 Open Access
Open Access
ARTICLE
Expert Consensus on Nutritional Support for Children with Congenital Heart Disease (2023 Edition)
1 Department of Cardiothoracic Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, 210008, China
2 Department of Pediatric Surgery and Nutrition, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200092, China
3 Department of Cardiothoracic Surgery, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127, China
4 Division of Pediatric Gastroenterology and Nutrition, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200092, China
5 Pediatric Cardiac Surgery Center, Fuwai Hospital, National Centre for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100037, China
6 Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, China
7 Cardiovascular Center, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, 510623, China
8 Department of Cardiac Surgery, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, 310052, China
9 Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, Guangzhou, 519041, China
10 Heart Center and Shanghai Institue of Pediatric Congenital Heart Disease, Shanghai Children’s Medical Center, National Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127, China
11 Heart Center, Qingdao Women and Children’s Hospital, Qingdao, 266034, China
12 Department of Cardiovascular Surgery, West China Hospital, Sichuan University, Chengdu, 610041, China
13 Department of Cardiac Surgery, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, 100045, China
14 Department of Pediatric Intensive Care Unit, Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100037, China
15 Department of ICU in Pediatric Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, China
16 Department of Pediatric Cardiac Surgery, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, China
17 Department of Cardiovascular Surgery, Central China Fuwai Hospital of Zhengzhou University, Zhengzhou, 451460, China
18 Pediatric Heart Disease Treatment Center of Jiangxi Province, Jiangxi Provincial Children’s Hospital, Nanchang, 330006, China
19 Department of Pediatric Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, 210008, China
20 Department of Clinical Nutrition, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127, China
21 Department of Cardiothoracic Surgery, Children’s Hospital of Fudan University, Shanghai, 201102, China
22 Department of Cardiovascular Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, 710032, China
23 Department of Pediatric Cardiology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, 518038, China
24 Department of Cardiac Surgery, Wuhan Asia Heart Hospital, Wuhan, 430056, China
25 Department of Cardiovascular Surgery, The Second Xiangya Hospital of Central South University, Changsha, 410011, China
26 Department of Cardiac Surgery, Fujian Children’s Hospital (Fujian Branch of Shanghai Children’s Medical Center), College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Medical University, Fuzhou, 350001, China
27 Department of Pediatric Surgery, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 611731, China
28 Heart Center, Dalian Municipal Women and Children’s Medical Center (Group), Dalian, 116699, China
29 Cardiac Intensive Care Unit, The Heart Center, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, 510623, China
30 Department of Cardiothoracic Surgery, Children’s Hospital Affiliated to Zhengzhou University, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, 451161, China
31 Department of Clinical Nutrition, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200092, China
32 Department of Cardiothoracic Surgery, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, China
33 Department of Cardiac Surgery, Qilu Children’s Hospital of Shandong University, Jinan, 250022, China
34 Department of Cardiology, Children’s Hospital of Shanxi, Women Health Center of Shanxi, Taiyuan, 030025, China
35 Department of Pediatric Cardiothoracic Surgery, Shanghai Children’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200062, China
36 Department of Cardiothoracic Surgery, Children’s Hospital of Soochow University, Suzhou, 215002, China
37 Department of Cardiothoracic Surgery, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430019, China
38 Department of Cardiac Intensive Care Unit, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, 310052, China
39 Department of Cardiothoracic Surgery, Hunan Children’s Hospital, Changsha, 410007, China
40 Department of Cardiothoracic Surgery, Harbin Children’s Hospital, Harbin Medical University, Harbin, 150010, China
41 Department of Cardiothoracic Surgery, Hainan Women and Children’s Medical Center, Haikou, 571103, China
42 Department of Cardiac Surgery, Children’s Hospital in Hebei Province, Shijiazhuang, 050200, China
* Corresponding Authors: Xuming Mo. Email: ; Wei Cai. Email:
Congenital Heart Disease 2023, 18(6), 571-593. https://doi.org/10.32604/chd.2024.048939
Received 22 December 2023; Accepted 29 December 2023; Issue published 19 January 2024
Abstract
The second edition of the expert consensus on pediatric nutrition was formed based on a global update of pediatric nutrition guidelines or consensus worldwide, the management of congenital heart disease, and the results of multi-center clinical nutrition research for congenital heart disease following the first Chinese consensus edition of 2016. The consensus was also shaped by the results of three discussion sessions and two questionnaires conducted by the 13-member collaboration group. This process was informed by both clinical guidelines and expert consensus. The quality of literature, both in English and Chinese, and the level of recommendations were evaluated using the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) system.Keywords
Congenital heart disease (CHD) is one of the most common birth defects in children and carries one of the highest mortality rates, accounting for 0.4% to 1% of all live births [1]. Hemodynamic abnormalities can lead to insufficient bodily reserves, depleted supply, and increased demand. Malnutrition is prevalent in children with CHD, particularly when high-risk factors such as cardiac failure, pulmonary hypertension, and infection are present; these factors significantly impact surgical timing, clinical outcomes, and the child’s growth and development [2–4]. However, the nutritional screening, assessment, and intervention for children with CHD remain challenging in clinical practice. Therefore, there is an urgent need to employ evidence-based methodologies to develop new clinical expert consensus on nutrition support for CHD, ensuring it is scientific, effective, and suitable for implementation in China.
The 2016 edition has shown a positive impact and guidance for clinical practical application in the whole country. However, the nutrition intervention for CHD has progressed rapidly in recent years, and it is necessary to evaluate the results, renew consensus and share successful experiences for developing countries.
Besides the results of China’s multicenter research on clinical nutrition in CHD since 2016 [5], we adopted the internationally accepted Delphi program and used the CNKI, Wanfang, Weipu, China biomedical full-text database (SinoMed), PubMed, Embase and the Cochrane Center for Evidence-Based Medicine database for the study on a systematic search of the literature from 2016 to 2023 at national and international. Meanwhile, the search keywords were “congenital heart disease”, “nutrition”, “nutritional screening”, “nutritional assessment”, “nutritional support”, “nutritional preparations”, “breast milk”, “enteral nutrition”, “parenteral nutrition”, “nutritional complications”, “home care”, “catch-up growth”. The second edition of the consensus was finalized by three offline discussion sessions among CHD experts and nutrition experts in October 2021 (20 experts), June 2022 (36 experts), and February 2023 (66 experts), and two online two questionnaires surveys in July 2022 (36 experts) and March 2023 (66 experts) to vote on the content and opinions of the consensus recommendations, respectively.
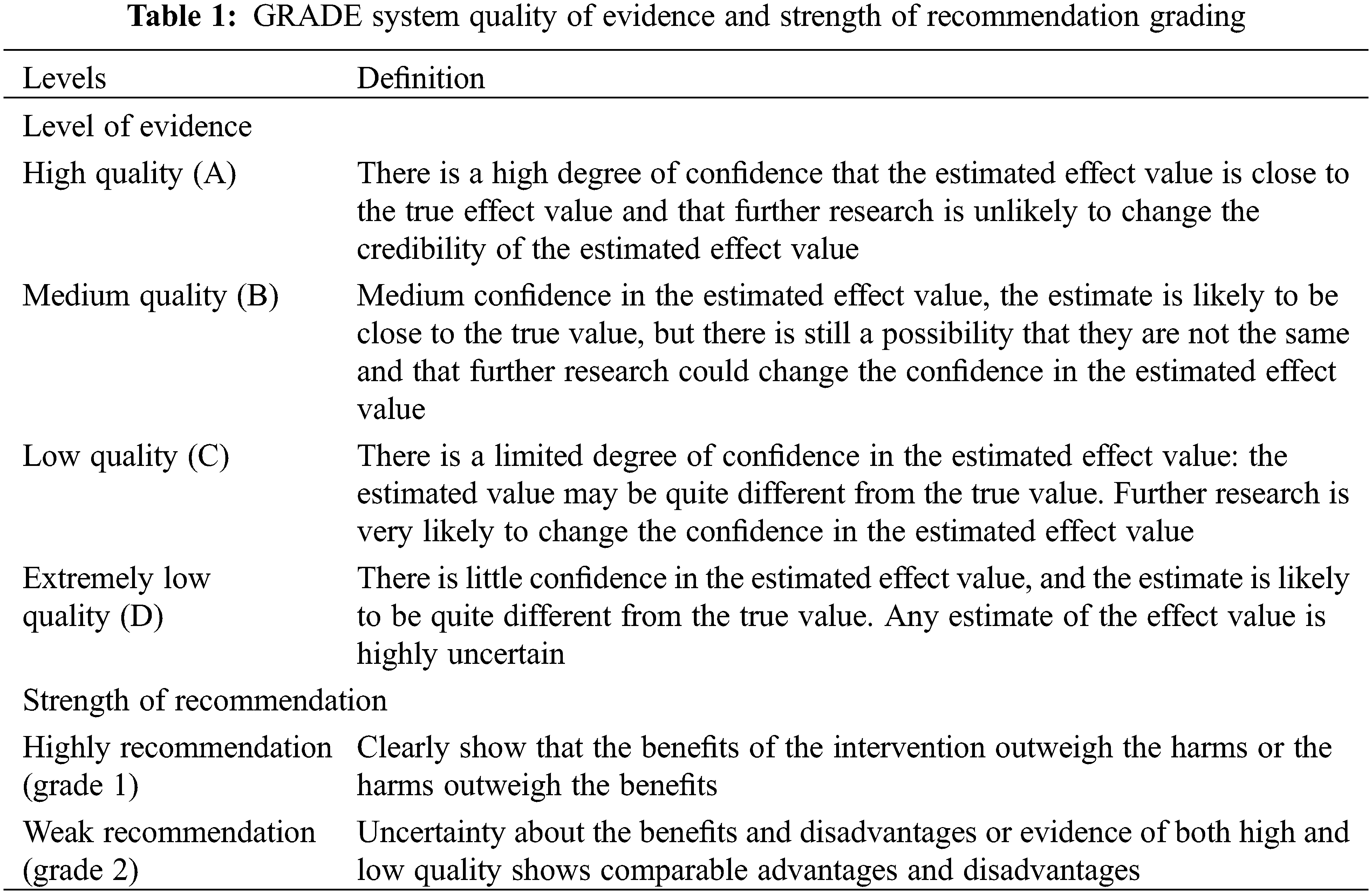
The “Basic Concepts and Formulation Specifications of Clinical Guidelines and Expert Consensus” [6], Group of Cardiac Surgery in Pediatric Surgery Society of Chinese Medical Association, and Group of Pediatric Nutrition in Parenteral Enteral Nutrition Society of Chinese Medical Association organized the national experts related to CHD and pediatric nutrition on the basis of the 2016 version of the “Expert Consensus on Nutritional Support for Children with Congenital Heart Disease,” among screened the 49 questions posed, summarizing 6 main topics and 25 questions. The second edition of the consensus was finally formed after online and offline discussions, as well as combining China’s multi-center pediatric cardiac surgery clinical experience. This consensus uses the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) system to assess the quality of evidence for evidence-based medicine and the level of recommendation opinions [7] (Table 1). Based on the content of the evaluation, the quality of the evidence was graded into four levels: high (A), moderate (B), low (C) and very low (D). Five downgrading factors-the methodological quality of the original study, imprecision, inconsistency, indirectness, and publication bias-and three upgrading factors-large effect size, dose-response relationship, and possible confounding factors (negative bias). Once the grading of the evidence was completed, the evidence was presented via an evidence summary (Table 1).
This consensus has completed registration (registration number: PREPARE-2023CN049) with the International Platform for Registration and Transparency of Practice Guidelines (http://www.guidelines-registry.cn/).
3.1 Nutritional Screening-Assessment and Multidisciplinary Team (MDT) for Children with CHD
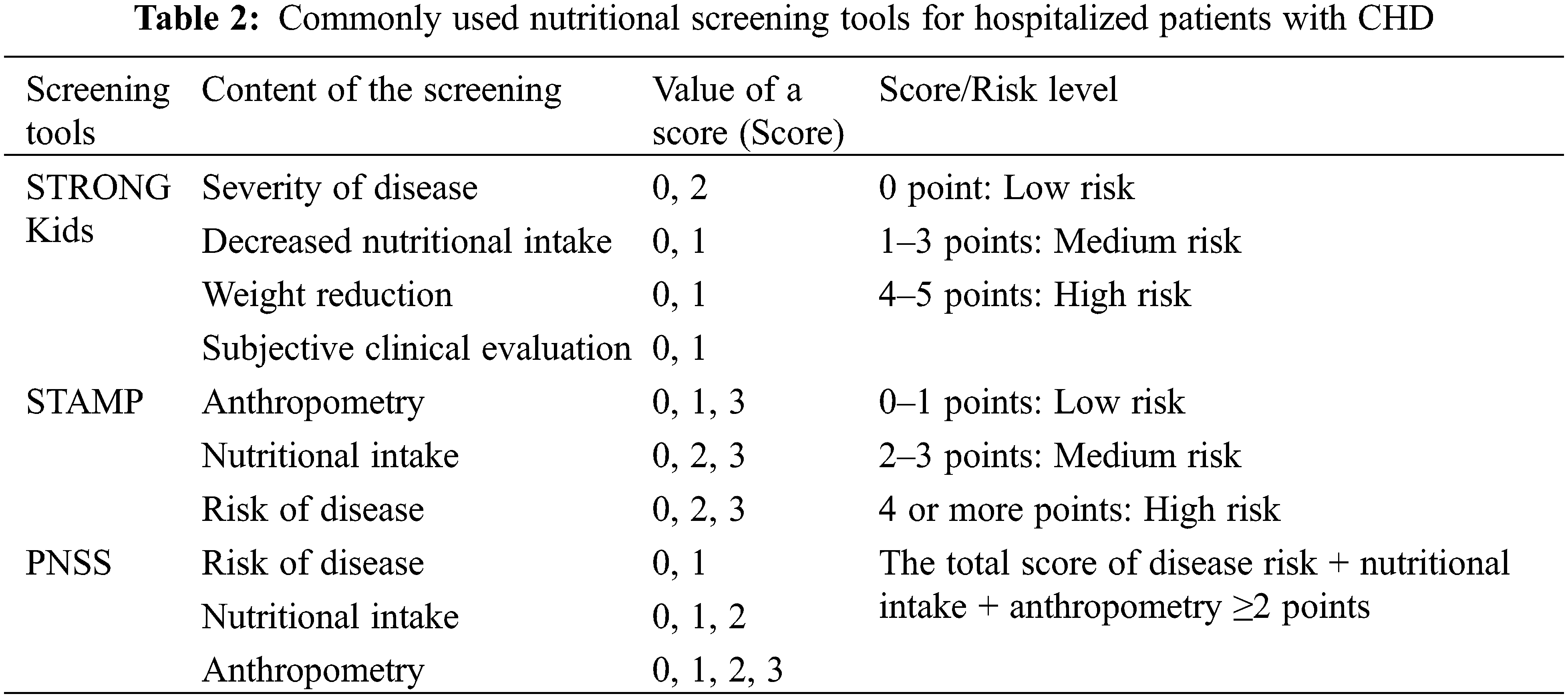
Chronic malnutrition in CHD can lead to growth retardation and poor physical and neuropsychiatric development [7]. Early identification of risk factors for malnutrition such as low birth weight, prematurity, cardiac failure, pulmonary hypertension, poor feeding capacity, and other malformations [8] is essential for nutritional screening and targeted interventions for CHD. Nevertheless, the comprehensive and standardized management of CHD throughout an individual’s lifetime must include nutritional assessments for children with CHD, enhanced perioperative nutritional support, and effective nutritional management post-discharge. Screening for malnutrition risk is recommended to be performed by non-nutritional healthcare professionals within 24 h of admission, using nutritional screening tools such as STRONG Kids, STAMP, or the Chinese Pediatric Nutritional Screening Score (PNSS) [9] (Table 2). Those hospitalized for more than 2 weeks should be screened weekly, and further comprehensive nutritional assessment by a nutritionist was recommended for children screened at more than moderate risk or with a total score ≥2. Among other things, anthropometric measurements (weight, height/length, and head circumference) are recommended to be based on the World Health Organization (WHO) Child Growth Standard Curve (http://www.who.int/childgrowth/standards/en/) [10,11].
(1) Target group: Children at moderate or higher risk of malnutrition on nutritional screening.
(2) Assessment content: Including medical history, nutritional history, feeding history, gastrointestinal function assessment, and physical measurements (height/length, weight, head circumference, mid-upper arm circumference, and skinfold thickness, etc.), laboratory indicators (total protein, prealbumin, retinol-binding protein, C-reactive protein, hemoglobin, electrolytes, etc.), and monitoring of micronutrients (calcium, iron, etc.) and vitamins (folic acid, B12, etcetera), when necessary; Physical measurements still refer to WHO standards, and Fenton 2013 (http://ucalgary.ca/fenton/2013chart) is recommended for preterm infants.
(3) Assessment tools: Subjective Global Nutritional Assessment (SGNA) is recommended for nutritional assessment.
(4) Assessment personnel: Relevant nutrition professionals who have been trained in standardized procedures [12,13].
3.1.3 MDT in Nutritional Support
Perioperative nutritional risk assessment, intervention and post-discharge nutritional management of children with CHD require MDT to regularly address nutritional issues, provide advice, offer programs, and provide appropriate guidance. The MDT team generally consists of multidisciplinary professionals from cardiac surgery, cardiology, CCU (cardiac intensive care unit), clinical nutrition, nursing, pharmacy, rehabilitation, etcetera, and make an integrated individualized plan including nutritional risk screening, nutritional assessment, enteral nutrition (EN), parenteral nutrition (PN) programs, nutritional preparation configuration, gastrointestinal function monitoring and intervention, nutritional knowledge education and post-discharge nutritional management [14]. Henceforth, multidisciplinary collaboration can standardize diagnosis and treatment, promote the effect of nutritional support in children with CHD, and improve the outcome.
Recommendation 1:
(1) Nutritional risk screening should be performed within 24 h of admission for hospitalized children with CHD, and children hospitalized for more than 2 weeks should be reassessed once a week, and those who are screened for moderate or higher risk of malnutrition need to be further evaluated for comprehensive nutritional assessment by a nutritional professional. The STRONG Kids, STAMP and PNSS tools are recommended for nutritional screening, and the Subjective Global Nutritional Assessment (SGNA) is recommended for nutritional assessment. Physical measurements recommend WHO’s Standardized Growth Curve for Children, and Fenton 2013 is recommended for preterm infants.
(Level of Evidence: B; Strength of recommendation: Strong).
(2) MDT of cardiac-related physicians, nursing, nutrition, pharmacy, rehabilitation, and other multidisciplinary personnel will be responsible for the nutritional management of patients with CHD, including the entire process of nutritional risk screening, nutritional assessment, nutritional supportive therapy, and post-discharge nutritional management.
(Level of Evidence: B; Strength of recommendation: Strong).
3.2 Perioperative Energy Requirements of Children with CHD
The daily energy expenditure of children with CHD (especially the complex and critical type) is increased compared with normal children, and malnourished children who need to catch up with growth in postoperative rehabilitation and discharge from the hospital, need to be supplemented with additional energy in order to meet the actual requirements. MDT should be involved in the whole process of nutritional management, including the assessment of swallowing ability, feeding ability, and therapeutic activities, such as gastroesophageal reflux [15,16].
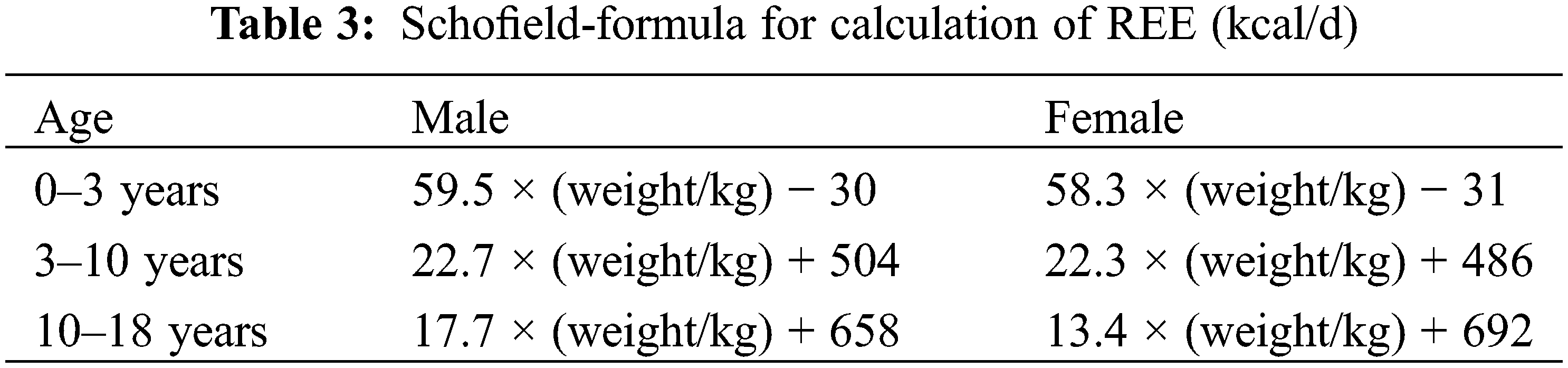
During the perioperative period of CHD, there are significant changes in the body’s energy requirements at different stages [17]. Some studies reported an indirect energy meter to detect resting energy expenditure (REE), hence, to individualize and guide the postoperative energy requirements [18]. If conditions do not permit this, the Schofield formula (Table 3) or the WHO formula can also be used to estimate energy requirements [19]. The selection of clinical energy measurement formulas needs to be individualized according to the actual nutritional status of children with CHD.
3.2.2 Stable Energy Requirements
The stabilization period of preoperative disease refers to the period of relatively stable conditions before surgery and discharge from the hospital. The preoperative energy requirements of some children are higher than those of normal children, but they tend to have insufficient intake. The energy requirements of most children return to the normal level in the first week after surgery [20], and then they enter into the phase of catching up with the growth, and the subsequent energy requirements are also higher than those of normal children in the same age group. Due to food-specific kinetic effects and fecal losses, the EN recommendation is higher than the PN recommendation, and the PN calorie recommendation is usually 70%–80% of the EN. PN calorie recommendation for stable neonates is 70–90 kcal/(kg.d) for term infants and 80–100 kcal/(kg.d) for preterm infants; the EN calorie recommendation is 105–130 kcal/(kg.d), 110–135 kcal/(kg.d) for preterm infants, and up to 150 kcal/(kg.d) for some ultra-low birth weight infants [21]. PN calorie requirements during the stabilization period of infants and toddlers are shown in Table 3 [22].
When EN is insufficient, PN supplemental calories are calculated by the following algorithm: PN required calories = (1 − EN intake calories/EN recommended calories) × PN recommended calories:
PN: PN required calories; a: intake calories; b: recommended calories; c: PN recommended calories.
3.2.3 Postoperative Critical Care Period Energy Requirements
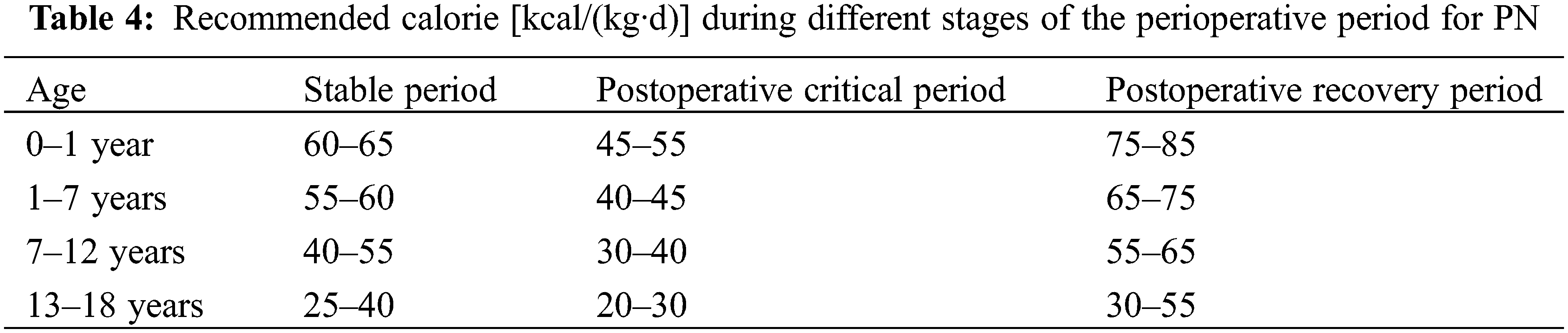
During the postoperative critical care period of CHD, an excessive energy supply carries an increased risk of complications [21,22]. The main goal of this phase is to maintain hemodynamic stability rather than to improve nutritional status, energy supply should not exceed REE, calories are gradually increased to 1.3 times REE after stabilization, and further increased during the recovery phase, the recommended PN calorie requirements for the corresponding phase [21,22] are shown in Table 4.
3.2.4 Post-Hospitalization Energy Requirements
During postoperative rehabilitation, a daily energy intake of 140–150 kcal/kg (actual body weight) or 110–120 kcal/kg (ideal body weight) is sufficient to meet the normal growth requirements of children 0–36 months of age with CHD in order to provide additional calories for “catch-up” growth. A daily energy intake of 140–150 kcal/kg (actual body weight) or 110–120 kcal/kg (ideal body weight) is sufficient for normal growth in children aged 0–36 months. When the growth assessment reaches the normal curve, the energy supply is returned to that of a normal child of the appropriate age.
Recommendation 2:
(1) Daily energy consumption of children with CHD is higher than that of normal children, and energy demand fluctuates greatly at different stages of the perioperative period; during the stabilization period, the energy supply can be supplemented according to the energy supply of normal children of the corresponding age, and energy intake should not exceed the REE of the corresponding age during the critical stage; calories gradually increase to 1.3 times of the REE after stabilization, and then increase further during the recovery period.
(Level of Evidence: B Strength of Recommendation: Strong).
(2) During the perioperative of CHD, if possible, dietitians may measure REE with an indirect energy meter to provide individualized energy requirement and when conditions are not available, the Schofield or WHO estimation formula can be used to estimate the energy requirement.
(Level of Evidence: A; Strength of recommendation: Strong).
3.3 Nutritional Support for Children with CHD
Children with CHD generally do not have congenital gastrointestinal malformations, while gastrointestinal function is affected by cardiac failure, intraoperative gastrointestinal ischemia-reperfusion injury, vasoactive drugs, and other impacts caused by dysfunction or obstacles. However, it is generally reversible, and gastrointestinal function improves correspondingly after the improvement of cardiac function status. Oral feeding is preferred for nutritional support in children with CHD, and EN via the naso-gastric (or jejunal) tube is recommended for those with dysphagia or gastroesophageal reflux [23], which helps to maintain the integrity of the gastrointestinal tract, increase secretion to promote gastrointestinal motility, reduce the incidence of infection, decrease postoperative complications, and shorten the length of hospitalization [23]. If EN cannot meet energy requirements partially supplemented by PN [23]. The amount of rehydration fluid should be matched with the cardiac function, the amount of fluid input and output should be relatively balanced, and the amount of input should be strictly controlled in some children with special types of CHD (e.g., Fontan) after surgery.
Indications, Relative Contraindications, and Contraindications
(1) Indications: 1) Preoperative preserved intestinal function can be given EN; 2) If postoperative day withdrawal of ventilator assistance, EN can be started 6 h after extubation; 3) If the postoperative day did not withdraw the ventilator assistance, hemodynamic stability, there is no contraindication to EN, EN can be started on the first postoperative day (within 24 h).
(2) Relative contraindications: 1) Postoperative hemodynamic instability: such as epinephrine greater than 0.1 µg/kg/min [24]; Dopamine/dobutamine dose greater than 5 µg/kg/min, milrinone greater than 0.5 µg/kg/min, and the inability to reduce the dosage suggests that the child’s hemodynamics is unstable; 2) Delayed closure of the chest, ECMO, VAD support, tracheal intubation in the early stage (within 6 h).
(3) Contraindications: 1) Severe gastrointestinal dysfunction: such as massive gastrointestinal bleeding, complete intestinal obstruction, necrotizing enterocolitis (NEC), high-flow enterocutaneous fistula, etc.; 2) Organic lesions of the gastrointestinal tract: such as the combination of uncorrected congenital esophagus, gastrointestinal malformations.
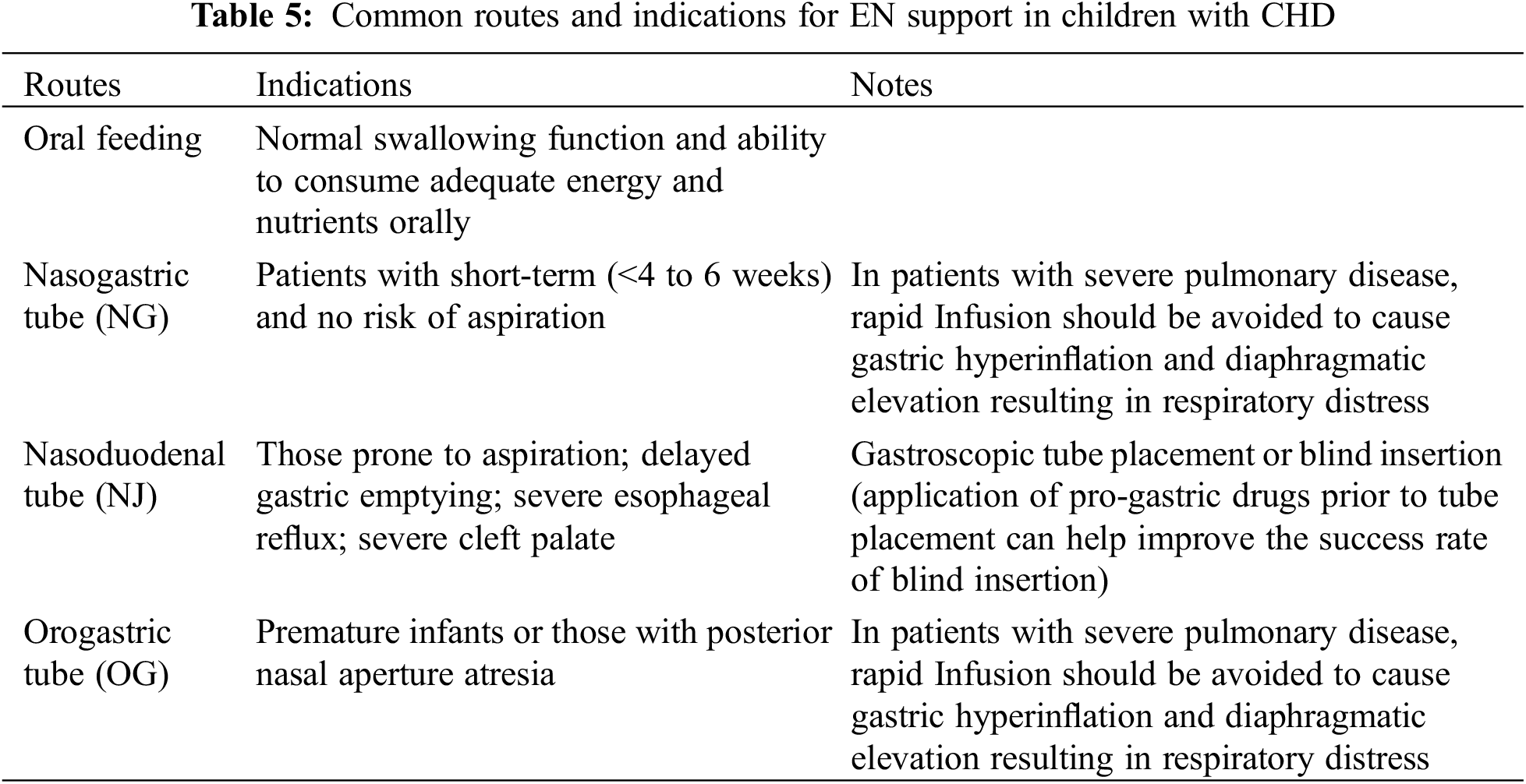
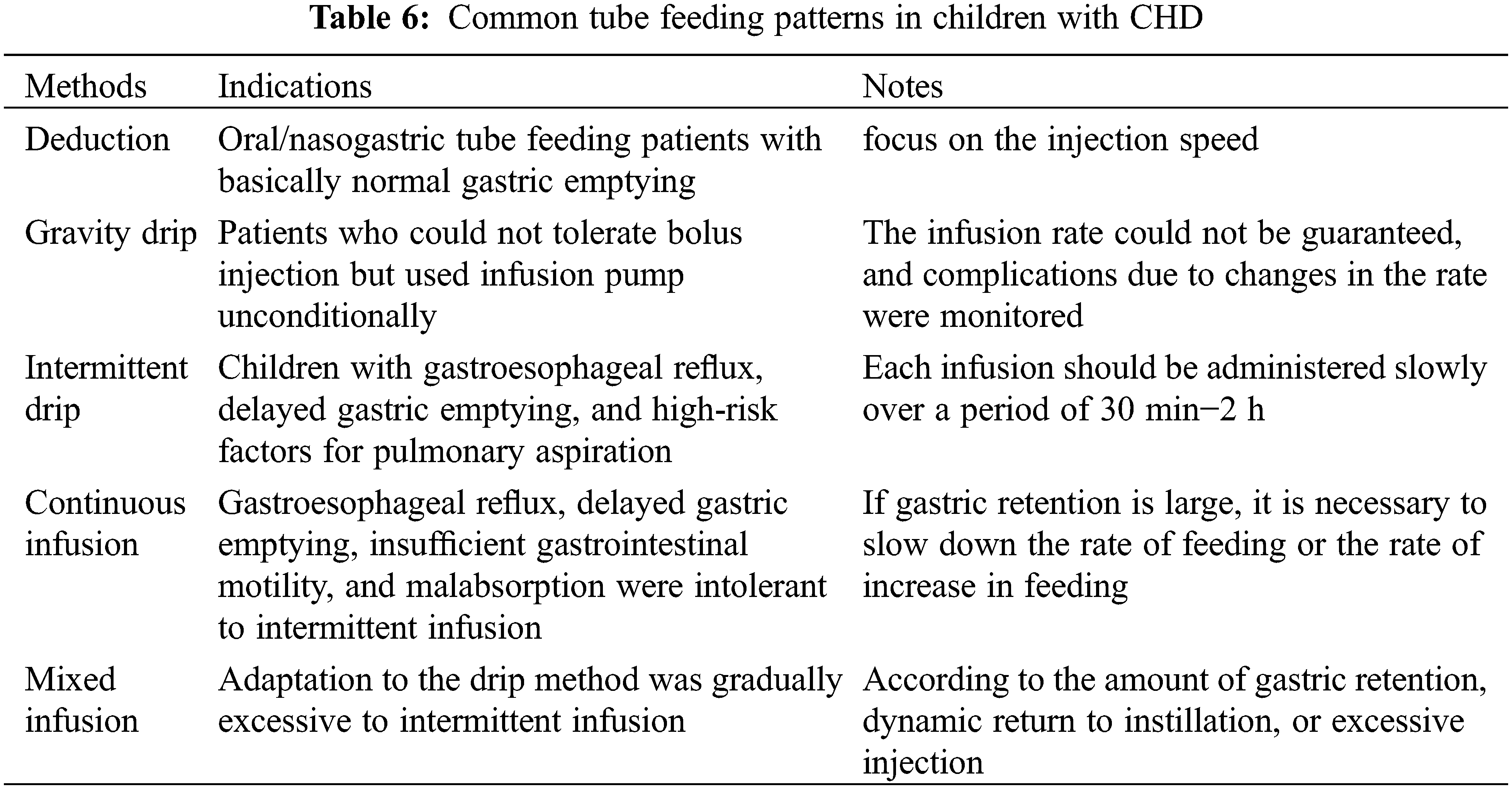
Appropriate EN was selected based on a comprehensive evaluation of the child’s age, cardiopulmonary function, with or without ventilator-assisted respiration, aspiration pneumonia, comorbidities, and duration of EN [25]. The commonly used EN routes are listed in Table 5, and tube feeding methods are listed in Table 6.
Breast milk is the best optimal food for infants and breastfeeding should be encouraged [26,27]. Preterm and very low birth weight infants are prone to feeding intolerance, and colostrum oral immunity treatment (OIT) is feasible [28]. Breast milk additives can be used to increase energy density in feeding-tolerant preterm infants [26,27]. If breastfeeding is insufficient, EN formulas should be selected according to age, disease state, gastrointestinal function, current feeding status, and the presence of food allergies (Table 7). Adult EN formulations can be selected for children over 10 years of age. Medium-chain fatty acid-enriched formulas are advised for postoperative children with developed chylothorax disease, and short peptide semi-essential formulas are selected for children with cow’s milk protein intolerance [17]. EN formulas with a high energy density can be selected for children with fluid limitations, and processed and stored using aseptic techniques [29].

EN Volume and Advanced Protocols
To start EN in a perioperative CHD period should follow the protocols of gradual increase in volume, acceleration, and concentration, specifically from low concentration to high concentration, adjusting the volume first and then adjusting the concentration, and not adjusting the volume and concentration at the same time. Nourishing type (micro) feeding can be used in the critical stage, with small amounts and multiple times or micro-pump micro-input, which can effectively prevent intestinal mucosal atrophy, maintain intestinal integrity, improve intestinal micro-ecology, and promote gastrointestinal dynamics. Nutritive feeding can be started if there is no contraindication to EN in 6–24 h after surgery, 12–24 ml/(kg.d) intermittent infusion by nasogastric tube is recommended. It can be changed to continuous infusion (0.5–1.0 ml/(kg.h)) if intolerance is not tolerated, and nourishing feeding is not included in the target calories.
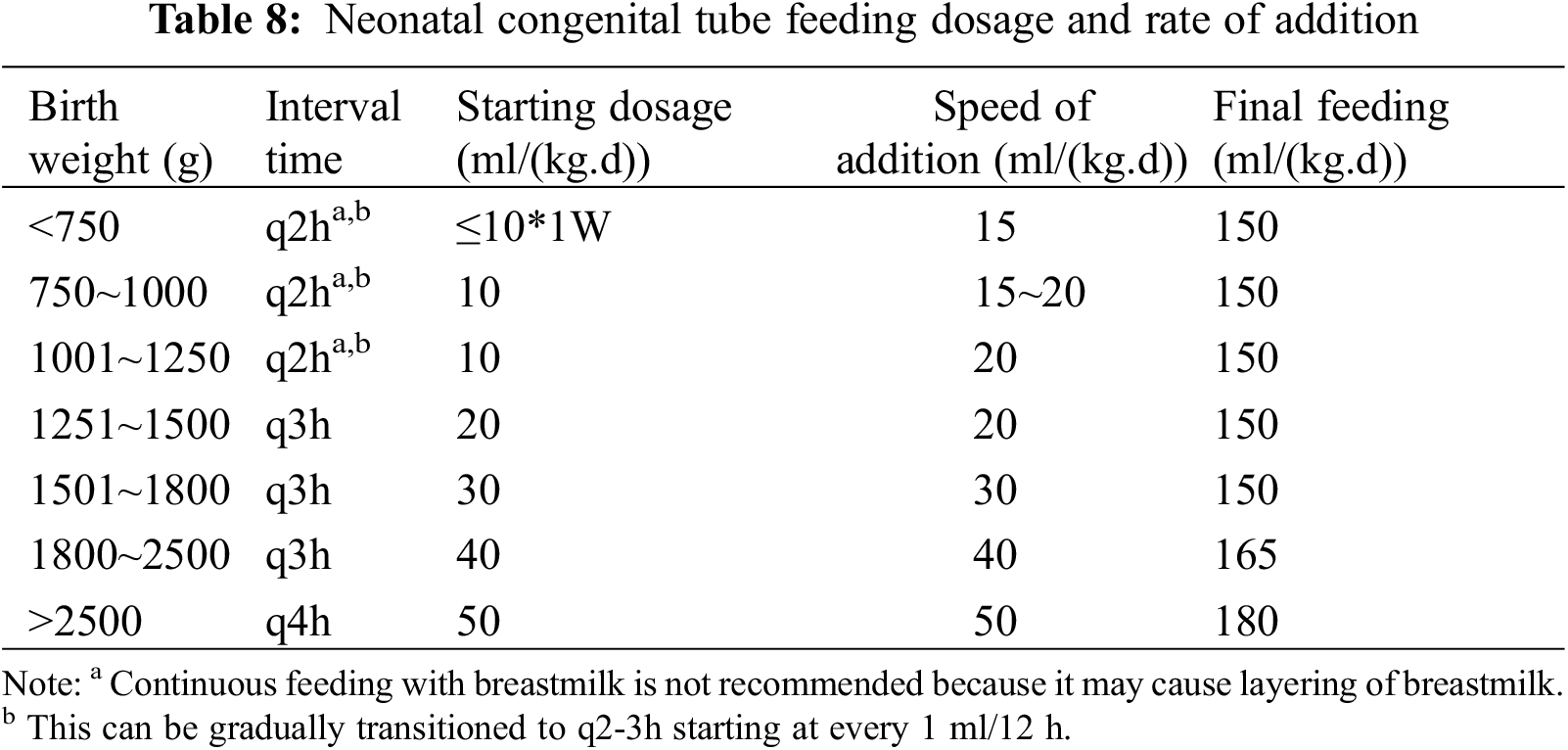
Neonatal feeding volume and rate refer to neonatal tube feeding dosage and addition rate as shown in Table 8. The addition of complementary foods in children of appropriate age should avoid the perioperative period, and the additional time can be delayed by 1–2 months compared with that of normal children appropriately, with the same types of food. Children with CHD usually have limited fluid requirements, and an increase in the calorie density of the formula may be considered, with the final target amount calculated in calories.
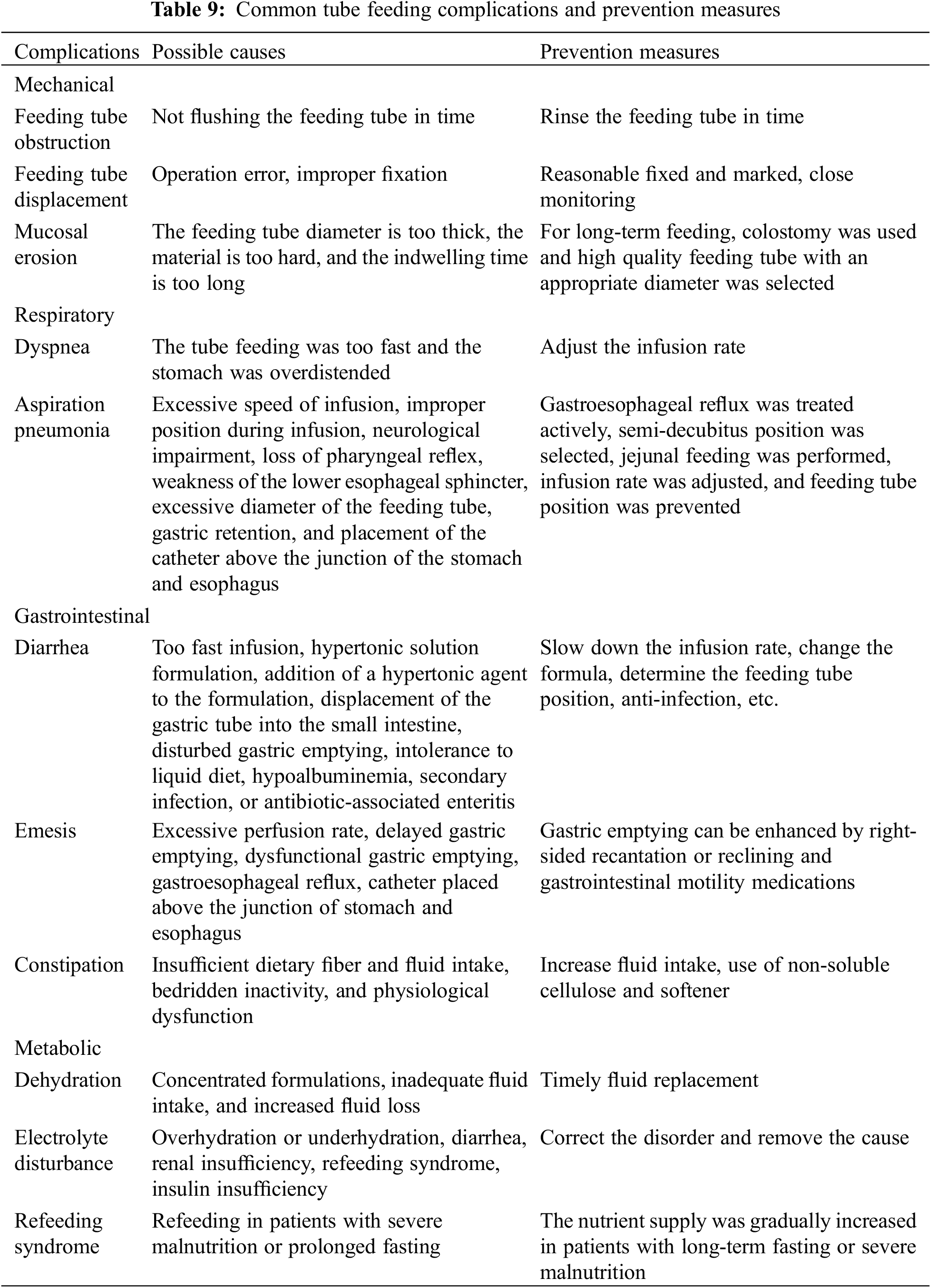
EN Complications, Prevention and Intervention [23,30]
Mechanical, respiratory, gastrointestinal, and metabolic complications can occur due to EN (Table 9), which should be prevented and treated in the context of the specific situation and characteristics of the CHD.
Recommendation 3:
(1) Breast milk is the preferred choice for EN in children with CHD. Colostrum OIT is feasible for preterm and very low birth weight children, and breast milk additives can also be used to increase energy density if necessary. For children who do not have breastfeeding conditions (if age below 1 year) or have special needs, appropriate nutritional preparations, and routes should be selected according to the condition of the child and the function of the gastrointestinal tract (for details, see Table 7).
(Level of Evidence: A; Strength of Recommendation: Strong).
(2) Preseved preoperative intestinal function can be given EN, and EN should be started as early as possible after preoperative surgery to exclude contraindications. EN is mainly given by oral feeding, and children with cardiac failure and low suction ability are fed by a mixture of oral + tube feeding, and all of them can be fed via naso-gastric tube or naso-duodenal feeding under special circumstances, and appropriate EN should be selected on the basis of assuring the number of feedings.
(Level of Evidence: B; Strength of Recommendation: Strong).
(3) Perioperative EN should follow the protocol of a gradual increase in volume, acceleration, and concentration, adjusted according to age, cardiac function, and other factors. Nourishment-type feeding can be used in the critical stage, and the principle of small amount and multiple times or micro pumping micro input can be used. Causes of feeding intolerance should be actively investigated, or the preparation of formula should be adjusted.
(Level of Evidence: B; Strength of Recommendation: Strong).
When EN is not feasible or the supplement of EN is insufficient in children with CHD, PN should be used on time [22].
Indications and Relative Contraindications [31]
(1) Indications: Firstly, EN is preferred, and if the amount of EN is insufficient, PN should be supplemented promptly in patients with CHD. Specifically, for neonates who receive less than 70% of the required EN for 3 consecutive days, and for children who receive less than 50% of the required EN for 3 consecutive days, timely supplementation with PN is recommended [22].
(2) Relative Contraindications: 1) Severe disturbances of water-electrolyte and acid-base balance; 2) Patients with hepatic and renal failure, and coagulation dysfunction.
(1) Central venous catheter (CVC);
(2) Peripherally inserted central catheter (PICC);
(3) Peripheral venous catheter (PVC).
The route of PN support should be determined by the number of days of infusion and the osmotic pressure of the nutrient solution formulation. For PN support exceeding 2 weeks, the use of a PICC is recommended. For PN support lasting 7 to 14 days, the use of a CVC or PICC is advised. For PN support less than 7 days, the use of a PVC or CVC is recommended. When the osmolality of the nutrient solution formulation exceeds 900 mosm/L, the CVC or PICC route is recommended [32].
(1) Amino acids: PN formulation for children requires more essential amino acids, and it is recommended to use pediatric-specific amino acids for children under 3 years old [21]. The recommended amount of amino acids for neonates: it can be started within 24 h after birth (except for those with renal insufficiency), and the starting dosage is 1.5~2.0 g/(kg.d), which can be increased to 3 g/(kg.d) for full-term infants and 3.5 g/(kg.d) for preterm infants.
(2) Fat emulsions: Children are advised to use novel fat emulsions that are not solely derived from soybean oil [33]. The recommended dosage of fat emulsion is less than 3 g/(kg.d) for children and less than 4 g/(kg.d) for neonates. Administration can begin within the first 24 h after birth. For preterm infants, start with 1.0 g/(kg.d) on the first day, and increase to up to 3 g/(kg.d) from the second to the seventh day. During rapid growth phases, the total amount should not exceed 4 g/(kg.d). During PN, blood triglyceride concentrations should be routinely monitored and the dose should be reduced if they exceed 227 mg/dL in infants or 400 mg/dL in older children. In cases of liver function abnormalities, the use of fat emulsions should be stopped or reduced. Fat emulsions should be used with caution in cases of total bilirubin >170 μmol/L (10 mg/dL), thrombocytopenia, respiratory failure, severe infections, and bleeding tendencies.
(3) Glucose: Stress hyperglycemia may occur during the postoperative critical period, and if the blood glucose is higher than 180 mg/dL, a “glycemic control strategy” should be adopted, including the application of insulin [22]. During PN, blood glucose levels should be maintained below 8.33 mmol/L. If levels exceed this threshold, the infusion rate should be gradually decreased. If hyperglycemia persists with an infusion rate of ≤4 mg/(kg.min), insulin at 0.05 IU/kg/d can be administered. Clinically, insulin dosages may be adjusted according to the individual patient’s response to treatment [34]. Recommended glucose dosage for neonates: initial glucose rate at 2.5~5 mg/(kg.min) and increase gradually at 1~2 mg/(kg.min), with the maximum dose not exceeding 12 mg/(kg.min). The glucose requirements for infants and young children with PN are shown in Table 10.
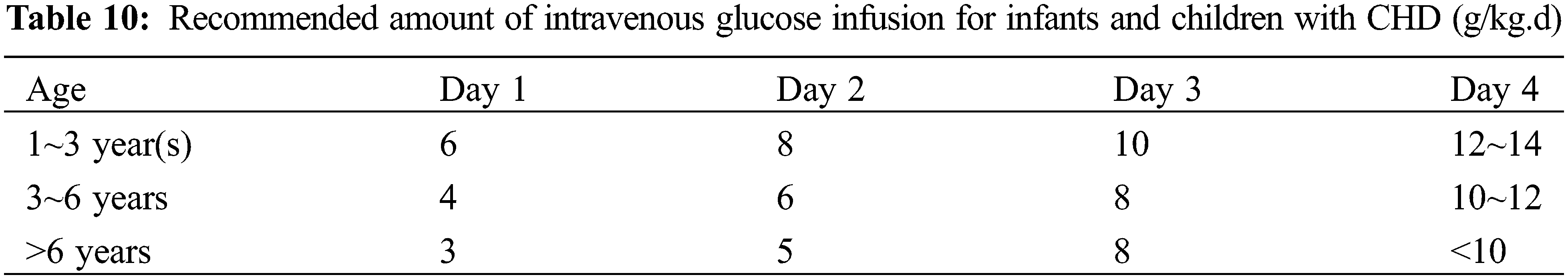
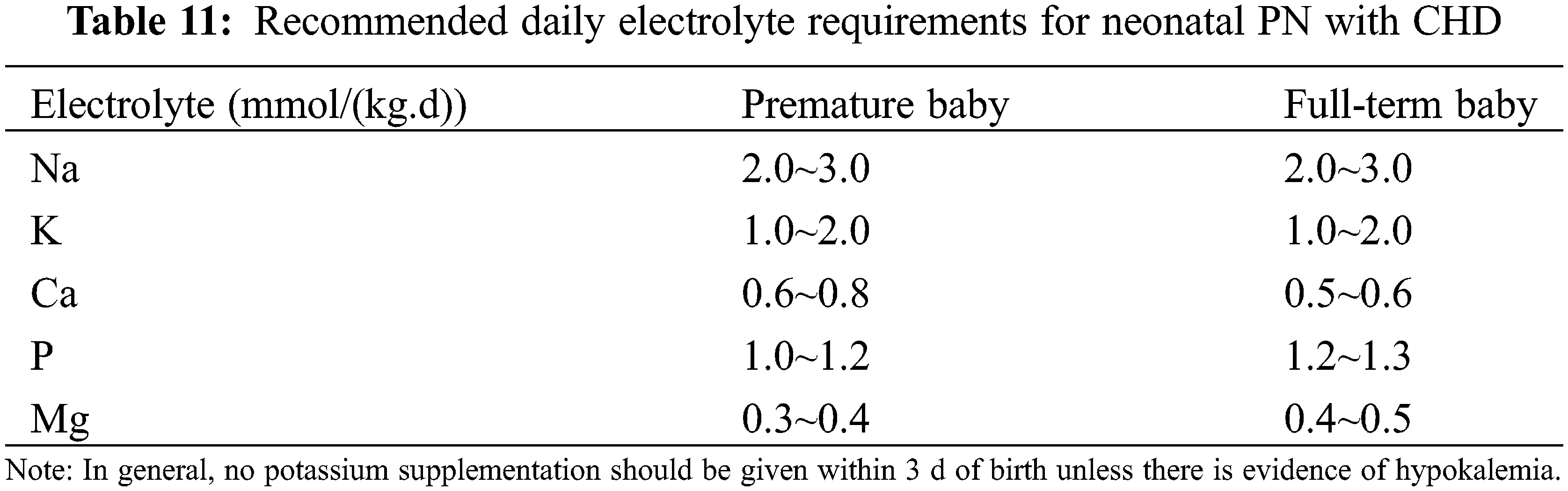
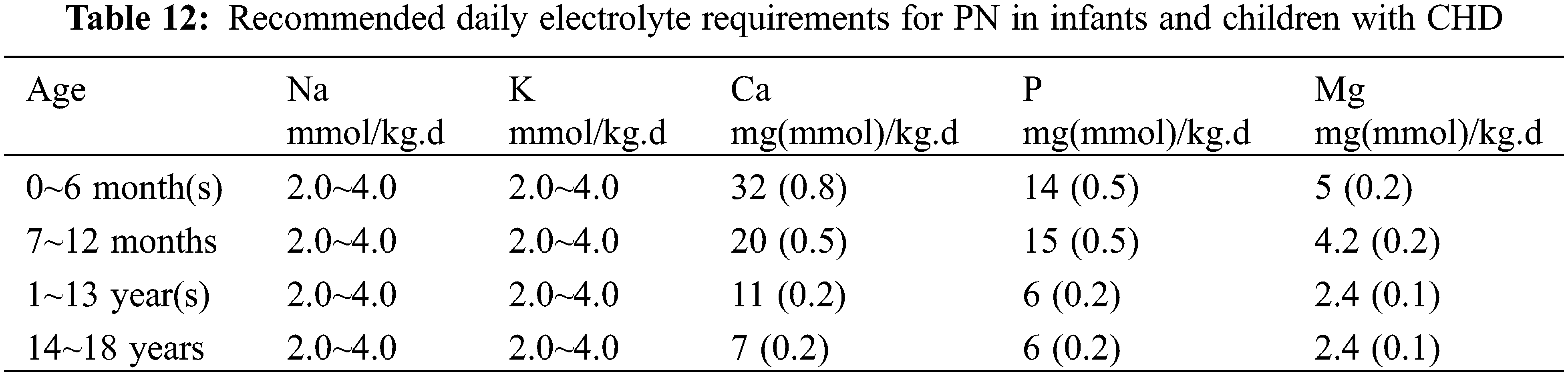
(4) Others: The recommended amount of electrolytes for neonates is shown in Table 11 and for infants and young children in Table 12. It is recommended to use water or fat-soluble vitamins and trace elements specific for children [35].
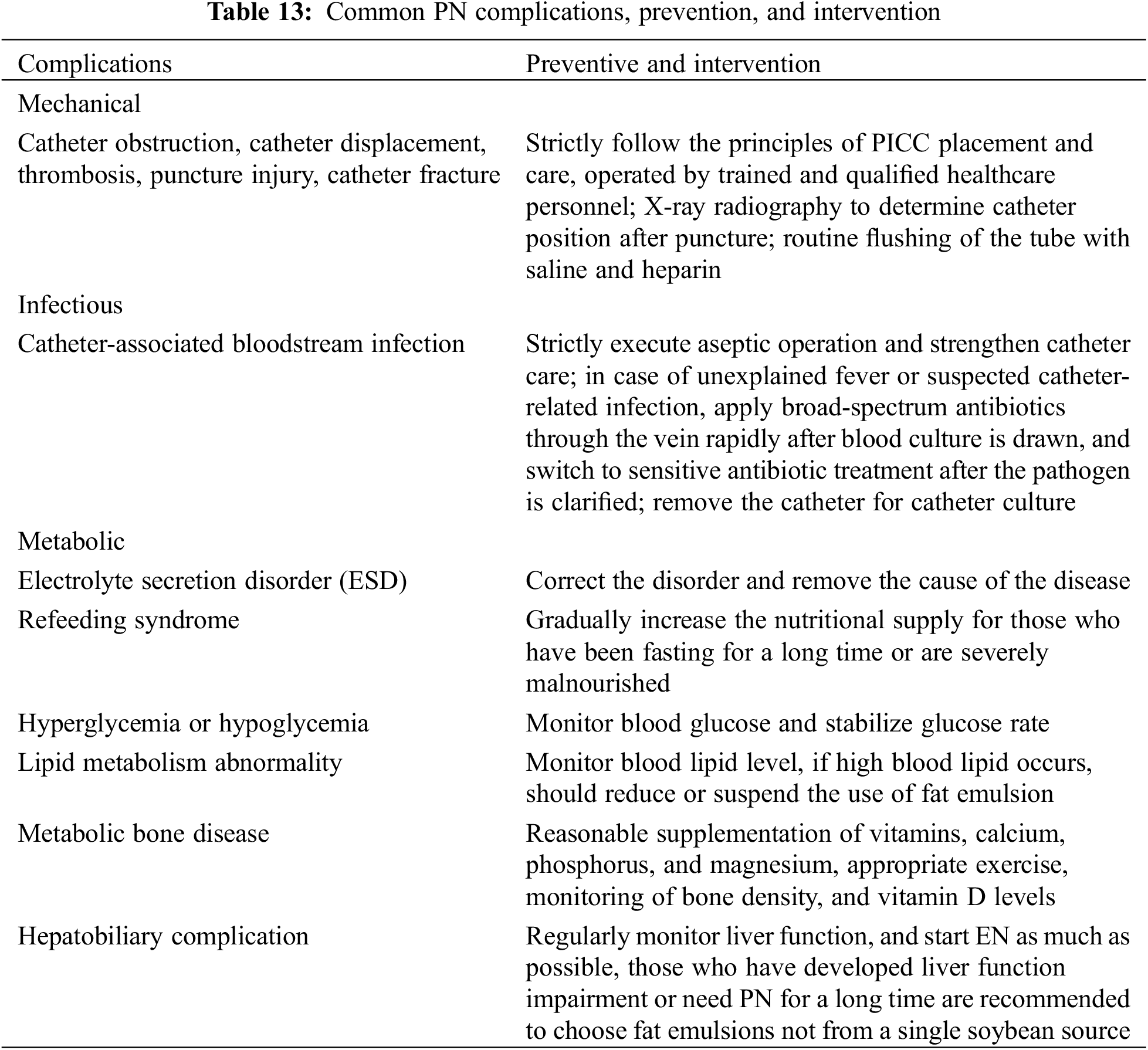
3.3.3 PN Complications, Prevention, and Intervention
Complications of PN support in CHD include catheter-related, infectious complications, metabolic complications, and organ dysfunction as shown in Table 13 [36].
3.3.4 PN Monitoring and Start-Up or Wean-Off Timing
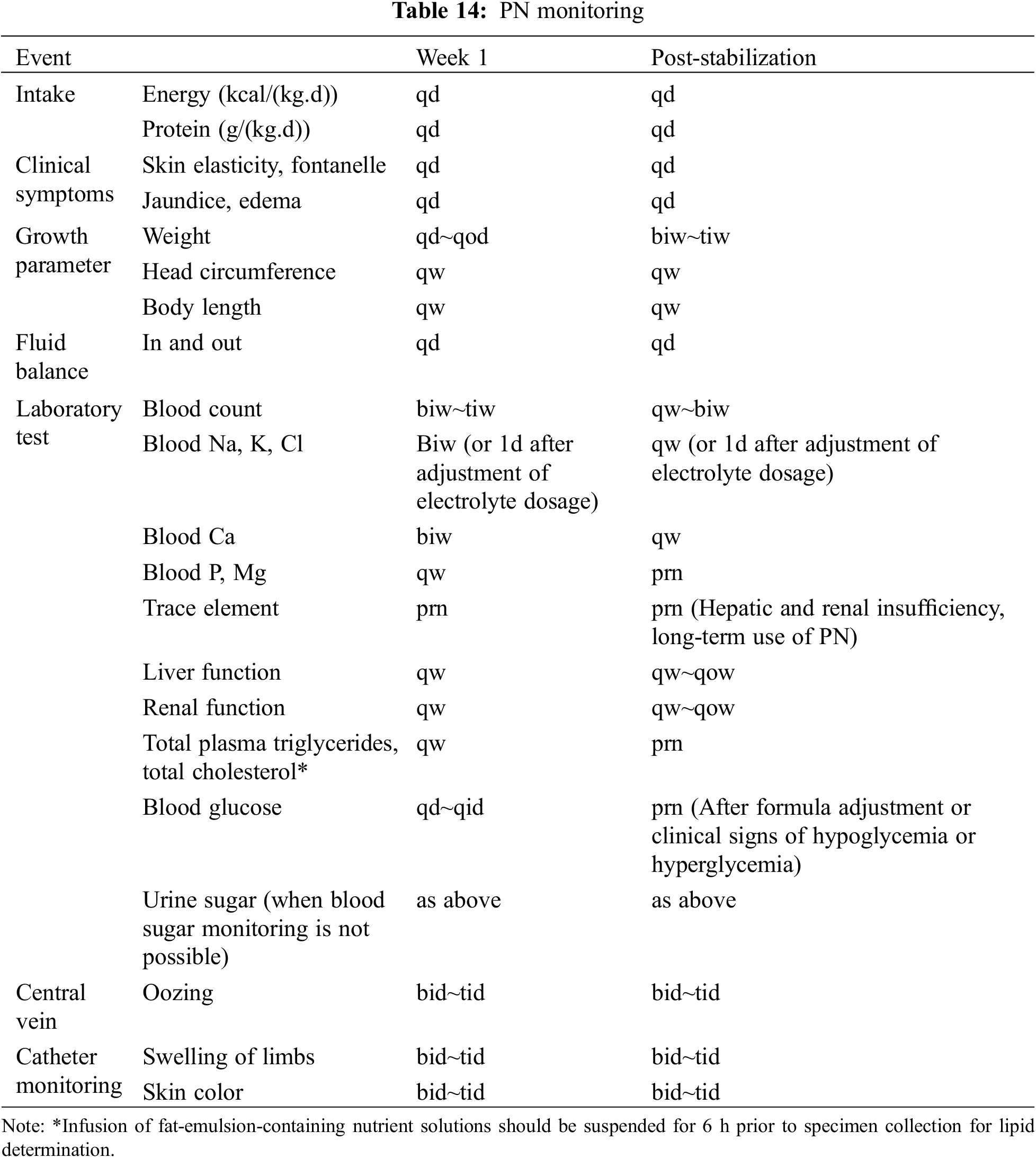
The PN monitoring is shown in Table 14, and the timing of PN on and off is individualized according to the degree of satisfaction with the EN supply [20].
Recommendation 4:
(1) For children with CHD, PN should be supplemented when EN is not feasible or when the EN is insufficient. If neonates receive less than 70% of required EN for 3 consecutive days, or if children receive less than 50% of required EN for 3 consecutive days, or in cases where EN is completely contraindicated, PN is necessary.
(Level of Evidence: B; Strength of Recommendation: Strong).
(2) When PN is required for more than 2 weeks, the use of a PICC is recommended. For PN lasting 7–14 days, the use of a CVC or PICC is advised. For PN support less than 7 days, the use of a PVC or CVC is recommended. If the osmolarity of the nutrition solution exceeds 900 mosm/L, the use of a CVC or PICC is recommended.
(Level of Evidence: A; Strength of recommendation: Strong).
(3) For infants and young children under 3 years of age, PN formulations should include pediatric amino acids solution, water-soluble or fat-soluble vitamins, and trace elements. Routine monitoring of serum triglycerides is recommended during fat emulsion infusion.
(Level of Evidence: B; Strength of Recommendation: Weak).
(4) Mechanical, infectious, and metabolic complications must be monitored during PN. Attention should be given to the possibility of stress-induced hyperglycemia in critically ill children during glucose infusion.
(Level of Evidence: B; Strength of Recommendation: Weak).
(5) Monitoring of PN should focus on clinical signs and laboratory indicators, and the initiation and discontinuation of PN should be personalized.
(Level of Evidence: A; Strength of Recommendation: Strong).
3.4 Nutritional Support for Special Conditions
3.4.1 Nutritional Support for Postoperative Chylothorax
In addition to medicine, improving hemodynamics and reducing venous pressure, postoperative chylothorax in CHD requires strict dietary control. It is recommended to consume foods that are either non-fat or low in fat. For formula milk, varieties rich in medium-chain fatty acids are advised. Defatted Hippuric Milk (DHM) is a feasible and safe way of providing breast milk for newborn infants. Depending on the patient’s condition, supplementation with albumin and high-glucose fluids is suggested to reduce fat consumption [37,38]. Ensuring adequate electrolyte and vitamin supplementation is crucial for maintaining a balanced nutrient supply. In the case of postoperative chylothorax, when the chyle output is ≤20 ml/kg/day, high medium-chain triglycerides (MCT) and low long-chain triglycerides (LCT) diet should be given for 1 week. If the chyle output remains ≤10 ml/kg/day, then the high MCT and low LCT diet should be continued for 6 weeks, starting from the initial occurrence of chylothorax post-surgery. In situations where postoperative chylothorax output is ≥20 ml/kg/day, total parenteral nutrition (TPN) combined with octreotide treatment should be administered for 7–10 days. If there is no reduction in chyle output, surgical intervention followed by continued total parenteral nutrition combined with octreotide should be carried out for another 7 days.
3.4.2 Nutritional Support in Extracorporeal Life Support (Including ECMO/VAD)
For patients under ECMO/VAD support, it is recommended to measure REE through an indirect calorimeter to provide individualized guidance for energy requirements. If the circumstances do not permit such measurements, refer to the recommended energy levels during the perioperative critical phase of CHD, as detailed in Table 4. During support, the body’s protein requirement increases. Nutritional supply can be estimated at 100~120 kcal/kg-d for calories and 3 g/(kg.d) for protein. Near-infrared gastrointestinal blood flow monitoring can be utilized for gastrointestinal function assessment during ECMO/VAD support. It is essential to initiate EN promptly after achieving hemodynamic stability, adhering to a protocol of gradual increase in volume, acceleration, and concentration. The reasonable goal is to achieve two-thirds of EN nutritional target in the first week of critical illness, with PN supplementing any deficit in EN [21,39]. Gastrointestinal motility is a common complication during nutritional support and should be considered to be assisted by gastrointestinal prokinetic agents. To safeguard the membrane lung, the use of fat emulsions is not recommended. If indeed necessary, it is advised to administer these emulsions through a dedicated intravenous line, ensuring a steady and continuous infusion over a period of 12 to 24 h per day [40].
3.4.3 Impact of Vasoactive Drugs on Nutritional Support
Following CHD surgery, the use of postoperative anesthesia and sedative medications can lead to decreased gastrointestinal motility. If hemodynamic stability is achieved, the dosage of vasoactive medications should be reduced as soon as possible until discontinued [41,42]. Early EN is recommended for children who maintain hemodynamic stability under vasoactive drug support and have no specific gastrointestinal contraindications.
3.4.4 Perioperative Nutritional Support for Specialized CHD
Protein-losing enteropathy (PLE) is one of the most challenging complications encountered in patients with single-ventricle physiology following Fontan palliative care. Nutritional management of PLE should take into account the disorder’s impact on gastrointestinal function, diarrhea, and malabsorption. A high-protein diet (≥2 g/(kg.d)) and a low-fat diet (≤25% of caloric intake) with medium-chain triglyceride supplementation are recommended to minimize the amount of intestinal protein loss and improve nutritional status [43]. Additionally, a low-fiber, low-salt diet should be considered. Adjustments to the gut microbiota and maintenance of electrolyte balance are important, along with supplementation of albumin, immunoglobulins, and fat-soluble vitamins. In cases of severe diarrhea, fasting and provision of parenteral nutrition are advisable to rest the gastrointestinal tract and reduce lymphatic flow, thereby facilitating mucosal repair.
Patients with ductus arteriosus-dependent CHD or CoA/IAA and preterm and newborn infants experience inadequate gastrointestinal perfusion and ischemia, which increases the risk of NEC. Breast milk exerts a protective effect on the infant’s microbiome. During the early stages of administering minimal EN through a micro-pump, the judicious addition of probiotics may help in reducing the risk of NEC [44,45]. Once NEC is diagnosed, EN should be discontinued and replaced with PN. Once abdominal symptoms have subsided, indicated by the absence of vomiting, abdominal distension, and signs of peritonitis such as high abdominal muscle tone, and with at least two consecutive negative occult blood tests, no red blood cells or pus cells present in the stool, and no evidence of pneumatosis intestinalis on an abdominal flat plate X-ray, trophic feeding can be initiated. This involves starting with small, frequent feeds or micro-pump infusion, and gradually transitioning to full EN support. The osmolarity of the EN formula should be carefully controlled to ensure it remains within appropriate limits.
Nutritional Support for Dysphagia
In individuals associated complications like cleft lip and palate anomalies, extended reliance on mechanical ventilation, damage to the recurrent laryngeal nerve post-surgery, and dysfunctional epiglottis can cause coughing, dysphagia, and elevated feeding risks. Regular evaluations of the vocal cords, employing nasogastric tube feeding, or using specialized nipples can offer significant benefits to these patients. The involvement of an MDT, encompassing rehabilitation specialists and nursing staff, is pivotal in providing targeted swallowing rehabilitation and feeding advice, which can effectively improve their nutritional status.
Recommendation 5:
(1) For postoperative chylothorax, strict dietary control is required, with non- or low-fat foods recommended. For formula milk, varieties rich in medium-chain fatty acids are advised. In the case of postoperative chylothorax, when the chyle output is ≤20 ml/kg/day, high medium-chain triglycerides (MCT) and low long-chain triglycerides (LCT) diet should be given for 1 week. If the chyle output remains ≤10 ml/kg/day, then the high MCT and low LCT diet should be continued for 6 weeks, starting from the initial occurrence of chylothorax post-surgery. In situations where postoperative chylothorax output is ≥20 ml/kg/day, TPN combined with octreotide treatment should be administered for 7–10 days. If there is no reduction in chyle output, surgical intervention followed by continued total parenteral nutrition combined with octreotide should be carried out for another 7 days.
(Level of Evidence: B; Strength of Recommendation: Strong).
(2) Patients with ECMO/VAD support refer to the recommended energy levels during the perioperative critical phase of CHD. During support, protein supply can be estimated at 3 g/(kg.d) Once hemodynamic stability is achieved, early initiation of EN should be prioritized. The reasonable goal is to achieve two-thirds of EN’s nutritional target in the first week of critical illness, with PN supplementing any deficit in EN.
(Level of Evidence: B; Strength of Recommendation: Weak).
(3) When patients are combined with NEC, enteral nutrition EN should be discontinued and replaced with PN. Once abdominal symptoms have subsided, indicated by the absence of vomiting, abdominal distension, and signs of peritonitis such as high abdominal muscle tone, and with at least two consecutive negative occult blood tests, no red blood cells or pus cells present in the stool, and no evidence of pneumatosis intestinalis on an abdominal flat plate X-ray, trophic feeding can be initiated. This involves starting with small, frequent feeds or micro-pump infusion, and gradually transitioning to full EN. The osmolarity of the EN formula should be carefully controlled to ensure it remains within appropriate limits.
(Level of Evidence: B; Strength of Recommendation: Weak).
(4) For children who have dysphagia, routine vocal cord evaluation and nasogastric tube feeding or special pacifier feeding are recommended. The involvement of an MDT, encompassing rehabilitation specialists and nursing staff, is pivotal in providing targeted swallowing rehabilitation and feeding advice, which can effectively improve their nutritional status.
(Level of Evidence: B; Strength of Recommendation: Weak).
3.5 Nutritional Management of CHD throughout the Lifecycle
Nutritional support is one of the most important aspects of the lifecycle management of CHD, which covers the continuous management from birth, infancy, adolescence to adulthood. In addition to the perioperative nutritional management, which is of high concern, attention should also be paid to preoperative nutritional management, post-discharge nutritional management, developmental period of adolescents aged 12~18 years old, and adult nutritional management, thus forming a nutritional support and management covering the whole life cycle.
3.5.1 Preoperative Nutritional Management
Children with preoperative CHD face nutritional risks after birth. The ESPEN Clinical Nutritional Guidelines for Surgery recommends that children with moderate or severe malnutrition should be given preoperative nutritional support for a certain period in non-emergency situations [46]. The duration of preoperative nutritional support should be determined based on the severity and urgency of the condition. Preoperative nutritional support should primarily consist of EN. For children with heart failure, a combination of oral feeding and fine nasal-gastric tube feeding can be adopted.
3.5.2 Post-Discharge Nutritional Management
The first 1~6 months after discharge is an important stage for children to catch up with the growth in weight and height. Children who have undergone satisfactory deformity correction and receive standardized nutritional guidance reach the nutritional status of their age-matched peers by 12 months after surgery. The joint outpatient service of cardiology and pediatric health care or clinical nutrition departments is crucial for guiding and managing post-discharge nutritional support.
3.5.3 Nutritional Management in Adolescence and Adulthood
The adolescent stage, from 12 to 18 years old, is the most optimal time for relay management from childhood to adulthood. During this period, patients should maintain appropriate exercise and adequate nutritional support, especially the supply of high-quality protein foods. For patients who continue to experience malnutrition and have undergone multiple surgeries, it is critical to conduct thorough nutritional assessments and provide comprehensive nutritional support and management through an MDT approach. As they enter adulthood, patients should maintain a healthy lifestyle, avoiding overeating and excessive obesity to prevent additional strain on the heart.
Recommendation 6:
Nutritional management of the whole life cycle of CHD covers preoperative nutritional management, perioperative nutritional management, adolescence, and adulthood. The first 12 months post-discharge is a critical period for catch-up growth in children who have had satisfactory deformity correction. It is recommended to carry out a joint outpatient service involving the cardiology department and the pediatric health care department or the clinical nutrition department.
(Level of Evidence: B; Strength of Recommendation: Strong).
This consensus refers to the latest evidence-based methodology of nutritional screening, assessment and intervention to produce a new clinical expert consensus on nutritional support for CHD, which is scientifically effective and suitable for China. Moreover, it helps children to recover and catch up with their growth and development, and the majority of the recommendations have received widespread agreement. Further large-sample, multi-center studies are needed to obtain more evidence-based methodology, while more consensuses should be reached to develop and promote the nutritional support guidelines for children with CHD.
Acknowledgement: We acknowledge all the experts of the Cardiac Surgery Group of Pediatric Surgery Society of Chinese Medical Association and Parenteral Enteral Nutrition Society of Chinese Medical Association for their contributions and vote in this consensus. We are grateful to Chongfan Zhang from Children’s Hospital of Fudan University, Fudan University GRADE Center for his advice on this consensus.
Funding Statement: This consensus was supported by the National Natural Science Foundation of China (81970265, 82270310); a Sub-Project of the National Key R&D Program “The recognition and Identification of Genetic Pathogenic Genes for Structural Birth Defects” (2021YFC2701002); Nanjing Science and Technology Development Project (2019060007); Jiangsu Provincial Key Research and Development Program (BE2023662).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: XM, WC; data collection: XM, WC, JQ, ZX, YW and WY; analysis and interpretation of results: XM, WC, JQ, ZX, YW and WY; draft manuscript preparation: XM, WC, JQ, ZX, YW, WY, SL, ND, XC, JL, QS, JC, HZ, HZ, QX, QA, XL, XW, YH, JS, TF, TM, WT, LH, JZ, MY, GS, YD, LT, YY, ZW, HC, QW, KY, LZ, PW, YC, BZ, YZ, QT, RC, CW, ZF, CL, YM, RZ, KL, XL, MP, XF, SS, PH, ZP, JQ, RC, ST, YS, HZ, LJ, MD, NP and LH. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data is available via the corresponding author’s email address attached.
Ethics Approval: Not applicable for this consensus.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Boyd, R., Johnson, A., Smith, C., Williams, D. (2022). Environmental exposures and congenital heart disease. Pediatrics, 149(1), 1098–1104. [Google Scholar]
2. Pan, F. (2022). China’s children’s development outline (2021–2030Setting clear goals for promoting the healthy growth of children in China. Women’s and Children’s Health Guide, 4, 9–11 (In Chinese). [Google Scholar]
3. Diao, J., Chen, L., Wei, J., Shu, J., Li, Y. et al. (2022). Prevalence of malnutrition in children with congenital heart disease: A systematic review and meta-analysis. Journal of Pediatrics, 242, 39–47.e4 [Google Scholar] [PubMed]
4. Capra, M. E., Pederiva, C., Viggiano, C., de Santis, R., Banderali, G. et al. (2021). Nutritional approach to prevention and treatment of cardiovascular disease in childhood. Nutrients, 13(7), 2359 [Google Scholar] [PubMed]
5. Chinese Medical Association of Cardiac Surgery Group of Pediatric Surgery Society, Parenteral Enteral Nutrition Society (2016). Expert consensus on nutritional support for children with congenital heart disease. Chinese Journal of Pediatric Surgery, 37(1), 3–8 (In Chinese). [Google Scholar]
6. Shan, S. S., Wang, J., Liu, Q. (2020). Basic concepts and norms of clinical guidelines and expert consensus. Chinese Journal of Pediatric Surgery, 41(2), 5 (In Chinese). [Google Scholar]
7. Zhang, M., Wang, L., Huang, R., Sun, C., Bao, N. et al. (2020). Risk factors of malnutrition in Chinese children with congenital heart defect. BMC Pediatrics, 20(1), 213 [Google Scholar] [PubMed]
8. Liu, J., Shi, H., Bai, S., Wu, Y. (2021). Nutritional assessment and nutritional intervention effect evaluation in children with congenital heart disease. Journal of Tianjin Medical University, 27(3), 4 (In Chinese). [Google Scholar]
9. Zhang, Y., Lu, L., Yang, L., Yan, W., Yu, Q. et al. (2023). Evaluation of a new digital pediatric malnutrition risk screening tool for hospitalized children with congenital heart disease. BMC Pediatrics, 23(1), 126 [Google Scholar] [PubMed]
10. Black, R. E., Allen, L. H., Bhutta, Z. A., Caulfield, L. E., de Onis, M. et al. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608), 243–260. [Google Scholar]
11. Liu, J., Yan, Y., Xi, B., Huang, G., Mi, J. and China Child and Adolescent Cardiovascular Health (CCACH) Study Group. (2019). Skeletal muscle reference for Chinese children and adolescents. Journal of Cachexia, Sarcopenia and Muscle, 10(1), 155–164 [Google Scholar] [PubMed]
12. Bang, Y. K., Park, M. K., Ju, Y. S., Cho, K. Y. (2018). Clinical significance of nutritional risk screening tool for hospitalized children with acute burn injuries: A cross-sectional study. Journal of Human Nutrition and Dietetics, 31(3), 370–378 [Google Scholar] [PubMed]
13. Pars, H., Açıkgöz, A., Erdoğan, B. D. (2020). Validity and reliability of the Turkish version of three screening tools (PYMS, STAMP, and STRONG-kids) in hospitalized children. Clinical Nutrition ESPEN, 39, 96–103 [Google Scholar] [PubMed]
14. Steeds, R., Sagar, V., Shetty, S., Oelofse, T., Singh, H. et al. (2019). Multidisciplinary team management of carcinoid heart disease. Endocrine Connections, 8(12), R184–R199 [Google Scholar] [PubMed]
15. Tume, L. N., Balmaks, R., da Cruz, E., Latten, L., Verbruggen, S. et al. (2018). Enteral feeding practices in infants with congenital heart disease across European PICUs: A European society of pediatric and neonatal intensive care survey. Pediatric Critical Care Medicine, 19(2), 137–144 [Google Scholar] [PubMed]
16. Singal, A., Sahu, M. K., Trilok Kumar, G., Kumar, A. (2022). Effect of energy- and/or protein-dense enteral feeding on postoperative outcomes of infant surgical patients with congenital cardiac disease: A systematic review and meta-analysis. Nutrition in Clinical Practice, 37(3), 555–566 [Google Scholar] [PubMed]
17. Marino, L. V., Johnson, M. J., Davies, N. J., Kidd, C. S., Fienberg, J. et al. (2020). Improving growth of infants with congenital heart disease using a consensus-based nutritional pathway. Clinical Nutrition, 39(8), 2455–2462 [Google Scholar] [PubMed]
18. Mehta, N. M., Skillman, H. E., Irving, S. Y., Coss-Bu, J. A., Vermilyea, S. et al. (2017). Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of critical care medicine and American society for parenteral and enteral nutrition. Pediatric Critical Care Medicine, 18(7), 675–715 [Google Scholar] [PubMed]
19. Roebuck, N., Fan, C. S., Floh, A., Harris, Z. L., Mazwi, M. L. (2020). A comparative analysis of equations to estimate patient energy requirements following cardiopulmonary bypass for correction of congenital heart disease. Journal of Parenteral and Enteral Nutrition, 44(3), 444–453 [Google Scholar] [PubMed]
20. Mills, K. I., Kim, J. H., Fogg, K., Goldshtrom, N., Graham, E. M. et al. (2022). Nutritional considerations for the neonate with congenital heart disease. Pediatrics, 150(Suppl 2), e2022056415G [Google Scholar] [PubMed]
21. Tume, L. N., Valla, F. V., Joosten, K., Jotterand Chaparro, C., Latten, L. et al. (2020). Nutritional support for children during critical illness: European society of pediatric and neonatal intensive care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Medicine, 46(3), 411–425 [Google Scholar] [PubMed]
22. Luca, A. C., Miron, I. C., Mîndru, D. E., Curpăn, A. Ş., Stan, R. C. et al. (2022). Optimal nutrition parameters for neonates and infants with congenital heart disease. Nutrients, 14(8), 1671 [Google Scholar] [PubMed]
23. Martini, S., Beghetti, I., Annunziata, M., Aceti, A., Galletti, S. et al. (2021). Enteral nutrition in term infants with congenital heart disease: Knowledge gaps and future directions to improve clinical practice. Nutrients, 13(3), 932 [Google Scholar] [PubMed]
24. del Castillo, S. L., McCulley, M. E., Khemani, R. G., Jeffries, H. E., Thomas, D. W. et al. (2010). Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatric Critical Care Medicine, 11(3), 373–377 [Google Scholar] [PubMed]
25. O’Neal Maynord, P., Johnson, M., Xu, M., Slaughter, J. C., Killen, S. A. S. (2021). A multi-interventional nutrition program for newborns with congenital heart disease. Journal of Pediatrics, 228, 66–73.e2 [Google Scholar] [PubMed]
26. Tsintoni, A., Dimitriou, G., Karatza, A. A. (2020). Nutrition of neonates with congenital heart disease: Existing evidence, conflicts and concerns. Journal of Maternal-Fetal & Neonatal Medicine, 33(14), 2487–2492. [Google Scholar]
27. Davis, J. A., Spatz, D. L. (2019). Human milk and infants with congenital heart disease: A summary of current literature supporting the provision of human milk and breastfeeding. Advances in Neonatal Care, 19(3), 212–218 [Google Scholar] [PubMed]
28. Pan, H., Li, H., Chen, X., Ren, Y. (2021). Effect of colostrum oral immunization care on growth and development of infants with very low birth weight. Journal of Clinical Medicine Practice, 25(15), 89–92+97 (In Chinese). [Google Scholar]
29. Pillai, A., Albersheim, S., Matheson, J., Lalari, V., Wei, S. et al. (2018). Evaluation of a concentrated preterm formula as a liquid human milk fortifier in preterm babies at increased risk of feed intolerance. Nutrients, 10(10), 1433 [Google Scholar] [PubMed]
30. Qi, J., Li, Z., Cun, Y., Li, X. (2017). Causes of interruptions in postoperative enteral nutrition in children with congenital heart disease. Asia Pacific Journal of Clinical Nutrition, 26(3), 402–405 [Google Scholar] [PubMed]
31. Marino, L. V., Johnson, M. J., Hall, N. J., Davies, N. J., Kidd, C. S. et al. (2018). The development of a consensus-based nutritional pathway for infants with CHD before surgery using a modified Delphi process. Cardiology in the Young, 28(7), 938–948 [Google Scholar] [PubMed]
32. Kolaček, S., Puntis, J. W. L., Hojsak, I., ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition (2018). ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Venous access. Clinical Nutrition, 37, 2379–2391 [Google Scholar] [PubMed]
33. Ishihara, T., Yoshida, M., Arita, M. (2019). Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. International Immunology, 31(9), 559–567 [Google Scholar] [PubMed]
34. Chinese Medical Association of Pediatrics Society of Parenteral Enteral Nutrition Branch, Pediatrics Society of Neonatology Group, Pediatric Surgery Society of Neonatal Surgery Group, Cai, W., Tang, Q. et al. (2013). Clinical application guide of neonatal nutrition support in China. Chinese Journal of Pediatric Surgery, 34(10), 782–787 (In Chinese). [Google Scholar]
35. Mihatsch, W., Fewtrell, M., Goulet, O., Molgaard, C., Picaud, J. C. et al. (2018). ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Calcium, phosphorus and magnesium. Clinical Nutrition, 37(6), 2360–2365 [Google Scholar] [PubMed]
36. Berlana, D. (2022). Parenteral nutrition overview. Nutrients, 14(21), 4480 [Google Scholar] [PubMed]
37. Neumann, L., Springer, T., Nieschke, K., Kostelka, M., Dähnert, I. (2020). ChyloBEST: Chylothorax in infants and nutrition with low-fat breast milk. Pediatric Cardiology, 41(1), 108–113 [Google Scholar] [PubMed]
38. Buckley, J. R., Graham, E. M., Gaies, M., Alten, J. A., Cooper, D. S. et al. (2017). Clinical epidemiology and center variation in chylothorax rates after cardiac surgery in children: A report from the pediatric cardiac critical care consortium. Cardiology in the Young, 27(9), 1678–1685. [Google Scholar]
39. Lee, A. E., Munoz, E., Al Dabbous, T., Harris, E., O’Callaghan, M. et al. (2022). Extracorporeal life support organization guidelines for the provision and assessment of nutritional support in the neonatal and pediatric ECMO patient. ASAIO Journal, 68(7), 875–880 [Google Scholar] [PubMed]
40. Farías, M. M., Olivos, C., Díaz, R. (2015). Nutritional implications for the patient undergoing extracorporeal membrane oxygenation. Nutricion Hospitalaria, 31(6), 2346–2351. [Google Scholar]
41. López-Herce, J., Santiago, M. J., Sánchez, C., Mencía, S., Carrillo, A. et al. (2008). Risk factors for gastrointestinal complications in critically ill children with transpyloric enteral nutrition. European Journal of Clinical Nutrition, 62(3), 395–400. [Google Scholar]
42. Khilnani, P., Rawal, N., Singha, C. (2020). Gastrointestinal issues in critically ill children. Indian Journal of Critical Care Medicine, 24(Suppl 4), S201–S204 [Google Scholar] [PubMed]
43. Al Balushi, A., Mackie, A. S. (2019). Protein-losing enteropathy following fontan palliation. Canadian Journal of Cardiology, 35(12), 1857–1860 [Google Scholar] [PubMed]
44. Kocjancic, L., Bührer, C., Berger, F., Boos, V. (2020). Effect of a dual-strain probiotic on necrotizing enterocolitis in neonates with ductal-dependent congenital heart disease: A retrospective cohort study. Neonatology, 117(5), 569–576 [Google Scholar] [PubMed]
45. Cognata, A., Kataria-Hale, J., Griffiths, P., Maskatia, S., Rios, D. et al. (2019). Human milk use in the preoperative period is associated with a lower risk for necrotizing enterocolitis in neonates with complex congenital heart disease. Journal of Pediatrics, 215, 11–16.e2 [Google Scholar] [PubMed]
46. Weimann, A., Braga, M., Carli, F., Higashiguchi, T., Hübner, M. et al. (2017). ESPEN guideline: Clinical nutrition in surgery. Clinical Nutrition, 36(3), 623–650 [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools