 Open Access
Open Access
ARTICLE
Surgical Repair of Ventricular Septal Defect in Neonates: Indications and Outcomes
1 Department of Thoracic and Cardiovascular Surgery, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
2 Department of Thoracic and Cardiovascular Surgery, Seoul National University Children’s Hospital, Seoul, Korea
3 Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea
* Corresponding Author: Sungkyu Cho. Email:
Congenital Heart Disease 2024, 19(1), 69-83. https://doi.org/10.32604/chd.2024.045137
Received 18 August 2023; Accepted 23 January 2024; Issue published 20 March 2024
Abstract
Background: The optimal surgical timing and clinical outcomes of ventricular septal defect (VSD) closure in neonates remain unclear. We aimed to evaluate the clinical outcomes of VSD closure in neonates (age ≤ 30 days). Methods: We retrospectively reviewed 50 consecutive neonates who underwent VSD closure for isolated VSDs between August 2003 and June 2021. Indications for the procedure included congestive heart failure/failure to thrive and pulmonary hypertension. Major adverse events (MAEs) were defined as the composite of all-cause mortality, reoperation, persistent atrioventricular block, and significant (≥grade 2) valvular dysfunction. Results: The median age and body weight at operation were 26.0 days (interquartile range [IQR], 18.8–28.3) and 3.7 kg (IQR, 3.3–4.2), respectively. The median follow-up duration was 110.4 months (IQR, 56.8–165.0). Seven patients required preoperative respiratory support, and five had significant (≥grade 2) preoperative valvular dysfunction. One early mortality occurred due to irreversible cardiogenic shock; no late mortality was observed. One reoperation was due to hemodynamically significant residual VSD at 103.8 months postoperatively. The overall survival, freedom from reoperation, and freedom from MAE at 15-years were 98.0%, 96.3%, and 94.4%, respectively. Preoperative mechanical ventilation was associated with a longer duration of postoperative mechanical ventilation (p < 0.001) and a longer length of intensive care unit stay (p < 0.001). Conclusions: VSD closure with favorable outcomes without morbidities is feasible even in neonates. However, neonates requiring preoperative respiratory support may require careful postoperative management considering the long-term postoperative risks. Overall, surgical VSD closure might be indicated earlier in neonates with respiratory compromise.Keywords
Surgical treatment for ventricular septal defect (VSD) is the most commonly performed procedure in pediatric cardiac surgery and has shown excellent clinical outcomes due to advances in technology, surgical technique, and postoperative management [1–3]. Consequently, surgical VSD closure is now performed in even younger patients [4].
Although postponing surgical treatment in neonates with symptomatic VSD may improve accessibility and convenience of surgical treatment, optimally timed early surgery for VSD can reduce the duration of therapy required to prevent heart failure, maintain growth, and minimize exposure to increased pulmonary pressures [4]. Moreover, a previous study revealed that early surgical repair (<3 months) of isolated VSDs can improve outcomes in infants with severe pulmonary hypertension (PH) [5].
However, neonatal surgery remains challenging, and few recent studies have addressed the optimal surgical timing and outcomes of isolated VSD closure in neonates. Furthermore, an ongoing debate persists on whether to surgically close isolated VSD in neonates with significant VSD-associated symptoms and signs or delay closure until after the neonatal period with medical treatment. Additionally, risk factors for the adverse outcomes of VSD closure remain unclear [2–4,6]. Therefore, we aimed to review and evaluate the clinical outcomes of symptomatic isolated VSD closure in neonates.
This retrospective study was approved by the Seoul National University Hospital Institutional Review Board (approval number: H-2106-179-1230). The requirement for informed consent was waived.
2.2 Study Population and Surgical Strategy
At our institution, VSD closure was performed for 1963 patients without more complex intra-cardiac lesions between August 2003 and June 2021. Among them, 1913 patients who underwent single-stage VSD closure after the neonatal period (n = 1900) and palliative procedure before the VSD closure (n = 13, four in the neonatal period and nine after the neonatal period) were excluded. Finally, we included 50 consecutive neonates (aged ≤ 30 days) who underwent isolated VSD closure in the study (Fig. 1).
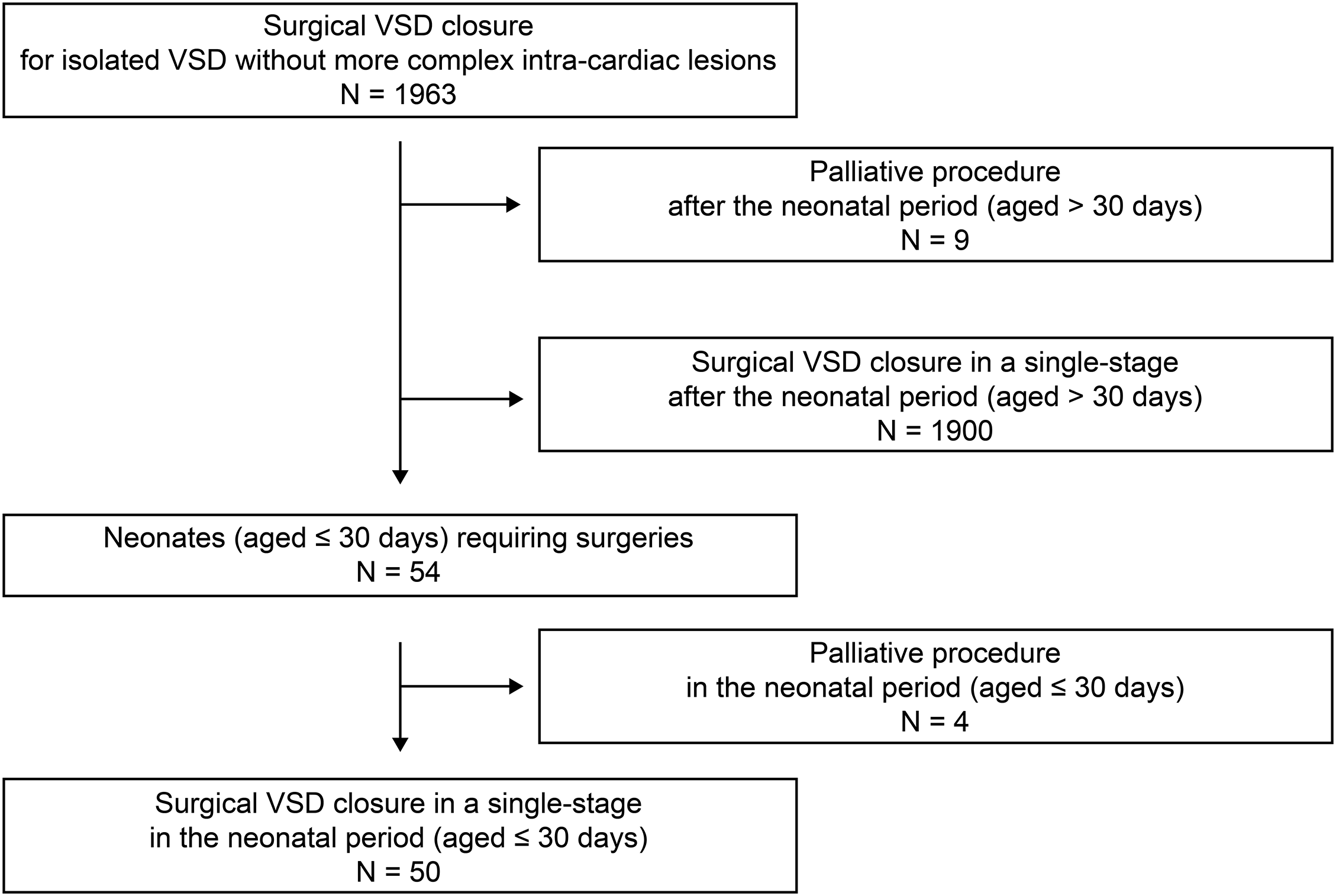
Figure 1: Flowchart depicting procedures and outcomes for patients who underwent surgical VSD closure for isolated VSD without more complex intra-cardiac lesions
VSD size was categorized as “small” (<3 mm), “moderate” (3–5 mm), and “large” (>6 mm). In the case of multiple VSDs, size was recorded as the sum of all sizes [2]. The diagnosis of restrictive VSD was based on echocardiographic findings, including the ratio between VSD diameter and aortic annulus diameter (<1/3), the pressure gradient between both ventricles (>60 mmHg), and VSD peak velocity (>4 m/s). Additionally, moderately restrictive VSD was diagnosed as VSD with the ratio between VSD diameter and aortic annulus diameter (1/3–2/3), the pressure gradient between both ventricles (25–60 mmHg), and VSD peak velocity (2.5–4 m/s) [7].
The indications for VSD closure in all patients were as follows: significant congestive heart failure (CHF)/failure to thrive and PH, despite proper medical management [2]. Significant CHF/failure to thrive was defined as symptomatic CHF of > class II based on the modified Ross classification [8,9]. The presence of PH was assessed using transthoracic echocardiography and diagnosed when a high or intermediate probability of PH was present based on the following guidelines: peak tricuspid regurgitation (TR) velocity of >3.4 m/s or peak TR velocity of 2.9–3.4 m/s with/without the presence of other echocardiographic signs suggestive of PH [10,11]. Additionally, the progression of aortic valve (AV) deformity, which may lead to aortic regurgitation (AR) was considered to determine the appropriate timing of surgery in consultation with cardiologists [2].
We prefer to perform single-stage VSD closure, even in neonates. However, during the same period (between August 2003 and June 2021), pulmonary artery banding as a palliative procedure was performed in 13 patients with multiple muscular VSDs (Swiss cheese-type VSDs) and/or left ventricular noncompaction (n = 9). Additionally, palliative procedures were considered in patients with VSDs more than half of the total ventricular septum (n = 2) if severe preoperative ventricular dysfunction was present or severe postoperative ventricular dysfunction was expected. Moreover, we performed palliative procedures in patients with contraindications for cardiopulmonary bypass (CPB), such as intracranial hemorrhage (n = 2) (Fig. 1). We performed 13 pulmonary artery bandings as palliative procedures instead of VSD closure in a single stage to delay VSD closure or choose an appropriate plan.
Lastly, after VSD closure, an additional concomitant mitral valve (MV) or tricuspid valve (TV) repair was performed to reduce the degree of valvular regurgitation based on intraoperative echocardiographic findings and saline injection test at the surgeon’s discretion.
2.3 Evaluation of Clinical Outcomes and End Points
Operative mortality was defined as death within 30 days postoperatively or during the same hospitalization. Late mortality was defined as death following discharge. Additionally, prolonged postoperative mechanical ventilation (PPMV) was defined as mechanical ventilation lasting ≥72 h postoperatively [12,13].
The primary endpoint was all-cause mortality after surgery. The secondary endpoint included reoperation and a composite of major adverse events (MAE). MAE included reoperation, left ventricular dysfunction, and VSD closure-related events, such as hemodynamically significant residual VSD, persistent atrioventricular block (AVB) requiring permanent pacemaker implantation, and the presence of significant (≥grade 2) valvular lesions, which may require reoperation. Long-term surgical outcomes, including overall survival, reoperation, and MAE, were evaluated and compared between the neonatal group (n = 50) and the other groups (4-6-month-old group [n = 210] and infant group [n = 1305]).
The perioperative and follow-up degrees of valvular regurgitation were evaluated using transthoracic echocardiography based on the following guidelines: 0, none-to-trivial; 1, mild; 2, mild-to-moderate; 3, moderate; 4, and severe [14,15]. Left ventricular dysfunction was defined as a left ventricular ejection fraction of <50% [16]. Regular postoperative follow-ups were performed at 3–6 months intervals.
Continuous variables are expressed as mean ± standard deviation for normally distributed variables or median (interquartile range [IQR]) for non-normally distributed variables. Categorical variables are presented as frequencies (%). Normality was assessed using the Shapiro-Wilk test.
Linear regression analysis was used to identify factors associated with the duration of mechanical ventilation and length of intensive care unit (ICU) stay. Assumptions of linear regression were examined using a plot of residuals vs. predicted values for linearity and homoscedasticity. Additionally, histogram, Q-Q plot, and Shapiro-Wilk test of residuals were used for normality. Furthermore, the duration of mechanical ventilation and length of ICU stay were log-transformed for linear regression analysis due to the right-skewed distribution. None of the plots showed evidence of violations of the linearity and homoscedasticity assumptions for log-transformed dependent variables. Additionally, the normality assumption was satisfied because the histograms appeared fairly bell-shaped and symmetric, the Q-Q plots were close to linear, and the Shapiro-Wilk tests were not significant for the normality assumption. Simple linear regression was used for an initial screening for candidate predictors. Variables with univariate p-value < 0.1 were included in a multiple linear regression model with backward selection and a significance threshold of <0.05. Survival and event-free survival rates were estimated using the Kaplan-Meier method. The log-rank test was performed to compare the survival curves between the groups.
Statistical analysis was performed using the IBM SPSS statistical software (version 25.0, IBM Inc., Armonk, NY, USA) and the SAS statistical software (SAS System for Windows, version 9.4; SAS Institute, Cary, NC, USA). All p-values were two-tailed, and statistical significance was set at p < 0.05.
3.1 Baseline Characteristics and Perioperative Data
The baseline characteristics and perioperative data are summarized in Tables 1 and 2, respectively. The median age and body weight at operation were 26 days (IQR, 19–28; range, 7–30) and 3.7 kg (IQR, 3.3–4.2; range, 1.7–4.7), respectively. Two (4%) and four (8%) patients were born preterm and with low birth weight (<2.5 kg), respectively. Four (8%) patients had genetic syndromes, including Down (n = 1), Edwards (n = 2), and CATCH 22 syndromes (n = 1). Seven patients required respiratory support and were preoperatively diagnosed with respiratory distress syndrome; three required mechanical ventilation and the others required continuous positive airway pressure. One patient had bronchopulmonary dysplasia (BPD). Perimembranous type VSD was the most common (n = 35, 70%), and 39 (78%) patients had large-sized (>6 mm) VSDs. Furthermore, in two patients with multiple VSDs, a patch or direct closure was performed at the surgeon’s discretion, depending on the status of the VSD. The median follow-up and echocardiographic follow-up durations were 110.4 (IQR, 56.8–165.0) and 52.3 months (IQR, 0.6–112.1), respectively.
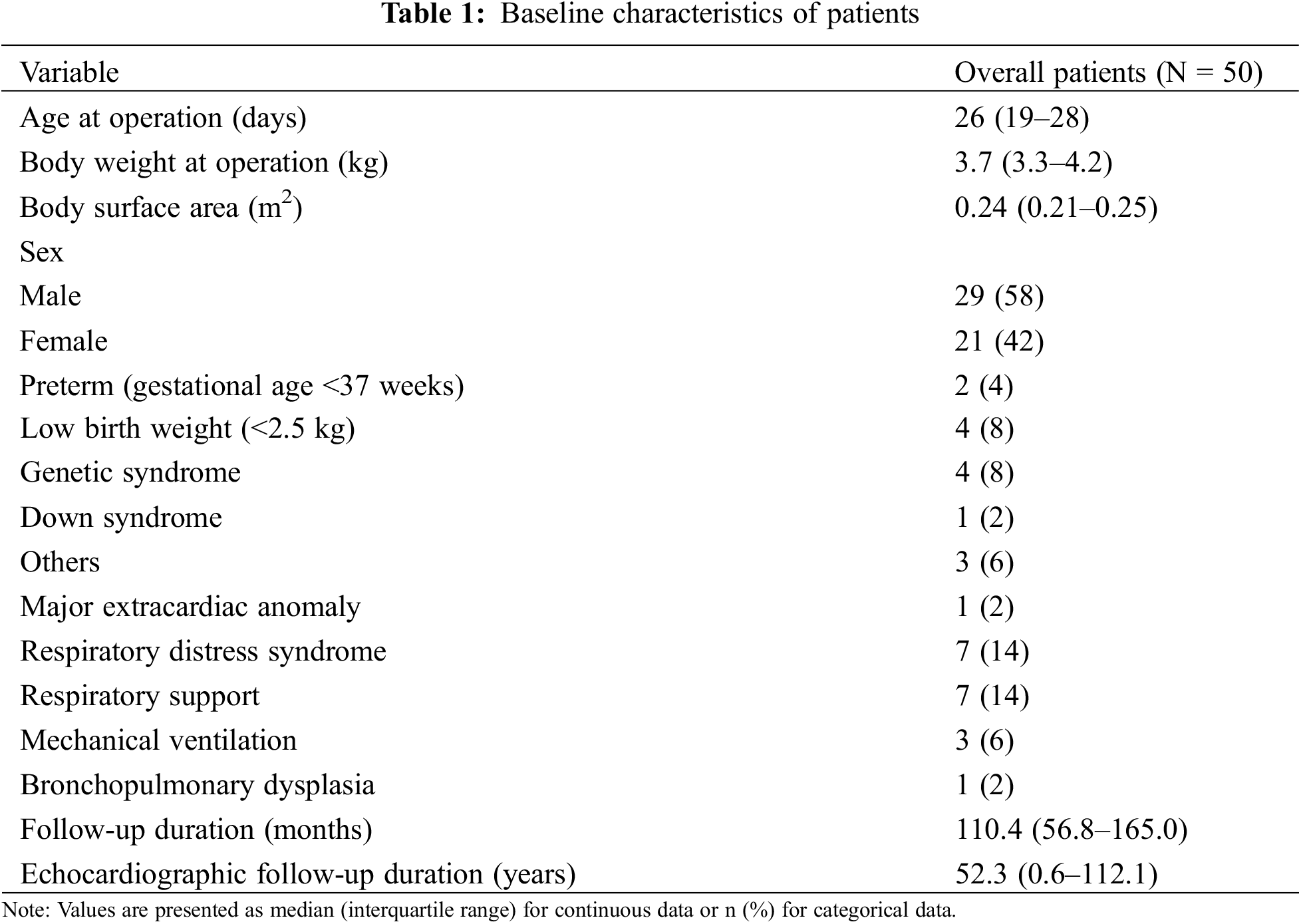
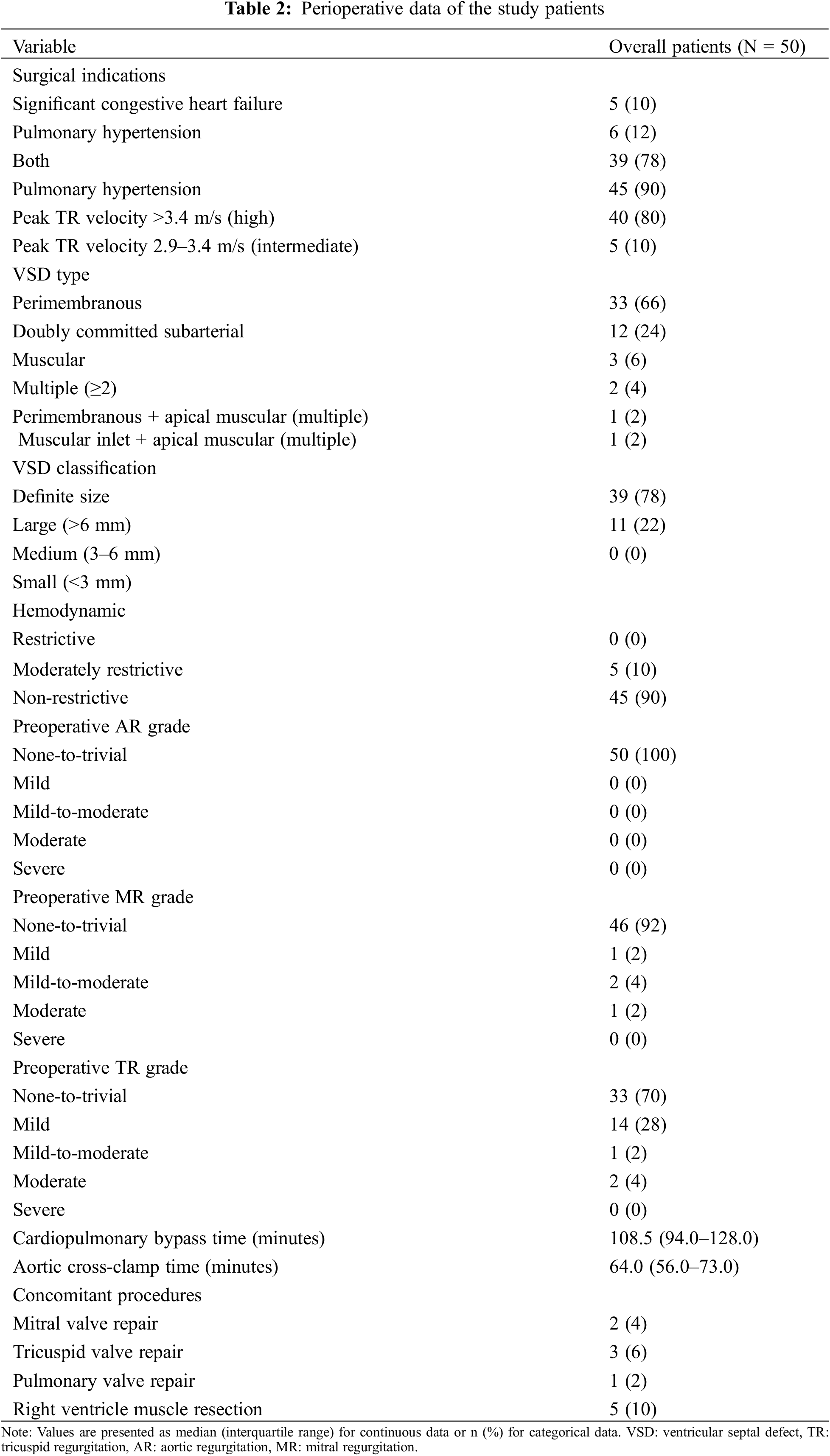
Among the 50 patients, 39 had both significant CHF and a high/intermediate probability of PH in the preoperative echocardiographic findings. No restrictive VSDs were found. Five patients developed significant valvular dysfunction prior to surgery: two with significant mitral regurgitation (MR), two with significant TR, and one patient with both significant MR and TR. Of these patients, TV repair was performed concomitantly in three patients and MV repair was performed concomitantly in two patients. In addition, in five patients, the hypertrophic muscle of the right ventricle was concomitantly resected at the surgeon’s discretion based on intraoperative findings to prevent the potential development of a double-chambered right ventricle. The median CPB and aortic cross-clamp (ACC) times were 108.5 (IQR, 94.0–128.0) and 64.0 min (IQR, 55.5–73.3), respectively.
Of the 50 patients, one (2.0%) early mortality occurred in the patient with a giant omphalocele on postoperative day 19. This patient was preterm (gestational age 29 + 3 weeks) and had a low birth weight (1.3 kg). At the time of surgery, the patient’s age and body weight were 29 days and 1.7 kg, respectively. Additionally, the patient had BPD that required mechanical ventilation for 29 days. The cause of death was an irreversible cardiogenic shock, which led to multi-organ failure.
PPMV occurred in 31 (62%) patients. The median duration of mechanical ventilation and length of ICU stay were 87.1 h (IQR, 65.8–118.5) and 6.0 days (IQR, 4.0–8.0), respectively. The postoperative morbidities are summarized in Table 3.
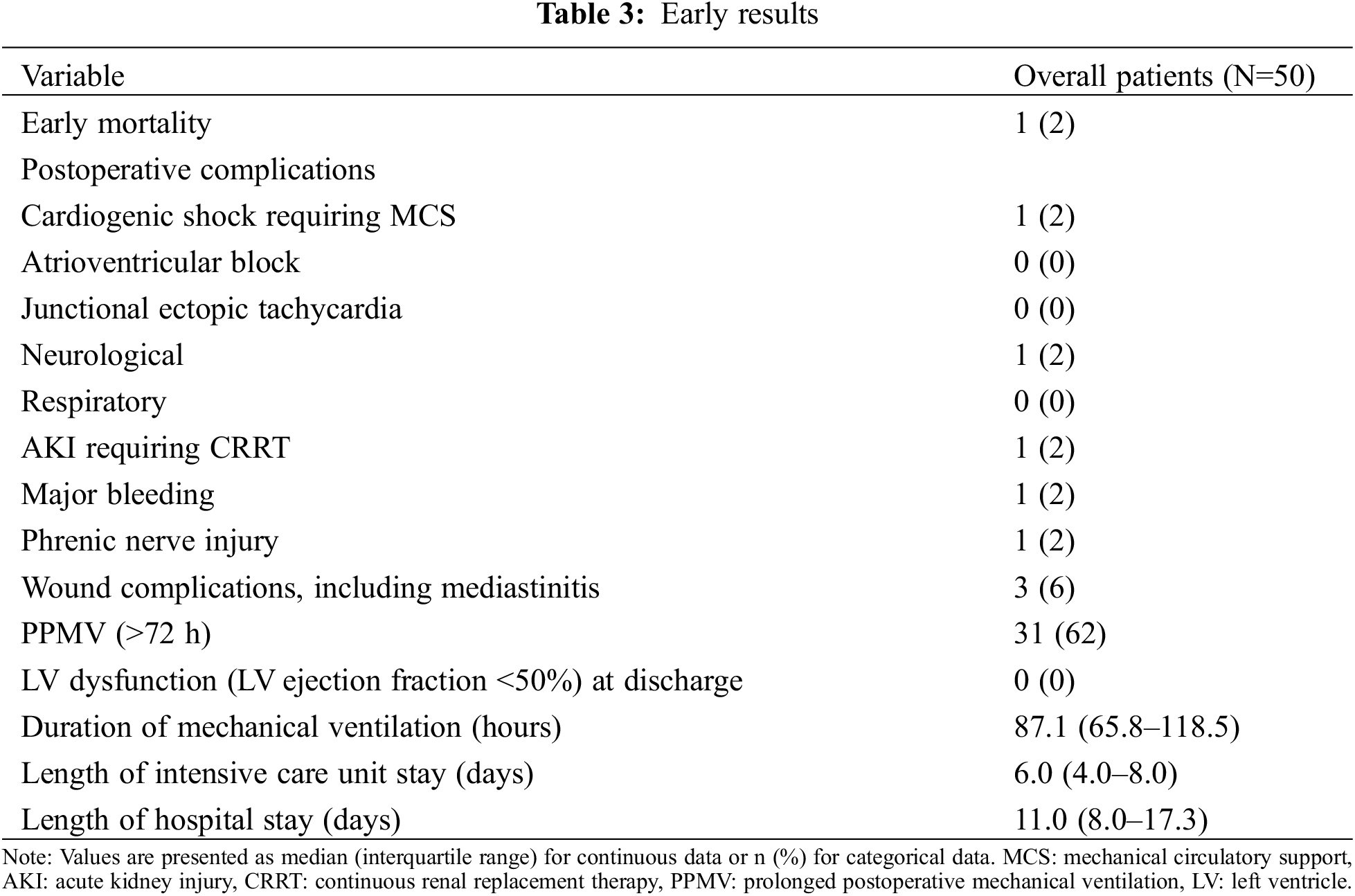
No late mortality was observed among the early survivors (n = 49). However, one patient required reoperation due to hemodynamically significant (Qp/Qs > 1.5:1 and right ventricular dilatation) residual perimembranous VSD at the 103.8-month follow-up; in addition to residual VSD closure, the patient underwent TV repair due to TR progression. In the overall cohort, the overall survival rates at 5, 10, and 15 years were 98.0%, 98.0%, and 98.0%, respectively. The rates of freedom from reoperation at 5, 10, and 15 years were 100.0%, 96.3%, and 96.3%, respectively (Fig. 2A). At the final follow-up echocardiographic assessment, no left ventricular dysfunction was reported except in one patient incidentally diagnosed with dilated cardiomyopathy 49.1 months postoperatively during follow-up. Residual VSD was observed in three patients; only one required reoperation. However, no AVBs that required permanent pacemaker implantation, and no significant regurgitation, or stenosis of the AV, MV, and TV were observed. The freedom from MAE rates at 5, 10, and 15 years were 97.5%, 94.1%, and 94.1%, respectively (Fig. 2B). Kaplan-Meier curve showed no significant differences in overall survival, reoperation, and MAE between the neonatal group and the other groups (4–6-month-old group and infant group) (Figs. 3–5).
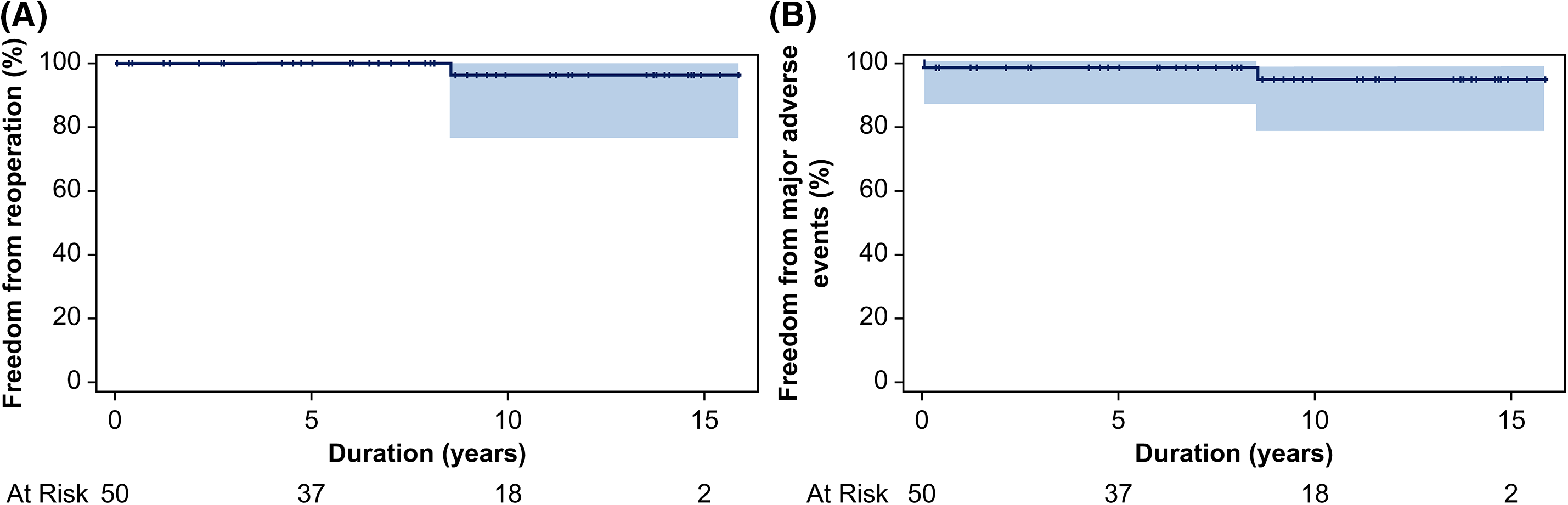
Figure 2: (A) Rate of freedom from reoperation and (B) freedom from major adverse events
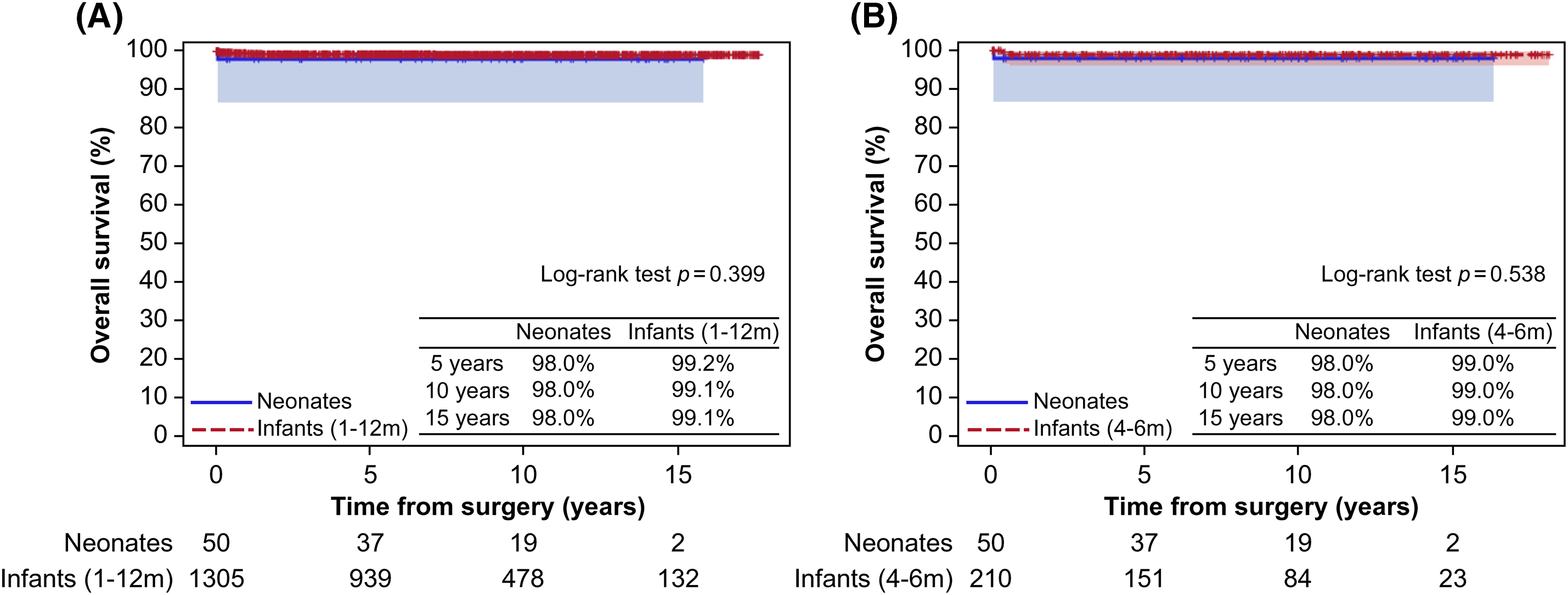
Figure 3: Comparison of overall survival (A) between the neonatal and infant groups and (B) between the neonatal and 4–6-month-old groups
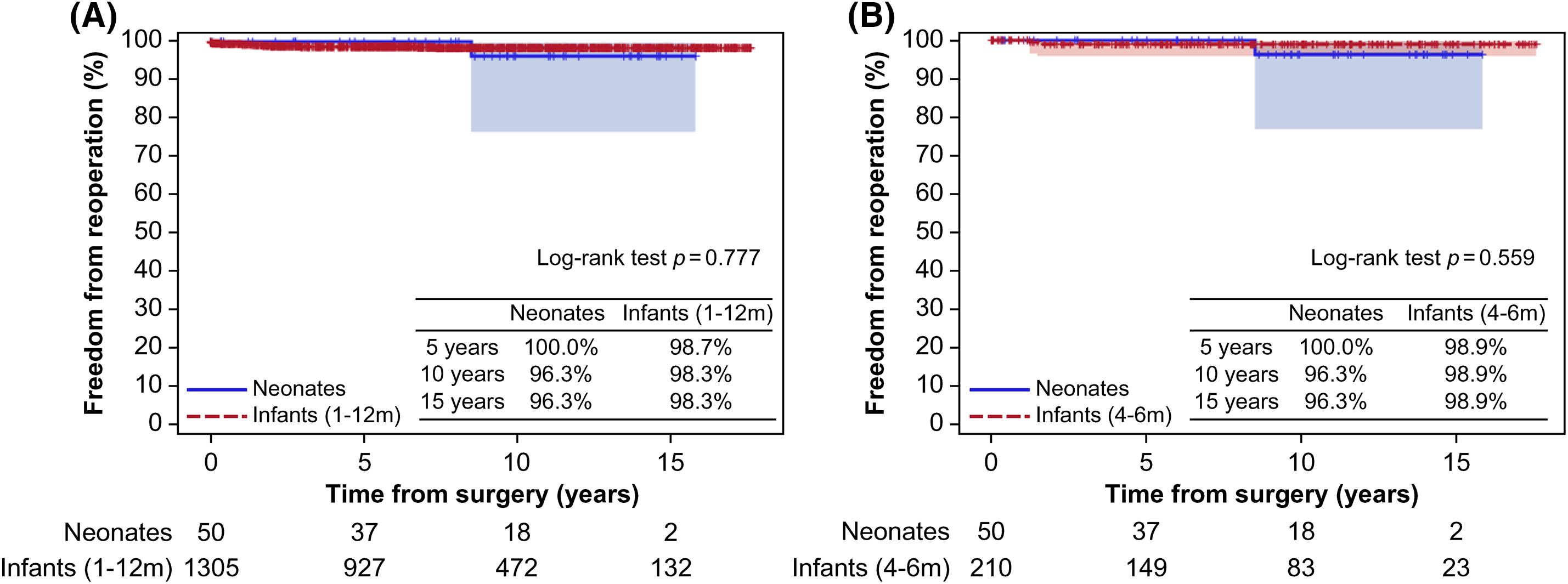
Figure 4: Comparison of freedom from reoperation (A) between the neonatal and infant groups and (B) between the neonatal and 4–6-month-old groups
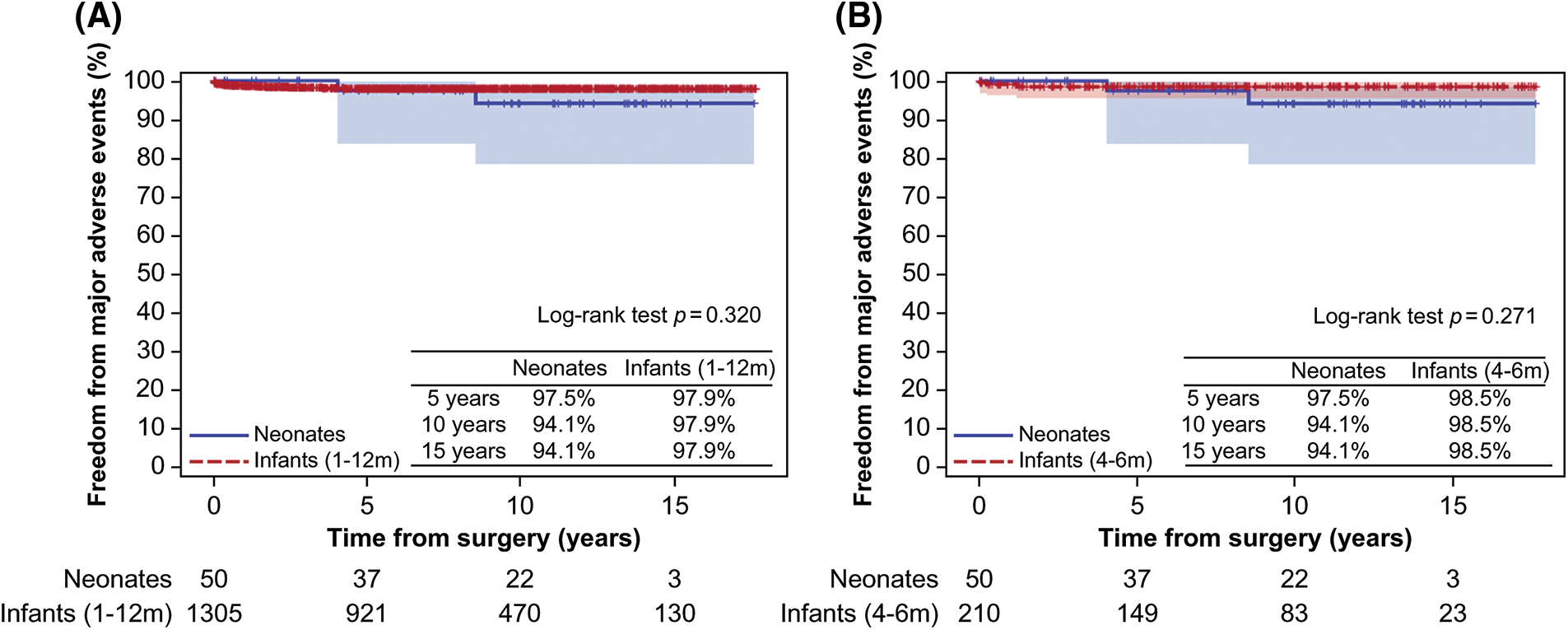
Figure 5: Comparison of major adverse events (A) between the neonatal and infant groups and (B) between the neonatal and 4–6-month-old groups
3.4 Postoperative Mechanical Ventilation and Intensive Care Unit Stay
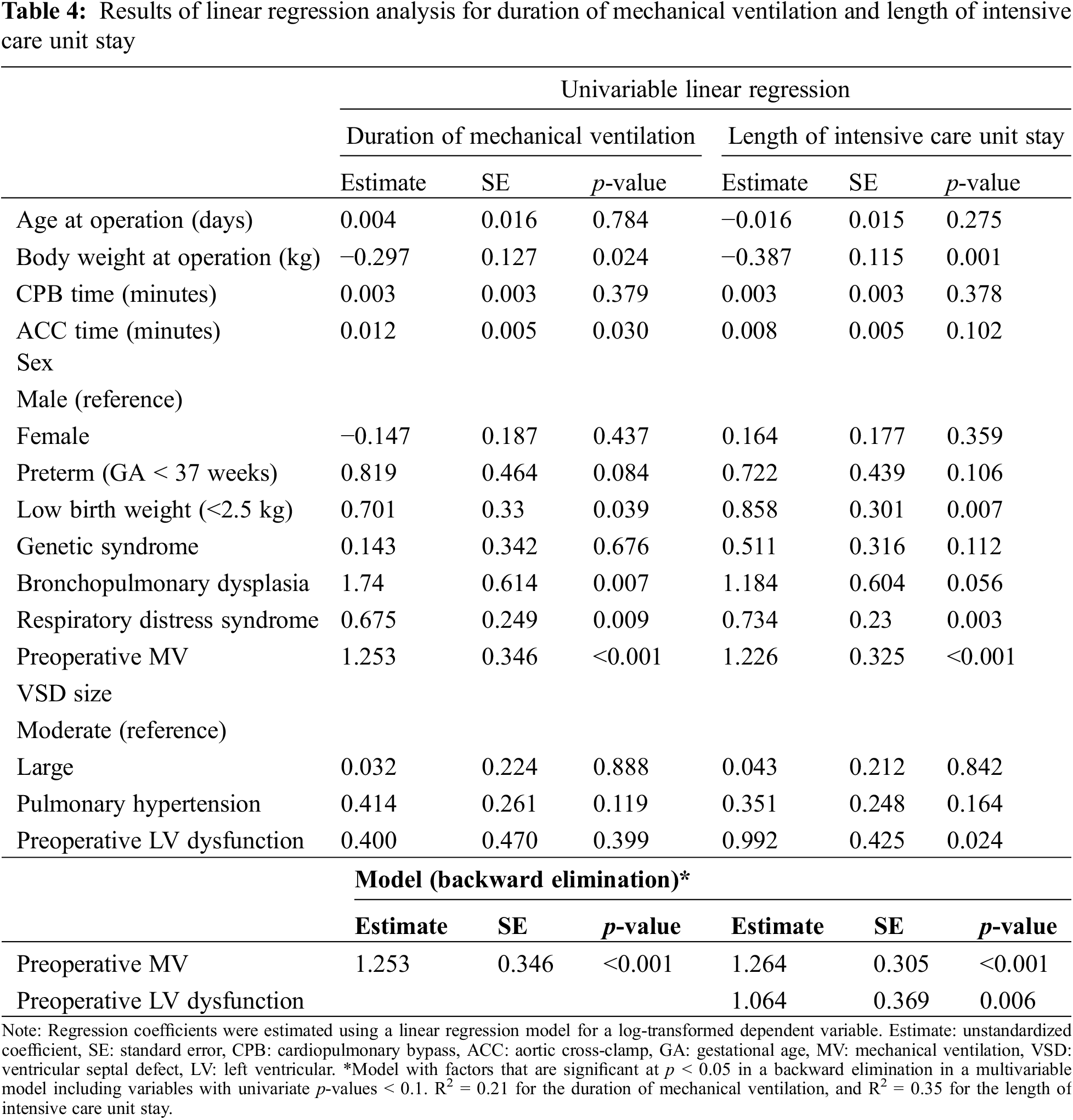
Multiple linear regression analysis showed that preoperative mechanical ventilation was a significant risk factor for longer postoperative mechanical ventilation (p < 0.001) and ICU stay (p < 0.001) (Table 4). In addition, preoperative left ventricular dysfunction was a significant risk factor for longer ICU stays (p = 0.006).
With multifaceted advances in technology and techniques, the number and clinical outcomes of neonatal cardiac surgeries have increased and improved, respectively [17–21]. Therefore, surgical VSD closure is now feasible in younger and smaller patients [4]. Although the clinical outcomes of isolated VSD closure are excellent, neonatal surgery is still challenging. Additionally, even considering the tendency to delay surgery for isolated VSDs in neonates to facilitate operative repair, there have been few studies on the optimal surgical timing and outcomes of isolated VSD closure in neonates [1–3].
Hence, the optimal surgical approach for VSD in symptomatic neonates remains unclear. Previous studies have suggested the safety and effectiveness of early surgery for symptomatic VSDs, particularly when performed before 3 months of age in terms of PH [4,5]. These results suggest that early repair can be performed for more favorable surgical results before PH progression. Accordingly, we aimed to evaluate the risk-benefit of early surgery in a single stage for symptomatic isolated VSDs even in neonates who may require surgeries instead of unnecessary aggressive medical management. Despite the challenging task of individualized risk-benefit assessment in neonates, we focused on the potential benefits of early surgery, including earlier definite and effective resolution of CHF and PH, and maintenance of growth [4,5]. Although patients who require palliative procedures before VSD closure should be considered, we expected that early VSD closure could be performed safely in neonates. In addition to its potential benefits, early VSD closure in a single stage has proven safe in neonates, suggesting it might be more advantageous than performing palliative procedures before VSD closure to avoid further surgeries.
The present study demonstrated two main findings. First, the surgical outcomes for isolated VSD repair in neonates were favorable. Second, the duration of mechanical ventilation and length of stay in the ICU was longer in patients who required preoperative mechanical ventilation.
In our study, all 50 neonates who underwent VSD closure presented signs of significant CHF (growth failure, marked tachypnea, or marked diaphoresis with feeding) or developed PH. This suggests that patients who underwent surgery for VSD had substantial symptoms caused by significant CHF or PH despite proper medical management. Therefore, neonates who underwent early surgery may have been in a clinically worse state with an abrupt exacerbation, given that surgical treatment tends to be postponed in neonates with isolated VSDs. Nevertheless, our findings may suggest that early surgery in neonates with VSDs can be safely performed if they have the appropriate indications for early surgery. Furthermore, early surgery can prevent and resolve the progression of CHF and PH by optimizing hemodynamics in neonates with symptomatic isolated VSD, which might be more beneficial than waiting until the patient’s age and body weight become suitable for surgery. Moreover, even considering the baseline characteristics of the study population with severe symptoms, surgical outcomes of VSD closure in neonates were excellent, irrespective of the severity of the disease. Notably, we observed only one case of early mortality due to intractable underlying disease and one reoperation due to residual VSD.
Several aspects are implicated in the favorable surgical outcomes of neonatal VSD closure observed in our study. First, it is important to determine the appropriate timing of surgery with reasonable surgical indications. Timely surgery based on a proper multidisciplinary approach involving cardiologists should be carefully considered. In our study, the VSD surgeries were performed mostly in patients with concurrent CHF and PH (78.0%). Although preoperative respiratory compromise affected postoperative mechanical ventilation and ICU stay, the long-term results after VSD closure in neonates were acceptable. Given our results, we cautiously suggest that early surgery for VSD closure should be considered before the progression of further respiratory failure in neonates who, despite proper medical treatment, have reached an impending compromised respiratory status following the progression of CHF or PH. Second, developing and revising various VSD closure strategies and techniques may have yielded excellent outcomes that were consistently maintained even in neonates. Jang et al. demonstrated the feasibility and durability of MV repair with growth potential in patients with VSD and accompanying MR [22]. Moreover, we showed that TV detachment could be a reasonable and valid option, providing better exposure to the VSD margin for successful VSD closure in patients with low body weight [23]. Furthermore, we have constantly attempted to improve the surgical outcomes for congenital heart disease and have previously demonstrated the excellent effects of minimized priming volume for open heart surgery in neonates and infants [24,25]. Consequently, these consistent efforts enabled favorable long-term outcomes after VSD closure in neonates.
In the present study, we could not perform a risk factor analysis for survival, reoperation, and MAE because of the shortage of cases; consequently, statistically significant results could not be obtained. Although the basic difference from other studies, including the low body weight of neonates, should be considered, there have been several studies on risk factors for postoperative morbidities after isolated VSD closure. There is still debate regarding risk factors for adverse outcomes, including low body weight at operation, genetic syndromes, or longer CPB time [2–4,6]. Although one mortality in our study occurred in the patient with the lowest body weight (1.7 kg), the patient had a giant omphalocele, which could not be surgically corrected at the time. Therefore, unlike other cases, postoperative management was considerably difficult and mortality might be mainly attributed to a giant omphalocele. In our study, residual VSD was found in three patients during the postoperative echocardiographic follow-up. Of these three patients, one required reoperation due to residual VSD (>5 mm), and the others with small residual VSDs (<2 mm) did not undergo further echocardiographic follow-up as the defects were expected to close spontaneously. These two patients developed no clinical signs suspicious of hemodynamically significant residual VSD during follow-up.
In the present study, preoperative mechanical ventilation was strongly associated with longer postoperative mechanical ventilation and ICU stays. However, contrary to previous studies, which reported that lower body weight is associated with longer mechanical ventilation and ICU stay, body weight at operation was not a statistically significant predictive factor of prolonged mechanical ventilation and ICU stay in our study [3,6]. Notably, there have been several studies on risk factors for prolonged mechanical ventilation following congenital heart surgery in neonates and infants [12,13,26,27]. Blinder et al. reported that mechanical ventilation to treat cardiac failure was a significant preoperative risk factor for a longer duration of mechanical ventilation [27]. This highlights the importance of proactive and timely surgery for symptomatic isolated VSDs, as despite adequate medical management, longer duration of mechanical ventilation and length of ICU stay can affect the quality of postoperative care in patients who require mechanical ventilation for preoperative respiratory failure.
Concerning neonatal cardiac surgery, Elassal et al. reported that postoperative extracorporeal membrane oxygenation support, postoperative intracranial hemorrhage, and acute kidney injury were significant risk factors for mortality [21]. Additionally, older age at surgery, lower body weight, lower birth weight, prematurity, higher complexity operations (RACHS-1), need for CPB, and necrotizing enterocolitis were reported as risk factors for a prolonged hospital stay [21]. Butts et al. suggested that the composite outcomes comprising clinical and laboratory signs were highly associated with early operative outcomes in neonates undergoing CPB [18].
Lastly, despite the relatively low RACHS-1 score and the specific characteristics of our study population, we believe that our favorable outcomes are a result of comprehensive efforts to manage the less developed and weaker cardiac structures of neonates with care. These efforts include minimizing CPB and ACC times, and exercising cautious perioperative care. In addition, we anticipate that our favorable outcomes might support the safety of neonatal VSD closure.
Nevertheless, our results do not suggest that early surgery for VSD closure should be performed in neonates. On the other hand, given the potential benefits of VSD closure, we cautiously recommend VSD closure as a feasible option with favorable outcomes if neonates possess reasonable surgical indications despite appropriate medical treatment.
This study has some limitations. First, this study was a non-randomized, retrospective study with a limited sample size conducted at a single center. Therefore, we did not compare our results with those of the groups who underwent palliative procedures in the neonatal period during the same period. Specifically, considering the non-identical clinical status between groups and the relatively smaller sample size of the group that underwent the palliative procedures, the surgical outcomes of neonatal VSD closure could not be compared with those of the group that underwent palliative procedures in the neonatal period. This is because we were unable to statistically control baseline characteristics and perioperative data. Moreover, we could not perform risk factor analysis for adverse outcomes such as mortality, reoperation, and MAE due to the small number of cases. Second, due to the limited number of studies on the outcomes of neonatal cardiac surgeries, especially surgical VSD closure for isolated VSD, it was difficult to verify and compare our studies with previous findings. Third, the surgical indications and techniques were not identical because the operations were performed by several surgeons with different experiences even though they shared and pursued the same surgical indications and strategies for VSD closure. In addition, changes over time, including the surgeon’s experience and surgical strategies of the center following the development of neonatal surgeries, should be considered. Fourth, our results might be biased considering the possible differences in the baseline characteristics of patients who underwent surgery for isolated VSDs during and after the neonatal period. This is because the patients who underwent early neonatal surgery might have undergone surgical treatment in a more clinically critical and worse situation than patients who underwent the procedure after the neonatal period. Fifth, pre- and postoperative catheterization data were lacking, which were required to demonstrate the preoperative patient status and potential benefits of early VSD closure. Lastly, potential bias may arise from variations in medical treatment strategies and the evaluation of patient status across different centers, given that the definition of significant CHF is based on clinical manifestations following the modified Ross classification. Therefore, despite the absence of significant differences in clinical outcomes between the groups, the results should be generalized cautiously and long-term studies with large sample sizes are required.
VSD closure in neonates, even when compared to infants, can be performed safely with favorable outcomes. However, neonates requiring preoperative respiratory support may require cautious postoperative management because of the potential risks during long-term postoperative care. Therefore, early VSD closure might be considered without delay in symptomatic neonates with respiratory compromise owing to the progression of CHF and PH, based on an individual risk-benefit analysis.
Acknowledgement: We thank the statistical team at the Seoul National University Hospital Medical Research Collaborating Center for the statistical evaluation and revision.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Jae Hong Lee, Sungkyu Cho; data collection: Jae Hong Lee; analysis and interpretation of results: Jae Hong Lee; draft manuscript preparation: Jae Hong Lee, Sungkyu Cho; manuscript review: Sungkyu Cho, Jae Gun Kwak; supervision: Woong-Han Kim; resources: Hye Won Kwon, Mi kyoung Song, Sang-Yun Lee, Gi Beom Kim, Eun Jung Bae. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data underlying this article will be shared upon reasonable request to the corresponding author.
Ethics Approval: This retrospective study was approved by the Seoul National University Hospital Institutional Review Board (approval number: H-2106-179-1230). The requirement for informed consent was waived.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Scully BB, Morales DL, Zafar F, McKenzie ED, Fraser CD, Heinle JS. Current expectations for surgical repair of isolated ventricular septal defects. Ann Thorac Surg. 2010;89(2):544–9. [Google Scholar] [PubMed]
2. Anderson BR, Stevens KN, Nicolson SC, Gruber SB, Spray TL, Wernovsky G, et al. Contemporary outcomes of surgical ventricular septal defect closure. J Thorac Cardiovasc Surg. 2013;145(3):641–7. [Google Scholar] [PubMed]
3. Schipper M, Slieker MG, Schoof PH, Breur JM. Surgical repair of ventricular septal defect; contemporary results and risk factors for a complicated course. Pediatr Cardiol. 2017;38(2):264–70. [Google Scholar] [PubMed]
4. Kogon B, Butler H, Kirshbom P, Kanter K, McConnell M. Closure of symptomatic ventricular septal defects: how early is too early. Pediatr Cardiol. 2008;29(1):36–9. [Google Scholar] [PubMed]
5. Aydemir NA, Harmandar B, Karaci AR, Sasmazel A, Bolukcu A, Saritas T, et al. Results for surgical closure of isolated ventricular septal defects in patients under one year of age. J Card Surg. 2013;28(2):174–9. [Google Scholar] [PubMed]
6. Ergün S, Genç SB, Yildiz O, Öztürk E, Kafalg HC, Ayyıldız P, et al. Risk factors for major adverse events after surgical closure of ventricular septal defect in patients less than 1 year of age: a single-center retrospective. Braz J Cardiovasc Surg. 2019;34(3):335–43. [Google Scholar]
7. Fusco F, Borrelli N, Palma M, Sarubbi B, Scognamiglio G. Imaging of ventricular septal defect: native and post-repair. Int J Cardiol Congenit Heart Dis. 2022;7(3):100335. [Google Scholar]
8. Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33(8):1295–300. [Google Scholar] [PubMed]
9. Das BB. Current state of pediatric heart failure. Child. 2018;5(7):88. [Google Scholar]
10. Meinel K, Koestenberger M, Sallmon H, Hansmann G, Pieles GE. Echocardiography for the assessment of pulmonary hypertension and congenital heart disease in the young. Diagnostics. 2020;11(1):49. [Google Scholar] [PubMed]
11. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. [Google Scholar] [PubMed]
12. Shi S, Zhao Z, Liu X, Shu Q, Tan L, Lin R, et al. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest. 2008;134(4):768–74. [Google Scholar] [PubMed]
13. Alrddadi SM, Morsy MM, Albakri JK, Mohammed MA, Alnajjar GA, Fawaz MM, et al. Risk factors for prolonged mechanical ventilation after surgical repair of congenital heart disease. Experience from a single cardiac center. Saudi Med J. 2019;40(4):367–71. [Google Scholar] [PubMed]
14. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. [Google Scholar] [PubMed]
15. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiog. 2009;22(1):1–23. [Google Scholar] [PubMed]
16. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [Google Scholar] [PubMed]
17. Kansy A, Tobota Z, Maruszewski P, Maruszewski B. Analysis of 14,843 neonatal congenital heart surgical procedures in the European Association for Cardiothoracic Surgery Congenital Database. Ann Thorac Surg. 2010;89(4):1255–9. [Google Scholar] [PubMed]
18. Butts RJ, Scheurer MA, Zyblewski SC, Wahlquist AE, Nietert PJ, Bradley SM, et al. A composite outcome for neonatal cardiac surgery research. J Thorac Cardiovasc Surg. 2014;147(1):428–33. [Google Scholar] [PubMed]
19. Amir G, Frenkel G, Bruckheimer E, Lowenthal A, Rotstein A, Katz J, et al. Neonatal cardiac surgery in the new era: lessons learned from 1000 consecutive cases. Isr Med Assoc J. 2016;18(11):645–8. [Google Scholar] [PubMed]
20. Hasegawa T, Masuda M, Okumura M, Arai H, Kobayashi J, Saiki Y, et al. Trends and outcomes in neonatal cardiac surgery for congenital heart disease in Japan from 1996 to 2010. Eur J Cardio-Thorac Surg. 2017;51(2):301–7. [Google Scholar] [PubMed]
21. Elassal AA, Al-Radi OO, Debis RS, Zaher ZF, Abdelmohsen GA, Faden MS, et al. Neonatal congenital heart surgery: contemporary outcomes and risk profile. J Cardiothorac Surg. 2022;17(1):80. [Google Scholar] [PubMed]
22. Jang WS, Kim WH, Cho JY, Choi K, Choi ES, Lee YO, et al. Surgical indications and results of mitral valve repair in pediatric patients with ventricular septal defects accompanied by mitral valve regurgitation. Ann Thorac Surg. 2015;99(3):891–7. [Google Scholar] [PubMed]
23. Lee JH, Cho S, Kwak JG, Kwon HW, Kwak Y, Min J, et al. Tricuspid valve detachment for ventricular septal defect closure in infants <5 kg: should we be hesitant. Eur J Cardio-Thorac Surg. 2021;60(3):544–51. [Google Scholar] [PubMed]
24. Chang HW, Nam J, Cho JH, Lee JR, Kim YJ, Kim WH, et al. Five-year experience with mini-volume priming in infants ≤5 kg: safety of significantly smaller transfusion volumes. Artif Organs. 2014;38(1):78–87. [Google Scholar] [PubMed]
25. Kim SY, Cho S, Choi E, Kim WH. Effects of mini-volume priming during cardiopulmonary bypass on clinical outcomes in low-bodyweight neonates: less transfusion and postoperative extracorporeal membrane oxygenation support. Artif Organs. 2016;40(1):73–9. [Google Scholar] [PubMed]
26. Polito A, Patorno E, Costello JM, Salvin JW, Emani SM, Rajagopal S, et al. Perioperative factors associated with prolonged mechanical ventilation after complex congenital heart surgery. Pediatr Crit Care Med. 2011;12(3):e122–6. [Google Scholar] [PubMed]
27. Blinder JJ, Thiagarajan R, Williams K, Nathan M, Mayer J, Kulik TJ. Duration of mechanical ventilation and perioperative care quality after neonatal cardiac operations. Ann Thorac Surg. 2017;103(6):1956–62. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools