 Open Access
Open Access
CASE REPORT
A 63-Year-Old Male with D-Transposition of the Great Arteries Who Had an Early Form of the Arterial Switch Operation
1 Division of Pediatric Cardiology, Department of Pediatrics, The University of Tennessee Health Science Center, The Heart Institute, Le Bonheur Children’s Hospital, Memphis, USA
2 College of Medicine, The University of Tennessee Health Science, Memphis, USA
3 Division of Pediatric Cardiothoracic Surgery, Department of Surgery, The University of Tennessee Health Science Center, Heart Institute, Le Bonheur Children’s Hospital, Memphis, USA
* Corresponding Author: Michael A. Rebolledo. Email:
Congenital Heart Disease 2024, 19(1), 65-68. https://doi.org/10.32604/chd.2024.046638
Received 09 October 2023; Accepted 24 January 2024; Issue published 20 March 2024
Abstract
We describe a 63-year-old male who appears to have undergone an early form of the arterial switch operation for D-transposition of the great arteries performed in the mid-1960s. We review the clinical and imaging data that support our conclusion. He had a diagnostic cardiac catheterization which demonstrated severe pulmonary hypertension responsive to epoprostenol and oxygen. Our case may represent one example of the experimental surgical work done prior to Dr. Adibe Jatene’s description of the first successful arterial switch performed in 1975.Keywords
Abbreviations
| ACHD | Adult congenital heart disease |
| CTA | Computed tomography angiogram |
| DAO | Descending aorta |
| d-TGA | Dextro-transposition of the great arteries |
| LPA | Left pulmonary artery |
| LV | Left ventricle |
| MRA | Magnetic resonance angiogram |
| MRI | Magnetic resonance imaging |
| neoAV | Neo-aortic valve |
| neoPV | Neo-pulmonary valve |
| RPA | Right pulmonary artery |
| RV | Right ventricle |
We present the case of a 63-year-old male with dextro-transposition of the great arteries (d-TGA) repaired at 4 years of age. The patient had limited follow-up upon referral to our adult congenital heart disease (ACHD) service. At the initial consultation, he reported shortness of breath while walking up stairs. He had no chest pain but described occasional fluttering in his chest. He denied smoking. His past medical history included a seizure disorder, sciatica, and hearing loss. There was no history of hyperlipidemia. He reported taking aspirin, phenobarbital, phenytoin, biotin, and omega-3 fatty acid supplements. The patient’s family history was remarkable for premature myocardial infarction. He worked full-time with moderate manual labor. Despite several attempts, surgical documentation could not be obtained. By self-report, he was born in the Philadelphia, PA area; he had cyanosis before the complete repair and underwent surgery in New York City, NY.
The patient’s physical exam demonstrated a heart rate of 68 bpm, respiratory rate of 18, blood pressure of 147/80 mm HG, and pulse oximetry of 96%. He appeared well and was in no distress. His lungs were clear to auscultation. He had a healed sternotomy scar and large left thoracotomy scar. Cardiac examination demonstrated regular rate and rhythm with normal S1 and S2. No murmur or gallop was detected. His abdomen was soft, nontender, and nondistended with no hepatosplenomegaly. His extremities revealed no cyanosis, clubbing, or edema.
An electrocardiogram demonstrated normal sinus rhythm and right bundle branch block. An echocardiogram demonstrated findings suggesting a prior arterial switch operation. Left ventricular (LV) size and systolic function were normal. There was mild bi-atrial dilation. and nonspecific LV diastolic dysfunction. There was a bicuspid aortic valve that was calcified but had no stenosis. There was mildly increased right ventricular chamber size with low normal systolic function. Right ventricular systolic pressure appeared mildly elevated qualitatively.
Cardiac magnetic resonance imaging (MRI) and chest computed tomography angiogram (CTA) were performed (Fig. 1). A diagnostic cardiac catheterization demonstrated severe pulmonary hypertension with a pulmonary vascular resistance of 20.2 Wood Units·m2 which decreased to 8 Wood Units·m2 while on epoprostenol and oxygen. He was started on diuretics and phosphodiesterase-5 inhibitor therapy. He did not tolerate endothelin receptor antagonist therapy because of fluid retention. He had a 6-min walk distance of 371 m. He was found to have restrictive lung disease by pulmonary function testing. In addition, he had obstructive sleep apnea and started on continuous positive airway pressure at night.
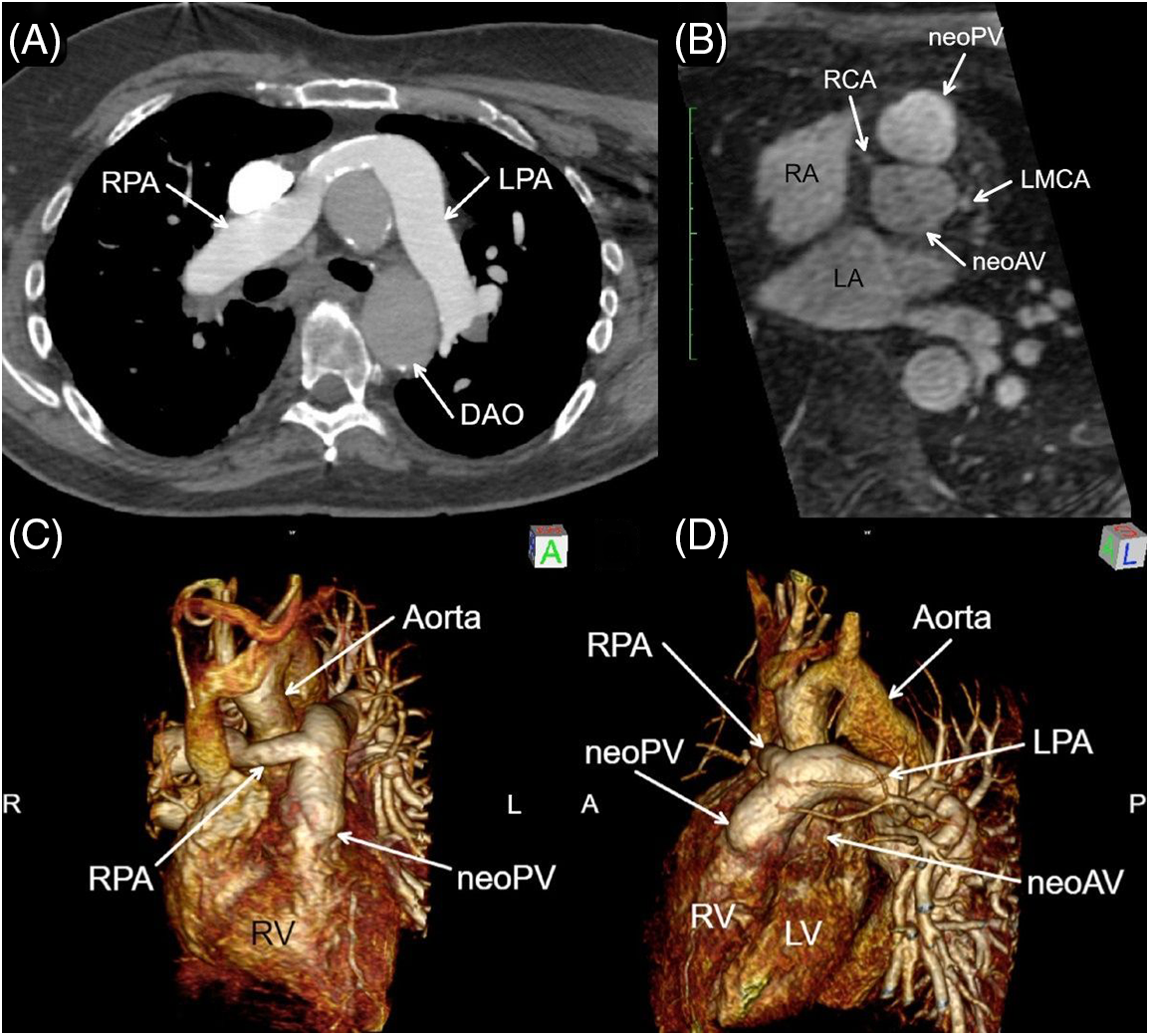
Figure 1: Axial CTA (A), bi-orthogonal en face neo-aortic valve (neoAV) view chest magnetic resonance angiogram (MRA) (B), and MRA 3D Reconstruction (C, D). Panels A, C, D: The proximal right pulmonary artery (RPA) is a surgical graft, which drapes anterior to aorta consistent with a form of the Le Compte maneuver. Panel B: The neo-pulmonary valve (neoPV) arises directly anterior and rightward to the neoAV, indicative of the pre-surgical anatomic relationship of the semilunar valves in D-transposition of great arteries. Anterior origins of the re-implanted right (RCA) and left main coronary artery (LMCA) are visible. RPA = Right pulmonary artery; LPA = left pulmonary artery; LV = left ventricle; RV = right ventricle; DAO = descending aorta
Surgical therapy for d-TGA has evolved over time [1]. Dr. Adib Jatene in Sao Paolo, Brazil, is credited with performing the first successful anatomical arterial switch operation in 1975 [2]. Jatene’s arterial switch procedure addressed the technical issue of the coronary transfer as “buttons” placed anteriorly on the aorta, which eliminated many long-term complications associated with the atrial switch operations, including baffle-associated complications, tricuspid and right ventricular failure, and sinus node dysfunction or ventricular arrhythmia, while restoring normal physiological blood flow [3]. Several reports have described long-term follow up after arterial switch operation [4–6]. One recent report described a 43-year follow-up, noting that right ventricular outflow tract obstruction and neoaortic valve regurgitation with aortic root dilation were the two most common reasons necessitating reoperation [7].
Based on clinical and imaging data, our patient appeared to have had an early form of an arterial switch operation performed in the mid-1960s. Cardiac MRI demonstrated a form of the Le Compte maneuver with the branch pulmonary arteries draped anteriorly over the aorta. The right pulmonary artery is a surgical graft which did not course under the aortic arch as in Jatenes’s original description. No ventricular septal defect patch was identified, calling into question how the LV remained conditioned prior to his definitive repair. Perhaps, he had a patent ductus arteriosus from birth, which kept LV volume and pressure loaded, but this cannot be confirmed. The direct anterior and rightward relationship of the neo-pulmonary valve to the neo-aortic valve supports the conclusion the preoperative anatomy was d-TGA. The neo-aortic valve is functionally bicuspid and has demonstrated remarkable longevity and durability.
The coronary arteries appeared widely patent with an anterior orientation from the aorta, as is commonly seen following arterial switch operation. There was mild residual right pulmonary artery stenosis. He had significant pulmonary artery hypertension due to pulmonary vascular obstructive disease, which likely occurred since the pulmonary vascular bed was not well protected early in life. The pulmonary artery hypertension was responsive to epoprostenol and oxygen and is currently managed on tadalafil. There was some evidence of LV diastolic dysfunction that required diuretic therapy. According to the 2018 American Heart Association/American College of Cardiology Guideline for the Management of ACHD, he is ACHD AP Class IIIC [8]. He was followed by our ACHD service but recently moved abroad and has since been lost to follow-up.
Acknowledgement: The authors thank Andrew J. Gienapp, MS (Children’s Foundation Research Institute, Le Bonheur Children’s Hospital, Memphis, TN) for copy and technical editing, preparation of the manuscript and figure for publishing, and publication assistance.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: MA Rebolledo, JS Yao, BR Waller; data collection: MA Rebolledo, JS Yao, JN Johnson, BR Waller; analysis and interpretation of results: MA Rebolledo, JS Yao, JN Johnson, US Boston, BR Waller; draft manuscript preparation: MA Rebolledo, JS Yao, JN Johnson, US Boston, BR Waller. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing not applicable–no new data generated.
Ethics Approval: Informed consent was granted by the patient to publish this case report.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Marathe SP, Talwar S. Surgery for transposition of great arteries: a historical perspective. Ann Pediatr Cardiol. 2015;8(2):122–8. [Google Scholar] [PubMed]
2. Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72(3):364–70. [Google Scholar] [PubMed]
3. Harrison D, Broda C, Ermis P, Opina A, Parekh D, Franklin WJ, et al. Very long-term complications in 132 patients with transposition of the great arteries who received the atrial switch operation. J Am Coll Cardiol. 2020;75(11_Supplement_1):650. [Google Scholar]
4. Moe TG, Bardo DME. Long-term outcomes of the arterial switch operation for d-transposition of the great arteries. Prog Cardiovasc Dis. 2018;61(3–4):360–4. [Google Scholar] [PubMed]
5. Baruteau AE, Vergnat M, Kalfa D, Delpey JG, Ly M, Capderou A, et al. Long-term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or aortic arch obstruction. Interact Cardiovasc Thorac Surg. 2016;23(2):240–6. [Google Scholar] [PubMed]
6. Fricke TA, Buratto E, Weintraub RG, Bullock A, Wheaton G, Grigg LE, et al. Long-term outcomes of the arterial switch operation. J Thorac Cardiovasc Surg. 2022;163(1):212–9. [Google Scholar] [PubMed]
7. van der Palen RLF, Blom NA, Kuipers IM, Rammeloo LAJ, Jongbloed MRM, Konings TC, et al. Long-term outcome after the arterial switch operation: 43 years of experience. Eur J Cardiothorac Surg. 2021;59(5):968–77. [Google Scholar] [PubMed]
8. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019, 2018;73(12):1494–563. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools