 Open Access
Open Access
REVIEW
Treatment and Clinical Management of Chronic Thromboembolic Pulmonary Hypertension: An Update of Literature Review
1 The Department of Cardiology, The First Affiliated Hospital of China Medical University, Shenyang, 110000, China
2 The Radiology Department, Shengjing Hospital of China Medical University, Shenyang, 110020, China
* Corresponding Author: Yanli Chen. Email:
Congenital Heart Disease 2024, 19(2), 157-176. https://doi.org/10.32604/chd.2024.047930
Received 22 November 2023; Accepted 08 March 2024; Issue published 16 May 2024
Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a chronic, progressive, debilitating, and life-threatening complication of pulmonary embolism (PE). Recent technological advances have permitted various treatment options for the treatment of CTEPH, including surgery, angioplasty, and medical treatment, depending on the location and characteristics of lesions. Pulmonary endarterectomy (PEA) is the treatment of choice for CTEPH, as it offers excellent long-term outcomes and a high probability of recovery. Moreover, various medical and interventional therapies are currently being developed for patients with inoperable CTEPH. This review mainly summarizes the current treatment approaches of CTEPH, offering more options for specialist physicians to, thus, better manage chronic thromboembolic syndromes.Keywords
Nomenclature
| PE | Pulmonary embolism |
| PH | Pulmonary hypertension |
| RHC | Right heart catheter |
| mPAP | Mean pulmonary artery pressure |
| PAWP | Pulmonary artery wedge pressure |
| PVR | Pulmonary vascular resistance |
| V/Q | Ventilation/perfusion |
| CTPA | Computed tomography pulmonary angiography |
| DOXT | Domiciliary oxygen therapy |
| VKAs | Vitamin K antagonists |
| NOACs | Novel oral anticoagulants |
| 6MWD | 6-min walking distance |
| ECMO | Extracorporeal membrane oxygenation |
Chronic thromboembolic pulmonary hypertension (CTEPH) is a long-term and life-threatening complication of pulmonary embolism (PE), which is classified in the fourth group of the European Society of Cardiology (ESC) Pulmonary Hypertension Guidelines. According to the ESC Guidelines for diagnosing and treating PH in 2022, CTEPH was defined as pulmonary hypertension (PH) following at least 3 months of effective anticoagulation treatment of PE. The haemodynamic characteristics are as follows: the mean pulmonary artery pressure (mPAP) measured by the right heart catheter (RHC) at rest is >20 mmHg (1 mmHg = 0.1333 kPa), the pulmonary artery wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >2 WU (Wood units) [1].
As a complication of acute PE, CTEPH is rare and potentially life-threatening. Its mortality rate is decided by timely diagnosis and subsequently appropriate treatment. CTEPH is characterized by unspecific symptoms involving multiple systems, and Multi-Disciplinary Treatment (MDT) including the respiratory department, interventional department, thoracic surgery department, and cardiovascular department is vital in clinical practice. Therefore, the prognosis varies greatly. In recent years, diagnostic methods and multiple therapies have made CTEPH potentially treatable, leading to increased interest. These therapeutic methods include pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), medical therapy, and multimodal therapy. To ensure the best outcomes, surgical candidates should be carefully selected by a multidisciplinary team in an expert center [2]. This review aims to comprise the novel diagnostic and therapeutic methods of CTEPH.
2 Epidemiology and Risk Factors of CTEPH
Systematic follow-up of PE can improve the diagnosis rate of CTEPH. When obvious transient major risk factors are absent, the CT characteristics of PH and chronic thromboembolic may serve as subsequent risk factors in diagnosing CTEPH in cases of acute PE [3]. According to recent studies, the incidence and prevalence rates of CTEPH are 2–6 and 26–38 cases per million adults, respectively [4–6]. Some prospective observational studies have shown that the cumulative incidence rate of CTEPH varies from 0.1% to 11.8% in the first 2 years before symptomatic PE [7–11]. The wide variation of incidence rate may be attributed to significant differences in the selection of the study populations, study design, and diagnostic standards used, i.e., RHC [12]. Ende-Verhaar published a systematic review involving 16 studies that included 4,047 PE patients [11]. The incidence rate varies from 0.56% to 3.2% in the three predefined subpopulations. The RHC might provide a higher incidence rate of CTEPH [11].
With the coronavirus disease 2019 (COVID-19) pandemic in full swing, it is expected that a higher incidence rate of CTEPH will occur and that a delayed diagnosis will result in the progression of this disease to more advanced stages. A brief report from the United Kingdom denoted that the number of patients had contracted by 32% from the 3 years before the onset of the COVID-19 pandemic in 2020 [13]. This decrease can be attributed to unprecedented pressure on the healthcare system, the lack of a more extended follow-up period, and a reduction in the prevalence rate of classical predisposing factors for thromboembolism [14]. However, in a multicenter cross-sectional study, no higher incidence of CTEPH was found in relation to COVID-19-related PE [15].
Numerous studies have investigated many risk factors for the development of CTEPH after PE. It has been found that age, unprovoked PE, recurrent PE, significant defect in pulmonary perfusion, proximal PE, initial pulmonary arterial systolic pressure, and a history of combined lower limb varicose veins are risk factors for CTEPH [9,16,17]. Meanwhile, the risk factors for CTEPH may be associated with many clinical conditions, including permanent intravascular devices (pacemakers, long-term central lines, and ventriculoatrial shunts), inflammatory bowel diseases, essential thrombocythaemia, polycythaemia vera, splenectomy, antiphospholipid syndrome, high-dose thyroid hormone replacement, and cancer [5,18,19]. Investigating these risk factors and baseline clinical conditions can promote the detection of CTEPH and provide effective treatments [4,5,20].
3 Clinical Presentation and Diagnosis
CTEPH is detected among participants of an average age of 63 years old, with no significant gender difference [19]. Symptoms of CTEPH are nonspecific until severe right heart failure. The period between symptom onset and diagnosis is 14 months on average [21]. CTEPH is characterized by dyspnea after exercise, accompanied by progressive aggravation or reduced tolerance to exercise. In addition, hemoptysis and syncope may occur [9,19,22,23]. Symptoms of PH and right heart failure may emerge as CTEPH progresses, including cyanosis of lips, distention of jugular vein, increased P2, edema in lower limbs, and even pleural and abdominal effusions [24].
3.2 Ventilation/Perfusion (V/Q) Lung Scan
Ventilation/perfusion (V/Q) lung scan, computed tomography pulmonary angiography (CTPA), RHC, and digital subtraction angiography (DSA) are important means for detecting CTEPH [18,25]. The V/Q test can detect pulmonary artery stenosis or occlusive illness (including embolic disease) in PH patients (Fig. 1). V/Q scans have high sensitivity (90%–100%) and specificity (94%–99%) in excluding CTEPH [26,27]. When pulmonary segmental perfusion does not match its ventilation and there is a perfusion defect, pulmonary artery stenosis or obstruction should be considered [12,25]. Furthermore, other causes of perfusion deficits can also occur in cases of pulmonary artery obstruction, such as pulmonary artery sarcomas, fibrinous mediastinitis, etc., which must be differentiated based on clinical and imaging findings.
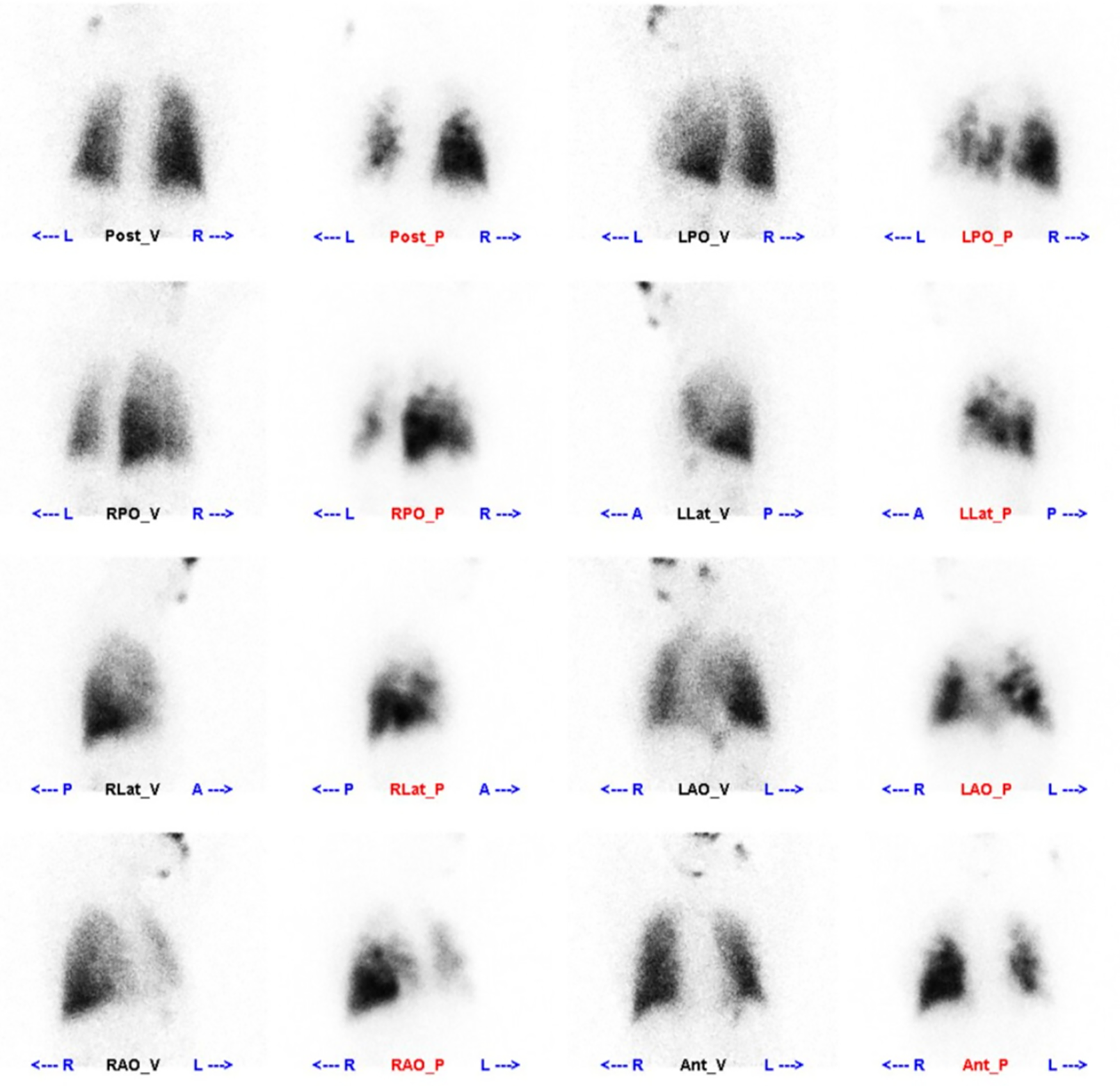
Figure 1: Perfusion images: multiple areas of sparse radiological distribution of defects are seen in both lungs, with the left lung and right lung’s upper lobe being the most prominent
3.3 Computed Tomography Pulmonary Angiography (CTPA)
CTPA is a crucial diagnostic tool for pulmonary vascular disorders (Fig. 2). It is employed to diagnose CTEPH by detecting both direct and indirect symptoms, such as filling defects (which may include thrombus adhering to the vascular wall), webs or bands in the pulmonary arteries, PA retraction/dilatation, mosaic perfusion, and enlarged bronchial arteries [28,29]. A recent meta-analysis encompassed 10 single-center studies involving a total of 734 patients. The combined sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and diagnostic odds ratio (DOR) of CTPA for CTEPH were found to be 0.98, 0.99, 0.94, 1.00, 0.96, and 292, respectively [28]. However, a basic limitation is that a negative CTPA test cannot rule out CTEPH because small vessel defects may be not found [27]. Nevertheless, CTPA can provide vital image guides for PEA to treat CTEPH [30].
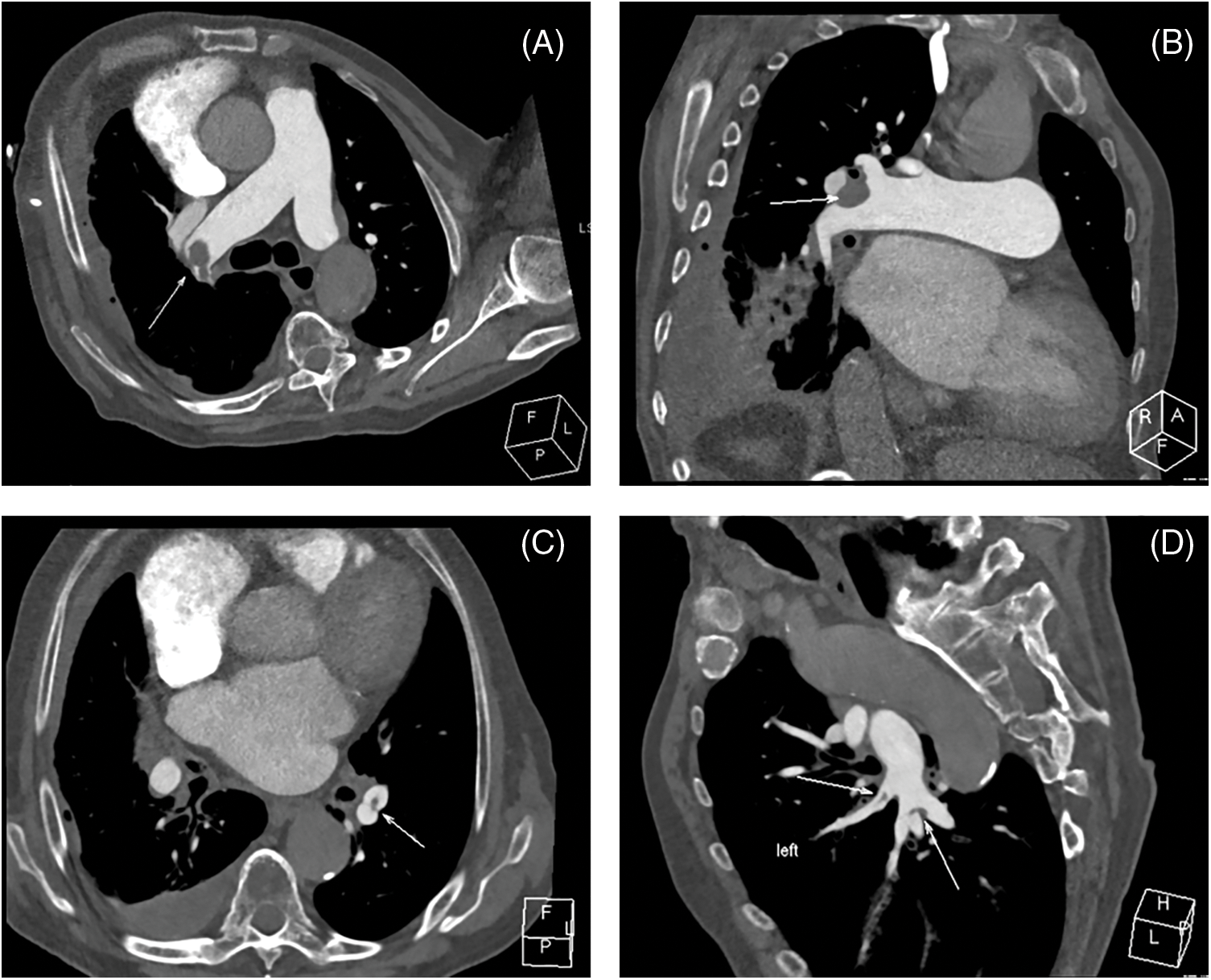
Figure 2: CTPA images from chronic thromboembolic disease (CTEPH) patient. (A) Axial view demonstrates the occlusion in the pulmonary trunk, (B) Multiplanar reformation visualizes this occlusion, (C) Axial representation shows the occlusion in the left inferior pulmonary artery branch, (D) The occlusion in the said branch is further depicted via multiplanar reformation
3.4 RHC and Digital Subtraction Angiography (DSA)
RHC is the gold method for diagnosing and categorizing PH (especially PAH or CTEPH) and may support treatment decisions [12]. RHC provides hemodynamic data, including right atrial pressure, right ventricular pressure (systolic, diastolic and mean), pulmonary artery pressure, PAWP, cardiac output, mixed venous oxygen saturation (SvO2), and PVR, which are important predictors of long-term outcome for PH [31].
DSA is used to diagnose CTEPH and evaluate therapeutic indications for pulmonary vascular surgeries, such as PEA or BPA (Fig. 3). In light of the development of CT technology, C-arm CT (CACT) of the pulmonary artery DSA may improve diagnostic work-up for patients with CTEPH by providing additional information through identifying more vessels as prospective BPA targets and giving guidance, especially before surgery or intervention [32,33].
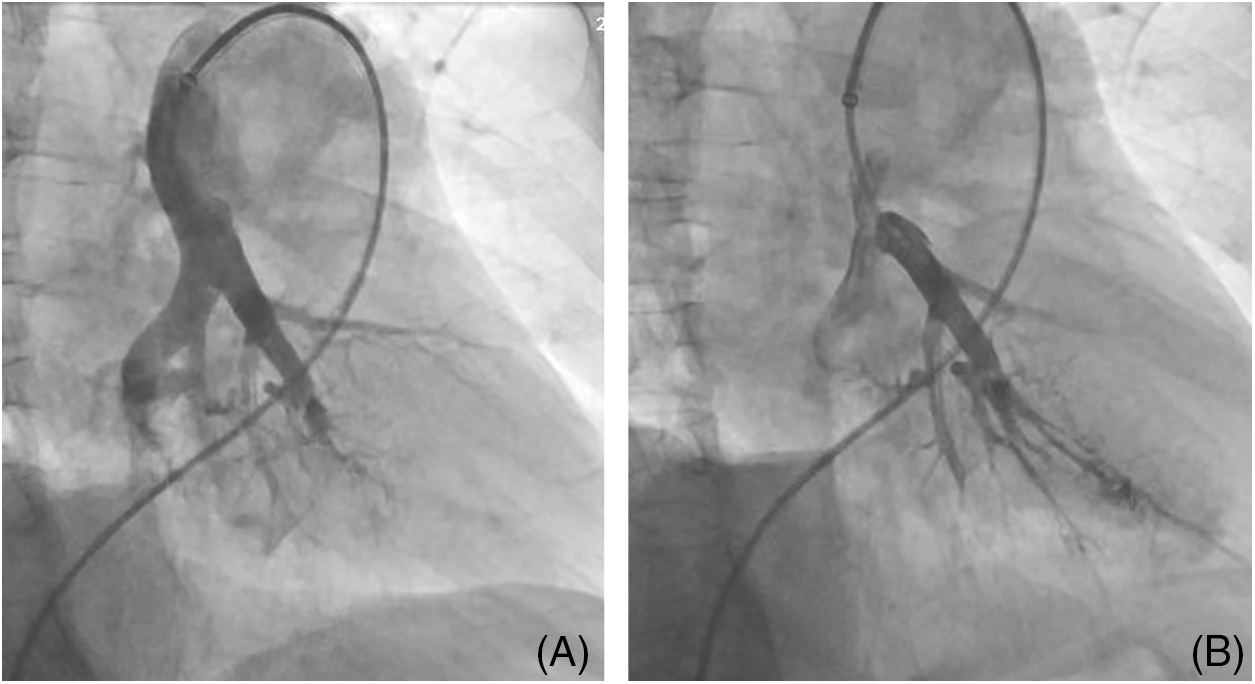
Figure 3: A patient with chronic thromboembolic pulmonary hypertension underwent balloon pulmonary angioplasty (BPA). (A) DSA of the pulmonary artery prior to BPA procedure, (B) DSA of the pulmonary artery post-BPA procedure
It is recommended that any patient with PH should be carefully evaluated for the possibility of CTEPH, regardless of whether an acute embolism has been documented [1]. For better screening and detecting CTEPH, patients with persistent or new-onset dyspnea or exercise limitation following PE should undergo a further diagnostic evaluation to assess for CTEPH/CTEPD according to the 2022 ESC/ERC Guidelines (Fig. 4). In addition, after 3 months of anticoagulation for acute PE, symptomatic patients with mismatched perfusion lung defects are recommended for referral to a PH/CTEPH center.
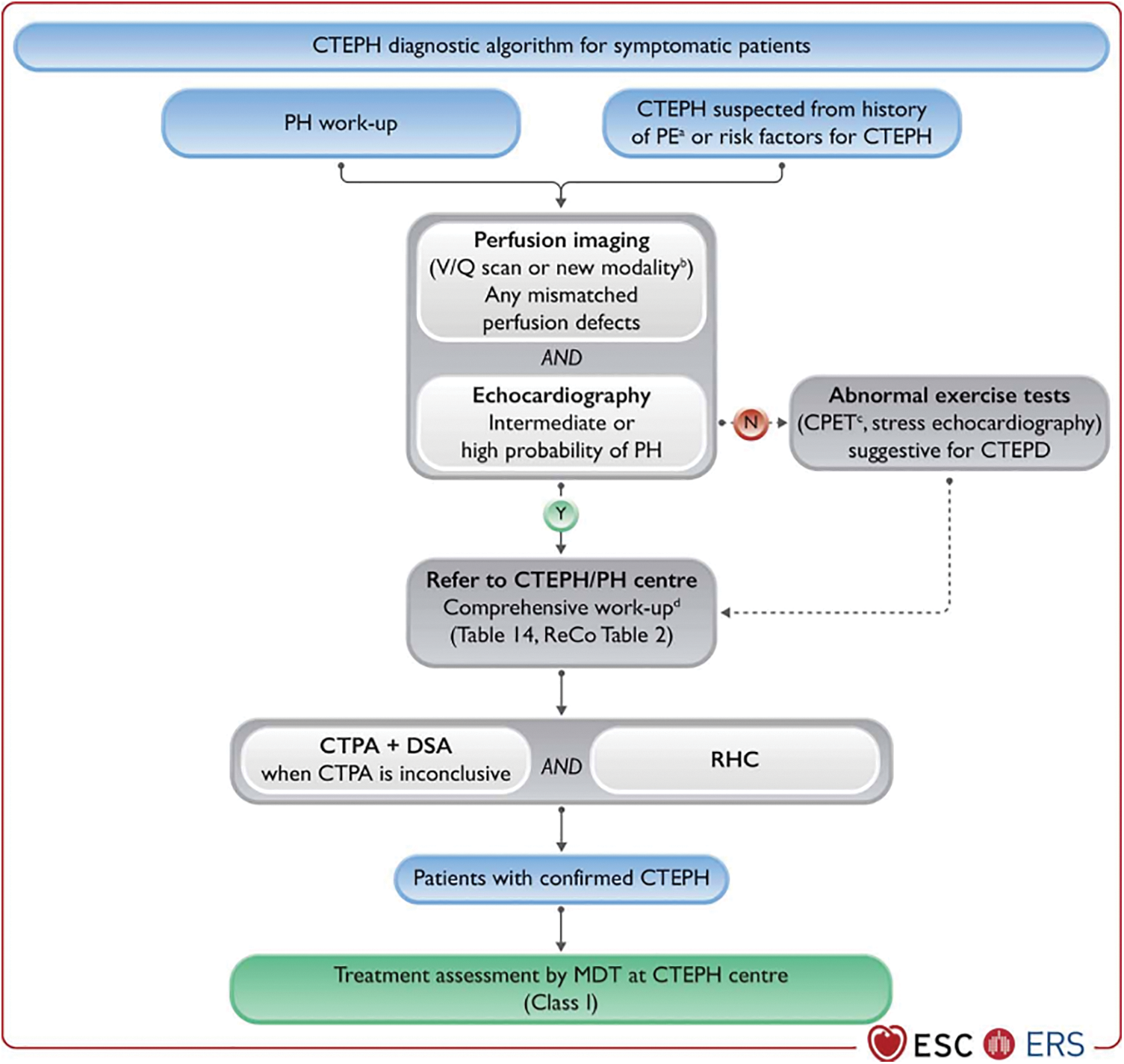
Figure 4: Diagnostic strategy in chronic thrombo-embolic pulmonary hypertension. CPET, cardiopulmonary exercise test; CTEPD, chronic thrombo-embolic pulmonary disease; CTEPH, chronic thrombo-embolic pulmonary hypertension; CTPA, computed tomography pulmonary angiography; DECT, dual-energy computed tomography; DSA, digital subtraction angiography; MDT, multidisciplinary team; MRI, magnetic resonance imaging; N, no; PE, pulmonary embolism; PETCO2, end-tidal partial pressure of carbon dioxide; PH, pulmonary hypertension; ReCo, recommendation; RHC, right heart catheterization; sPAP, systolic pulmonary arterial pressure; V/Q, ventilation/perfusion; VE/VCO2, ventilatory equivalents for carbon dioxide; VO2/HR, oxygen pulse; VO2, oxygen uptake; Y, yes. aCTEPH suspected from history of PE, including elevated sPAP on echocardiography and signs suggesting CTEPH on CTPA performed at the time of the acute PE. bAlternative perfusion imaging techniques—such as iodine subtraction mapping, DECT, and MRI perfusion—are currently under evaluation. cTypical pattern, including low PETCO2, high VE/VCO2, low VO2/HR, and low peak VO2. dComprehensive work-up after 3 months of therapeutic anticoagulation or sooner in unstable or rapidly deteriorating patients. Ideally, CTPA, DSA, and RHC are performed in CTEPH centres, but they are sometimes performed in PH centres, depending on the country and organization. Reproduced with permission of the © European Society of Cardiology & European Respiratory Society 2023: European Respiratory Journal 61(1) 2200879; DOI: 10.1183/13993003.00879-2022 Published 06 January 2023
The treatment of CTEPH involves a multimodal approach that combines PEA, BPA, and pharmacologic therapy to target a diverse anatomical pattern of lesions, including proximal, distal, and microvascular involvement (Fig. 5).
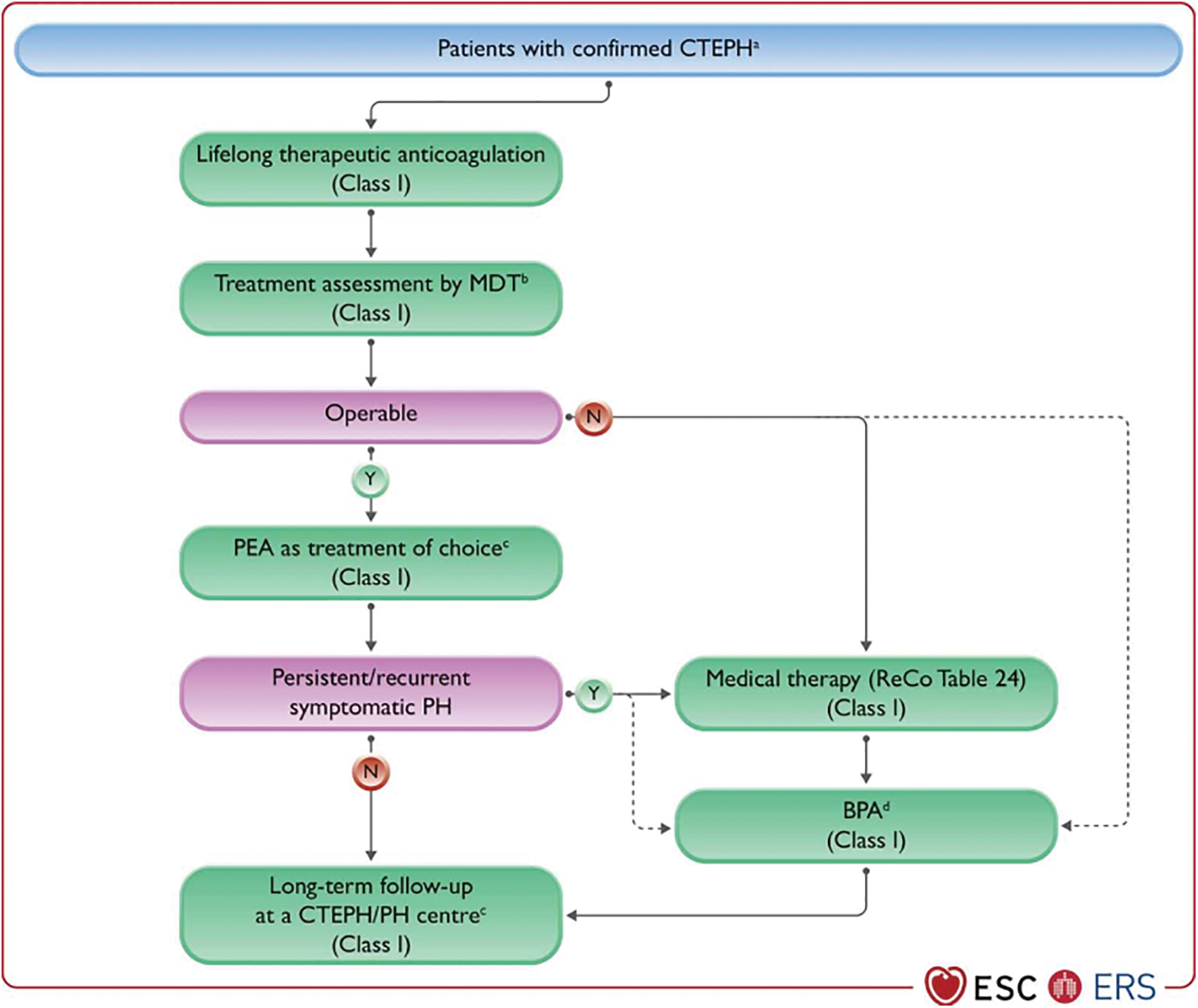
Figure 5: Management strategy in chronic thrombo-embolic pulmonary hypertension. BPA, balloon pulmonary angioplasty; CTEPD, chronic thrombo-embolic pulmonary disease; CTEPH, chronic thrombo-embolic pulmonary hypertension; MDT, multidisciplinary team; N, no; PAH, Pulmonary arterial hypertension; PEA, pulmonary endarterectomy; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; ReCo, recommendation; WU, Wood units; Y, yes. aSelected symptomatic patients with CTEPD without PH can also be treated by PEA and BPA. bMDT meeting can be virtual. cTreatment assessment may differ, depending on the level of expertise in PEA and BPA. dFor inoperable patients with PVR > 4 WU, medical therapy should be considered prior to BPA; there are limited data on BPA as first-line therapy. Reproduced with permission of the © European Society of Cardiology & European Respiratory Society 2023: European Respiratory Journal 61(1) 2200879; DOI: 10.1183/13993003.00879-2022 Published 06 January 2023
For patients with confirmed CTEPH, treatment should be implemented. Basic treatment involves long-term anticoagulation, diuretics, domiciliary oxygen therapy (DOXT), and rehabilitation [18].
Patients with CTEPH should receive anticoagulant therapy for the duration of their lives. CTEPD patients without PH who are at intermediate or high risk of PE recurrence or who do not have a history of VTE should receive long-term anticoagulation [1]. The most commonly used anticoagulants in clinical practice are vitamin K antagonists (VKAs) and novel oral anticoagulants (NOACs). Recently, NOACs (also known as direct oral anticoagulants, DOACs) have become increasingly popular as alternatives to VKAs [34–36]. The DOACs selectively inhibit the active sites of coagulation factors, such as dabigatran targeting factor IIa and rivaroxaban and apixaban targeting factor Xa, thereby directly blocking key components in the coagulation cascade to achieve an anticoagulant effect. A meta-analysis included a total of ten studies, which demonstrated that NOACs were non-inferior to VKAs in terms of all-cause mortality, venous thromboembolism, and major bleeding. This meta-analysis provides support for the utilization of NOACs in patients with CTEPH [37]. Although NOACs are increasingly used in the treatment of CTEPH, they do not benefit patients with renal insufficiency or antiphospholipid syndrome [38,39]. Before administering anticoagulants, individuals with CTEPH should undergo antiphospholipid syndrome testing to guarantee a safe anticoagulant medication [1].
CTEPH patients benefit from long-term DOXT. It was uncovered in a randomized, placebo-controlled trial conducted in patients with CTEPH who have exercise-induced hypoxemia that 5 weeks of DOXT improves exercise performance, quality of life (QoL), and the New York Heart Association (NYHA) functional class. Furthermore, DOXT can also serve as an adjunct to PH-targeted drug treatment for CTEPH patients who suffer from mild hypoxemia at rest and exacerbated conditions during exercise [40]. CTEPH patients who develop right heart failure may experience hepatic stasis, plasma cavity effusion, and other symptoms that can aggravate their overall health. Diuretics may be effective in improving symptoms.
CTEPH patients can also benefit from the general measures recommended for PH, including supervised exercise training, which is effective and safe for patients who are inoperable, and those who are still recovering from PEA [41]. To date, the majority of exercise training studies in PH have adopted the Heidelberg model [42], which entails an intensive inpatient induction phase lasting for 3 weeks, followed by a 12-week outpatient monitoring period. Exercise is performed on a minimum of 5 days per week, encompassing aerobic training, resistance training, and respiratory training. An extensive randomized, controlled study has shown that exercise training is a feasible and safe adjunct to medical management in treating PH and CTEPH [43]. Fukui et al. conducted a prospective randomized study to determine the effects of rehabilitation following BPA [44]. Despite the small sample size, the results demonstrated that the combination of BPA plus rehabilitation is more beneficial than BPA alone. A prospective cohort study examined the effects of exercise training on patients with CTEPH who underwent PEA. The results revealed that a significant improvement is seen in the cardiopulmonary output, mPAP, right heart size, 6-min walking distance (6MWD), QoL, and oxygen saturation within the first 3 weeks following PEA, as well as during the following 19 weeks of exercise training [41]. Currently, the majority of programs are still primarily situated within hospital settings, thereby limiting accessibility. A recent study has demonstrated that a fully remote pulmonary hypertension (PH) and home-based (PHAHB) physical activity program is both safe and feasible, while effectively enhancing functional capacity, promoting levels of physical activity, and improving patients’ quality of life [45]. In conclusion, exercise training can be practical as a supplement to CTEPH medication and perioperative care.
CTEPH patients eligible for surgery may benefit from PEA, which involves removing thromboembolic material from surgically accessible pulmonary arteries. The 2022 ESC/ERS Guidelines for the diagnosis and treatment of PH recommend that CTEPH patients with fibrotic obstructions within pulmonary arteries accessible by surgery should undergo PEA [1]. The procedure entails the excision of fibrous obstructive tissue from the pulmonary artery during circulatory arrest under deep hypothermia. Complications following PEA encompass infection (including ventilator-associated pneumonia, mediastinitis, catheter-associated sepsis, etc.), persistent PH, neurological deficits, hemorrhage, reperfusion pulmonary edema, pericardial effusion and the requirement for extracorporeal membrane oxygenation. Furthermore, mortality is a significant complication post-surgery. It should be noted that the incidence and severity of these complications may vary depending on individual differences and specific procedural circumstances [46]. Some evidence indicates that PEA is effective in treating symptomatic CTEPD patients without PH, with improvements in clinical and haemodynamic parameters at rest and during exercise [47,48].
During PEA, the patient will be placed on a heart-lung machine, with deep hypothermia and circulatory arrest (DHCA). The evaluation of surgical operability and the selection of the final treatment should be performed by a multidisciplinary expert team consisting of experienced PEA surgeons (on-site or closely collaborating) [25]. The feasibility of PEA is affected by several factors, including the team’s experience, the accessibility to PA lesions, the correlation between the severity of PH and the degree of PA obstructions, and comorbidities [49].
A well-established CTEPH center has a positive surgical outcome with a perioperative mortality rate of 2.5%, attributed to the improved management of cardiac and pulmonary complications and the use of extracorporeal membrane oxygenation (ECMO) [50]. According to the CTEPH task force at the 2013 World Symposium on Pulmonary Hypertension (WSPH), PEA centers should have ECMO available in case of unforeseen complications following surgery [17]. A retrospective analysis conducted in the UK over a 17-year period revealed that patients who underwent PEA surgery and received ECMO support had a 5-year survival rate of 73.9% (SE: 6.1%) and a 10-year survival rate of 58.2% (SE: 9.5%) [51].
Despite decades of progress, PEA continues to encounter a variety of obstacles. It leads to such complications as neurological issues under DHCA, recurrent PH, and reperfusion pulmonary edema. For instance, a meta-analysis showed that 25% of CTEPH patients have residual pulmonary hypertension (RPH) after PEA [52]. However, long-term outcomes following PEA are excellent regarding survival (an average 3-year survival of 90%) and QoL, even in patients suffering from distal PA obstructions [20,53–55]. Even though the procedure is optimal, residual CTEPH persists in 10% to 40% of patients [46,56–58]. According to a random study, pulmonary artery denervation (PADN) significantly reduces pulmonary artery pressure (PAP) and PVR after PEA in patients with CTEPH. During the following 12 months, the patients experience improved 6-min walking test (6MWT) results and reduced hospitalization needs [59].
It is essential to note that, despite the criteria for determining whether a patient is operable, the decision on undergoing surgery is still influenced by the experience of the center responsible for the intervention. Experience within a surgical or multidisciplinary team is crucial for evaluating which vascular lesions are proximal and thus surgically accessible [2,52]. High PVR values, right heart dysfunction, and distal pulmonary vascular obstruction combined with high PVR values are not absolute contraindications to surgery [60]. Therefore, all CTEPH patients should undergo an evaluation in an expert center to determine their eligibility for endarterectomy.
After the reports from Japan in 2012, BPA has become a crucial part of the CTEPH therapy regimen [61–63], which improves the distal blood supply to the stenosis by dilating the pulmonary artery stenosis through mechanical methods. Patients who are technically inoperable or who have residual PH after PEA, as well as those with distal blockages, are candidates for BPA. In addition, technically operable patients with a high proportion of distal illness and an unfavorable risk-to-benefit ratio for PEA may also be candidates for BPA [1]. It takes 4.8 sessions on average for a patient’s hemodynamic profile to improve [2]. A multicenter study conducted in Japan revealed that 1,408 BPA operations have been performed on 308 participants. The following hemodynamic parameters are improved after BPA: mPAP and PVR, whereas targeted therapy and oxygen therapy needs are reduced significantly [64].
According to studies, BPA improves haemodynamics, symptoms, exercise capacity, and right ventricular function [65]. It is true that BPA is easier to perform than PEA and that it imposes fewer restrictions on the patient. However, BPA is still associated with risks and complications, such as hemoptysis, lung injury, pulmonary artery perforation, and pericardial effusion [25,66]. BPA-related lung injury was previously described as a result of reperfusion pulmonary edema, but current consensus indicates that mechanical vascular injuries are caused by wire manipulation or balloon over-dilation rather than reperfusion pulmonary edema [67]. To achieve the best outcomes, this treatment should be performed in high-volume CTEPH-experienced centers. According to a randomized controlled experiment and an auxiliary follow-up analysis, medical pretreatment can minimize the incidence rate of interventional complications; therefore, patients with CTEPH should consider medical therapy before receiving BPA [68].
In a study published in 2018, Wasdenroth et al. demonstrated that BPA improves World Health Organization functional class (WHO-FC), 6MWD, PVR, pulmonary artery compliance, and decreases NT-proBNP concentrations in symptomatic CTEPD patients without PH [69]. While the text notes that these results need to be confirmed by a more extensive, forward-looking international registry study. The new guidelines clearly state that PEA or BPA should be considered for patients with selected symptoms of CTEPD without PH [1].
A prospective pilot cohort research examined the efficacy and safety of bronchial artery embolization (BAE) for the treatment of hemoptysis in patients with CTEPH [23]. The results uncovered that the oxygenation index and right heart function are not significantly different between BAE-treated and non-treated subjects. Compared to non-treated subjects, BAE-treated subjects have significantly reduced hemoptysis relapse (20% vs. 80%; p = 0.025) and hemoptysis-related mortality rates (0% vs. 40%; p = 0.032) and significantly increased overall survival (90% vs. 40%; p = 0.040) [23]. The findings of this study might require further confirmation by prospective multicenter studies with a large sample size in the future.
4.4 PH-Targeted Medical Therapy
The possibility of microvascular arteriopathy arising from CTEPH provides a rationale for adopting PH-targeted therapy in treating CTEPH [70,71]. Phosphodiesterase-5 inhibitors (PDE5i), dual endothelin receptor antagonists (ERA), prostacyclin analogs, and soluble guanylate cyclase stimulators (sGCs), prostacyclin receptor agonist are some of the most common drugs used to treat CTEPH.
Riociguat, a stimulator of Guanylate Cyclase (an sGC), is the only drug approved by Food and Drug Administration (FDA) for treating patients with inoperable CTEPH or persistent/recurrent PH after PEA (Recommendation IB) [72]. Based on results from the CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1), a phase III randomized controlled trial (RCT), riociguat (targeting the nitric oxide pathway) significantly increases 6MWD and reduces PVR in 261 patients at 16 weeks after initiation of treatment compared to placebo. A bridging therapy trial examining the efficacy and safety of riociguat is currently being conducted (NCT 03273257). Riociguat is also being evaluated in comparison to BPA in patients with inoperable CTEPH [68].
In the CTREPH (Subcutaneous Treprostinil for Severe Non-operable Chronic Thromboembolic Pulmonary Hypertension) trial, a comparison was made between low-dose (3 ng/kg/min) and high-dose (30 ng/kg/min) treprostinil subcutaneous infusions in a mixed population of CTEPH patients. Treprostinil (prostacyclin analog) treatment results in a 40-m improvement in 6MWD after 24 weeks [73]. Subcutaneous infusion of treprostinil may be considered for patients in WHO-FC III–IV who have inoperable CTEPH or persistent/recurrent PH after PEA (Recommendation IIb B) [1].
The MERIT-1 (Macitentan in the Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension) trial is a phase II RCT study, and only patients with inoperable CTEPH were included. Compared to the placebo, 10 mg macitentan (dual ERA) improves PVR and 6MWD at 16 and 24 weeks, respectively [74]. Unlike previous studies, 61% of patients received PDE5i or inhaled/oral prostanoid therapies as background treatments. In an ongoing phase III RCT, the safety and efficacy of 75 mg macitentan were evaluated in patients with inoperable, persistent, or recurrent CTEPH (NCT 04271475) [1].
In addition to the three large controlled trials described previously, several smaller studies have also been published in the literature, see Table 1. As for other medical treatments, PDE5is (such as sildenafil) and ERAs (such as bosentan) have been used off-label because their efficacy in inoperable CTEPH has not been validated by RCTs or registry studies [53,75,76]. Combination therapy with PDE5is and ERAs is commonly prescribed in CTEPH patients with severe hemodynamic impairment [77]. The 2022 ESC/ERC Guidelines revised the recommendation that off-label use of drugs approved for PAH may be considered for symptomatic patients who have inoperable CTEPH (Recommendation IIb B). For patients with inoperable CTEPH, combining sGCs/PDE5is, ERA, or parenteral prostacyclin analogues may be considered now (Recommendation IIb C) [1].
Anatomical lesions may be mixed with lobar, segmental, or microvascular lesions in many patients. Multimodal therapy involves using PEA, BPA, and pharmaceutical therapy in conjunction with each other to address proximal lesions, distal lesions, and microvascular alterations (Fig. 6). Patients with a high preoperative PVR are often prescribed medication to improve their pulmonary hemodynamics prior to PEA. This practice remains controversial because it is thought to delay the referral for surgery and eventual treatment [85–87].
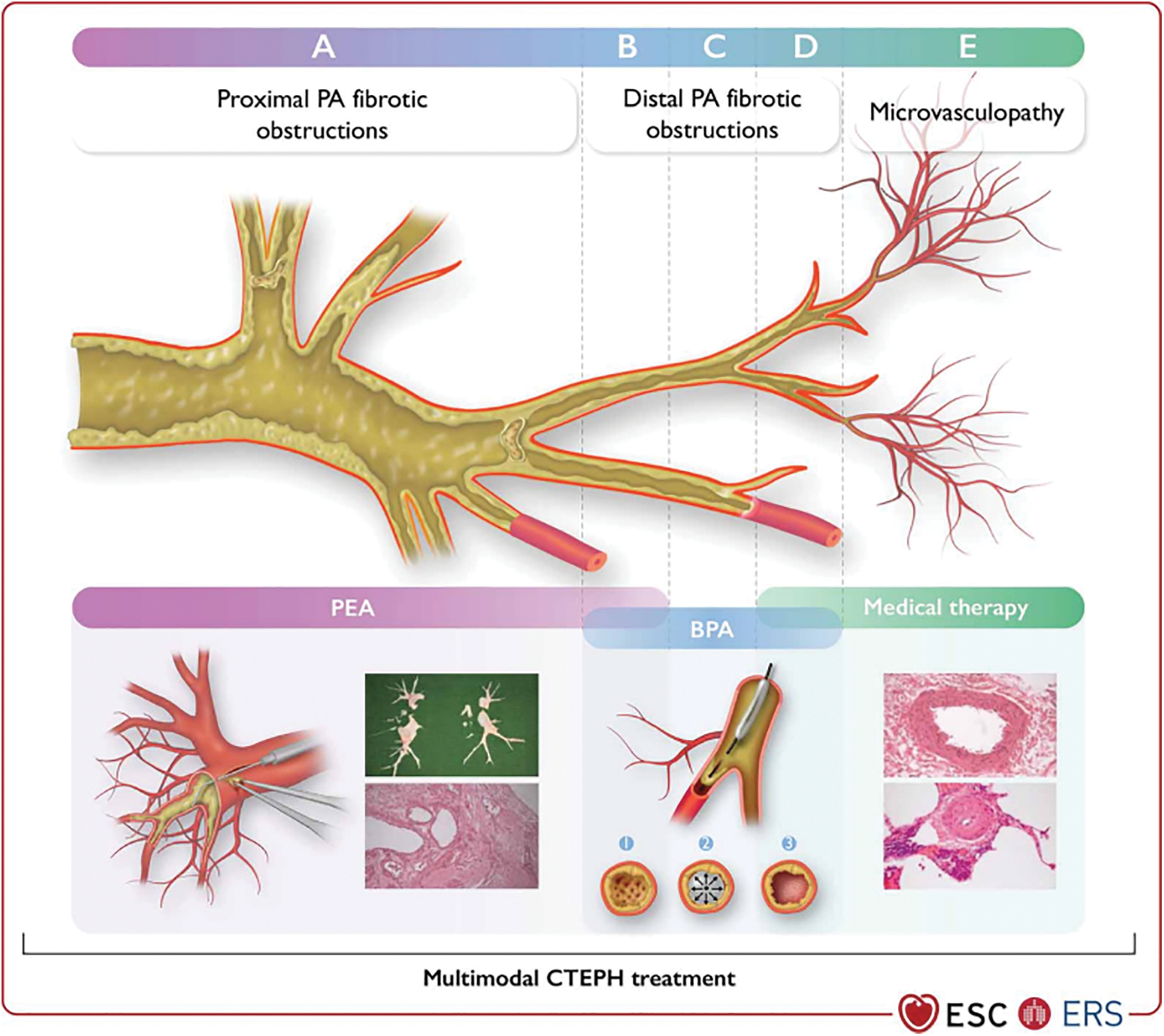
Figure 6: Overlap in treatments/multimodality approaches in chronic thrombo-embolic pulmonary hypertension. BPA, balloon pulmonary angioplasty; CTEPH, chronic thrombo-embolic pulmonary hypertension; PA, pulmonary artery; PEA, pulmonary endarterectomy. Top panels: (A) Proximal PA fibrotic obstructions (vessel diameter 10–40 mm). (B) Distal segmental and subsegmental PA fibrotic obstruction is potentially suitable for both PEA and BPA interventions (vessel diameter 2–10 mm). (C) Distal subsegmental PA fibrotic obstructions form a web-lesion in a subsegmental branch of the PA suitable for BPA interventions (vessel diameter 0.5–5 mm). (D) Distal subsegmental PA fibrotic obstructions form web-like lesions, which might be accompanied by microvasculopathy (vessel diameter < 0.5 mm). (E) Microvasculopathy (vessel diameter < 0.05 mm) treated with medical therapy. Bottom panels: (A) bottom left: PEA; vessel diameter (0.2–3 cm). The right PA is opened and the suction dissector is introduced between the artery wall and fibrosis. Following the inside of the artery down to segmental and subsegmental levels, the fibrotic material is subsequently freed from the wall and removed with forceps. (A) bottom right: PEA specimen with ‘tails’ to subsegmental branches of the PA; cross-section of partially organized and permeabilized thrombotic lesion of the large PA dissected during PEA. (B–D) The wire is introduced between the fibrotic material (1), then the balloon is inflated, leading to a rupture of the web (2). Fibrotic material is connected to the vessel wall (3). (E) Small muscular PA displaying eccentric intimal fibrosis involving intimal thickening and proliferation—target for medical therapies. Reproduced with permission of the © European Society of Cardiology & European Respiratory Society 2023: European Respiratory Journal 61(1) 2200879; DOI: 10.1183/13993003.00879-2022 Published 06 January 2023
Recent research evaluated three groups of 418 CTEPH patients: those undergoing PEA alone, those receiving PEA combined with targeted healing therapy induction, and those treated with PEA after BPA induction. Regardless of the type of preoperative treatment received, PEA leads to a similar reduction in mPAP. However, early survival and prognosis are worse in patients who had surgery following medical induction. With the adoption of BPA, elderly patients can undergo shorter procedures with satisfactory results. It was concluded that multimodal therapy used to treat CTEPH patients does not affect the effectiveness of PEA. Medical therapy and BPA may serve as a synergistic treatment approach with surgery in some more challenging cases [88].
Multimodal therapy should be considered for patients with persistent PH following PEA and those with inoperable CTEPH, according to new suggestions in the guidelines [1].
The term CTEPD with or without PH has been introduced in group 4 PH, acknowledging the presence of similar symptoms, perfusion defects, and organized fibrotic obstructions in patients both with and without resting PH.
The guidelines advocate a multimodal, multidisciplinary approach (including surgery, intervention, and medication) for patients diagnosed with confirmed CTEPH, see Table 2.
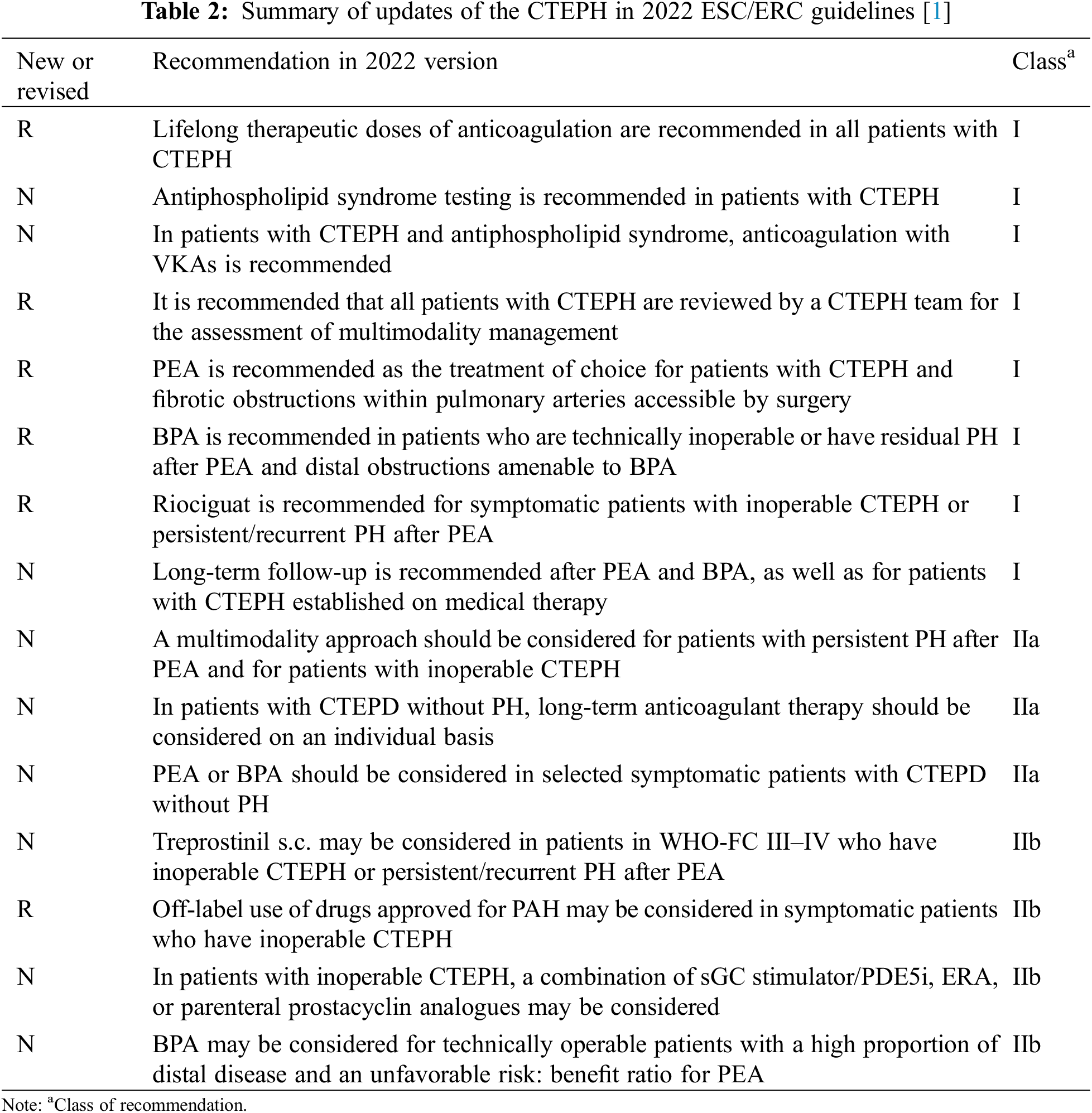
In summary, PEA remains the gold standard treatment for patients with CTEPH. In suitable candidates, PEA can significantly improve quality of life and exercise tolerance, reduce pulmonary vascular resistance, and enhance long-term survival. However, some patients continue to have residual pulmonary hypertension after surgery, while others are not candidates for PEA due to the location or extent of thromboembolic lesions. For these patients, BPA has emerged as a promising treatment option. Available evidence indicates BPA can effectively improve hemodynamics and functional capacity.
Additionally, medical therapy with soluble guanylate cyclase (sGC) stimulators has demonstrated benefits in patients who are inoperable. A multimodality approach, combining PEA, BPA and pharmacotherapy, may provide comprehensive treatment tailored to each CTEPH patient’s lesion profile. Importantly, most studies on BPA and medical therapy for CTEPH have been small non-randomized trials. Large-scale randomized controlled trials are warranted to determine the long-term efficacy and safety of these therapies. Further research is also needed to identify optimal patient selection criteria for BPA and drug treatment.
In conclusion, while PEA remains the gold standard treatment, BPA and medical treatment offer new hope for CTEPH patients who are not surgical candidates or have persistent pulmonary hypertension despite surgery. Continued research to refine patient selection and treatment algorithms will be key to maximizing outcomes in this complex patient population.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: YLC, YXS; data collection: ZGY; analysis and interpretation of results: YR; draft manuscript preparation: YR. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: With publication, the data set used for this review will be shared upon request from the study authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879. doi:10.1183/13993003.00879-2022. [Google Scholar] [PubMed] [CrossRef]
2. Ruaro B, Baratella E, Caforio G, Confalonieri P, Wade B, Marrocchio C, et al. Chronic thromboembolic pulmonary hypertension: an update. Diagnostics. 2022;12(2):235–46. doi:10.3390/diagnostics12020235. [Google Scholar] [PubMed] [CrossRef]
3. Durrington C, Hurdman JA, Elliot CA, Maclean R, Veen JV, Sacccullo G, et al. Systematic pulmonary embolism follow-up increases diagnostic rates of chronic thromboembolic pulmonary hypertension and identifies less severe disease: results from the ASPIRE Registry. Eur Respir J. 2024;63(3):2300846. doi:10.1183/13993003.00846-2023. [Google Scholar] [PubMed] [CrossRef]
4. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):2045894020977300. [Google Scholar] [PubMed]
5. Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57(6):2002828. doi:10.1183/13993003.02828-2020. [Google Scholar] [PubMed] [CrossRef]
6. Kramm T, Wilkens H, Fuge J, Schafers HJ, Guth S, Wiedenroth CB, et al. Incidence and characteristics of chronic thromboembolic pulmonary hypertension in Germany. Clin Res Cardiol. 2018;107(7):548–53. doi:10.1007/s00392-018-1215-5. [Google Scholar] [PubMed] [CrossRef]
7. Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–64. doi:10.1056/NEJMoa032274. [Google Scholar] [PubMed] [CrossRef]
8. Golpe R, Perez-de-Llano LA, Castro-Anon O, Vazquez-Caruncho M, Gonzalez-Juanatey C, Veres-Racamonde A, et al. Right ventricle dysfunction and pulmonary hypertension in hemodynamically stable pulmonary embolism. Respir Med. 2010;104(9):1370–6. doi:10.1016/j.rmed.2010.03.031. [Google Scholar] [PubMed] [CrossRef]
9. Guerin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112(3):598–605. [Google Scholar] [PubMed]
10. Simonneau G, Hoeper MM. Evaluation of the incidence of rare diseases: difficulties and uncertainties, the example of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2017;49(2):1602522. doi:10.1183/13993003.02522-2016. [Google Scholar] [PubMed] [CrossRef]
11. Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu AT, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49(2):1601792. doi:10.1183/13993003.01792-2016. [Google Scholar] [PubMed] [CrossRef]
12. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERSendorsed by: association for European Paediatric and Congenital Cardiology (AEPCInternational Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75. [Google Scholar] [PubMed]
13. Newman J, Boubriak I, Jenkins D, Ng C, Ruggiero A, Screaton N, et al. Rising COVID-19 related acute pulmonary emboli but falling national chronic thromboembolic pulmonary hypertension referrals from a large national dataset. ERJ Open Research. 2021;7(4):00431–2021. doi:10.1183/23120541.00431-2021. [Google Scholar] [PubMed] [CrossRef]
14. Ciurzyński M, Kurzyna M, Kopeć G, Błaszczak P, Chrzanowski Ł, Kamiński K, et al. An expert opinion of the polish cardiac society working group on pulmonary circulation on screening for chronic thromboembolic pulmonary hypertension patients after acute pulmonary embolism: update. Kardiologia polska. 2022;80(6):723–32. doi:10.33963/KP.a2022.0141. [Google Scholar] [PubMed] [CrossRef]
15. de Jong CMM, Visser C, Bemelmans RHH, Boersma WG, van den Borst B, Burggraaf JLI, et al. Chronic thromboembolic pulmonary hypertension and clot resolution after COVID-19-associated pulmonary embolism. Eur Respir J. 2023;61(4):2300171. doi:10.1183/13993003.00171-2023. [Google Scholar] [PubMed] [CrossRef]
16. Yang S, Yang Y, Zhai Z, Kuang T, Gong J, Zhang S, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis. 2015;7(11):1927–38. [Google Scholar] [PubMed]
17. Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25_Suppl.):D92–9. [Google Scholar] [PubMed]
18. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi:10.1093/eurheartj/ehz405. [Google Scholar] [PubMed] [CrossRef]
19. Bonderman D, Wilkens H, Wakounig S, Schafers HJ, Jansa P, Lindner J, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33(2):325–31. [Google Scholar] [PubMed]
20. Newnham M, Bunclark K, Abraham N, Ali S, Amaral-Almeida L, Cannon JE, et al. CAMPHOR score: patient-reported outcomes are improved by pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020;56(4):1902096. doi:10.1183/13993003.02096-2019. [Google Scholar] [PubMed] [CrossRef]
21. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69. doi:10.1093/eurheartj/ehu283. [Google Scholar] [PubMed] [CrossRef]
22. Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, et al. Chronic thromboembolic pulmonary hypertension (CTEPHresults from an international prospective registry. Circulation. 2011;124(18):1973–81. doi:10.1161/CIRCULATIONAHA.110.015008. [Google Scholar] [PubMed] [CrossRef]
23. Yang S, Wang J, Kuang T, Gong J, Ma Z, Shen YH, et al. Efficacy and safety of bronchial artery embolization on hemoptysis in chronic thromboembolic pulmonary hypertension: a pilot prospective cohort study. Crit Care Med. 2019;47(3):e182–e89. doi:10.1097/CCM.0000000000003578. [Google Scholar] [PubMed] [CrossRef]
24. Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24(136):246–52. doi:10.1183/16000617.00001115. [Google Scholar] [PubMed] [CrossRef]
25. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1):1801915. doi:10.1183/13993003.01915-2018. [Google Scholar] [PubMed] [CrossRef]
26. Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48(5):680–4. doi:10.2967/jnumed.106.039438. [Google Scholar] [PubMed] [CrossRef]
27. He J, Fang W, Lv B, He JG, Xiong CM, Liu ZH, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun. 2012;33(5):459–63. doi:10.1097/MNM.0b013e32835085d9. [Google Scholar] [PubMed] [CrossRef]
28. Lambert L, Michalek P, Burgetova A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension-systematic review and meta-analysis. Eur Radiol. 2022;32(11):7927–35. doi:10.1007/s00330-022-08804-5. [Google Scholar] [PubMed] [CrossRef]
29. Chen R, Liao H, Deng Z, He Z, Zheng Z, Lu J, et al. Efficacy of computed tomography in diagnosing pulmonary hypertension: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:966257. doi:10.3389/fcvm.2022.966257. [Google Scholar] [PubMed] [CrossRef]
30. McInnis MC, Wang D, Donahoe L, Granton J, Thenganatt J, Tan K, et al. Importance of computed tomography in defining segmental disease in chronic thromboembolic pulmonary hypertension. ERJ Open Res. 2020;6(4):00461–2020. doi:10.1183/23120541.00461-2020. [Google Scholar] [PubMed] [CrossRef]
31. Marin-Romero S, Ballaz-Quincoces A, Gomez-Cuervo C, Marchena-Yglesias PJ, Lopez-Miguel P, Francisco-Albesa I, et al. Symptom-related screening programme for early detection of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the SYSPPE study. Thorax. 2024;79(2):144–52. doi:10.1136/thorax-2023-220580. [Google Scholar] [PubMed] [CrossRef]
32. Maschke S, Werncke T, Becker LS, Renne J, Dewald CLA, Olsson KM, et al. Motion reduction for C-arm computed tomography of the pulmonary arteries: image quality of a motion correction algorithm in patients with chronic thromboembolic hypertension during balloon pulmonary angioplasty. Rofo. 2021;193(9):1074–80. doi:10.1055/a-1354-6736. [Google Scholar] [PubMed] [CrossRef]
33. Hinrichs JB, Renne J, Hoeper MM, Olsson KM, Wacker FK, Meyer BC. Balloon pulmonary angioplasty: applicability of C-Arm CT for procedure guidance. Eur Radiol. 2016;26(11):4064–71. doi:10.1007/s00330-016-4280-z. [Google Scholar] [PubMed] [CrossRef]
34. Hosokawa K, Watanabe H, Taniguchi Y, Ikeda N, Inami T, Yasuda S, et al. A multicenter, single-blind, randomized, warfarin-controlled trial of edoxaban in patients with chronic thromboembolic pulmonary hypertension: KABUKI trial. Circulation. 2024;149(5):406–9. doi:10.1161/CIRCULATIONAHA.123.067528. [Google Scholar] [PubMed] [CrossRef]
35. Ishisaka Y, Watanabe A, Takagi H, Steiger D, Kuno T. Anticoagulation in chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Thromb Res. 2023;231:91–8. doi:10.1016/j.thromres.2023.10.003. [Google Scholar] [PubMed] [CrossRef]
36. Kido K, Shimizu M, Shiga T, Hashiguchi M, Jalil B, Caccamo M, et al. Meta-analysis comparing direct oral anticoagulants versus vitamin K Antagonists in patients with chronic thromboembolic pulmonary hypertension. Am J Cardiol. 2024;210:172–6. doi:10.1016/j.amjcard.2023.10.017. [Google Scholar] [PubMed] [CrossRef]
37. Salazar AM, Panama G, Kim AG, Rayamajhi S, Abela GS. Clinical outcomes between direct oral anticoagulants versus vitamin K antagonists in chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Curr Probl Cardiol. 2024;49(3):102377. doi:10.1016/j.cpcardiol.2024.102377. [Google Scholar] [PubMed] [CrossRef]
38. Ordi-Ros J, Saez-Comet L, Perez-Conesa M, Vidal X, Riera-Mestre A, Castro-Salomo A, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: aa randomized noninferiority trial. Ann Intern Med. 2019;171(10):685–94. doi:10.7326/M19-0291. [Google Scholar] [PubMed] [CrossRef]
39. Stammler R, Legendre P, Cacoub P, Blanche P, Piette JC, Costedoat-Chalumeau N. Catastrophic antiphospholipid syndrome following the introduction of rivaroxaban. Lupus. 2020;29(7):787–90. doi:10.1177/0961203320914363. [Google Scholar] [PubMed] [CrossRef]
40. Ulrich S, Saxer S, Hasler ED, Schwarz EI, Schneider SR, Furian M, et al. Effect of domiciliary oxygen therapy on exercise capacity and quality of life in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension: a randomised, placebo-controlled trial. Eur Respir J. 2019;54(2):1900276. doi:10.1183/13993003.002762019. [Google Scholar] [PubMed] [CrossRef]
41. Nagel C, Nasereddin M, Benjamin N, Egenlauf B, Harutyunova S, Eichstaedt CA, et al. Supervised exercise training in patients with chronic thromboembolic pulmonary hypertension as early follow-up treatment after pulmonary endarterectomy: a prospective cohort study. Respiration. 2020;99(7):577–88. doi:10.1159/000508754. [Google Scholar] [PubMed] [CrossRef]
42. Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–9. doi:10.1161/CIRCULATIONAHA.106.618397. [Google Scholar] [PubMed] [CrossRef]
43. Grunig E, MacKenzie A, Peacock AJ, Eichstaedt CA, Benjamin N, Nechwatal R, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J. 2021;42(23):2284–95. doi:10.1093/eurheartj/ehaa696. [Google Scholar] [PubMed] [CrossRef]
44. Fukui S, Ogo T, Takaki H, Ueda J, Tsuji A, Morita Y, et al. Efficacy of cardiac rehabilitation after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Heart. 2016;102(17):1403–9. doi:10.1136/heartjnl-2015-309230. [Google Scholar] [PubMed] [CrossRef]
45. McCormack C, Kehoe B, Cullivan S, McCaffrey N, Gaine S, McCullagh B, et al. Safety, feasibility and effectiveness of the remotely delivered Pulmonary Hypertension and Home-Based (PHAHB) physical activity intervention. ERJ Open Res. 2024;10(1):00608–2023. doi:10.1183/23120541.00608-2023. [Google Scholar] [PubMed] [CrossRef]
46. Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–10. doi:10.1016/j.jtcvs.2010.11.024. [Google Scholar] [PubMed] [CrossRef]
47. Guth S, Wiedenroth CB, Rieth A, Richter MJ, Gruenig E, Ghofrani HA, et al. Exercise right heart catheterisation before and after pulmonary endarterectomy in patients with chronic thromboembolic disease. Eur Respir J. 2018;52(3):1800458. doi:10.1183/13993003.00458-2018. [Google Scholar] [PubMed] [CrossRef]
48. Taboada D, Pepke-Zaba J, Jenkins DP, Berman M, Treacy CM, Cannon JE, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J. 2014;44(6):1635–45. doi:10.1183/09031936.00050114. [Google Scholar] [PubMed] [CrossRef]
49. Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94(1):97–103, discussion 03. doi:10.1016/j.athoracsur.2012.04.004. [Google Scholar] [PubMed] [CrossRef]
50. Lankeit M, Krieg V, Hobohm L, Kolmel S, Liebetrau C, Konstantinides S, et al. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2017;37(2):250–58. [Google Scholar]
51. Chia AXF, Valchanov K, Ng C, Tsui S, Taghavi J, Vuylsteke A, et al. Perioperative extracorporeal membrane oxygenation support for pulmonary endarterectomy: a 17-year experience from the UK national cohort. J Heart Lung Transplant. 2024;43(2):241–50. doi:10.1016/j.healun.2023.09.008. [Google Scholar] [PubMed] [CrossRef]
52. Hsieh WC, Jansa P, Huang WC, Niznansky M, Omara M, Lindner J. Residual pulmonary hypertension after pulmonary endarterectomy: a meta-analysis. J Thorac Cardiovasc Surg. 2018;156(3):1275–87. doi:10.1016/j.jtcvs.2018.04.110. [Google Scholar] [PubMed] [CrossRef]
53. Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133(9):859–71. doi:10.1161/CIRCULATIONAHA.115.016522. [Google Scholar] [PubMed] [CrossRef]
54. Vuylsteke A, Sharples L, Charman G, Kneeshaw J, Tsui S, Dunning J, et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOGa randomised controlled trial. Lancet. 2011;378(9800):1379–87. doi:10.1016/S0140-6736(11)61144-6. [Google Scholar] [PubMed] [CrossRef]
55. D’Armini AM, Morsolini M, Mattiucci G, Grazioli V, Pin M, Valentini A, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148(3):1005–11. doi:10.1016/j.jtcvs.2014.06.052. [Google Scholar] [PubMed] [CrossRef]
56. Bonderman D, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Dunkler D, Taghavi S, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115(16):2153–8. doi:10.1161/CIRCULATIONAHA.106.661041. [Google Scholar] [PubMed] [CrossRef]
57. Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the united kingdom national cohort. Circulation. 2016;133(18):1761–71. doi:10.1161/CIRCULATIONAHA.115.019470. [Google Scholar] [PubMed] [CrossRef]
58. Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383–7. doi:10.1016/j.jtcvs.2009.12.056. [Google Scholar] [PubMed] [CrossRef]
59. Romanov A, Cherniavskiy A, Novikova N, Edemskiy A, Ponomarev D, Shabanov V, et al. Pulmonary artery denervation for patients with residual pulmonary hypertension after pulmonary endarterectomy. J Am Coll Cardiol. 2020;76(8):916–26. doi:10.1016/j.jacc.2020.06.064. [Google Scholar] [PubMed] [CrossRef]
60. Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12(4):213–18. doi:10.14797/mdcj-12-4-213. [Google Scholar] [PubMed] [CrossRef]
61. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):748–55. doi:10.1161/CIRCINTERVENTIONS.112.971077. [Google Scholar] [PubMed] [CrossRef]
62. Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):756–62. doi:10.1161/CIRCINTERVENTIONS.112.971390. [Google Scholar] [PubMed] [CrossRef]
63. Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76(2):485–8. doi:10.1253/circj.CJ-11-1217. [Google Scholar] [PubMed] [CrossRef]
64. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10(11):e004029. doi:10.1161/CIRCOUTCOMES.117.004029. [Google Scholar] [PubMed] [CrossRef]
65. Zoppellaro G, Badawy MR, Squizzato A, Denas G, Tarantini G, Pengo V. Balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension-a systematic review and meta-analysis. Circ J. 2019;83(8):1660–67. doi:10.1253/circj.CJ-19-0161. [Google Scholar] [PubMed] [CrossRef]
66. Brenot P, Jais X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(5):1802095. doi:10.1183/13993003.02095-2018. [Google Scholar] [PubMed] [CrossRef]
67. Ejiri K, Ogawa A, Fujii S, Ito H, Matsubara H. Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2018;11(12):e005884. doi:10.1161/CIRCINTERVENTIONS.117.005884. [Google Scholar] [PubMed] [CrossRef]
68. Jais X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACEa multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med. 2022;10(10):961–71. doi:10.1016/S2213-2600(22)00214-4. [Google Scholar] [PubMed] [CrossRef]
69. Wiedenroth CB, Olsson KM, Guth S, Breithecker A, Haas M, Kamp JC, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ. 2018;8(1):2045893217753122. [Google Scholar] [PubMed]
70. Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103(3):685–92. doi:10.1378/chest.103.3.685. [Google Scholar] [PubMed] [CrossRef]
71. Galie N, Kim NH. Pulmonary microvascular disease in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3(7):571–6. doi:10.1513/pats.200605-113LR. [Google Scholar] [PubMed] [CrossRef]
72. Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29. doi:10.1056/NEJMoa1209657. [Google Scholar] [PubMed] [CrossRef]
73. Sadushi-Kolici R, Jansa P, Kopec G, Torbicki A, Skoro-Sajer N, Campean IA, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPHa double-blind, phase 3, randomised controlled trial. Lancet Respir Med. 2019;7(3):239–48. doi:10.1016/S2213-2600(18)30367-9. [Google Scholar] [PubMed] [CrossRef]
74. Ghofrani HA, Simonneau G, D’Armini AM, Fedullo P, Howard LS, Jais X, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–94. doi:10.1016/S2213-2600(17)30305-3. [Google Scholar] [PubMed] [CrossRef]
75. Jais X, D’Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertensiona randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–34. doi:10.1016/j.jacc.2008.08.059. [Google Scholar] [PubMed] [CrossRef]
76. Reichenberger F, Voswinckel R, Enke B, Rutsch M, El Fechtali E, Schmehl T, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2007;30(5):922–7. doi:10.1183/09031936.00039007. [Google Scholar] [PubMed] [CrossRef]
77. Guth S, D’Armini AM, Delcroix M, Nakayama K, Fadel E, Hoole SP, et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res. 2021;7(3):00850–2020. doi:10.1183/23120541.00850-2020. [Google Scholar] [PubMed] [CrossRef]
78. Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134(2):229–36. doi:10.1378/chest.07-2681. [Google Scholar] [PubMed] [CrossRef]
79. Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–903. doi:10.1161/CIRCULATIONAHA.108.839274. [Google Scholar] [PubMed] [CrossRef]
80. Escribano-Subias P, Bendjenana H, Curtis PS, Lang I, Vonk Noordegraaf A. Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH). Pulm Circ. 2019;9(2):2045894019846433. [Google Scholar] [PubMed]
81. Simonneau G, D’Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2015;45(5):1293–302. doi:10.1183/09031936.00087114. [Google Scholar] [PubMed] [CrossRef]
82. McLaughlin VV, Jansa P, Nielsen-Kudsk JE, Halank M, Simonneau G, Grunig E, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med. 2017;17(1):216. doi:10.1186/s12890-017-0563-7. [Google Scholar] [PubMed] [CrossRef]
83. Marra AM, Halank M, Benjamin N, Bossone E, Cittadini A, Eichstaedt CA, et al. Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Respir Res. 2018;19(1):258. doi:10.1186/s12931-018-0957-y. [Google Scholar] [PubMed] [CrossRef]
84. Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347(5):322–9. doi:10.1056/NEJMoa020204. [Google Scholar] [PubMed] [CrossRef]
85. Bresser P, Fedullo PF, Auger WR, Channick RN, Robbins IM, Kerr KM, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23(4):595–600. doi:10.1183/09031936.04.00020004. [Google Scholar] [PubMed] [CrossRef]
86. Nagaya N, Sasaki N, Ando M, Ogino H, Sakamaki F, Kyotani S, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest. 2003;123(2):338–43. doi:10.1378/chest.123.2.338. [Google Scholar] [PubMed] [CrossRef]
87. Reesink HJ, Surie S, Kloek JJ, Tan HL, Tepaske R, Fedullo PF, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2010;139(1):85–91. doi:10.1016/j.jtcvs.2009.03.053. [Google Scholar] [PubMed] [CrossRef]
88. Mercier O, Dubost C, Delaporte A, Genty T, Fabre D, Mitilian D, et al. Pulmonary thromboendarterectomy: the marie lannelongue hospital experience. Ann Cardiothorac Surg. 2022;11(2):143–50. doi:10.21037/acs. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools