 Open Access
Open Access
CASE REPORT
Prenatal Diagnosis of an Apically Located Congenital Left Ventricular Aneurysm: A Rare Case
1 Department of Perinatology, Antalya Education and Research Hospital, University of Health Sciences, Antalya, Turkey
2 Department of Perinatology, Faculty of Medicine, Süleyman Demirel University, Isparta, Turkey
* Corresponding Author: Yücel Kaya. Email:
Congenital Heart Disease 2024, 19(1), 123-129. https://doi.org/10.32604/chd.2024.048145
Received 29 November 2023; Accepted 07 February 2024; Issue published 20 March 2024
Abstract
Congenital ventricular aneurysm is a very rare cardiac anomaly. A diagnosis can be made during the prenatal period using fetal echocardiography. This study presents a very rare apically located left ventricular aneurysm case, and the relevant literature was reviewed and discussed. In this case, a 35-year-old, gravida 2, parity 1 pregnant woman at 24 weeks of gestation, displayed a wide aneurysmal image in the left ventricular apical wall on fetal echocardiography. There was a 1.79 mm muscular ventricular septal defect at the apical region of the interventricular septum. In the course of the color Doppler ultrasonography examination, an aberrant fibrous band within the left ventricle and consequent turbulent flow during systole were observed. The baby, born via cesarean section at 37 weeks of gestation, is now in its postnatal seventh month. However, during echocardiographic follow-ups, changes have been observed, including mild to moderate mitral insufficiency and a decrease in systolic function. Despite these findings, the clinical condition remains asymptomatic. It is of great importance to use a multidisciplinary approach in managing these rare cases that could lead to potential adverse outcomes during the antenatal or postnatal periods.Keywords
Congenital Ventricular Aneurysm (CVA) is a congenital heart condition that can be identified on an ultrasonagram as a protrusion from a portion of the ventricular wall, presenting as an outpouching. During fetal echocardiography (ECHO) examinations performed in the prenatal period, in addition to the four-chamber image, it can be observed that it opens to the ventricle with a broad connection in this section, and there is asynchronous contractility in the ventricle [1]. There is no statistically precise data on the incidence of this extremely rare congenital malformation, but one study reported that the incidence of CVA is 1 in 200000 births, indicating that it is very rarely encountered [2]. Although the etiopathogenesis of the disease is not yet fully understood, it has been emphasized in some studies that it may be related to defects in the embryological development process [3]. Another proposed theory suggests that myocardial hypokinesia, which arises due to impaired blood flow supplying the myocardium as a result of ischemia, may lead to the development of an aneurysm [4].
The clinical presentation of CVA can range from being asymptomatic to potentially progressing severely in both the prenatal and postnatal periods, leading to adverse outcomes such as heart failure, myocardial ischemia, arrhythmias, thromboembolism, and even death [5–7]. For this reason, emphasizing the value of making a prenatal diagnosis in order to deal with some of these potential risks, it is crucial to highlight that with prenatal or postnatal treatment strategies, the course of the disease can be alleviated [7].
In our paper, an infrequent case of apically located left ventricular aneurysm (LVA) is presented and discussed with a review of the relevant literature.
A 35-year-old patient, gravida 2, parity 1, pregnant in the 24th gestational week was referred for abnormal cardiac morphology. The patient, who had had a previous cesarean section, had no history of congenital heart disease or any other genetic disease in her medical and family history. Fetal ECHO revealed a large aneurysmatic image opening from the apical wall of the left ventricle (LV) into the left ventricular cavity (Fig. 1).
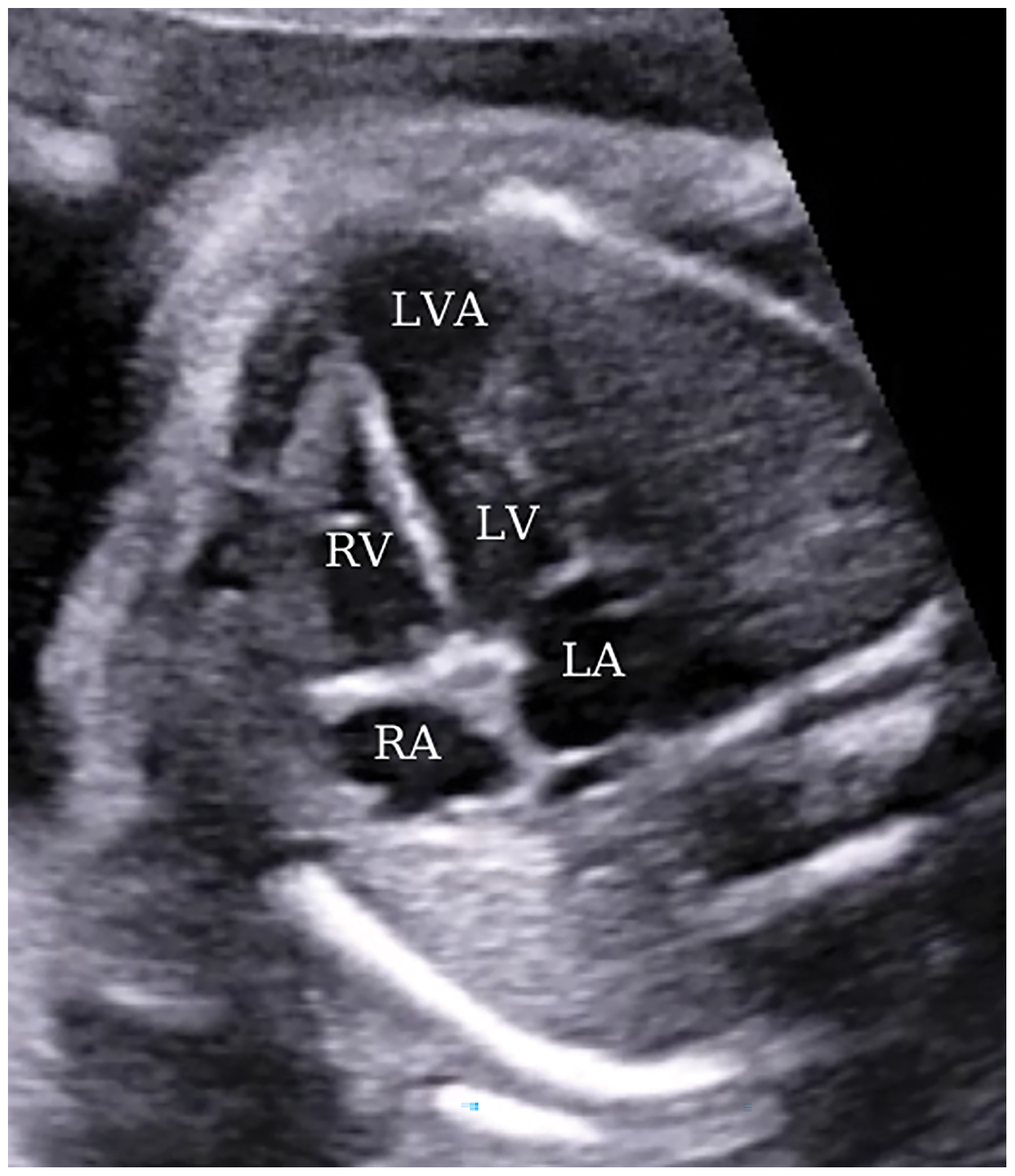
Figure 1: A view from a four-chamber section displaying an apically located left ventricular aneurysm at 24 weeks of gestation. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; LVA, left ventricular aneurysm
This aneurysmatic image was 11 mm × 13 mm in size, and in addition, there was a 1.79 mm muscular ventricular septal defect in the apical region of the interventricular septum (Fig. 2).
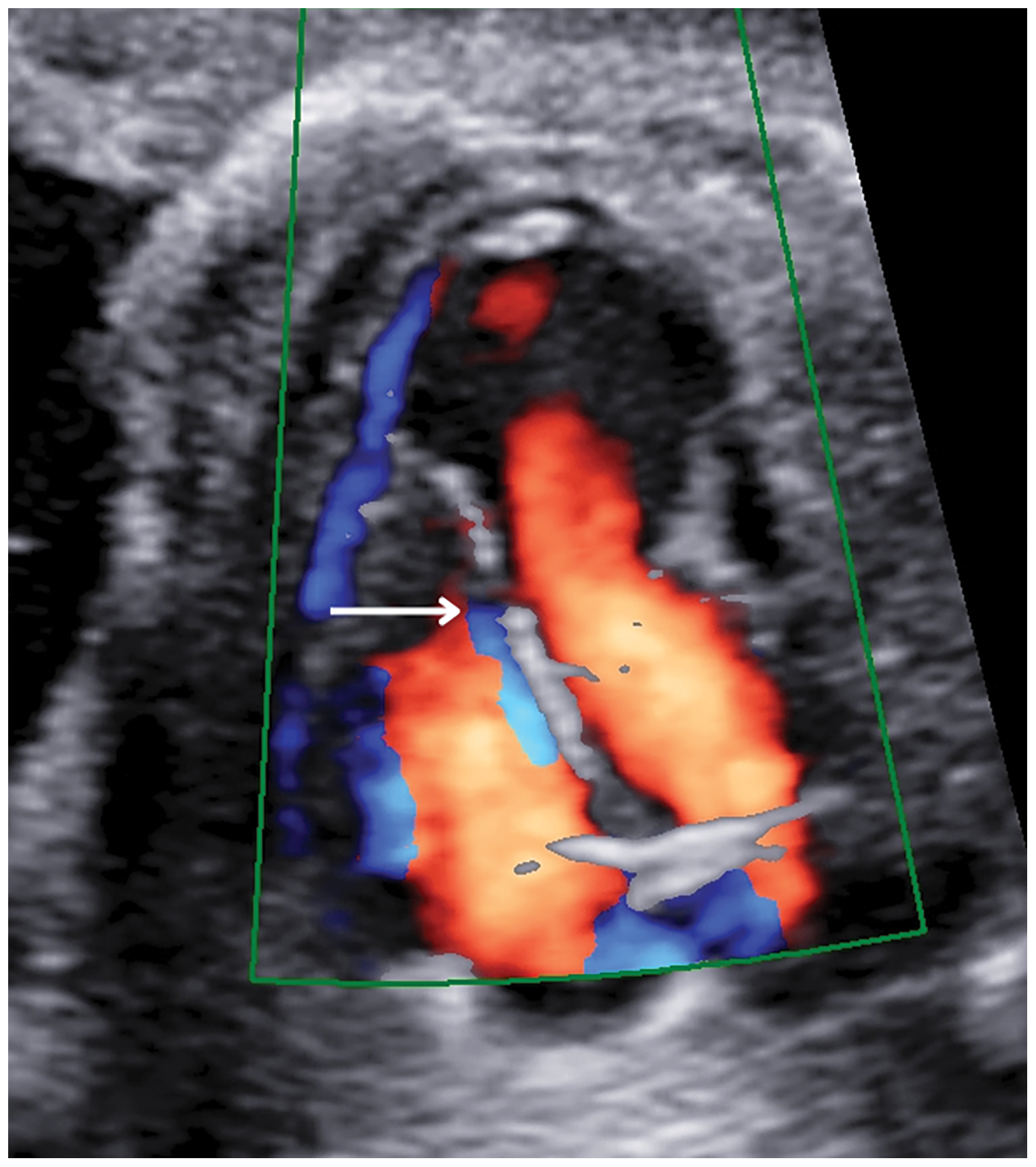
Figure 2: A four-chamber cross-section at 24 weeks of pregnancy showed a muscular ventricular septal defect (white arrow)
Color Doppler ultrasonography showed an aberrant fibrous band in the LV and turbulent flow during systole, which was thought to be related to this band (Fig. 3).
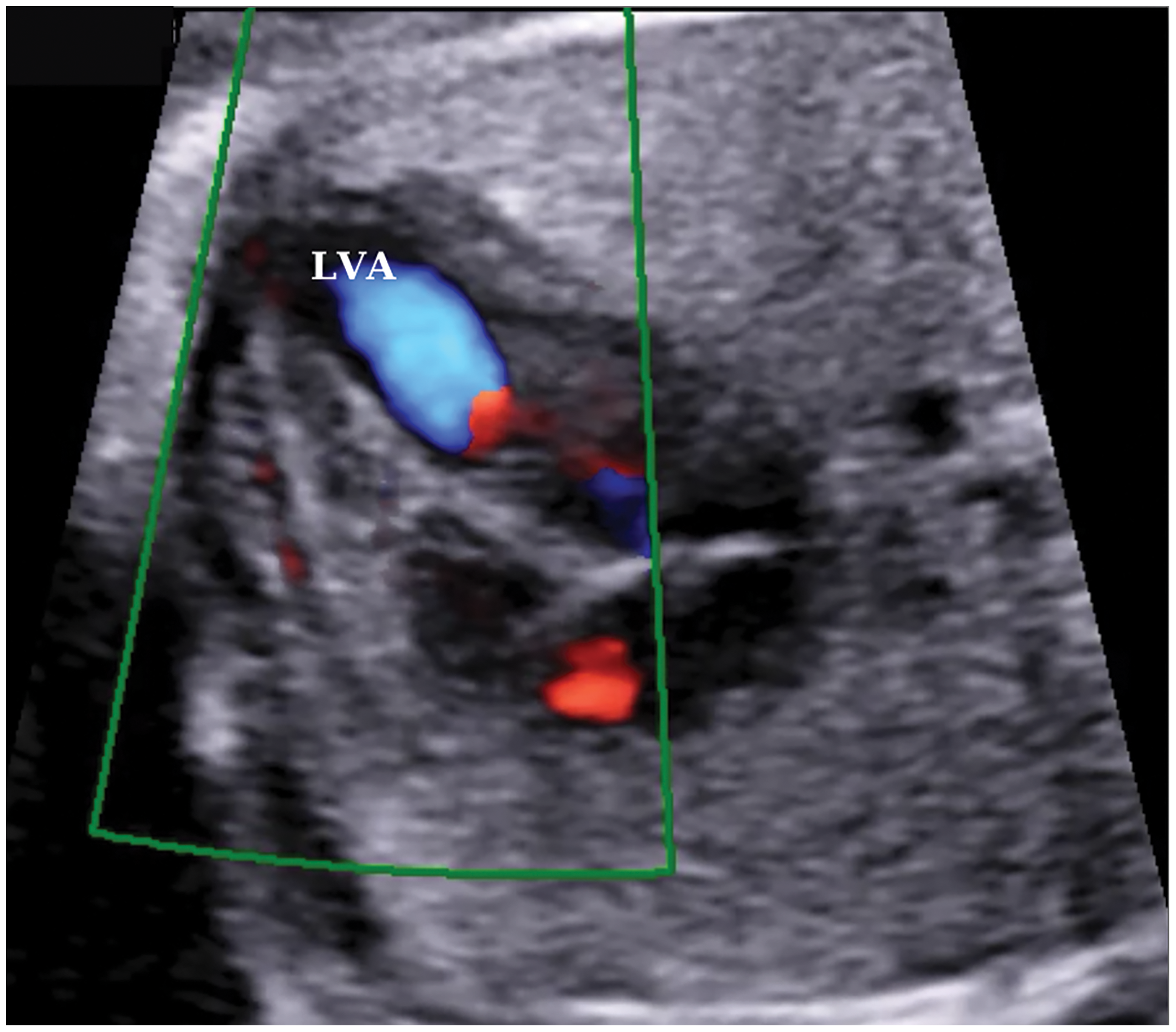
Figure 3: A color doppler ultrasound image of turbulent flow in the left ventricle at 24 weeks of gestation. LVA, left ventricular aneurysm
The myocardial layer in the aneurysmatic area was thinned and measured 0.9 mm in thickness. Myocardial contractility was within normal function; however, contractions of the LVA were not synchronized with those of the left ventricle. Additionally, the cardiothoracic ratio was observed to be 0.46, which is within the normal range. Other than these findings, no evidence of arrhythmia, intracardiac thrombosis, heart failure, pericardial effusion, or hydrops was detected in the fetal ECHO.
The extracardiac examination included a thorough evaluation of all organs, particularly the thoracic and abdominal regions, in order to identify potential signs of abnormalities and exclude the possibility of Cantrell pentalogy.
In order to investigate the etiology of the aneurysm, a thorough examination of teratogenic exposure was conducted by questioning about medication use during early pregnancy, but there was no history of any medication usage. Additionally, a potential maternal infection was explored by assessing infection parameters for toxoplasma, cytomegalovirus, rubella, and parvovirus B19. However, the test results did not reveal any evidence of infection. Following an extensive consultation with the family regarding genetic disorders and potential adverse outcomes, the decision was made to proceed with the pregnancy without opting for genetic testing.
Throughout the course of the pregnancy, a biweekly series of ultrasonography was conducted with the primary objective of identifying antenatal adverse events, including fetal arrhythmia, pericardial effusion, aneurysm rupture, or fetal loss. Furthermore, a meticulous examination of fetal biometric measurements, aneurysm dimensions, and cardiothoracic ratio to assess mass effect on the lungs, and the determination of the presence of polyhydramnios and oligohydramnios was undertaken during ultrasound follow-ups to ascertain the potential occurrence of fetal growth restriction. In the later stages of pregnancy, the dimensions of the aneurysm increased to 14 mm × 16 mm; however, no additional unfavorable findings, such as pericardial effusion or aneurysm rupture, were observed (Fig. 4).
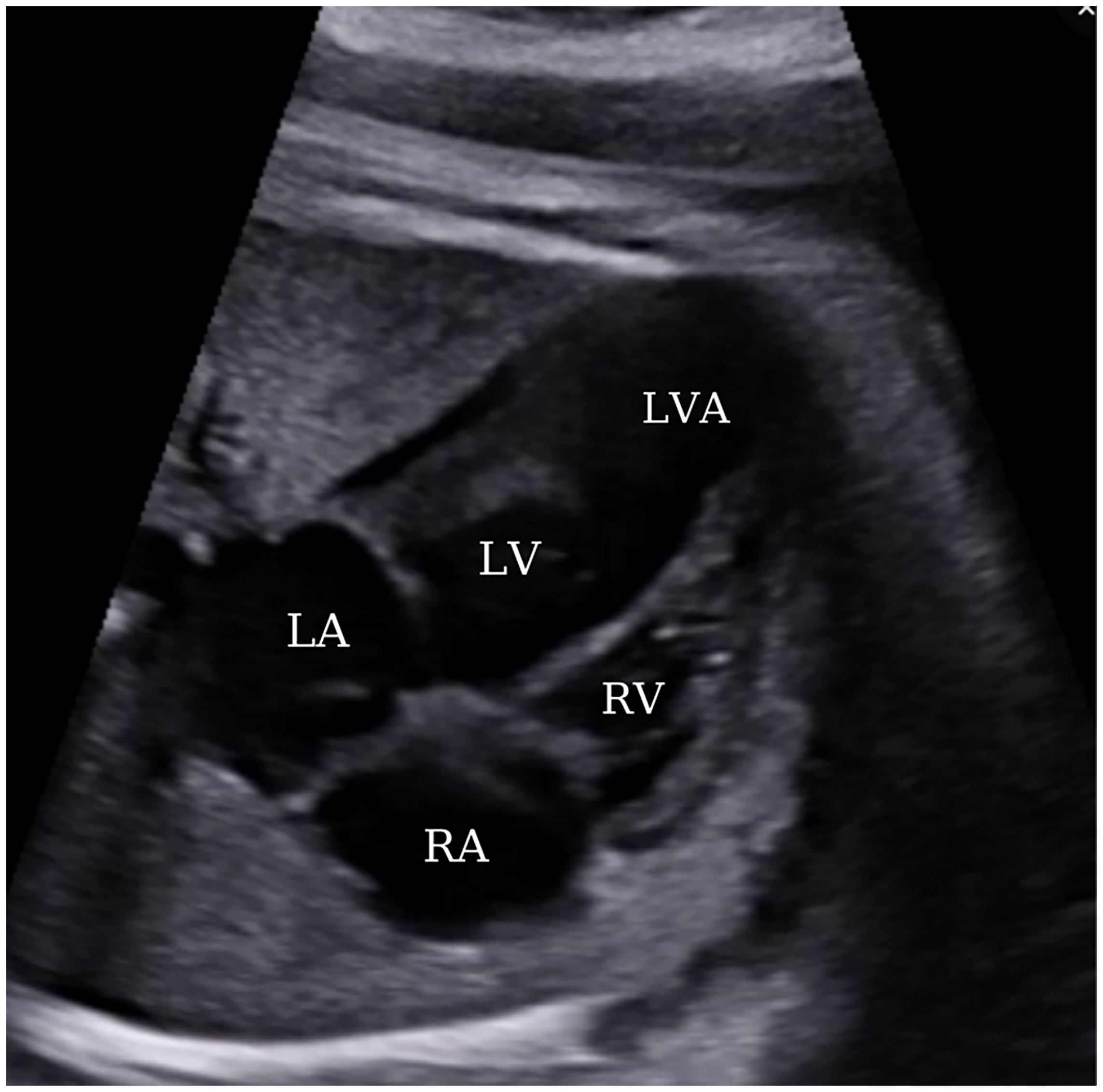
Figure 4: A view from a four-chamber section displaying an apically located left ventricular aneurysm at 35 weeks of gestation. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; LVA, left ventricular aneurysm
At the 37th week of gestation, the decision for delivery was prompted by the manifestation of pain complaints, the observation of contractions in the non-stress test, and a documented history of prior cesarean section. Subsequently, a cesarean section was performed, resulting in the delivery of a live male infant weighing 3200 grams, with a 1-min Apgar score of 9. In the postnatal period, the newborn was hemodynamically stable and presented with an asymptomatic clinical condition. The postnatal ECHO findings were similar to those obtained during the prenatal period, with no signs of heart failure. The baby, who was followed up without planning surgical intervention, is now seven months old, and the aneurysm size has remained the same in ECHO follow-ups, but mild to moderate mitral regurgitation and decreased systolic function developed as a change. Still, the asymptomatic clinical condition is continuing, and the baby is receiving acetylsalicylic acid and digoxin treatments.
Despite improved imaging models, CVA, one of the fetal cardiac diseases that can be diagnosed in the prenatal period, is not easy to differentiate from congenital ventricular diverticulum (CVD). This is because CVD, similar to CVA, presents on fetal echocardiography by protruding from the ventricular wall, forming a finger-like pouch [8]. Therefore, the terminology of CVA and CVD is a matter of debate in the literature, with some authors using the common term ventricular protrusion to refer to these two cardiac malformations, while others refer to them as true aneurysms and pseudoaneurysms [8,9]. Moreover, regarding this issue, different methods have thus far been proposed by many authors to classify it. Rad et al. [10] have designed a classification system based on left ventricular (LV) geometry, emphasizing its mechanics, arguing that terms such as LVA, left ventricular diverticulum (LVD), accessory LV, double-chambered LV, and accessory-chambered LV do not provide sufficient information regarding treatment strategies and prognosis. In contrast, Austen et al. [11] have proposed an anatomical classification, providing more detailed distinctions for LVA and LVD. The widely accepted view is that, although classified based on morphological and histological characteristics, CVD, unlike CVA, is reported to have a narrower connection to the ventricle, synchronized ventricular movements, and histologically includes all three layers of the heart [1,6,7,12]. In our opinion, as the number of cases of both CVA and CVD increases, diagnostic and medical treatment strategies and the prognosis of the disease will be better understood, and a common typology will be proposed in future studies.
Although the incidence of CVAs, which are rarely encountered in fetal life, cannot be clearly defined, it has come to our attention that the relevant publications are generally case-based. To the best of our knowledge, we have not come across a publication that provides clear statistical data, except for a previously published study that reported a prevalence of 1 in 200000 births. In a systematic review by Shuplock et al. [9], they identified a total of 86 cases in the antenatal period from 1990 to 2015, of which 47 (55%) were diagnosed as aneurysms and 39 (45%) as diverticula. Of 47 patients in the aneurysm group, 39 were in the LV and 8 in the right ventricle. In a comprehensive systematic review by Ohlow et al. [12], 664 patients had been identified since 1816, when LVA or LVD was first reported, and only 42 of them (26 with LVA and 16 with LVD) were diagnosed in the prenatal period. In the aforementioned systematic reviews, the localization of aneurysms and diverticula predominantly originated from the apical heart region. According to our research, based on information obtained from the PubMed database, we identified 52 cases diagnosed with CVA that have been published to date, of which 38 were LVA and 14 were right ventricular aneurysms. In light of this information and taking into account the studies performed so far, it is observed that the CVA anomaly is extremely rare and, as in our case, it tends to occur more frequently in the left ventricle and at the apex of the heart.
After the diagnosis of CVA, close monitoring of the aneurysmal segment is imperative during the antenatal period through fetal ECHO follow-ups. This is crucial due to the inherent risk of adverse outcomes associated with CVA, some of which include fetal arrhythmia, pericardial effusion, aneurysm rupture, fetal hydrops, and fetal demise [12,13]. Similarly, in cases of a ventricular aneurysm in the postnatal period, hemodynamic instability may develop in the newborn; in addition to thromboembolic events, angina pectoris due to coronary artery spasm, subacute bacterial endocarditis and congestive heart failure are other possible adverse outcomes. Although there is no definite scientific consensus on the medical and surgical treatment of this disease, digoxin can be administered in cases of fetal tachyarrhythmia, and in cases of bradyarrhythmia, maternal β-sympathomimetic therapy may be considered. These interventions address adverse outcomes that may develop in the fetus during the antenatal period. Slightly differently, in the postnatal period, medical management typically involves the administration of anticoagulants to prevent thromboembolic events, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers to manage congestive heart failure, as well as digoxin, diuretics and β-blockers [14,15]. Additionally, surgical procedures such as aneurysmectomy, ventricular restoration and heart transplantation are other options for treatment in selected symptomatic patients [15]. Based on this information, in our case, since no arrhythmia developed during antenatal follow-up, additional treatment methods were not deemed necessary, and pregnancy follow-up continued without encountering any adverse obstetric outcomes in both antenatal and perinatal periods. Drawing upon literature data highlighting the wide spectrum of clinical courses observed in CVA, this case report will enrich our understanding of the natural course and effects of the disease.
During routine ultrasound examinations in the prenatal period, the identification of abnormal four-chambered heart structures should raise suspicion for this rarely encountered condition. Establishing the diagnosis of these congenital heart diseases facilitates closer monitoring of fetuses at high risk to mitigate potential adverse outcomes. Delivery planning should be conducted in a tertiary center, and postnatal echocardiographic follow-ups should be consistently maintained. Optimal perinatal management requires the adoption of a multidisciplinary approach, necessitating collaboration among specialists such as perinatologists, pediatric cardiologists and pediatric surgeons.
Acknowledgement: We appreciate the valuable support of the nurses working in our clinic.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: And Yavuz, HB Sayal; data collection: Yücel Kaya, KN Özcan; analysis and interpretation of results: Büşra Tsakir, Gökalp Kabacaoğlu; draft manuscript preparation: Yücel Kaya. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The dataset for the research presented in this article is on record. The corresponding author can be contacted to access the necessary data network.
Ethics Approval: The informed consent is obtained, and the patient granted permission to publish the images for this article.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Zhao L, Wu P, Jiao X, Zhang M, Jing W, Wu Y, et al. Characteristics and outcomes of fetal ventricular aneurysm and diverticulum: combining the use of a new technique, fetal HQ. Front Pediatr. 2023;11:1165972. [Google Scholar] [PubMed]
2. Gonçalves LF, Sims J, Jeanty P. Aneurysm of the left ventricle. Fetus. 1992;1(7):10. [Google Scholar]
3. Soynov IA, Gorbatykh YN, Kulyabin YY, Kornilov IA, Nichay NR, Gorbatykh AV, et al. Diverticula and congenital aneurysms of the left ventricle: Anatomical features, pathophysiology, clinical presentation and treatment strategy. Kardiologiia; 2018. doi:10.18087/cardio.2018.2.10066. [Google Scholar] [CrossRef]
4. Weichert J, Chiriac A, Axt-Fliedner R. Fetal diagnosis of left ventricular aneurysm of the free wall and the interventricular septum: report of two cases and review of the literature. J Matern Neonatal Med. 2010;23(12):1510–5. [Google Scholar]
5. Rychik J, Tian Z. Diverticulum or aneurysm of the ventricle. In Fetal cardiovascular imaging: a disease based approach. Philadelphia: Elsevier/Saunders; 2012. p. 359. [Google Scholar]
6. Gowda M, Bharathi S, Thiagarajan M, Aneja T. Prenatal diagnosis of fetal right and left congenital ventricular aneurysms. J Matern Neonatal Med. 2018;31(17):2367–70. [Google Scholar]
7. Dipak NK, Venkatesh S, Prabhu S, Rao S. Evolution of ventricular outpouching through the fetal and postnatal periods: unabating dilemma of serial observation or surgical. J Saudi Hear Assoc. 2016;29(3):203–10. [Google Scholar] [PubMed]
8. Morin C, Ponzio A, Guirgis M, Benzouid C, Beyler C, Rosenblatt J. Prenatal diagnosis of congenital ventricular aneurysm and diverticulum: prenatal features and perinatal management. Prenat Diagn. 2022;42(4):428–34. [Google Scholar] [PubMed]
9. Shuplock JM, Kavanaugh-McHugh A, Parra D. Prenatally diagnosed congenital ventricular outpouchings: an institutional experience and review of the literature. Pediatr Cardiol. 2020;41(2):272–81. doi:10.1007/s00246-019-02252-7. [Google Scholar] [PubMed] [CrossRef]
10. Malakan Rad E, Awad S, Hijazi ZM. Congenital left ventricular outpouchings: a systematic review of 839 cases and introduction of a novel classification after two centuries. Congenit Heart Dis. 2014;9(6):498–511. [Google Scholar] [PubMed]
11. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc committee for grading of coronary artery disease, council on cardiovascular surgery, american heart association. Circ. 1975;51:5–40. [Google Scholar]
12. Ohlow MA, Brunelli M, Lauer B. Characteristics and outcome of primary congenital left ventricular aneurysm and diverticulum: analysis of cases from the literature. Prenat Diagn. 2014;34(9):893–9. [Google Scholar] [PubMed]
13. Abuhamad A, Chaoui R. Rare cardiac anomalies. In: A practical guide to fetal echocardiography normal and abnormal hearts. 4th ed2022. Philadelphia: Lippincott Williams & Wilkins. p. 1300–1. [Google Scholar]
14. Friedman BM, Dunn MI. Postinfarction ventricular aneurysms. Clin Cardiol. 1995;18(9):505–11. doi:10.1002/clc.4960180905. [Google Scholar] [PubMed] [CrossRef]
15. Sattar Y, Alraies MC. Ventricular aneurysm. In: StatPearls. Florida: StatPearls; 2023. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools