 Open Access
Open Access
ARTICLE
Association of Congenital Heart Defects (CHD) with Factors Related to Maternal Health and Pregnancy in Newborns in Puerto Rico
1 Biochemistry & Pharmacology Department, San Juan Bautista School of Medicine, Caguas, 00726, Puerto Rico
2 Hispanic Alliance for Clinical and Translational Research, University of Puerto Rico Medical Sciences Campus, San Juan, 00936, Puerto Rico
3 Department of Internal Medicine, University of Puerto Rico Medical Sciences Campus, San Juan, 00936, Puerto Rico
* Corresponding Author: Yamixa Delgado. Email:
Congenital Heart Disease 2024, 19(1), 19-31. https://doi.org/10.32604/chd.2024.046339
Received 27 September 2023; Accepted 09 January 2024; Issue published 20 March 2024
Abstract
Background: Given the pervasive issues of obesity and diabetes both in Puerto Rico and the broader United States, there is a compelling need to investigate the intricate interplay among body mass index (BMI), pregestational, and gestational maternal diabetes, and their potential impact on the occurrence of congenital heart defects (CHD) during neonatal development. Methods: Using the comprehensive System of Vigilance and Surveillance of Congenital Defects in Puerto Rico, we conducted a focused analysis on neonates diagnosed with CHD between 2016 and 2020. Our assessment encompassed a range of variables, including maternal age, gestational age, BMI, pregestational diabetes, gestational diabetes, hypertension, history of abortion, and presence of preeclampsia. Results: A cohort of 673 patients was included in our study. The average maternal age was 26 years, within a range of 22 to 32 years. The mean gestational age measured 39 weeks, with a median span of 38 to 39 weeks. Of the 673 patients, 274 (41%) mothers gave birth to neonates diagnosed with CHD. Within this group, 22 cases were linked to pre-gestational diabetes, while 202 were not; 20 instances were associated with gestational diabetes, compared to 200 without; and 148 cases exhibited an overweight or obese BMI, whereas 126 displayed a normal BMI. Conclusion: We identified a statistically significant correlation between pre-gestational diabetes mellitus and the occurrence of CHD. However, our analysis did not show a statistically significant association between maternal BMI and the likelihood of CHD. These results may aid in developing effective strategies to prevent and manage CHD in neonates.Keywords
Abbreviations
| CHD | Congenital Heart Defect |
| BMI | Body Mass Index |
Congenital heart defects (CHD) can range from mild to severe and can consist of damage to the newborn heart’s walls and/or valves, the arteries, and veins near the heart [1,2]. CHD is one of the most common types of birth defects. It is the most common congenital malformation associated with the highest mortality among them [2,3]. In Puerto Rico, CHD is diagnosed in 9.5 out of 1000 live births [4].
CHD is associated with lifelong comorbidity and increased health service utilization [5]. The cost of utilizing these services will not decrease in the distant future, as the incidence of CHD has not changed, but the overall prevalence has increased [6]. Due to improvements in medical technology, physicians are now aware of several different risk factors that can lead to a child being born with a CHD [5,6]. While non-modifiable risk factors may be present and account for the heritable bases of CHD, genetic etiology was only found in less than 20% of all CHD cases [2,6]. This suggests that modifiable risk factors, in which patients can take measures to change or prevent health outcomes, play a major role in CHD.
Several studies have suggested an association between maternal obesity and increased risk of CHD in the offspring [7–9]. Maternal obesity is linked to various cardiac defects, including septal defects, aortic arch defects, persistent ductus arteriosus, conotruncal defects, and ventricular outflow defects [8]. Moreover, high maternal BMI predisposes women to develop other health risk factors, such as an elevated risk of developing Type 2 diabetes and gestational diabetes [10,11]. Diabetes mellitus is characterized by insufficient insulin production (Type 1) or abnormal insulin response, leading to hyperglycemia (Type 2). Studies have also indicated a potential correlation between pre-gestational diabetes and the risk of congenital health anomalies like CHD [9–11]. In addition, diseases during pregnancy may influence the development and viability of the fetus [12]. Thus, gestational diabetes could also be a risk factor for CHD in the fetus. Fig. 1 presents non-genetic risk factors associated with CHD, as identified in previous studies. These findings suggest a significant association between maternal obesity, diabetes mellitus, and the risk of CHD, warranting further investigation. However, the specific analysis of these factors within the population of Puerto Rico remains limited.
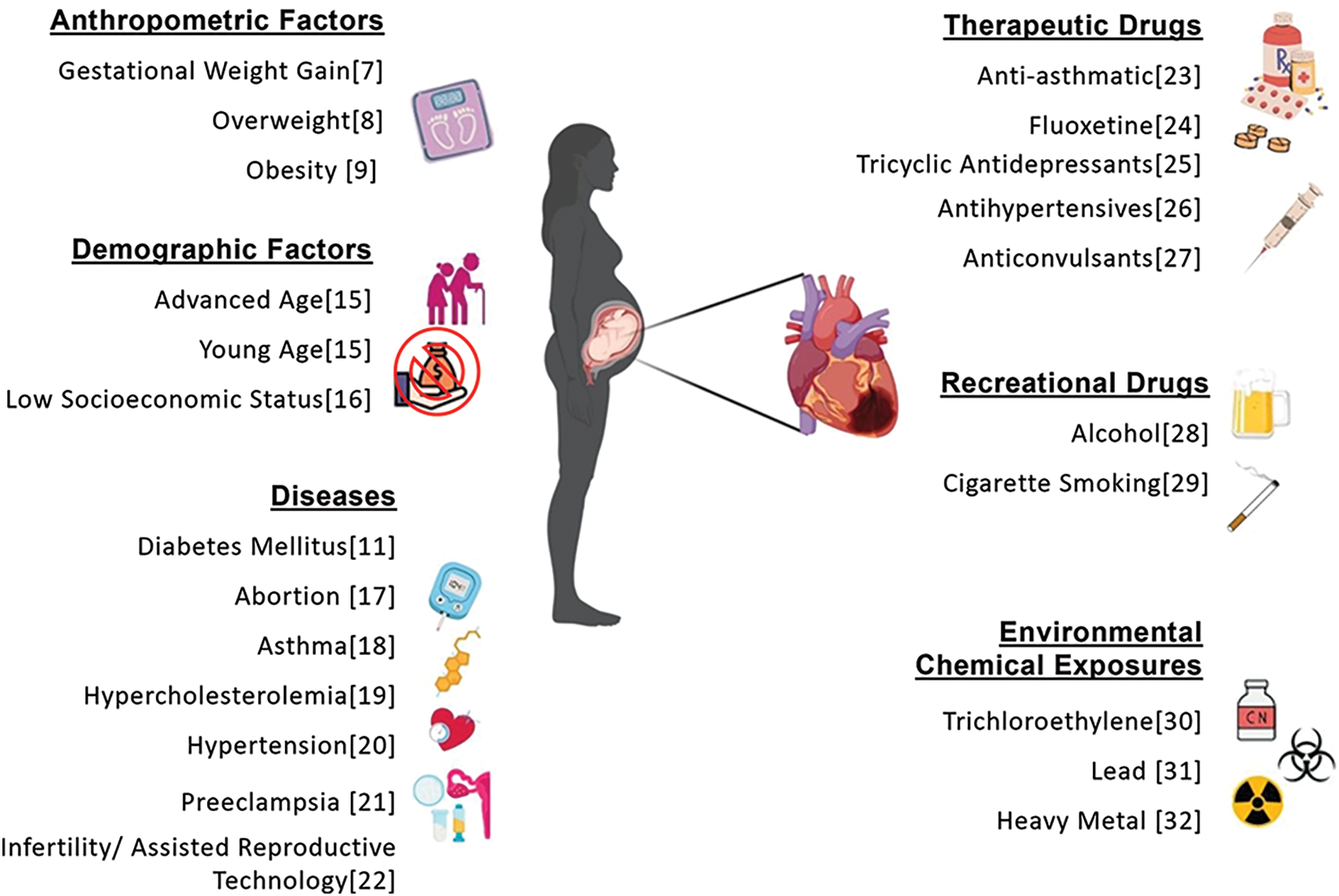
Figure 1: Risk factors for congenital heart defects (CHDs) from previous studies conducted in different countries [7–9,11,15–32]
Puerto Rico exhibits an unusually high obesity rate, with a prevalence of 41.5% among adults, particularly affecting women compared to men [13]. Additionally, the prevalence of diagnosed diabetes mellitus among adults in Puerto Rico is reported to be 20.1% [14], nearly double the national average in the United States. Consequently, this study aims to investigate the influence of maternal BMI and diabetes before and during pregnancy, along with other non-genetic factors, within a sample of Puerto Rico from 2016 to 2020.
2.1 Study Design and Objective
This study is a cross-sectional study with an observational comparative focus conducted on samples of newborns presenting congenital defects, and how specifically, heart defects have potential associations with maternal pre-existing conditions and pregnancy-related factors, including diabetes and overweight status.
In our study, convenience sampling was from a secondary database obtained from the Birth Defects Surveillance and Prevention System database of the Puerto Rico Department of Health, which was established in 2008. This surveillance system monitors around 51 different types of congenital disabilities, including CHD. This system collects information from mothers and their newborns with CHD. Data from mothers of the newborns with congenital defects from 2016–2020 were included in this study. The anonymity and confidentiality of all participating mothers and newborns were guaranteed by the Birth Defects Surveillance and Prevention System of the Puerto Rico Department of Health. Participation in this study did not pose significant risks. Informed consent was obtained from the participants through the Puerto Rico Department of Health. The Institutional Review Board of the San Juan Bautista School of Medicine evaluated and granted approval (#EMSJBIRB-7-2021) for the study. We focused on two main groups: mothers of children born with congenital heart defects (CHD) and mothers of children born with other congenital abnormalities (excluding the heart). Other inclusion criteria contained in this study were live births, full-term newborns (≥37 weeks of gestation), newborns in Puerto Rico, and those born with congenital defects. Exclusion criteria included stillbirth, premature infants (<37 week’s gestation), multiple gestation, and newborns not born in Puerto Rico. The criteria for stillbirth, premature infants and multiple gestation were excluded due to their potential inherent impact on the occurrence of congenital defects. The sample size originally received from the surveillance system was 1,286 neonates. Still, during the database cleaning process, we excluded 537 because the record did not include the mother’s height and weight measurement to calculate BMI. Furthermore, 76 neonates were found to have a diagnosis of chromosomal defects and were therefore excluded from our data. Chromosomal defects can have a wide range of effects on various organ systems, including the heart, and by excluding these cases, we minimize the potential for confounding effects on our endpoint of CHD specifically. After applying all the exclusion factors, we obtained a final sample size of 673 mothers with newborns with congenital disabilities. We performed three types of post-hoc tests (1. t tests-Means: Wilcoxon-Mann-Whitney test (two groups); 2. χ² tests-Goodness-of-fit tests: Contingency tables; 3. z tests-Logistic regression) to determine the statistical power of our sample using the G*Power software, version 3.1.9.7 and with a level alpha of 0.05. All of them resulted in enough (>80%) statistical power (1-β err prob = 99%).
The variables of interest chosen from the Surveillance and Prevention System of Congenital Defects database from the Puerto Rico Department of Health for the study were age, gestational age, BMI, pre-gestational diabetes, gestational diabetes, hypertension, abortion, and pre-eclampsia. We chose these variables due to their relevance and association as risk factors in previous studies [7–12,15–17,20–22]. We defined pre-gestational diabetes as diabetes mellitus diagnosed in a woman before pregnancy, encompassing both type 1 and type 2 diabetes. In contrast, gestational diabetes develops during pregnancy in women who did not have diabetes before becoming pregnant. By studying these variables, we gain a comprehensive understanding of the cases present in our participants. The age of each participant was recorded as a continuous variable in years at the time of enrollment. Age was utilized in statistical analysis to control potential gestational age-related CHD. Gestational age was measured as the number of weeks of the participants’ pregnancy state. The body mass index (BMI) was calculated using the standard formula: weight (kg) divided by height (m2). For this study, BMI was categorized as under/normal (≤24.9) or overweight/obese (>24.9) and used as a covariate to account for potential influence on CHD in neonates. Hypertension was defined using the American Heart Association’s standard values of resting systolic blood pressure (SBP) greater than or equal to 130 mmHg and/or diastolic blood pressure (DBP) greater than or equal to 80 mmHg. Participants were classified as having hypertension if diagnosed with high blood pressure prior to pregnancy. We defined Preeclampsia as participants experiencing the onset of hypertension (SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg) after 20 weeks of gestation. Participants were categorized for abortion based on their history of pregnancy termination. Abortion was defined as participants who had previously undergone termination of pregnancy through spontaneous, medical, or surgical means. By studying these variables, we gain a comprehensive understanding of the cases present in our participants.
Characteristics of participants were evaluated using frequency distribution and percentages. Missing information was described among study groups, and their percentages were compared to determine whether the groups were similar or not. A difference of over 5 units was considered different for study purposes. Associations among selected characteristics and groups of congenital defects (CHD vs. other congenittal defects) were performed using the chi-square test for categorical variables and the Mann-Whitney test for continuous variables for all complete cases on each variable. Logistic Regression Analysis, with their odds ratio (OR) and 95% Confidence Intervals, was used to determine if maternal BMI and pre-gestational diabetes were associated with the development of CHD in the newborn. Statistical significance was determined with a p-value of <0.05. All analysis was performed using the STATA program (StataCorp. 2022. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
From 2016 to 2020, a total of 673 participants with newborns with congenital disabilities from the System of Vigilance and Surveillance of Congenital Defects of Puerto Rico were included in this study. The total cases of participants with CHD infants made up 41% of the original sample size (Fig. 2A). Among these participants, 29% were considered obese, 25% were considered overweight, 40% were normal, and 5% were underweight according to their BMI (Fig. 2B). Among the participants, 6% were considered to have pre-gestational diabetes (Fig. 2C). The mean maternal age of the study population was 26 years, with a median range of 22 to 32 years. The mean gestational age was 39 weeks with a median range of 38–39 weeks. Both mean maternal age and gestational age were consistent across participants with and without CHD neonates (Table 1). The data for each variable was split into two characteristics: YES/NO (Table 1). The characteristic of Yes represented the frequency of cases of neonates that did have CHD which was 274 cases. The characteristic of No represented the frequency of cases that did not have neonates with CHD, which was 399 (these cases represent congenital defects not from the heart).
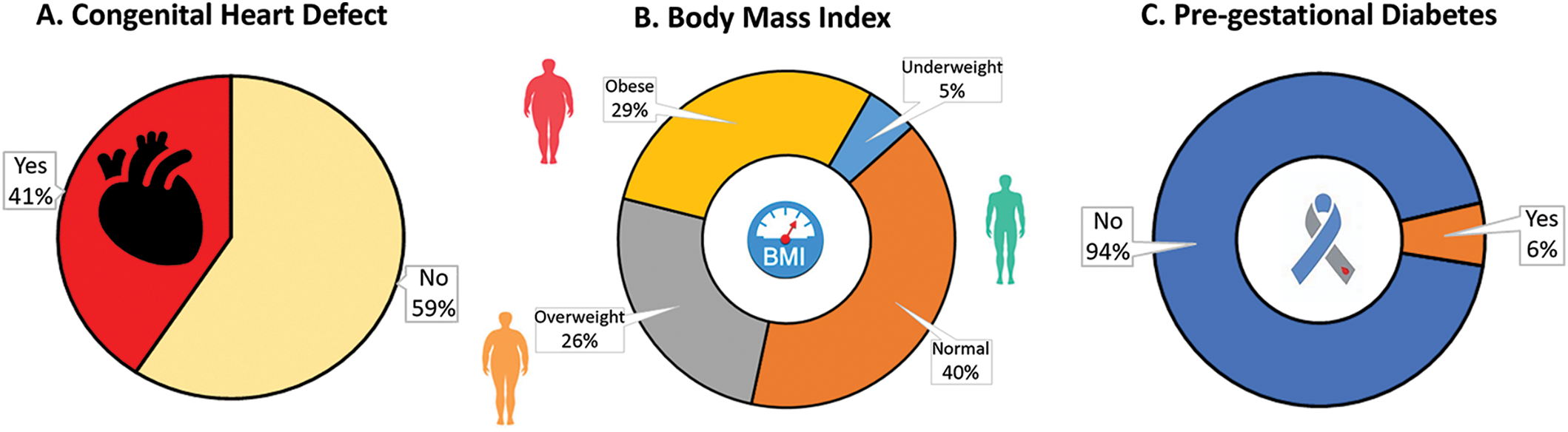
Figure 2: Sample of participants and variables analyzed in this study (N = 673). (A) total cases of children with CHD, (B) distribution of BMI in the sample of women, and (C) total cases of maternal pre-gestational diabetes
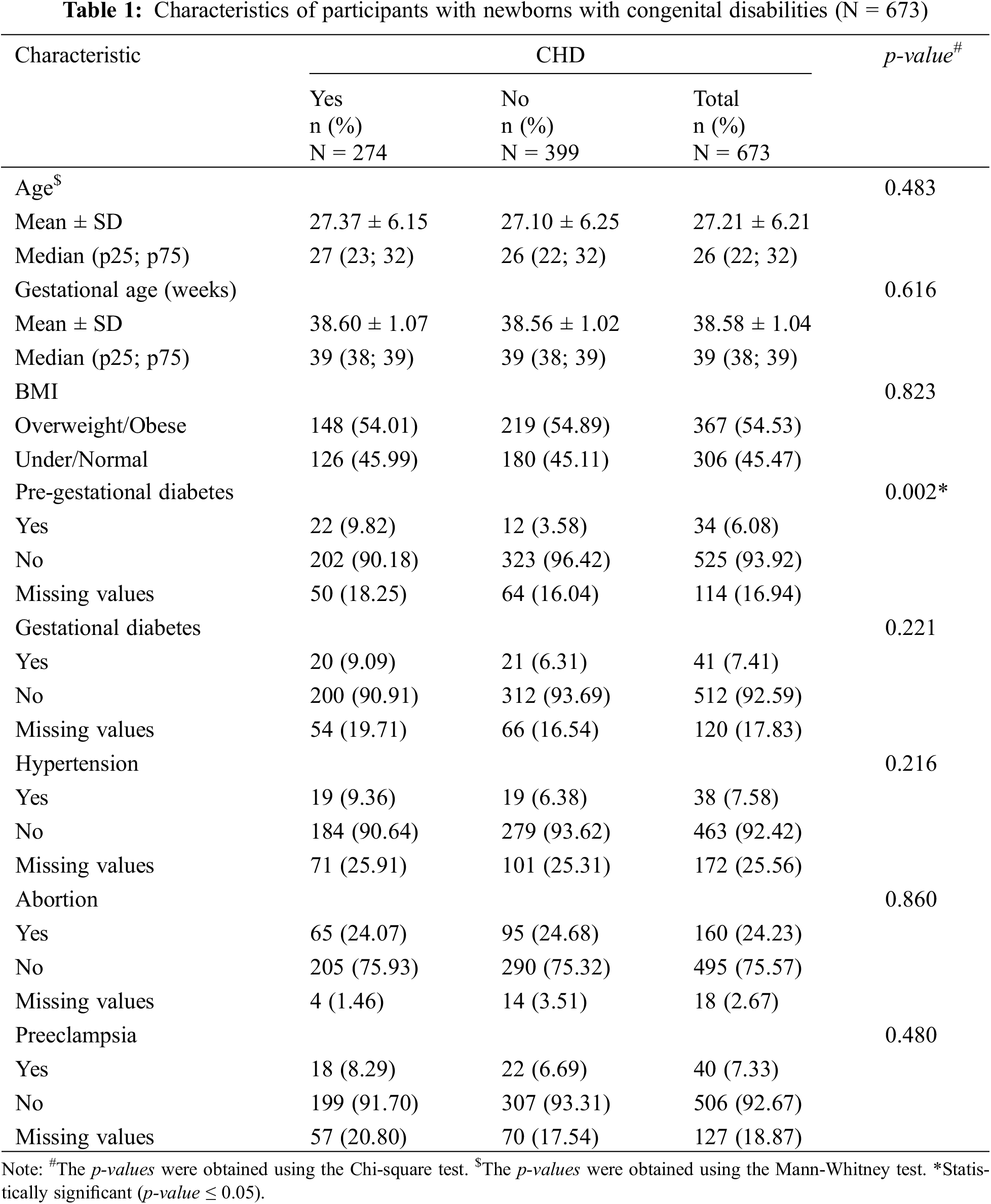
BMI level. For BMI, we decided to continue our analysis by combining overweight/obese and normal/underweight. Of the participants, out of the total number of cases, 367 (55%) cases were overweight/obese, and 306 (45%) cases were normal/underweight. Out of the overweight/obese participants, 148 (54%) reported having neonates with CHD, and 219 (55%) participants reported neonates without CHD. Among the normal-weight participants, 126 (46%) reported having neonates with CHD and 180 (45%) of cases reported having neonates without CHD.
Pre-gestational diabetes. For pre-gestational diabetes, 34 (6%) cases reported having pre-gestational diabetes, and 525 (94%) cases reported they did not have pre-gestational diabetes. Among the participants with pregestational diabetes, 22 (10%) cases reported having a neonate with CHD, and 12 (4%) reported neonates without CHD. Among the participants without pregestational diabetes, 202 (90%) reported neonates with CHD and 323 (96%) reported neonates without CHD. Among the 673 participants, a total of 114 (17%) cases with missing values for the pre-gestational diabetes diagnosis, where 50 (18%) were amongst those with CHD and 64 (16%) amongst those without CHD.
Gestational diabetes. For gestational diabetes, of the total volume of participants, 41 (7%) cases reported having gestational diabetes, and 512 (93%) cases reported they did not have gestational diabetes. Among the participants that had gestational diabetes, 20 (9%) had neonates with CHD and 21 (6%) reported neonates without CHD. Among the participants without gestational diabetes, 200 (91%) reported neonates with CHD, and 312 (94%) reported neonates without CHD. Among the total participants (N = 673) a total of 120 (18%) cases had missing information for the gestational diabetes diagnosis, where 54 (20%) were amongst those with CHD and 66 (17%) amongst those without CHD.
Hypertension. For hypertension, of the total number of participants, 38 (8%) cases reported having hypertension, and 463 (92%) participants reported they did not have hypertension. Among the participants with hypertension, 19 (9%) reported having a neonate with CHD, and 19 (6%) reported having neonates without CHD. Among the participants without hypertension, 184 (91%) of participants reported neonates with CHD and 279 (94%) reported having neonates without CHD. Among the total participants (N = 673), a total of 172 (26%) cases had missing information for hypertension diagnosis, where 71 (26%) were among those with CHD and 101 (25%) among those without CHD.
Abortion. For abortion, 160 (24%) participants reported having an abortion, and 495 (76%) reported they did not have an abortion. Among the participants who had an abortion, 65 (24%) participants reported having a neonate with CHD and 95 (25%) reported having neonates without CHD. Among participants without an abortion, 205 (76%) participants reported neonates with CHD, and 290 (75%) reported neonates without CHD. Among the total participants (N = 273), 18 (3%) cases had missing values for abortion, where 4 (1%) were those with CHD and 14 (4%) were among those without CHD.
Preeclampsia. For preeclampsia, 40 (7%) cases reported having preeclampsia, and 506 (93%) cases reported they did not have preeclampsia. Among participants with preeclampsia, 18 (8%) reported having neonates with CHD, and 22 (7%) reported having neonates without CHD. Among the cases without preeclampsia, 199 (92%) reported having neonates with CHD and 307 (93%) reported having neonates without CHD. Among the total participants (N = 673), there were 127 (19%) cases with missing values for preeclampsia, where 57 (21%) were among those with CHD and 70 (18%) among those without CHD.
Missing data was accounted for each characteristic and variable measured (Table 1). These missing values are being distributed almost equally for both groups (with and without CHD) in each variable measured, except for abortion.
Comparison analysis. A Chi-squared test was then performed across all variables measured, with the exception of age, which underwent a Mann-Whitney test. Pregestational diabetes was the only variable determined to have a statistically significant result (p = 0.002).
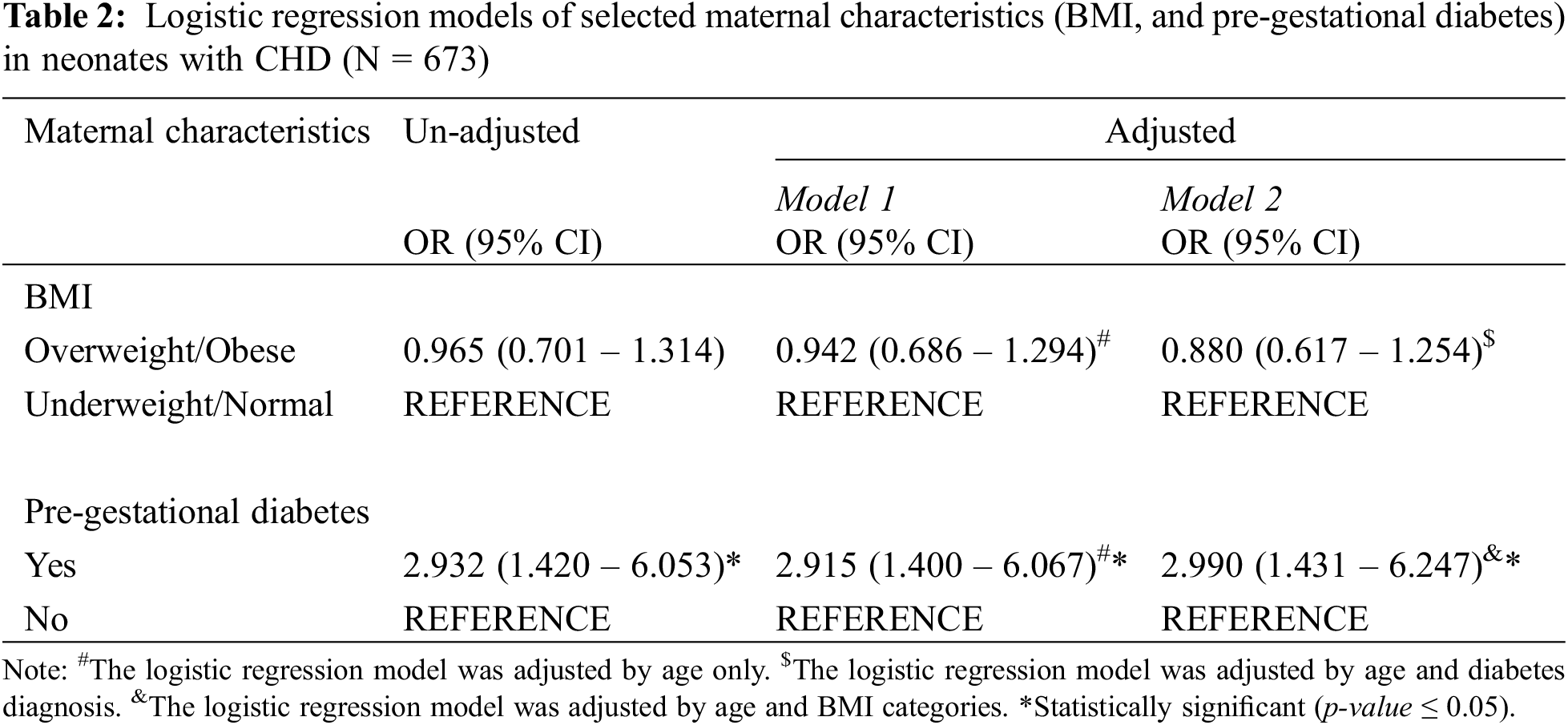
Logistic Regression analysis. Three logistic regression models, with their OR and 95% Confidence Intervals, were performed for all available cases (without missing values) using the dependent CHD groups and the independent variables of pregestational diabetes and BMI (Table 2). Model 1 represents the “unadjusted” estimates, Model 2 represents the age-adjusted estimates, and Model 3 represents the age, pre-gestational diabetes and BMI adjusted estimates. For Model 3, BMI was only adjusted for age and diabetes diagnosis, while pre-gestational diabetes was only adjusted for age and BMI. Results from these models showed no association between CHD groups and BMI. On the contrary, pre-gestational diabetes showed a significant association with CHD groups. All models behaved similarly (Table 2). Participants with pre-gestational diabetes, had almost 3 times (95% CI (1.431–6.247)) the possibility of having a newborn with CHD compared to participants without pre-gestational diabetes.
Our study on the potential associations between CHD and characteristics related to maternal health and pregnancy is of great importance, as it provides valuable insights into the underlying risk factors contributing to CHD occurrence in Puerto Rico. In this study, we examined the relationship between CHD and two key maternal elements: pre-gestational diabetes and BMI, in newborns in Puerto Rico. Our results indicate a significant association between pre-gestational diabetes and the occurrence of CHD in newborns. This finding aligns with previous studies highlighting the role of maternal diabetes as a risk factor for the development of CHD [9–11]. The biochemical mechanistic link between maternal diabetes and CHD development remains complex and multifaceted. It has been proposed that glucose imbalance during early embryogenesis can increase the levels of oxidative stress, leading to structural anomalies in the fetus [33–35]. In a deeper mechanistic context, maternal diabetes prompts vascular abnormalities in the placenta, contributing to persistent fetal hypoglycemia, hypoxemia, hyperinsulinemia, and polycythemia [36]. These imbalances collectively lead to an increase in fetal cardiac mass, hypertrophy of the myocardium, diastolic dysfunction, and a thickened cardiac wall [37]. On the other hand, maternal diabetes may induce epigenetic modifications in the developing fetus. DNA methylation changes, for example, can impact gene expression patterns relevant to heart development [10,38]. In addition, inflammatory mediators and immune dysregulation characteristic of diabetes can negatively impact heart development in fetus [39]. These results emphasize the need for vigilant monitoring and management of maternal diabetes during pregnancy to potentially mitigate the risk of CHD in offspring. Further research is needed to elucidate these mechanisms fully and explore potential targets for intervention and prevention.
Remarkably, our analysis did not reveal a statistically significant association between maternal BMI and the likelihood of CHD. However, this finding is consistent with some previous studies that have reported inconclusive results regarding the impact of maternal BMI on CHD risk [40,41]. On the other hand, a high number of studies in literature have shown associations between high maternal BMI and newborns with CHD [7–9]. However, Gilboa et al. argued that most of them show a weak association between these variables [42]. For example, in a large case-control study conducted by Cedergren et al. on mothers giving birth to infants with CHD, a positive association was found between maternal BMI > 29 kg/m2 in early pregnancy and CHD in their offspring [43]. Although the study notes this possible association, there is a potential undetected type 2 diabetes during the early stages of pregnancy because obese people prompt impaired glucose metabolism [44]. Diabetes and obesity share several phenotypes, which could be transmissible from mother to fetus. This means that an increase in maternal glucose could be responsible for the association of CHD in newborns of obese women, as suggested by others [45]. This implies that pregnant women with high BMI and an unhealthy diet could lead to a great imbalance in glucose levels affecting the fetus. This discussion also opens up the importance of the early days of gestation and how maternal health status can initiate congenital defects. According to this research, the most critical period for fetal defects occurs during days 14–16 after conception, interestingly when the heart structures begin to differentiate.
Futhermore, the origins of CHD are multifactorial. It is important to consider the complexity of CHD etiology, which likely involves a combination of factors. While maternal diabetes may exert a direct effect on cardiac development, the relationship between maternal BMI and CHD could be modulated by intricate interplays with other genetic and environmental factors. Correa and Marcinkevage [46] conducted an extensive meta-analysis focusing on obesity, high maternal BMI, and birth defects. They applied the Bradford-Hill criteria [47] to identify potential causal relationships. The analysis indicates that the degree of uncertainty concerning a causal link between pre-pregnancy obesity and birth defects is considerable for CHD, as it satisfies only two of the seven criteria. In contrast, maternal pre-pregnancy diabetes and CHD appear to fulfill six out of the seven criteria, indicating a weaker level of uncertainty in their potential causality. In agreement with this study, another article designed as a dose-response metanalysis showed that 9 studies reported maternal obesity as a risk factor for CHD, and 10 reported no significant association [48].
The observed association between maternal pre-gestational diabetes and CHD underscores the significance of preconception care for women with diabetes. Healthcare providers may consider intensifying preconception counseling, emphasizing the meticulous management of blood glucose levels before pregnancy. Closer monitoring and tailored interventions during the preconception period could potentially mitigate the risk of CHD in newborns. Women who do not receive prenatal care are five times more likely to experience infant mortality compared to those who have received prenatal care [49]. The lack of prenatal care is particularly pronounced in Puerto Rico due to the current shortage of physicians. Over the last decade, there has been a 46% decrease in practicing physicians, especially specialists like OBGYNs, making it challenging for women to access timely prenatal healthcare [50]. This shortage presents a significant determinant to the overall health of both the mother and the fetus, especially during the first trimester when the occurrence of congenital defects is at a high potential risk. These findings advocate for the integration of targeted public health initiatives aimed at diabetes prevention and management, especially among women of childbearing age. We recommend the development of preconception guidelines for women with diabetes, focusing on optimizing glycemic control and nutritional support to potentially mitigate the risk of CHD in newborns. By integrating these findings into broader healthcare frameworks, we aspire to contribute to proactive strategies that enhance maternal and fetal health outcomes.
For this study, we utilized a secondary database obtained from the Birth Defect Surveillance Prevention System database of the Puerto Rico Health Department. We opted for a convenience sampling approach due to data availability. It is important to acknowledge that this choice represents a limitation, as convenience sampling is a non-probability method in which the sample population is not systematically selected, thus lacking equal chances of representation for each subject in the target population [51]. Nevertheless, convenience sampling remains a commonly employed method in clinical research due to its practical applicability and widespread use. Although confounding variables were adjusted to achieve a statistical association between maternal BMI, diabetes, and neonate CHD outcomes, additional confounding variables were not adjusted in this study. Such variables include those that may have affected the intrauterine development of the fetus, such as smoking exposure during pregnancy, alcohol consumption during pregnancy, uncontrolled diabetes mellitus throughout pregnancy, and uncontrolled hypertension throughout pregnancy. Another possible limitation is the exclusion of stillbirths in our study. CHD is one of the most common causes of stillbirths, and we had no data on these mothers who could have strengthened our results. Also, in 114 cases of a total of 673, there are missing values for the diabetes mellitus (pre-gestational diabetes) diagnosis.
This study aimed to investigate the impact of pregestational diabetes and maternal BMI on the development of neonatal CHD within the Puerto Rican population. The results from this study provide valuable evidence supporting the link between pregestational diabetes and an elevated risk of CHD in neonates. It underscores the importance of preconception care and managing pregestational diabetes to reduce the likelihood of CHD in infants. These findings establish a groundwork for forthcoming research and could contribute to the formulation of efficient approaches for the prevention and management of congenital heart defects in newborns. Consequently, further research involving diverse populations and exploring additional factors is warranted to gain a more comprehensive understanding of CHD etiology and prevention. Future studies in Puerto Rico should aim to encompass a more diverse and comprehensive array of demographic, socioeconomic, and geographical factors to elucidate potential modifiers and enhance the capturing of population heterogeneity. This expanded scope should consider additional variables, including an assessment of therapeutic and recreational drug use. Moreover, the integration of multi-omics approaches, such as genomics, transcriptomics, and metabolomics, holds promise in advancing our understanding of the molecular mechanisms that underlie the observed associations. This holistic perspective may unveil novel biomarkers and therapeutic targets, thereby paving the way for precision medicine approaches in the prevention and management of CHD.
Acknowledgement: The authors would like to acknowledge the support from the following: Biostatistics, Epidemiology, and Research Design Core (BERD) from Alliance NIGMS U54GM133807, which assisted with the statistical analysis, and the Puerto Rico Department of Health Birth Defects Surveillance and Prevention System which collaborated with the access of the data. We also extend our appreciation to Dr. Estela S. Estapé for the scientific writing review and our medical students in SJBSM: Kavina Jani, Gabriela M. Díaz Rivera, Joanneth M. Padró Serrano, Andrea Pereira Rodríguez, and Luis G. Sánchez Rodríguez for their assistance during the IRB documents submission and first contacts with the Puerto Rico Department of Health.
Funding Statement: None.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: YD; data collection: BCM; analysis and interpretation of results: CG, NP, EM, LPL, YD; draft manuscript preparation: CG, NP, EM, YD. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Responses without personal identifiers from participants are available upon request to the authors.
Ethics Approval: The Puerto Rico Department of Health Birth Defects Surveillance and Prevention System ensured the anonymity and confidentiality of all the participants. There were no significant risks involved in the participation of this study. Informed consent was obtained from the participants through the Puerto Rico Department of Health. The San Juan Bautista School of Medicine’s Institutional Review Board approved the study (EMSJBIRB-7-2021).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Arjmandnia M, Besharati M, Rezvan S. Studying the determinant factors leading to congenital heart disease in newborns. J Educ Health Promot. 2018;7:53. doi:10.4103/jehp.jehp_146_17. [Google Scholar] [PubMed] [CrossRef]
2. Wang T, Chen L, Yang T, Huang P, Wang L, Zhao LJ, et al. Congenital heart disease and risk of cardiovascular disease: a meta-analysis of cohort studies. J Am Heart Assoc. 2019;8(10):e012030. doi:10.1161/JAHA.119.012030. [Google Scholar] [PubMed] [CrossRef]
3. Varela-Chinchilla CD, Sánchez-Mejíab DE, Trinidad-Calderón PA. Congenital heart disease: the state-of-the-art on its pharmacological therapeutics. J Cardiovasc Dev Dis. 2022;9(7):201. doi:10.3390/jcdd9070201. [Google Scholar] [PubMed] [CrossRef]
4. Martínez-Quiæones A, Rivera-Sánchez S, Cruz-Pagán C. Sistema de Vigilancia y prevención defectos congønitos-departamento de salud de Puerto Rico. In: Vigilancia de Defectos Congénitos de Puerto Rico: Informe Anual 2014. Puerto Rico: Estado Libre Asociado de Puerto Rico Departmento de Salud; 2014. [Google Scholar]
5. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circ. 2016;134(2):101–9. doi:10.1161/CIRCULATIONAHA.115.019307. [Google Scholar] [PubMed] [CrossRef]
6. Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circ. 2007;115(23):2995–3014. doi:10.1161/CIRCULATIONAHA.106.183216. [Google Scholar] [PubMed] [CrossRef]
7. Wang J, Du B, Wu Y, Li Z, Chen Q, Zhang X, et al. Association of maternal gestational weight gain with left ventricle geometry and function in offspring at 4 years of age: a prospective birth cohort study. Front Pediatr. 2021;9:722385. doi:10.3389/fped.2021.722385. [Google Scholar] [PubMed] [CrossRef]
8. Persson M, Razaz N, Edstedt Bonamy AK, Villamor E, Cnattingius S. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol. 2019;73(1):44–53. doi:10.1016/j.jacc.2018.10.050. [Google Scholar] [PubMed] [CrossRef]
9. Helle E, Priest JR. Maternal obesity and diabetes mellitus as risk factors for congenital heart disease in the offspring. J Am Heart Assoc. 2020;9(8):e011541. doi:10.1161/JAHA.119. [Google Scholar] [CrossRef]
10. Basu M, Zhu JY, LaHaye S, Majumdar U, Jiao K, Han Z, et al. Epigenetic mechanisms underlying maternal diabetes-associated risk of congenital heart disease. JCI Insight. 2017;2(20):e95085. doi:10.1172/jci.insight.95085. [Google Scholar] [PubMed] [CrossRef]
11. Chen ZY, Mao SF, Guo LH, Qin J, Yang LX, Liu Y. Effect of maternal pregestational diabetes mellitus on congenital heart diseases. World J Pediatr. 2023;19(4):303–14. doi:10.1007/s12519-022-00582-w. [Google Scholar] [PubMed] [CrossRef]
12. Muglia LJ, Benhalima K, Tong S, Ozanne S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. 2022;20(1):418. doi:10.1186/s12916-022-02632-6. [Google Scholar] [PubMed] [CrossRef]
13. Pérez CM, Sánchez H, Ortiz AP. Prevalence of overweight and obesity and their cardiometabolic comorbidities in Hispanic adults living in Puerto Rico. J Commun Health. 2013;38(6):1140–6. doi:10.1007/s10900-013-9726-5. [Google Scholar] [PubMed] [CrossRef]
14. Puerto Rico-International Diabetes Federation. 2021. Available from: https://idf.org/our-network/regions-and-members/south-and-central-america/members/puerto-rico/ (accessed on 01/07/2023). [Google Scholar]
15. Miller A, Riehle-Colarusso T, Siffel C, Frías JL, Correa A. Maternal age and prevalence of isolated congenital heart defects in an urban area of the United States. Am J Med Genet A. 2011;155A(9):2137–45. doi:10.1002/ajmg.a.34130. [Google Scholar] [PubMed] [CrossRef]
16. Agha MM, Glazier RH, Moineddin R, Moore AM, Guttmann A. Socioeconomic status and prevalence of congenital heart defects: does universal access to health care system eliminate the gap? Birth Defects Res Part A, Clin Mol Teratol. 2011;91(12):1011–8. doi:10.1002/bdra.22857. [Google Scholar] [PubMed] [CrossRef]
17. Li NN, Chen XL, Liu Z, Li XH, Deng Y, Zhu J. Maternal abortion history and the risk of congenital heart defects. A case-control study. J Reprod Med. 2015;60(5–6):236–42. [Google Scholar] [PubMed]
18. Lin S, Herdt-Losavio M, Gensburg L, Marshall E, Druschel C. Maternal asthma, asthma medication use, and the risk of congenital heart defects. Birth Defects Res Part A, Clin Mol Teratol. 2009;85(2):161–8. doi:10.1002/bdra.20523. [Google Scholar] [PubMed] [CrossRef]
19. Smedts HP, van Uitert EM, Valkenburg O, Laven JS, Eijkemans MJ, Lindemans J, et al. A derangement of the maternal lipid profile is associated with an elevated risk of congenital heart disease in the offspring. Nutr Metab Cardiovasc Dis. 2012;22(6):477–85. doi:10.1016/j.numecd.2010.07.016. [Google Scholar] [PubMed] [CrossRef]
20. Ramakrishnan A, Lee LJ, Mitchell LE, Agopian AJ. Maternal hypertension during pregnancy and the risk of congenital heart defects in offspring: a systematic review and meta-analysis. Pediatr Cardiol. 2015;36(7):1442–51. doi:10.1007/s00246-015-1182-9. [Google Scholar] [PubMed] [CrossRef]
21. Ferreira BD, Barros T, Moleiro ML, Guedes-Martins L. Preeclampsia and fetal congenital heart defects. Curr Cardiol Rev. 2022;18(5):80–91. doi:10.2174/1573403X18666220415150943. [Google Scholar] [PubMed] [CrossRef]
22. Wang C, Lv H, Ling X, Li H, Diao F, Dai JC, et al. Association of assisted reproductive technology, germline de novo mutations and congenital heart defects in a prospective birth cohort study. Cell Res. 2021;31:919–28. doi:10.1038/s41422-021-00521-w. [Google Scholar] [PubMed] [CrossRef]
23. Howley MM, Papadopoulos EA, van Bennekom CM, van Zutphen AR, Carmichael SL, Munsie JPW, et al. Asthma medication use and risk of birth defects: national birth defects prevention study, 1997–2011. J Allergy Clin Immunol Pract. 2020;8(10):3490–9.e9. doi:10.1016/j.jaip.2020.07.033. [Google Scholar] [PubMed] [CrossRef]
24. Bakker MK, de Walle HEK, Wilffert B, de Jong-van den Berg LTW. Fluoxetine and infantile hypertrophic pylorus stenosis: a signal from a birth defects—Drug exposure surveillance study. Pharmacoepidemiol Drug Saf. 2010;19:808–13. doi:10.1002/pds.1964. [Google Scholar] [PubMed] [CrossRef]
25. Sun M, Zhang S, Li Y, Chen L, Diao J, Li JQ, et al. Effect of maternal antidepressant use during the pre-pregnancy/early pregnancy period on congenital heart disease: a prospective cohort study in central China. Front Cardiovasc Med. 2022;9:916882. doi:10.3389/fcvm.2022.916882. [Google Scholar] [PubMed] [CrossRef]
26. Fisher SC, van Zutphen AR, Werler MM, Lin AE, Romitti PA, Druschel CD, et al. Maternal antihypertensive medication use and congenital heart defects: updated results from the national birth defects prevention study. Hypertension. 2017;69(5):798–805. doi:10.1161/HYPERTENSIONAHA.116.08773. [Google Scholar] [PubMed] [CrossRef]
27. Güveli BT, Rosti RÖ., Güzeltao A, Tuna EB, Ataklg D, Sencer S, et al. Teratogenicity of antiepileptic drugs. Clin Psychopharm Neurosci. 2017;15(1):19–27. doi:10.9758/cpn.2017.15.1.19. [Google Scholar] [PubMed] [CrossRef]
28. Mateja WA, Nelson DB, Kroelinger CD, Ruzek S, Segal J. The association between maternal alcohol use and smoking in early pregnancy and congenital cardiac defects. J Women’s Health. 2012;21(1):26–34. doi:10.1089/jwh.2010.2582. [Google Scholar] [PubMed] [CrossRef]
29. Correa A, Levis DM, Tinker SC, Cragan JD. Maternal cigarette smoking and congenital heart defects. J Pediatr. 2015;166(4):801–4. doi:10.1016/j.jpeds.2015.01.013. [Google Scholar] [PubMed] [CrossRef]
30. Bukowski J. Critical review of the epidemiologic literature regarding the association between congenital heart defects and exposure to trichloroethylene. Crit Rev Toxicol. 2014;44(7):581–9. doi:10.3109/10408444.2014.910755. [Google Scholar] [PubMed] [CrossRef]
31. Liu Z, Yu Y, Li X, Wu A, Mu M, Li N, et al. Maternal lead exposure and risk of congenital heart defects occurrence in offspring. Adv Exp Med Biol. 2015;51:1–6. doi:10.1016/j.reprotox.2014.11.002. [Google Scholar] [PubMed] [CrossRef]
32. Li S, Wang Q, Luo W, Jia S, Liu D, Ma W, et al. Relationship between maternal heavy metal exposure and congenital heart defects: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2022;29(37):55348–55366. doi:10.1007/s11356-022-21071-7. [Google Scholar] [PubMed] [CrossRef]
33. Jaime-Cruz R, Sánchez-Gómez C, Villavicencio-Guzmán L, Lazzarini-Lechuga R, Patiæo-Morales CC, García-Lorenzana M, et al. Embryonic hyperglycemia disrupts myocardial growth, morphological development, and cellular organization: an in vivo experimental study. Life. 2023;13(3):768. doi:10.3390/life13030768. [Google Scholar] [PubMed] [CrossRef]
34. Basu M, Garg V. Maternal hyperglycemia and fetal cardiac development: clinical impact and underlying mechanisms. Birth Defects Res. 2018;110(20):1504–16. doi:10.1002/bdr2.1435. [Google Scholar] [PubMed] [CrossRef]
35. Choudhury TZ, Majumdar U, Basu M, Garg V. Impact of maternal hyperglycemia on cardiac development: insights from animal models. Genesis. 2021;59(11):e23449. doi:10.1002/dvg.23449. [Google Scholar] [PubMed] [CrossRef]
36. Zhao Z, Reece EA. New concepts in diabetic embryopathy. Clin Lab Med. 2013;33(2):207–33. doi:10.1016/j.cll.2013.03.017. [Google Scholar] [PubMed] [CrossRef]
37. Al-Biltagi M, El Razaky O, El Amrousy D. Cardiac changes in infants of diabetic mothers. World J Diabetes. 2021;12(8):1233–47. doi:10.4239/wjd.v12.i8.1233. [Google Scholar] [PubMed] [CrossRef]
38. Xu P, Dong S, Wu L, Bai Y, Bi X, Li Y, et al. Maternal and placental DNA methylation changes associated with the pathogenesis of gestational diabetes mellitus. Nutr. 2022;15(1):70. doi:10.3390/nu15010070. [Google Scholar] [PubMed] [CrossRef]
39. Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52(12):2951–8. doi:10.2337/diabetes.52.12.2951. [Google Scholar] [PubMed] [CrossRef]
40. Rankin J, Tennant PWG, Stothard KJ, Bythell M, Summerbell CD, Bell R. Maternal body mass index and congenital anomaly risk: a cohort study. Int J Obesity. 2010;34(9):1371–80. doi:10.1038/ijo.2010.66. [Google Scholar] [PubMed] [CrossRef]
41. Ghaderian M, Emami-Moghadam AR, Khalilian MR, Riahi K, Ghaedi F. Prepregnancy maternal weight and body mass index of children with and without congenital heart disease. Iran J Pediatr. 2014;24(3):313–8. [Google Scholar] [PubMed]
42. Gilboa SM, Correa A, Botto LD, Rasmussen SA, Waller DK, Hobbs CA, et al. Association between prepregnancy body mass index and congenital heart defects. Am J Obstet Gynecol. 2010;202(1):51.E1–10. doi:10.1016/j.ajog.2009.08.005. [Google Scholar] [PubMed] [CrossRef]
43. Cedergren MI, Källén BA. Maternal obesity and infant heart defects. Obes Res. 2003;11(9):1065– 71. doi:10.1038/oby.2003.146. [Google Scholar] [PubMed] [CrossRef]
44. Mazzoni SE, Hill PK, Webster KW, Heinrichs GA, Hoffman MC. Group prenatal care for women with gestational diabetes. J Maternal-Fetal Neonatal Med. 2016;29(17):2852–6. doi:10.3109/14767058.2015.1107541. [Google Scholar] [PubMed] [CrossRef]
45. Wu XX, Ge RX, Huang L, Tian FY, Chen YX, Wu LL, et al. Pregestational diabetes mediates the association between maternal obesity and the risk of congenital heart defects. J Diabetes Invest. 2022;13:367–74. [Google Scholar]
46. Correa A, Marcinkevage J. Prepregnancy obesity and the risk of birth defects: an update. Nutr Rev. 2013;71(suppl_1):S68–77. doi:10.1111/nure.12058. [Google Scholar] [PubMed] [CrossRef]
47. Hilla B. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. [Google Scholar]
48. Liu X, Ding G, Yang W, Feng X, Li Y, Liu H, et al. Maternal body mass index and risk of congenital heart defects in infants: a dose-response meta-analysis. Biomed Res Int. 2019;2019:1315796. doi:10.1155/2019/1315796. [Google Scholar] [PubMed] [CrossRef]
49. State Approaches to Ensuring Healthy Pregnancies Through Prenatal Care. 2021. Available from: https://www.ncsl.org/health/state-approaches-to-ensuring-healthy-pregnancies-through-prenatal-care#:~:text=Babies%20of%20mothers%20who%20do,pregnancy%20complications%20can%20be%20significant (accessed on 20/11/2023). [Google Scholar]
50. Exodus of Doctors: A Problem That Defies Simple Solutions. 2023. Available from: https://grupocne.org/2023/03/05/exodus-of-doctors-a-problem-that-defies-simple-solutions/#:~:text=According%20to%20Health%20Department%20officials,little%20more%20than%20one%20decade (accessed on 20/11/2023). [Google Scholar]
51. Elfil M, Negida A. Sampling methods in clinical research; an educational review. Emergency. 2017;5(1):e52. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools