosus stenting for all ductal-waning use of Blalock-Taussig shunts. Circ Cardiovasc Interv. 2021;14(3):272–81. doi:10.1161/CIRCINTERVENTIONS.120.009520. [Google Scholar] [PubMed] [CrossRef]
13. Mini N, Schneider MBE, Asfour B, Mikus M. Duct stenting vs. Modified Blalock-Taussig shunt: New insights learned from high-risk patients with duct-dependent pulmonary circulation. Front Cardiovasc Med. 2022;9:1–9. doi:10.3389/fcvm.2022.933959. [Google Scholar] [PubMed] [CrossRef]
14. Ghaderian M, Behdad S, Mokhtari M, Salamati L. Comparison of patent ductus arteriosus stenting and Blalock‐Taussig shunt in ductal dependent blood flow congenital heart disease and decreased pulmonary blood flow. Hear Views by Wolters Kluwer. 2023;24:11–6. doi:10.4103/heartviews.heartviews_84_22. [Google Scholar] [PubMed] [CrossRef]
15. Lemley BA, Wu L, Roberts AL, Shinohara RT, Gillespie MJ, Rome JJ, et al. Trends in ductus arteriosus stent versus ductal-dependent pulmonary blood flow: an observational study using the pediatric health information systems database. Am Hear Assoc. 2023;12(23):1–10. [Google Scholar]
16. Sirisani JD, Haranal M, Soo KW, Sivalingam S, Khalid KFM. Comparison of immediate intensive care outcomes of patent ductus arteriosus stenting versus modified Blalock-Taussig-Thomas shunt in infants with ductal-dependent pulmonary circulation. Pediatr Cardiol. 2023;35(1):1–15. doi:10.21203/rs.3.rs-3256682/v1. [Google Scholar] [CrossRef]
17. Qureshi AM, Goldstein BH, Glatz AC, Agrawal H, Aggarwal V, Ligon RA, et al. Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting. Catheter Cardiovasc Interv. 2019;93:933–43. doi:10.1002/ccd.28125. [Google Scholar] [PubMed] [CrossRef]
18. Mini N, Schneider MBE, Zartner PA. Use of the ductal curvature index to assess the risk of ductal stenting in patients with duct-dependent pulmonary circulation. Transl Pediatr. 2021;10:1307–16. doi:10.21037/tp-21-17. [Google Scholar] [PubMed] [CrossRef]
19. Oofuvong M, Tanasansuttiporn J, Wasinwong W, Chittithavorn V, Duangpakdee P, Jarutach J, et al. Predictors of death after receiving a modified Blalock-Taussig shunt in cyanotic heart children: a competing risk analysis. PLoS One. 2021;16:1–18. doi:10.1371/journal.pone.0245754. [Google Scholar] [PubMed] [CrossRef]
20. Sivakumar K. PDA stenting in duct-dependent ductal stenting for pulmonary circulation. Card Catheter Congenit Hear Dis. 2014;8(2):375–99. [Google Scholar]
21. Tseng SY, Truong VT, Peck D, Kandi S, Brayer S, Jason DP, et al. Patent ductus arteriosus stent versus surgical aortopulmonary shunt for initial palliation of cyanotic congenital heart. Am Hear Assoc. 2022;11(13):1–12. doi:10.1161/JAHA.121.024721. [Google Scholar] [PubMed] [CrossRef]
22. Alsagheir A, Koziarz A, Makhdoum A, Contreras J, Alraddadi H, Abdalla T, et al. Duct stenting versus modified Blalock-Taussig shunt in neonates and infants with duct-dependent pulmonary blood flow: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2020. doi:10.1016/j.jtcvs.2020.06.008. [Google Scholar] [PubMed] [CrossRef]
23. Abdelmassih AF, Menshawey R, Menshawey E, El-maghraby AE, Sabry AO, Kamel A, et al. Blalock-Taussig shunt versus ductal stent in the palliation of duct dependent corresponding author information: Rahma menshawey. Curr Probl Cardiol. 2021:100885. doi:10.1016/j.cpcardiol.2021.100885. [Google Scholar] [PubMed] [CrossRef]
24. Illner J, Reinecke H, Baumgartner H, Kaleschke G. Stenting of modified Blalock–Taussig shunt in adult with palliated pulmonary atresia and ventricular septal defect: a case report. Eur Soc Cardiol. 2019;3(4):1–4. doi:10.1093/ehjcr/ytz201. [Google Scholar] [PubMed] [CrossRef]
25. Gopalakrishnan A, Sasidharan B, Menon S, Krishnamoorthy KM. Drug-eluting stent for acute Blalock-Taussig shunt thrombosis in a child—case report. Egypt Hear J. 2020;72(1):0–2. doi:10.1186/s43044-020-00084-y. [Google Scholar] [PubMed] [CrossRef]
26. Lale P, Aggarwal N, Agarwal M, Joshi RK, Joshi R, Vivek BS. Endovascular stent implantation as a primary method of treatment for blocked modified Blalock–Taussig shunt. Ann Pediatr Cardiol. 2022;15(2):2021–3. doi:10.4103/apc.apc_170_21. [Google Scholar] [PubMed] [CrossRef]
27. Bonnet M, Petit J, Lambert V, Brenot P, Riou JY, Angel CY, et al. Catheter-based interventions for modified Blalock–Taussig shunt obstruction: a 20-year experience. Pediatr Cardiol. 2014;36(4):835–41. doi:10.1007/s00246-014-1086-0. [Google Scholar] [PubMed] [CrossRef]
28. Euringer C, Schaeffer T, Heinisch PP, Burri M, Georgiev S, Lemmer J, et al. Changes in pulmonary artery index and its relation to outcome after stage II palliation in patients with hypoplastic left heart syndrome. Eur J Cardio-Thoracic Surg. 2023;63:1–15. doi:10.1093/ejcts/ezad077. [Google Scholar] [PubMed] [CrossRef]
29. Itatani K, Miyaji K, Nakahata Y, Ohara K. The lower limit of the pulmonary artery index for the extracardiac Fontan circulation. J Thorac Cardiovasc Surg. 2011;142:127–35. doi:10.1016/j.jtcvs.2010.11.033. [Google Scholar] [PubMed] [CrossRef]
30. Koneti N, Bakhru S, Dhulipudi B, Rajan S, Sreeram N. Stent strut dilation in branch pulmonary artery stenosis following stenting of arterial duct in duct dependent pulmonary circulation. Pediatr Cardiol. 2023;35(1):1–10. doi:10.1007/s00246-023-03319-2. [Google Scholar] [PubMed] [CrossRef]
31. Valencia E, Staffa SJ, KUntz MT, Zaleski KL, Kaza AK, Maschietto N, et al. Transcatheter ductal stents versus surgical systemic-pulmonary artery shunts in neonates with congenital heart disease with ductal-dependent pulmonary blood flow: trends and associated outcomes from the pediatric health information system database. Am Hear Assoc. 2023;12(17):1–9. doi:10.1161/JAHA.123.030528. [Google Scholar] [PubMed] [CrossRef]
Appendix
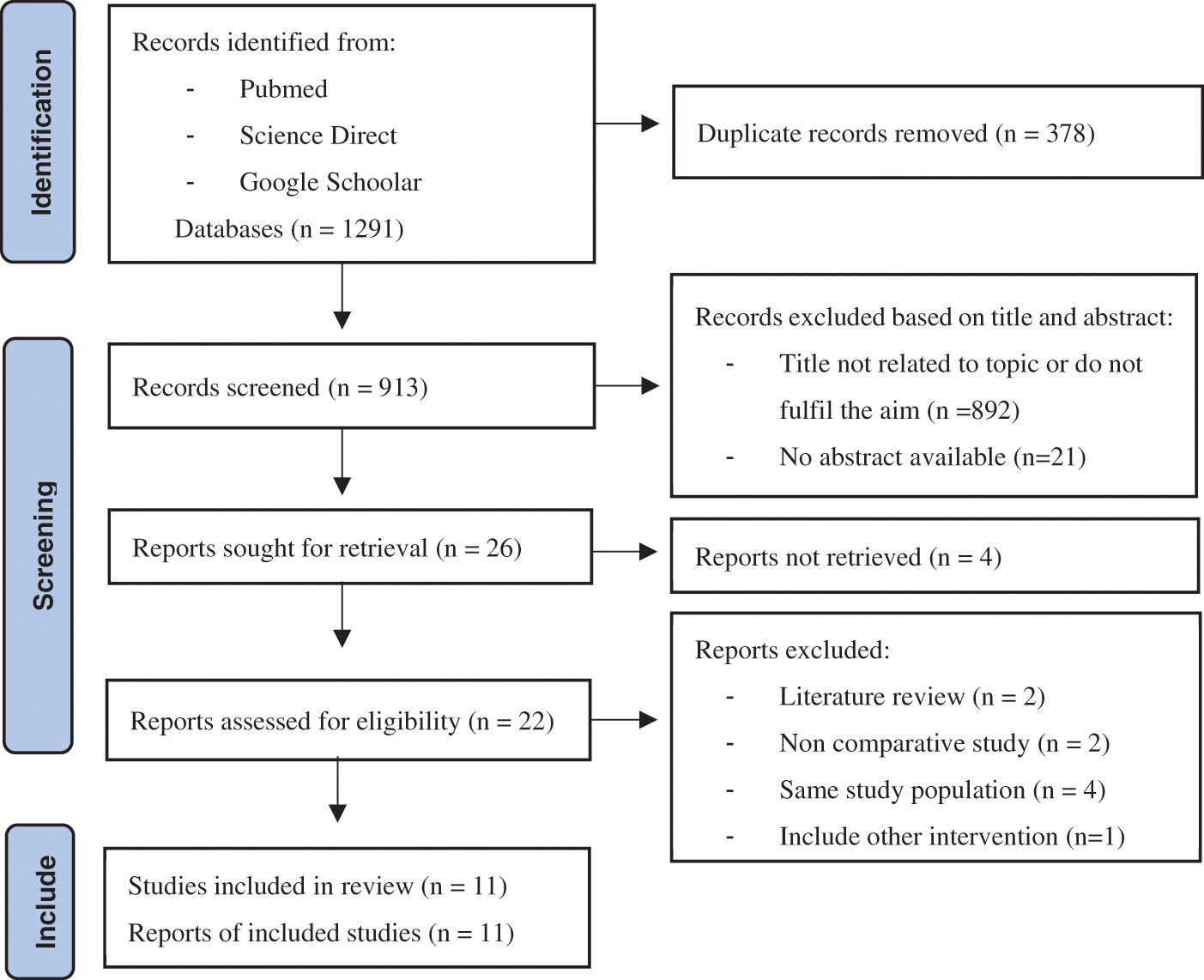
Appendix A: Prisma flow diagram of study selection process

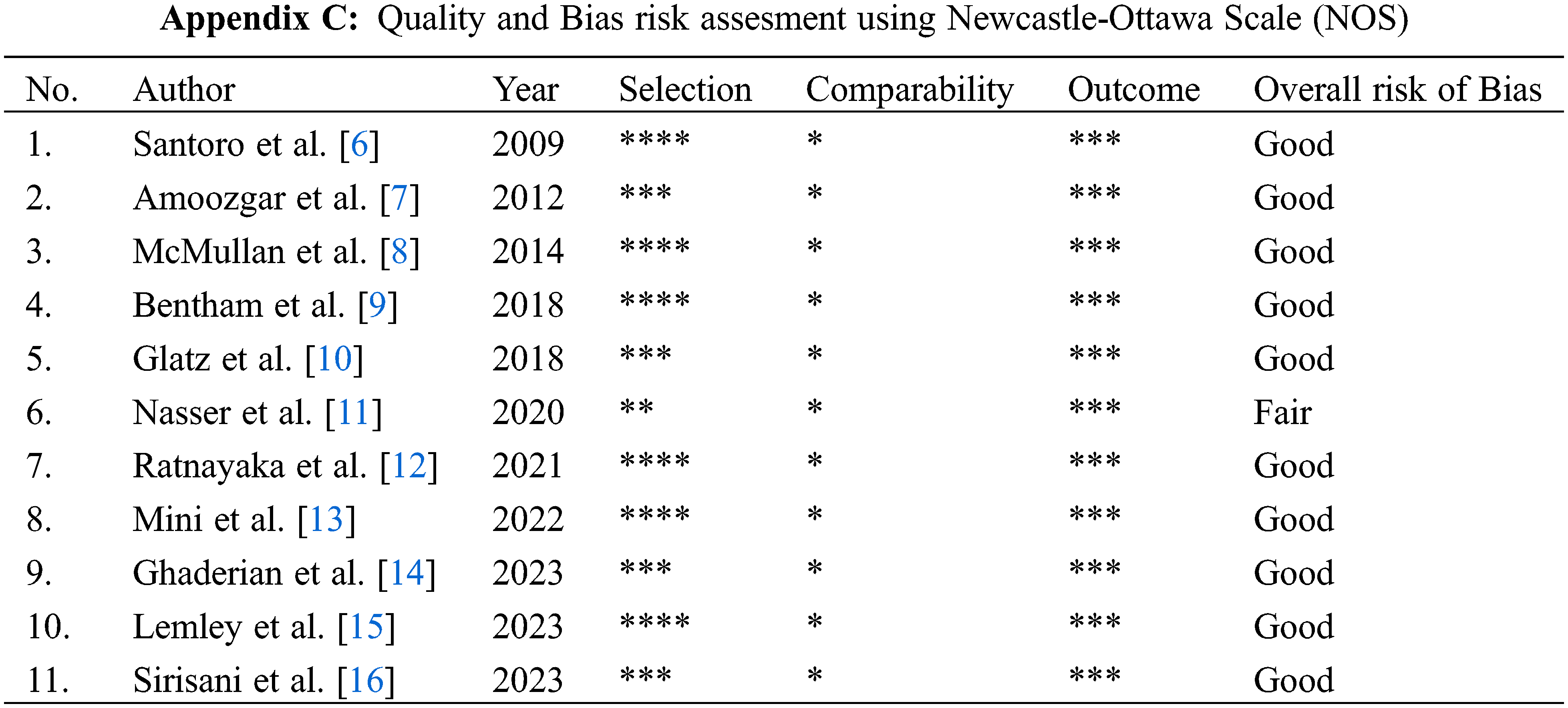
Appendix D
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools