 Open Access
Open Access
ARTICLE
High Prevalence of Anatomical Variations and Anomalies of the Coronary Arteries Detected by CT Angiography in Symptomatic Patients
Department of Radiology, College of Medicine, King Khalid University, Abha, 61421, Saudi Arabia
* Corresponding Author: Ghazi A. Alshumrani. Email:
Congenital Heart Disease 2024, 19(2), 197-206. https://doi.org/10.32604/chd.2024.049401
Received 06 January 2024; Accepted 21 March 2024; Issue published 16 May 2024
Abstract
Objective: Coronary artery anatomical variations and anomalies are an important topic due to their potential clinical manifestations. This study aims to investigate the prevalence of coronary artery anatomical variations and anomalies in symptomatic patients with coronary computed tomography angiography (CCTA). Methods: This is a retrospective study that included all symptomatic patients who had CCTA in a tertiary care hospital in Saudi Arabia during a period of seven years. Results: The total number of included patients was 507 (60% males) with a mean age of 57.4 years. Approximately 41% had luminal stenoses, averaging 49.7%. The total number of patients with coronary anatomical variations (CAV) and coronary artery anomalies (CAA) was 217 (43%). CAV prevalence was 26%, which included 14% non-right coronary dominance, 5% short left main coronary artery (LMCA), and 7% division variations (trifurcation and quadrifurcarion) of the LMCA. The prevalence of CAA was 29%, which included 5% origin anomalies, 22% myocardial bridge, and 2% course anomalies. Conclusions: A high prevalence of coronary artery anatomic variations and anomalies in symptomatic patients is reported in this study. Systematic reviews, meta-analyses, reporting guidelines, and unified definitions and classifications of coronary variations and anomalies are lacking in the literature, presenting potential opportunities for future research and publications.Keywords
Anatomical variations and anomalies of coronary arteries (AVACA) are uncommon but important findings to recognize [1]. Imaging of coronary arteries by coronary computed tomography angiography (CCTA) has emerged as a noninvasive alternative to the diagnostic coronary catheter angiogram [2]. It is an accurate diagnostic modality with high spatial and temporal resolutions along with capabilities of three-dimensional and extraluminal evaluation of the coronary arteries [3–5].
The definitions of normal morphology, anatomical variation, and anomaly vary from one reference to another. Angelini and coworkers defined them based on the prevalence among an unselected population [6]. They defined “normal” as any morphologic feature observed in >1% of an unselected population, “normal variant” as a relatively unusual alternative morphological feature seen in >1% of that population, and “anomaly” as a morphological feature present in <1% of the same population. They reported that the incidence of coronary anomalies was 5.6% in a continuous series of 1950 angiograms. In another study that utilized CCTA, the prevalence was 18% [7]. Awareness of AVACA is crucial, given several related possible consequences, including misdiagnosis, myocardial ischemia, increased risk of coronary atherosclerotic disease and bacterial endocarditis, secondary aortic valve disease, ischemic cardiomyopathy, volume overload, technical difficulties during coronary angioplasty, complications during cardiac surgery, and sudden cardiac death [6].
1.2 Rationale and Aim of the Study
AVACA has been linked to increased symptoms and sequelae, such as chest pain, dyspnea, syncope, ventricular fibrillation, myocardial infarction, cardiomyopathy, and sudden death [6]. Yet, the opposite may not necessarily be accurate. Investigating symptomatic patients for the presence of AVACA may or may not reveal a significant increase in its prevalence compared to an asymptomatic population. The consequences of AVACA mentioned above increase the chance of developing symptoms, which may lead to a higher discovery of anomalies among symptomatic patients. Therefore, it was hypothesized in this study that symptomatic cardiac patients have a higher prevalence of AVACA. This study aimed to investigate this hypothesis in a large number of symptomatic patients and compare the results to published results of asymptomatic populations.
The Institutional Research Ethics Committee approved this study and waived informed consent for patients’ anonymous data. This retrospective study included all symptomatic patients with CCTA in a tertiary care hospital in South Saudi Arabia (Assir Province) for seven years, from January 01, 2010, to December 31, 2016. The patients’ symptoms included one or more of the following: chest pain, shortness of breath, palpitation, and syncope. Patients with a coronary artery bypass graft (CABG) surgery or poor scan quality were excluded, because in such cases native coronary artery anatomy may be altered or inaccurately evaluated. To eliminate interobserver variations, all CCTAs were interpreted by one radiologist with ten years of post-board experience.
All studies were performed on a 64-slice CT scanner (Lightspeed VCT, General Electric, Milwaukee, WI, USA). The targeted heart rate in order to perform the scans was less than 65 beats per minute. Beta blockers (or, if contraindicated, calcium channel blockers) were prescribed by cardiologists to achieve the targeted heart rate as needed. In some cases, the target heart rate was not achieved, and they were excluded from this study if the radiologist deemed the scan quality poor. The test bolus technique was used for the timing of the scans. A dual-head power injector was used to inject 20 milliliters (ml) of Iobitridol (350 milligrams of iodine/ml) intravenous (IV) contrast (Xenetix, Guerbet, Roissy, France) at a rate of 5 ml/second, followed by 40 ml of normal saline at a rate of 5 ml/second for the bolus timing phase. For the CCTA phase, 50–70 ml of the same type of IV contrast was injected, followed by 40 ml of normal saline at the same rate. The scanning of CCTA was prospectively ECG-gated during breath-holding with a target phase of data acquisition at 75% of the R-R interval. Other parameters included a tube rotation time of 0.35 s, slice thickness of 0.625 mm, beam collimation of 40 mm at 120 kV, and tube current of 300–700 mA depending on the patient’s body size.
The CCTA images of all patients were viewed, processed, and analyzed on the same workstation (Advantage Window, General Electric, Milwaukie, WI, USA). An electronic data collection sheet was created with Google Sheets software (Google LLC, Mountain View, CA, USA), which was used to perform the statistical calculations by creating different formulas for the needed results. For each patient; age, gender, and percentage of the maximum stenosis of the coronary arteries were recorded.
The evaluated coronary anatomical variations (CAVs) included short left main coronary artery (LMCA) of ≤5 mm, division pattern of LMCA with the presence of ramus intermedius (trifurcation and quadrifurcation), and non-right coronary dominance (Fig. 1).
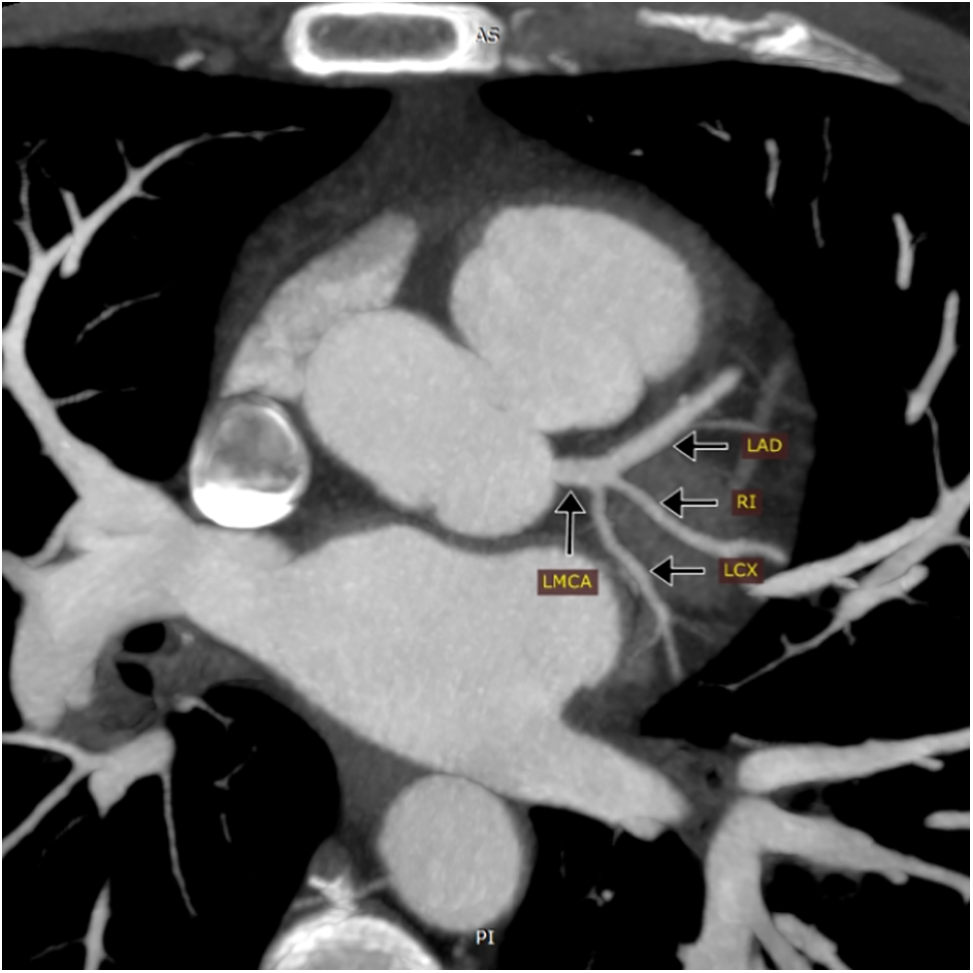
Figure 1: Maximum intensity projection (MIP) of coronary CT angiography showing trifurcation of the left main coronary artery (LMCA) into the left anterior descending artery (LAD), Ramus intermedius artery (RI), and left circumflex artery (LCX)
The right coronary dominance is the posterior descending artery (PDA) supplied by the right coronary artery (RCA). Left-sided dominance is defined as PDA supplied by the left circumflex artery (LCX), while bilateral dominance is when PDA is supplied by both RCA and LCX.
Coronary artery anomalies (CAAs) were classified into five groups. Group 1 included anomalies of the origin of the coronary arteries, defined as an origin, not from the corresponding sinus of Valsalva or higher than the junctional zone. Absent LMCA, with two separate origins of LAD and LCX, was classified in group 1 (Fig. 2).
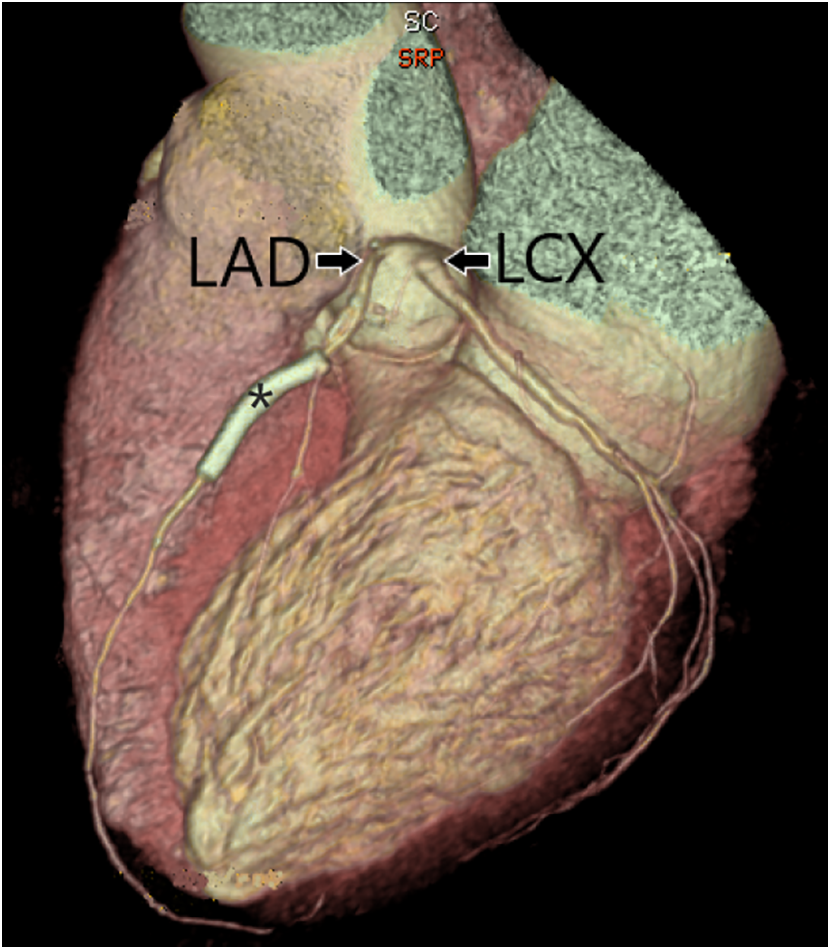
Figure 2: Three-dimensional surface rendering of coronary CT angiography showing two separate origins of the left anterior descending artery (LAD) and left circumflex artery (LCX) from the left coronary cusp, with an absent left main coronary artery. Stent (*) is present in the LAD
Group 2 included a myocardial bridge (MB), defined as a major epicardial artery running into the myocardium (Fig. 3).
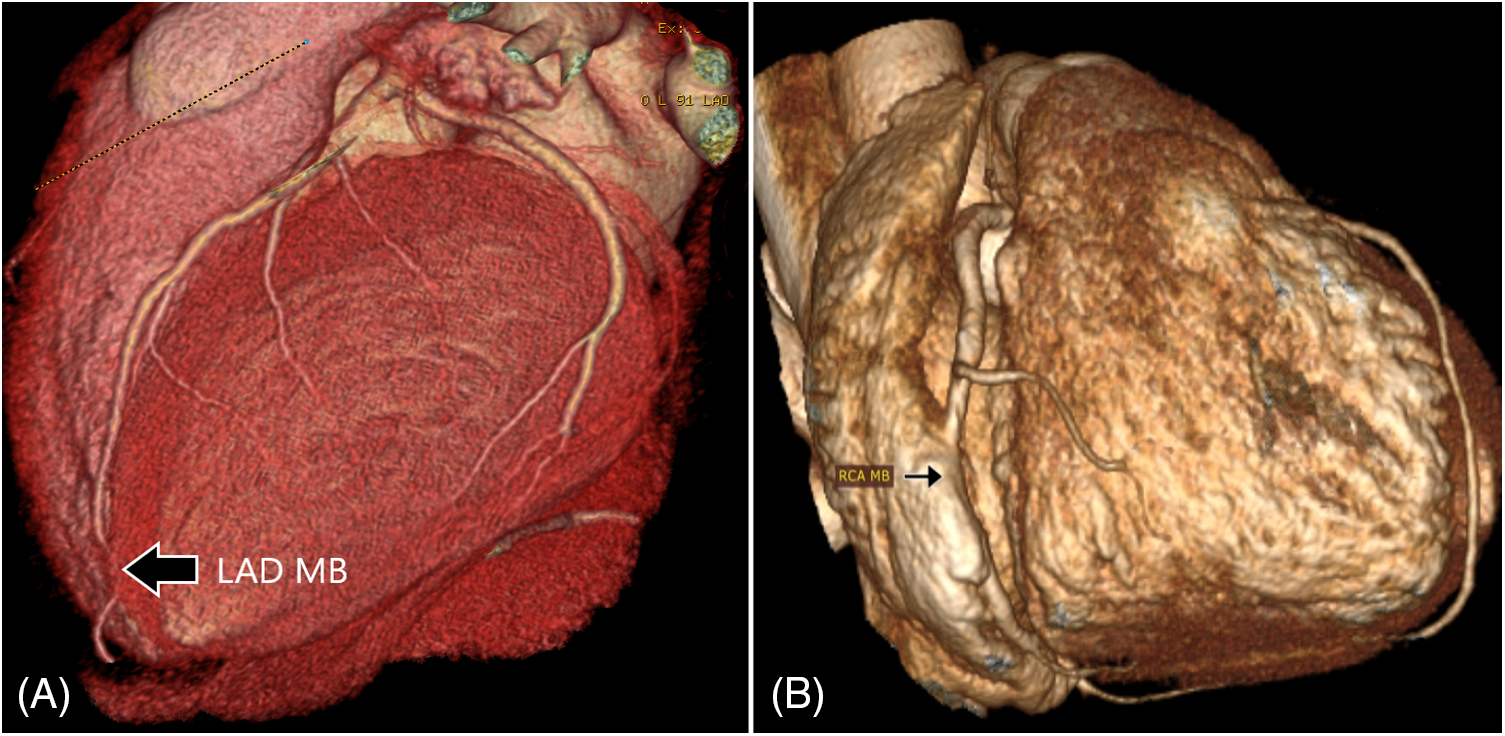
Figure 3: Three-dimensional surface rendering of coronary CT angiography showing myocardial bridges. (A) Distal left anterior descending artery myocardial bridge (LAD MB). (B) The RCA passes within the wall of the right atrium (RCA MB) rather than the usual course in the right atrioventricular groove
Group 3 included the anomalous course of the coronary arteries (Fig. 4).
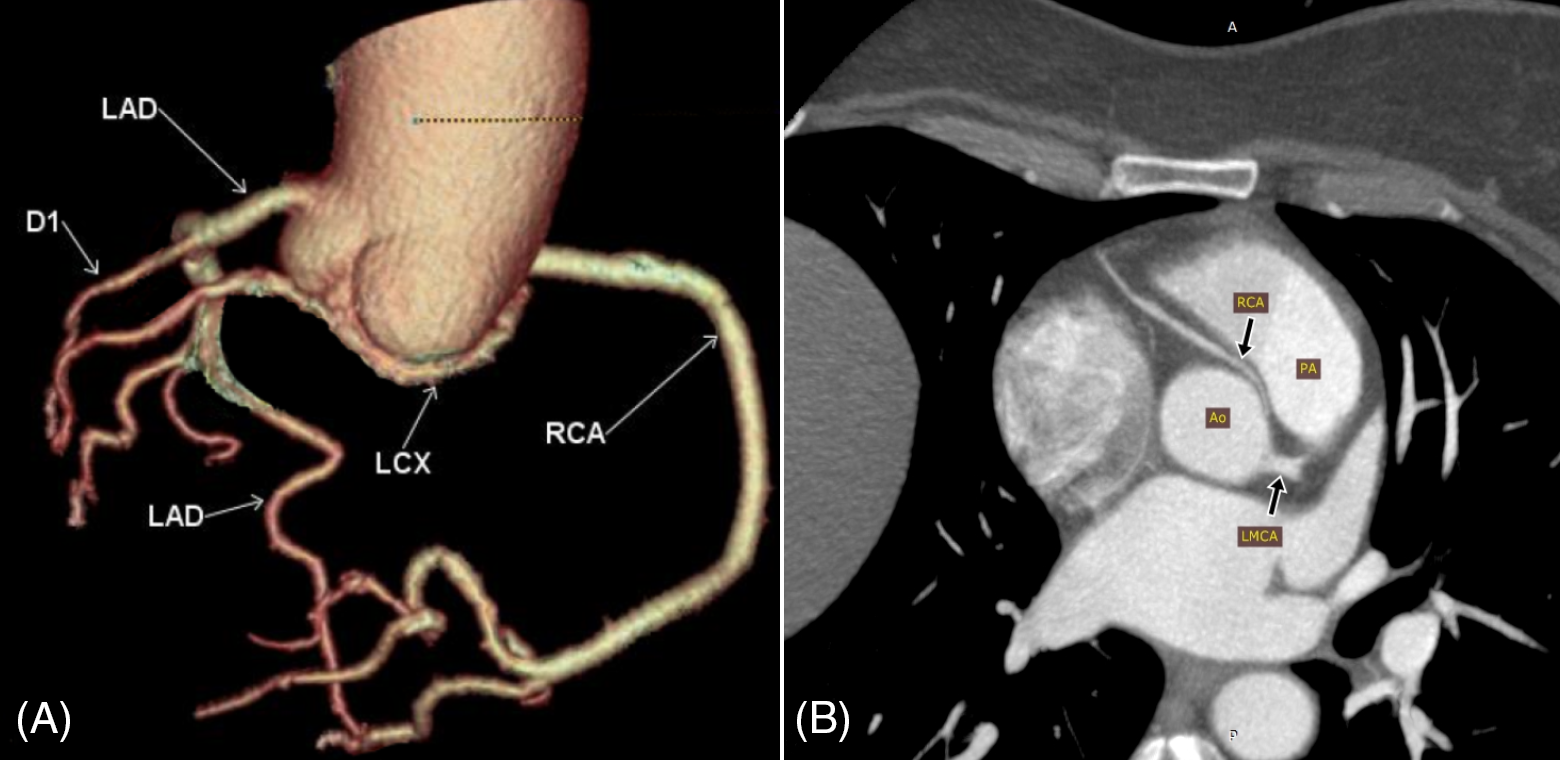
Figure 4: Abnormal course (and origin) of coronary arteries. (A) Volume rendering (VR) of the coronary artery tree showing the left circumflex artery (LCX) arising from the right coronary cusp and coursing inferior to the posterior aortic cusp towards the left side (RCA; right coronary artery, LAD; left anterior descending artery arising directly from the left cusp, D1; first diagonal branch). (B) Maximum intensity projection (MIP) showing the right coronary artery (RCA) arising anterior to the left coronary cusp and passing between the aorta (Ao) and the pulmonary artery (PA) in the interarterial groove (LMCA; left main coronary artery)
Excessive tortuosity of the coronary arteries was classified in group 3. Intrinsic anomalies, including ectasia and aneurysm of coronary arteries, were classified in group 4. Ectasia and aneurysm were defined as a dilatation of more than 1.5 times the normal diameter of the artery, diffusely (≥50% of the artery length) or focally (<50% of the artery length), respectively. Group 5 included termination anomalies (fistulas) of the coronary artery. If a CAA definition applied to more than one group, it would be classified in all corresponding groups; for example, an RCA, originating from the left coronary cusp and passing in the interarterial groove, was classified in groups 1 and 3.
3.1 Results of the Study Population
A total of 527 symptomatic patients had CCTA during a period of seven years, from January 01, 2010, to December 31, 2016. After applying the two exclusion criteria (patients with CABG and poor scan quality), 20 patients were excluded, leaving 507 patients in this study. There were 306 (60.4%) males and 201 (39.6%) females with a mean age of 57.4 years (SD 12.7) ranging from 23–95 years. A total of 297 (58.6%) patients had no stenosis of the coronary arteries in the CCTA, and 210 (41.4%) had stenosis caused by atherosclerosis, with luminal stenosis averaging 49.7% and ranging from 15%–100%.
The total number of patients with CAV and CAA was 217 (42.8%): this included 79 (36.4%) females and 138 (63.6%) males with 277 variations and anomalies.
3.2 Results of Anatomical Variations
Table 1 summarizes the results of CAV. Right coronary dominance was present in 437 patients (86.2%), left side dominance was present in 54 patients (10.6%), and bilateral dominance, when RCA and LCX supply the PDA, was present in 16 patients (3.2%).
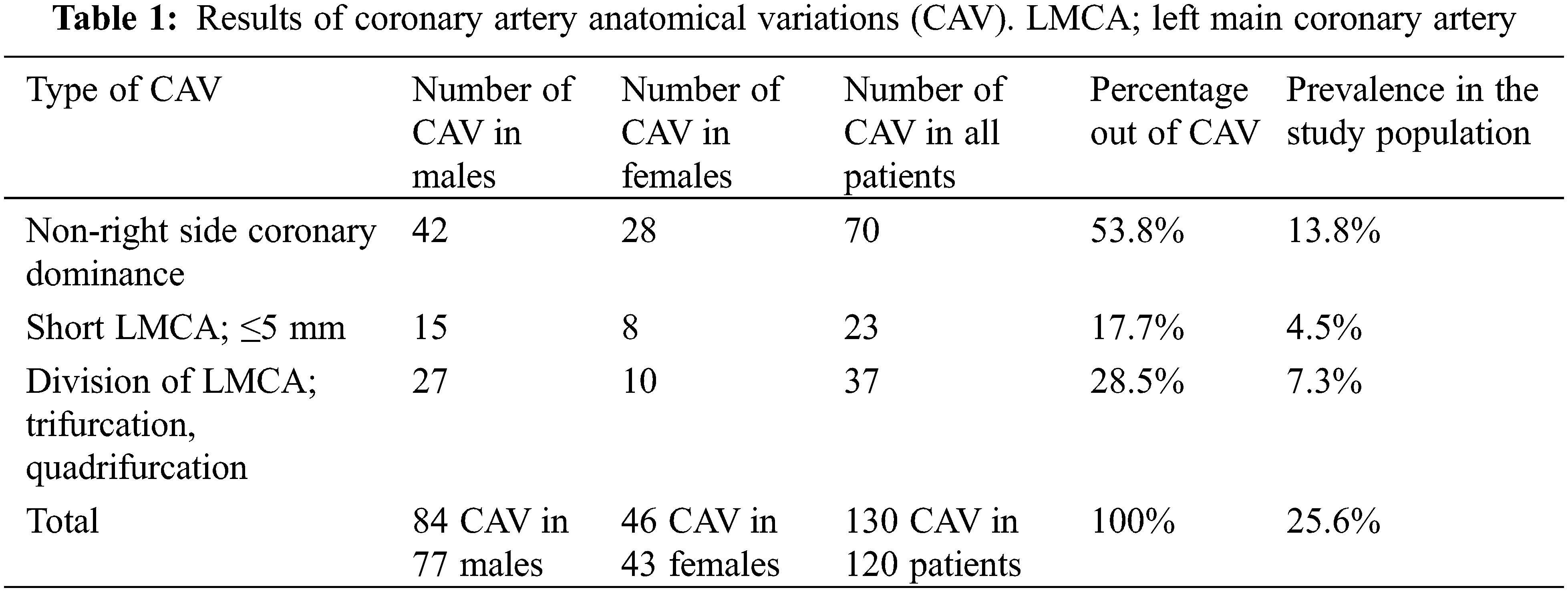
The mean length of the left main coronary artery (LMCA) was 10 mm (SD 4.8), ranging from zero (absent LMCA with two separate origins of the left anterior descending (LAD) artery and LCX) to 25 mm. If we exclude the cases of absent LMCA, the mean length would be 10.4 mm (range 3–25 mm). The LMCA was absent in 9 (1.8%) patients. Short LMCA, defined as a length of 5 mm or less, was present in 23 (4.5%) patients. The total number of patients with absent or short LMCA was 32 (6.3%). In this study, absent LMCA was counted in group 1 of CAA, whereas short LMCA was counted as CAV. Trifurcation and quadrifurcation of LMCA were present in 36 (7.1%) patients and one patient (0.2%), respectively.
3.3 Results of Coronary Artery Anomalies
There were 137 (27%) patients with CAA [90 (65.7%) males, 47 (34.3%) females, mean age 55.4 years, range 26–86 years], with a total of 147 CAAs (29%) because 10 (2%) patients had more than one type of CAA. Of 137 patients with CAA, 54 (39.4%) had stenosis in CCTA (mean stenosis 45.7%, range 15%–95%).
The CAA was classified into five groups (Table 2).
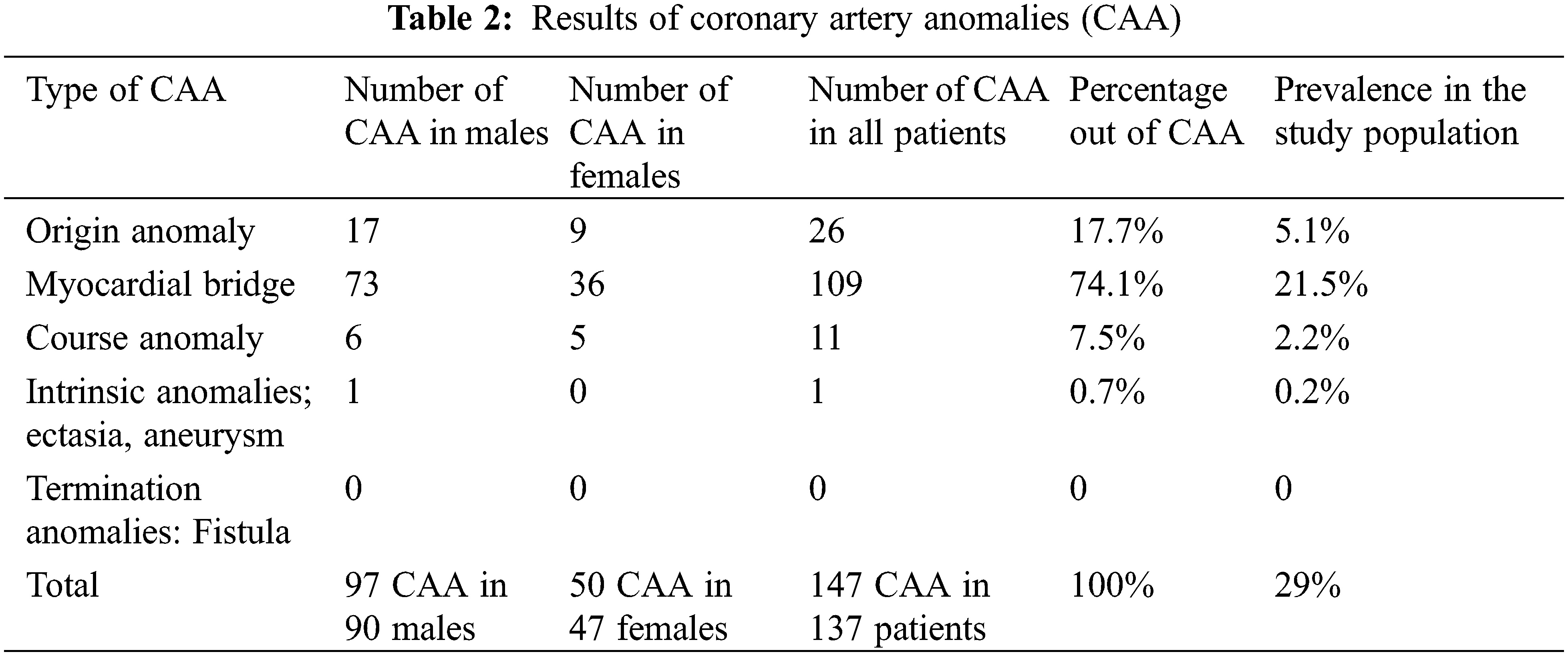
The most common CAA was MB (group 2), which was seen in 109 patients, representing 79.6% of CAA and 21.5% of the total study population. It was present in 23.9% (73/306) of males and 17.9% (36/201) of females. MB of LAD was the most common (in 104 patients, 95.4%), followed by ramus intermedius bridging in three (2.8%) patients and RCA bridging in two (1.8%) (Fig. 2). If MB is excluded, the prevalence of CAA in this study population would be 7.1% (36 anomalies). Coronary ectasia was present in one (0.2%) patient, representing (0.7%) of all CAA patients. There were no cases of coronary artery aneurysms or fistulas.
The present study showed a higher prevalence of CAA (29%) than reported. Previous studies in different parts of the world reported a prevalence ranging from 1% to 18% [6–12]. There are possible reasons for such high prevalence in the current study. Symptomatic patients may have a higher prevalence because CAA increases the chance of developing symptoms, which may lead to a higher discovery of the anomalies among those patients. The variability in the definition of anomalies is another possible reason. For example, some studies considered MB as an anomaly while others did not [7,13–16]. The present study did consider MB an anomaly, which was relatively common (21.5% of the study population). Without MB, the prevalence of CAA was 7% in this study. MB was considered an anomaly because of its potential to cause remarkable compression with subsequent angina and myocardial infarction even when there is no evidence of coronary atherosclerosis by angiography or autopsy, in addition to other potentially serious consequences, such as left ventricular dysfunction, arrhythmias, and sudden cardiac death [17–19]. A recent study showed that MB was the most common abnormality among young adults presenting with chest pain [20]. Another possible cause of the high prevalence of CAA in this study could be geographic and higher ethnic prevalence in Southern Saudi Arabia. The only study found in the literature of a large number of patients from Saudi Arabia was from Qassim Province, which showed a prevalence of 1% of CAA (according to their definitions) and 11% of all AVACA [21]. CCTA has a higher sensitivity to detect CAA compared to the coronary catheter angiogram, particularly for MB detection, which is another possible explanation for the higher prevalence in the present study [22,23].
There are several known anatomical variations of coronary arteries. In this study, three types are reported; non-right-sided dominance, short LMCA, and division variations of LMCA. The prevalence of coronary dominance (right, left, and balanced) in this study is close to that reported in the literature by CCTA or catheter angiogram [7,9]. Short LMCA, present in 4.5% of patients in this study, may lead to serious coronary consequences. Patients with short LMCA developed stenosis more frequently near the ostium and were found to have a higher incidence of acute myocardial infarction in the left coronary artery [24,25]. The branching pattern of LMCA is commonly a bifurcation into LAD and LCX, with a prevalence of ramus intermedius between 21% and 33% [7,15,26]. However, it was reported in some studies that trifurcation, with the presence of the ramus intermedius branch, was the most common branching pattern in up to 69% [27,28]. In this study, the prevalence of ramus intermedius was 7%, which is much lower than reported in the literature.
The prevalence of origin anomalies was 5% in the current study, which is higher than the 2% reported in another study of more than 6,000 consecutive patients evaluated by invasive angiography [13]. However, a prevalence of more than 7% was reported in a previous study using CCTA [7].
MB was the most common anomaly in this study, reaching about 21%. This is close to (but less than) the prevalence in other studies with 64-slice CCTA, in which MB was found in 26%–37% of patients [13,22]. However, the prevalence of MB in this study is double that reported in other studies [10]. Several studies showed that CCTA is more sensitive than conventional angiography in detecting MB [22,23]. The published studies classified MB differently; as an intrinsic anomaly, a course anomaly, in a separate group of anomalies, or as a variation rather than an anomaly [7,9,15,21]. The present study opted to report MB in a separate group of anomalies to keep the data presentation more detailed.
Intrinsic (aneurysm and ectasia) and termination (fistulas) anomalies of coronary arteries are very rare, and only one anomaly in those two groups was found in the current study.
The anomalies of the course were present in 2% of patients in this study. The prevalence of the course anomalies is variable in the literature depending on their definition and classification, as some studies group them with the origin anomalies. The reported origin and course anomalies are around 2%–8% [7,15].
A study comparing symptomatic and asymptomatic patients found no statistically significant difference in the prevalence of MB (31% vs. 42%, p = 0.51) and CAA (7% vs. 3%, p = 0.26), although the prevalence of CAA was slightly higher in the symptomatic patients [29]. Another study evaluated asymptomatic patients with ST-segment anomalies during maximal exercise tests and found a prevalence of 15% of CAA, which is similar or even higher than the reported prevalence in other studies of symptomatic patients [30]. Those two studies may support the null hypothesis of the present study, i.e., rejecting the relation between the higher prevalence of CCA and symptomatic patients. However, the larger sample size in this study will stand against rejecting its hypothesis based on two smaller studies. Large studies of CAA prevalence in asymptomatic patients still are lacking in the literature. The higher prevalence of CAA in this study of symptomatic patients may still be attributed to the other causes mentioned above, including the definition of CAA cases and the ethnic background of the study population.
The main limitation of this study is its retrospective single-center design. Lack of correlation with coronary catheter angiogram is another possible limitation, although CCTA is superior in some aspects of coronary artery evaluation, as mentioned in the introduction and discussion.
A high prevalence of coronary artery anatomic variations and anomalies among symptomatic patients is reported in this study. Systematic reviews and meta-analyses of this important topic are lacking in the literature which presents an opportunity to conduct further larger studies. The lack of unified definitions and classifications for coronary variations and anomalies makes it harder to compare results with published studies. Therefore, greater collaboration between different scientific societies is recommended to develop more unified definitions, classifications, and reporting guidelines for coronary anomalies and variations.
Acknowledgement: Not applicable.
Funding Statement: The author received no specific funding for this study.
Author Contributions: Ghazi A. Alshumrani: Study concept and design, data collection and analysis, manuscript writing and editing.
Availability of Data and Materials: Available upon request.
Ethics Approval: The Institutional Research Ethics Committee approved this study and waived informed consent for patients’ anonymous data.
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
References
1. Kastellanos S, Aznaouridis K, Vlachopoulos C, Tsiamis E, Oikonomou E, Tousoulis D. Overview of coronary artery variants, aberrations and anomalies. World J Cardiol. 2018;10(10):127–40. doi:10.4330/wjc.v10.i10.127. [Google Scholar] [PubMed] [CrossRef]
2. Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018;39(41):3689–98. [Google Scholar] [PubMed]
3. Clemente A, Seitun S, Mantini C, Gentile G, Federici D, Barison A, et al. Cardiac CT angiography: normal and pathological anatomical features–a narrative review. Cardiovasc Diagn Ther. 2020;10(6):1918–45. doi:10.21037/cdt. [Google Scholar] [CrossRef]
4. Díaz R, Vega J. Role of coronary computed tomography angiography for the diagnosis of coronary anomalies. Rev Med Chil. 2016;144(10):1277–86. [Google Scholar]
5. Gräni C, Benz DC, Schmied C, Vontobel J, Possner M, Clerc OF, et al. Prevalence and characteristics of coronary artery anomalies detected by coronary computed tomography angiography in 5634 consecutive patients in a single centre in Switzerland. Swiss Med Wkly. 2016;146:w14294. [Google Scholar]
6. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105(20):2449–54. doi:10.1161/01.CIR.0000016175.49835.57. [Google Scholar] [PubMed] [CrossRef]
7. Cademartiri F, La Grutta L, Malagò R, Alberghina F, Meijboom WB, Pugliese F, et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol. 2008;18(4):781–91. doi:10.1007/s00330-007-0821-9. [Google Scholar] [PubMed] [CrossRef]
8. Szymczyk K, Polguj M, Szymczyk E, Majos A, Grzelak P, Stefańczyk L. Prevalence of congenital coronary artery anomalies and variants in 726 consecutive patients based on 64-slice coronary computed tomography angiography. Folia Morphol. 2014;73(1):51–7. doi:10.5603/FM.2014.0007. [Google Scholar] [PubMed] [CrossRef]
9. Altin C, Kanyilmaz S, Koc S, Gursoy YC, Bal U, Aydinalp A, et al. Coronary anatomy, anatomic variations and anomalies: a retrospective coronary angiography study. Singapore Med J. 2015;56(6):339–45. doi:10.11622/smedj.2014193. [Google Scholar] [PubMed] [CrossRef]
10. Sirasapalli CN, Christopher J, Ravilla V. Prevalence and spectrum of coronary artery anomalies in 8021 patients: a single center study in South India. Indian Heart J. 2018;70(6):852–6. doi:10.1016/j.ihj.2018.01.035. [Google Scholar] [PubMed] [CrossRef]
11. Şahin T, Ilgar M. Investigation of the frequency of coronary artery anomalies in MDCT coronary angiography and comparison of atherosclerotic involvement between anomaly types. Tomography. 2022;8(3):1631–41. doi:10.3390/tomography8030135. [Google Scholar] [PubMed] [CrossRef]
12. Ganga KP, Goyal A, Ojha V, Deepti S, Sharma S, Kumar S. Prevalence rates of Congenital coronary anomalies and coronary variations in adult Indian population using dual-source computed tomography coronary angiography: analysis of regional distribution of coronary anomalies and the need for standardized reporting formats. Indian J Radiol Imaging. 2021;31(1):138–49. [Google Scholar] [PubMed]
13. Erdem K, Ozbay Y. Prevalence and characteristics of coronary artery anomalies using invasive coronary angiography in 6237 consecutive patients in a single center in Turkey. Arch Iran Med. 2018;21(6):240–5. [Google Scholar] [PubMed]
14. Mathai RT, Fahmy DM, Sadek HL, Renno WM. Congenital coronary artery anomalies in adult population detected using dual source ECG-gated CTA in a single institution. Folia Morphol. 2017;76(2):208–18. doi:10.5603/FM.a2016.0053. [Google Scholar] [PubMed] [CrossRef]
15. Rao A, Pimpalwar Y, Yadu N, Yadav RK. A study of coronary artery variants and anomalies observed at a tertiary care armed forces hospital using 64-slice MDCT. Indian Heart J. 2017;69(1):81–6. doi:10.1016/j.ihj.2016.05.018. [Google Scholar] [PubMed] [CrossRef]
16. Chaosuwannakit N. Anatomical variants and coronary anomalies detected by dual-source coronary computed tomography angiography in North-Eastern Thailand. Pol J Radiol. 2018;83:e372–e378. doi:10.5114/pjr.2018.78420. [Google Scholar] [PubMed] [CrossRef]
17. De Rosa R, Sacco M, Tedeschi C, Pepe R, Capogrosso P, Montemarano E, et al. Prevalence of coronary artery intramyocardial course in a large population of clinical patients detected by multislice computed tomography coronary angiography. Acta radiol. 2008;49(8):895–901. doi:10.1080/02841850802199825. [Google Scholar] [PubMed] [CrossRef]
18. Erbel R, Ge J, Möhlenkamp S. Myocardial bridging: a congenital variant as an anatomic risk factor for myocardial infarction? Circulation. 2009;120(5):357–9. doi:10.1161/CIRCULATIONAHA.109.881367. [Google Scholar] [PubMed] [CrossRef]
19. Lee MS, Chen CH. Myocardial bridging: an up-to-date review. J Invasive Cardiol. 2015;27(11):521–8. [Google Scholar] [PubMed]
20. Burt JR, O’Dell MC, Yacoub B, Chamberlin J, Waltz J, Wallace C, et al. Prevalence of abnormal coronary findings on coronary computed tomography angiography among young adults presenting with chest pain. J Thorac Imaging. 2020. doi:10.1097/RTI.0000000000000564. [Google Scholar] [PubMed] [CrossRef]
21. Smettei OA, Sayed S, Abazid RM. The prevalence of coronary artery anomalies in Qassim Province detected by cardiac computed tomography angiography. J Saudi Heart Assoc. 2017;29(2):84–9. doi:10.1016/j.jsha.2016.07.006. [Google Scholar] [PubMed] [CrossRef]
22. Leschka S, Koepfli P, Husmann L, Plass A, Vachenauer R, Gaemperli O, et al. Myocardial bridging: depiction rate and morphology at CT coronary angiography–comparison with conventional coronary angiography. Radiology. 2008;246(3):754–62. doi:10.1148/radiol.2463062071. [Google Scholar] [PubMed] [CrossRef]
23. Javadrashid R, Tarzamni MK, Aslanabadi N, Ghaffari M, Salehi A, Sorteji K. Myocardial bridging and coronary artery anomalies detected by ECG-gated 64-row multidetector computed tomography angiography in symptomatic patients. Folia Morphol. 2009;68(4):201–6. [Google Scholar]
24. Maehara A, Mintz GS, Castagna MT, Pichard AD, Satler LF, Waksman R, et al. Intravascular ultrasound assessment of the stenoses location and morphology in the left main coronary artery in relation to anatomic left main length. Am J Cardiol. 2001;88(1):1–4. doi:10.1016/S0002-9149(01)01575-2. [Google Scholar] [PubMed] [CrossRef]
25. Dong Z, Gong K, Xin P, Shen Y, Wu P, Zhu W, et al. Predictive value of the angiographic anatomic characteristics of the left main coronary on acute myocardial infarction in patients with coronary atherosclerosis. J Invasive Cardiol. 2013;25(9):449–54. [Google Scholar] [PubMed]
26. Koşar P, Ergun E, Oztürk C, Koşar U. Anatomic variations and anomalies of the coronary arteries: 64-slice CT angiographic appearance. Diagn Interv Radiol. 2009;15(4):275–83. [Google Scholar] [PubMed]
27. da Costa Sobrinho OP, de Lucena JD, Pessoa RS, Veríssimo NA, Nunes LM, Rojas PK et al. Anatomical study of length and branching pattern of main trunk of the left coronary artery. Morphologie. 2019;103(341):17–23. doi:10.1016/j.morpho.2018.10.002. [Google Scholar] [PubMed] [CrossRef]
28. Kultida CHY, Ruedeekorn SW, Keerati HS. Anatomic variants and anomalies of coronary arteries detected by computed tomography angiography in southern Thailand. Med J Malaysia. 2018;73(3):131–6. [Google Scholar] [PubMed]
29. Tsai RJ, Lai HY, Ni CF, Tsao SM, Lan GY, Hsieh KLC. Young adult cardiovascular diseases: a single center coronary computed tomography angiography study. Clin Imaging. 2018;52:343–9. doi:10.1016/j.clinimag.2018.09.013. [Google Scholar] [PubMed] [CrossRef]
30. Ermolao A, Roman F, Gasperetti A, Varnier M, Bergamin M, Zaccaria M. Coronary CT angiography in asymptomatic middle-aged athletes with ST segment anomalies during maximal exercise test. Scand J Med Sci Sports. 2016;26(1):57–63. doi:10.1111/sms.2016.26.issue-1. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools