 Open Access
Open Access
ARTICLE
Dynamic Changes in Left and Right Cerebral Oxygen Saturation during Selective Cerebral Perfusion in Young Infants
1 Department of Anesthesiology and Pain Medicine, Laboratory for Cardiovascular Dynamics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, 05505, Korea
2 Department of Physician Education and Training, Asan Medical Center, Seoul, 05505, Korea
* Corresponding Author: Won-Jung Shin. Email:
Congenital Heart Disease 2023, 18(6), 639-647. https://doi.org/10.32604/chd.2023.030065
Received 21 March 2023; Accepted 24 October 2023; Issue published 19 January 2024
Abstract
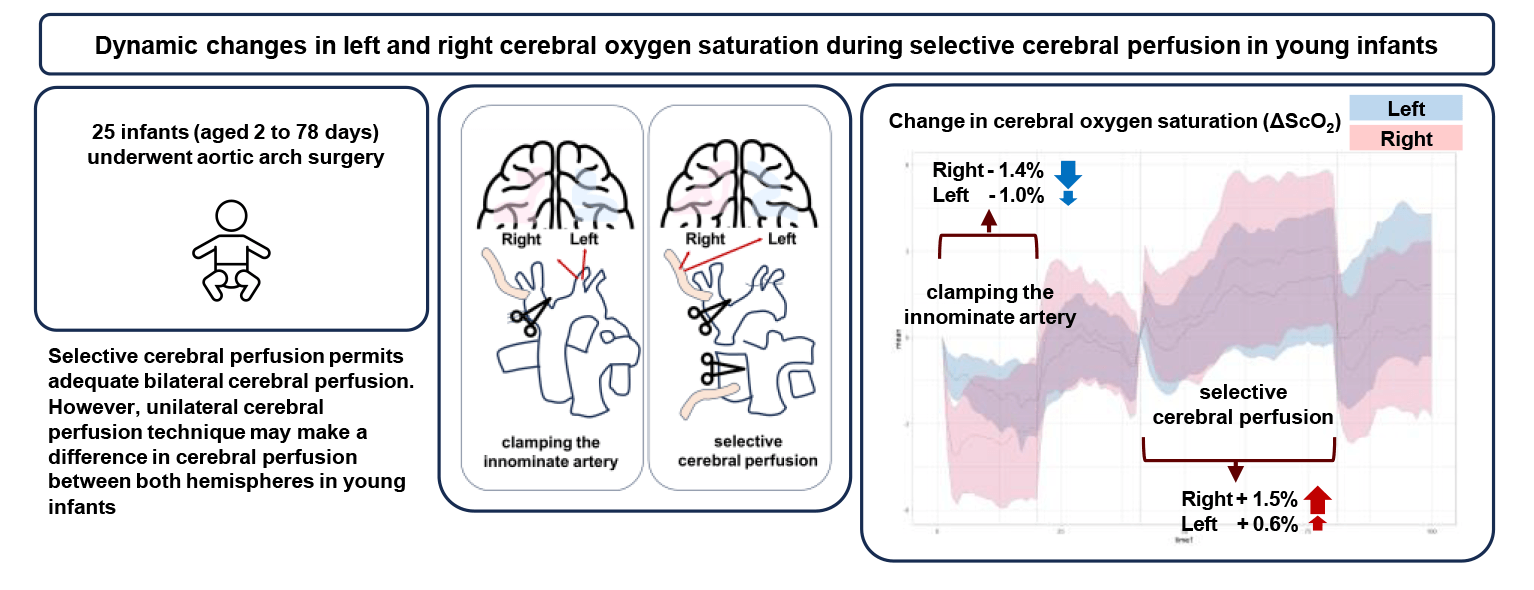
Objectives: We investigated whether the selective cerebral perfusion (SCP) technique causes differences in changes in cerebral perfusion between both hemispheres in young infants, using cerebral oxygen saturation (ScO2) as an index. Further, we determined the association between the discrepancy in ScO2 and cerebral perfusion pressure during SCP. Methods: The difference in ScO2 between the left and right cerebral hemispheres (ΔScO2 Rt-Lt) was calculated during clamping of the innominate artery (IA) and during SCP. Results: In 25 infants (aged 2 to 78 days), the left and right ScO2 were well maintained (median 63.2% and 60.9% during IA clamping, respectively; 64.0% and 65.6% during SCP, respectively). During IA clamping, right and left ScO2 decreased (median −1.4% and −1.0%, respectively). During SCP, right ΔScO2 was higher compared to left ΔScO2 (median 1.5% vs. 0.6%; p < 0.001). Eight patients had a higher right ΔScO2 than left ΔScO2 throughout SCP. They had lower ΔScO2 Rt-Lt during IA clamping (median −3.2% vs. 0.0%; p < 0.001) and higher ΔScO2 Rt-Lt during SCP than others (median 5.0% vs. −0.8%; p < 0.001). During and after SCP, the correlation coefficient between right ΔScO2 and change in the mean arterial pressure was higher in patients with a discrepancy than in others (r = 0.731 vs. r = 0.519; p < 0.001). Conclusions: This study suggests that SCP permits adequate bilateral cerebral perfusion. However, the unilateral cerebral perfusion technique may cause a difference in cerebral perfusion between both hemispheres in young infants; this may depend on the perfusion pressure.Graphic Abstract
Keywords
Selective antegrade cerebral perfusion (SCP) is performed to save the brain from neurological injury during aortic arch surgery requiring cardiopulmonary bypass [1–3]. During interrupted blood flow to the left carotid and vertebral arteries due to clamping of the aortic arch, SCP ensures sustained cerebral perfusion in both hemispheres through the collateral vessels of the brain. Unfortunately, an investigation using magnetic resonance angiography recently revealed that the completion rate of the circle of Willis was only 40% in neonates and infants [4]. Moreover, infants, especially neonates, are more vulnerable to brain hypoxia during aortic arch surgery since they are still in the process of development [5]. Prolonged hypoxic and ischemic injury leads to cellular necrosis, apoptosis, and excitotoxicity, especially in the basal ganglia, brain stem, and sensory cortex of neonates [5]. Fluctuations in cerebral blood flow can worsen neurodevelopmental outcomes, especially during aortic arch repair [6].
Assessment of the adequacy of cerebral perfusion can be investigated during SCP for aortic arch surgery using transcranial doppler, electroencephalography, and near-infrared spectroscopy (NIRS) [7]. There are few studies on whether cerebral perfusion is adequately maintained through unilateral SCP [8,9]. Although SCP is generally believed to be safer than deep hypothermic circulatory arrest, whether unilateral cerebral perfusion results in an even distribution of blood to both hemispheres remains unresolved. In other words, cerebral blood flow to the right side may be more than that to the left side in a perfusion pressure-dependent manner [10].
Therefore, we investigated whether there is a difference between changes in left and right cerebral perfusion based on the SCP technique used during aortic arch surgery in young infants, using continuous monitoring of cerebral oxygen saturation (ScO2) in both hemispheres. In addition, we also determined whether the difference between changes in left and right cerebral oxygen saturation is associated with the perfusion pressure during SCP.
This retrospective study was performed using the electronic medical records and database of patients who underwent aortic arch surgery for conditions including coarctation of aorta (CoA), hypoplastic left heart syndrome (HLHS), and interruption of aortic arch (IAA). All patients underwent arch reconstruction with cardiopulmonary bypass (CPB). Of 150 patients younger than 3 months from February 2017 to March 2022, 125 patients were excluded. 124 either lacked ScO2 data on one side or had incomplete medical records. One patient was excluded due to undergoing deep hyperthermic circulatory arrest. All included patients underwent antegrade SCP during arch reconstruction.
2.2 General Anesthesia and Monitoring
All patients were operated under general anesthesia, maintained by continuous infusion of midazolam 0.1–0.2 mg/kg/hr, remifentanil 0.1–0.2 μg/kg/min, and rocuronium 1 mg/kg/hr. Invasive arterial blood pressure monitoring was performed simultaneously at the radial and femoral arteries, and central venous catheterization was performed for central venous pressure monitoring and the infusion of vasoactive and inotropic agents. All intraoperative variables, including arterial blood pressure, central venous pressure, body temperature, oxygen saturation (SpO2; measured using a pulse oximeter), end-tidal carbon dioxide concentration, and electrocardiographic data, were obtained from a prospectively established database by real-time recording using Vital Recorder [11].
The surgical procedures were performed according to our institutional protocol of pediatric cardiac surgery [12]. For the SCP, a 3.5-mm polytetrafluoroethylene prosthetic graft (WL Gore & Associates, Inc., Flagstaff, AZ) was connected end-to-side to the innominate artery (IA). The right brachiocephalic circulation had to be transiently interrupted because the IA was clamped during the anastomosis. Following this, an 8-Fr arterial cannula was inserted into the IA via the prosthetic graft. Under moderate hypothermia (27°C–28°C), SCP was initiated through the right IA cannulation, at a flow rate of 50–70 ml/kg/min. During arch repair with aorta cross-clamping, only the brain and right arm received perfusion. Depending on the surgeon’s discretion, selective myocardial perfusion was done if necessary. After completion of the arch repair, the full flow rate of the CPB was restored.
2.4 Measurement and Analysis of ScO2
Using near-infrared spectroscopy (O3® Regional Oximeter, Masimo, Irvine, CA), ScO2 was measured in both hemispheres by neonatal sensors placed on the forehead. ScO2 was also recorded bilaterally at time intervals of 2 s simultaneously with other intraoperative variables and stored in the Vital Recorder database. The ScO2 data of each side of the cerebral hemisphere were extracted and averaged at 30-s intervals, and divided into the following four periods: 1) 10 min immediately after the IA was clamped for arterial cannulation 2) 10 min immediately after declamping of the IA 3) 20 min during the SCP for arch reconstruction 4) 10 min after full CPB flow was restored (Fig. 1).
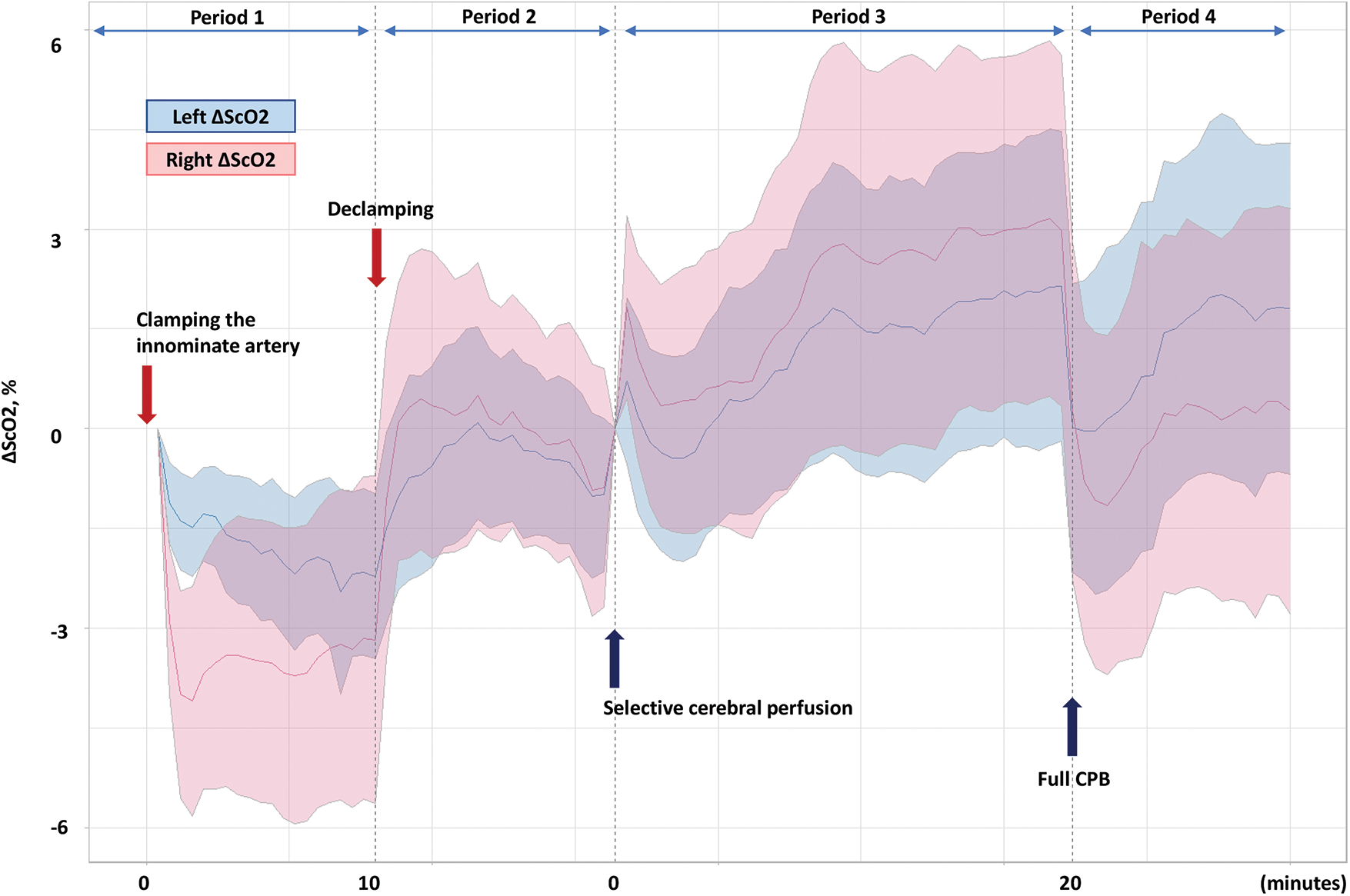
Figure 1: Changes in cerebral oxygen saturation (ΔScO2) of left (light blue) and right (pink) hemispheres. Lines represent median ScO2 values measured throughout each period and shading around the median ScO2 lines indicates 95% confidence intervals
The changes in ScO2 in each hemisphere and mean radial arterial pressure (ΔScO2 and ΔMAP, respectively) were calculated: ΔScO2 (ΔMAP) = the mean ScO2 (MAP) measured throughout each period-the reference value of the ScO2 (MAP) of each period. The reference values of the ScO2 (MAP) were defined as the mean ScO2 (MAP) for 1 min before IA clamping (periods 1 and 2) and 1 min before starting SCP (periods 3 and 4), respectively (Fig. 1). The MAP was measured from the femoral artery during IA clamping and from the radial artery during the SCP.
To identify patients at risk of higher right brain than left brain perfusion, patients were classified into 2 groups: 1) those with a difference in ScO2 between the left and right hemispheres (ΔScO2 Rt-Lt), defined as higher right ΔScO2 than left ΔScO2 throughout the SCP period, and 2) others.
All data are presented as mean (SD, standard deviation), median (IQR, interquartile range), and number (%). Difference between right and left ScO2 parameters were analyzed using Wilcoxon signed rank test. Comparison between the two groups based on whether the patient had a difference in ScO2 between both sides was performed using the Mann-Whitney test. A Linear regression was performed to investigate the relationship between ΔScO2 and ΔMAP. Fisher’s z-transformation test was performed to compare correlation coefficients between patients with and without any ScO2 discrepancy. A p-value below 0.05 was considered statistically significant. R 4.2.0 (http://www.r-project.org) was used for all statistical analysis.
In this study, the data of 25 patients were finally analyzed: 15 had CoA, 7 had HLHS, and 3 had IAA. The median age of HLHS patients (median 30 days [IQR 18–48 days]) was higher compared to those with CoA (median 7 days [IQR 6–9 days]) and IAA (median 6 days [IQR 6–15 days]) (p = 0.011). Among the patients with CoA or IAA, 13 had a concomitant ventricular septal defect (VSD). Of these, 11 underwent VSD closure, while 2 underwent pulmonary artery banding following arch reconstruction. The median duration of aortic cross-clamping was 54 min [IQR 24–64 min].
Before CPB, the median left ScO2 was 64.4% (IQR 58.7%–67.5%), and the median right ScO2 was 64.0% (IQR 60%–67.5%). Throughout the surgical procedures, the absolute values of ScO2 in both sides were within normal range (Table 1). While there was no difference in left ScO2 based on each period of surgery, right ScO2 significantly differed between clamping and declamping of the IA and between SCP and post-SCP periods (p < 0.001 in both cases) (Table 1).
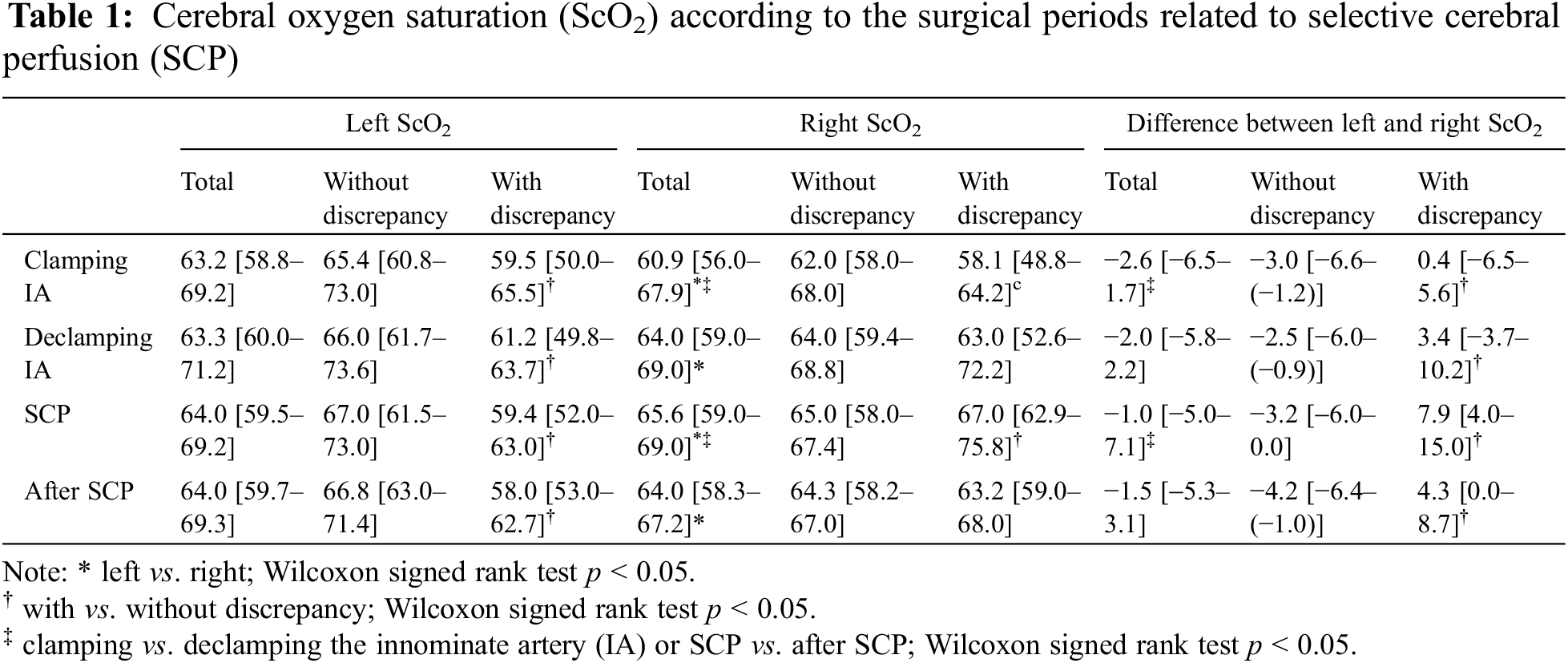
While the IA was clamped for arterial cannulation (median duration of 14 min [IQR 12–16 min]), the decrease in right ΔScO2 was more pronounced than that of left ΔScO2, reaching a median nadir of −4.1% at two min after clamping. Following the declamping of the IA, both left and right ΔScO2 showed transient overshoot and returned to baseline levels. With starting SCP (median duration of 28 min [IQR 22–31 min]), the ΔScO2 in both sides increased; in particular, the right ΔScO2 was higher than the left ΔScO2. After cessation of SCP and full CPB flow, the decrease in right ΔScO2 was greater than that of the left ΔScO2 (Table 2 and Fig. 1).
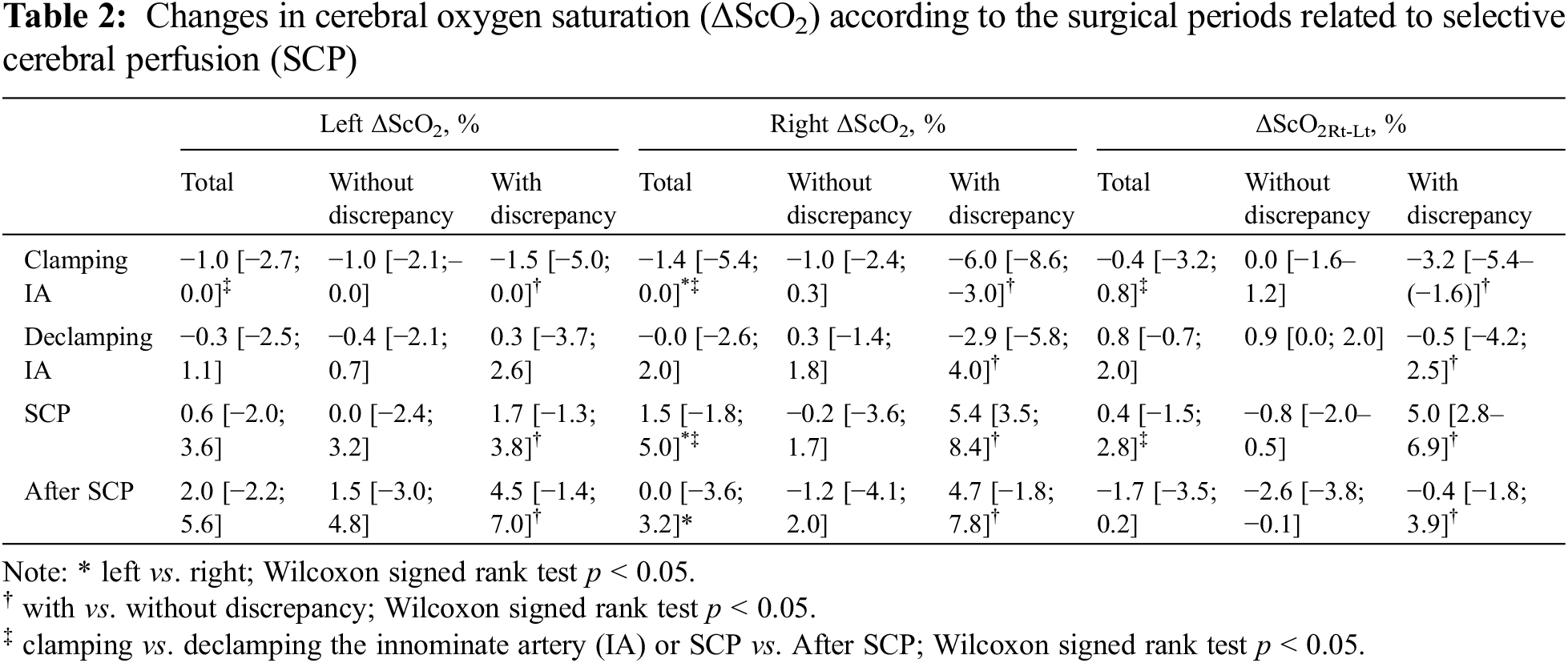
Eight patients consistently showed higher right ΔScO2 than left ΔScO2 throughout SCP (Fig. 2), indicating a discrepancy in ΔScO2 Rt-Lt. ΔScO2 Rt-Lt during SCP was higher in patients with this discrepancy than in others (median 5.0% [IQR 2.8%–6.9 %] vs. −0.8% [−2.0%–0.5%]; p < 0.001).
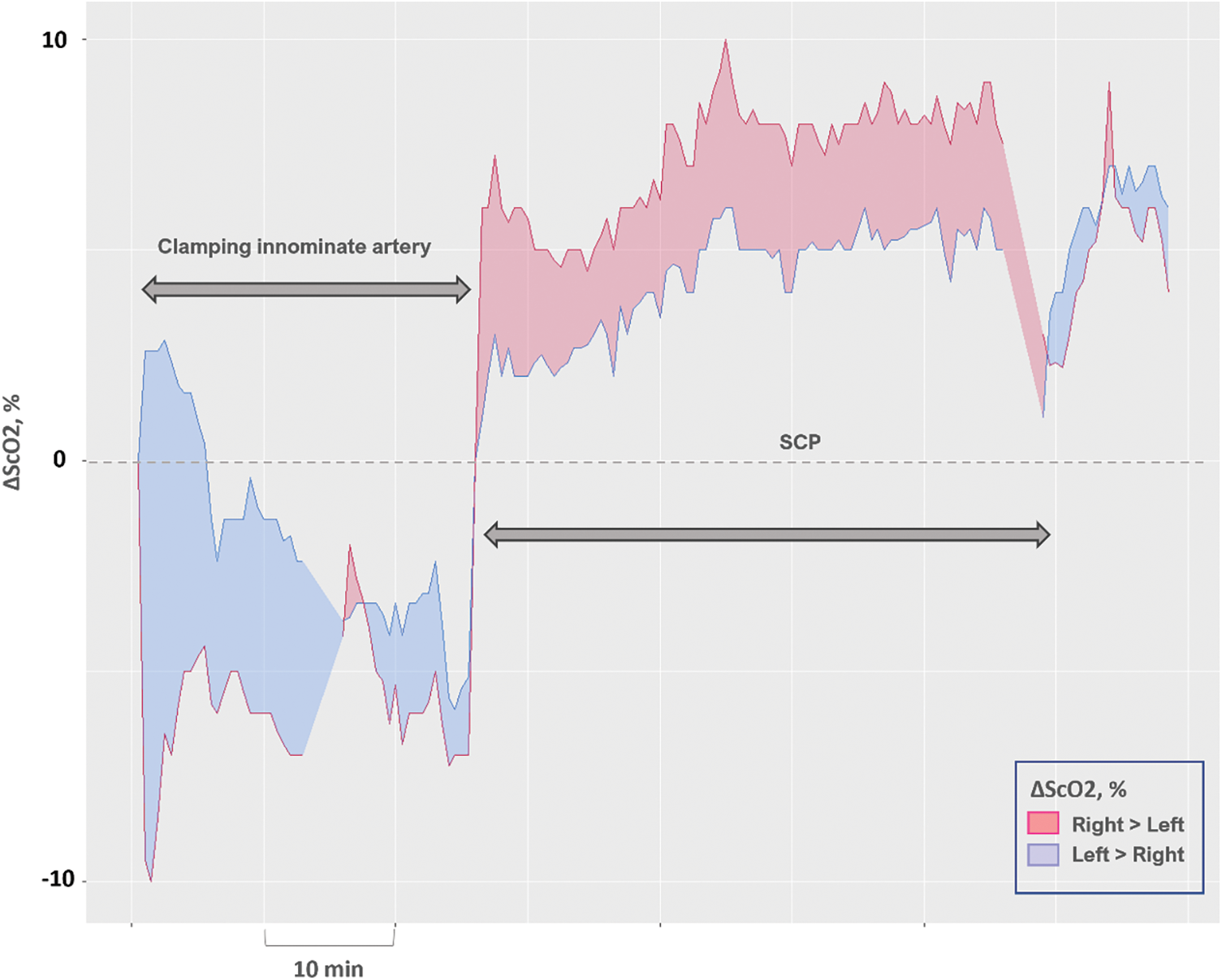
Figure 2: A representative plot tracking the changes in cerebral oxygen saturation in the left and right hemispheres, simultaneously recorded during surgical periods related to selective cerebral perfusion (SCP). This patient has a higher right ΔScO2 than left ΔScO2 throughout the SCP period
Patients with a difference in ΔScO2 had significantly lower right ΔScO2 during IA clamping than others (median −6.0% [IQR −8.6%–−3.0%] vs. −1.0% [−2.4%–0.3%]; p < 0.001). Moreover, patients with a difference in ΔScO2 exhibited a significantly higher correlation coefficient of linear regression between right ΔScO2 and ΔMAP compared to others (r = 0.735 vs. r = 0.510, Fisher’s Z = 6.53; p < 0.001) (Fig. 3).
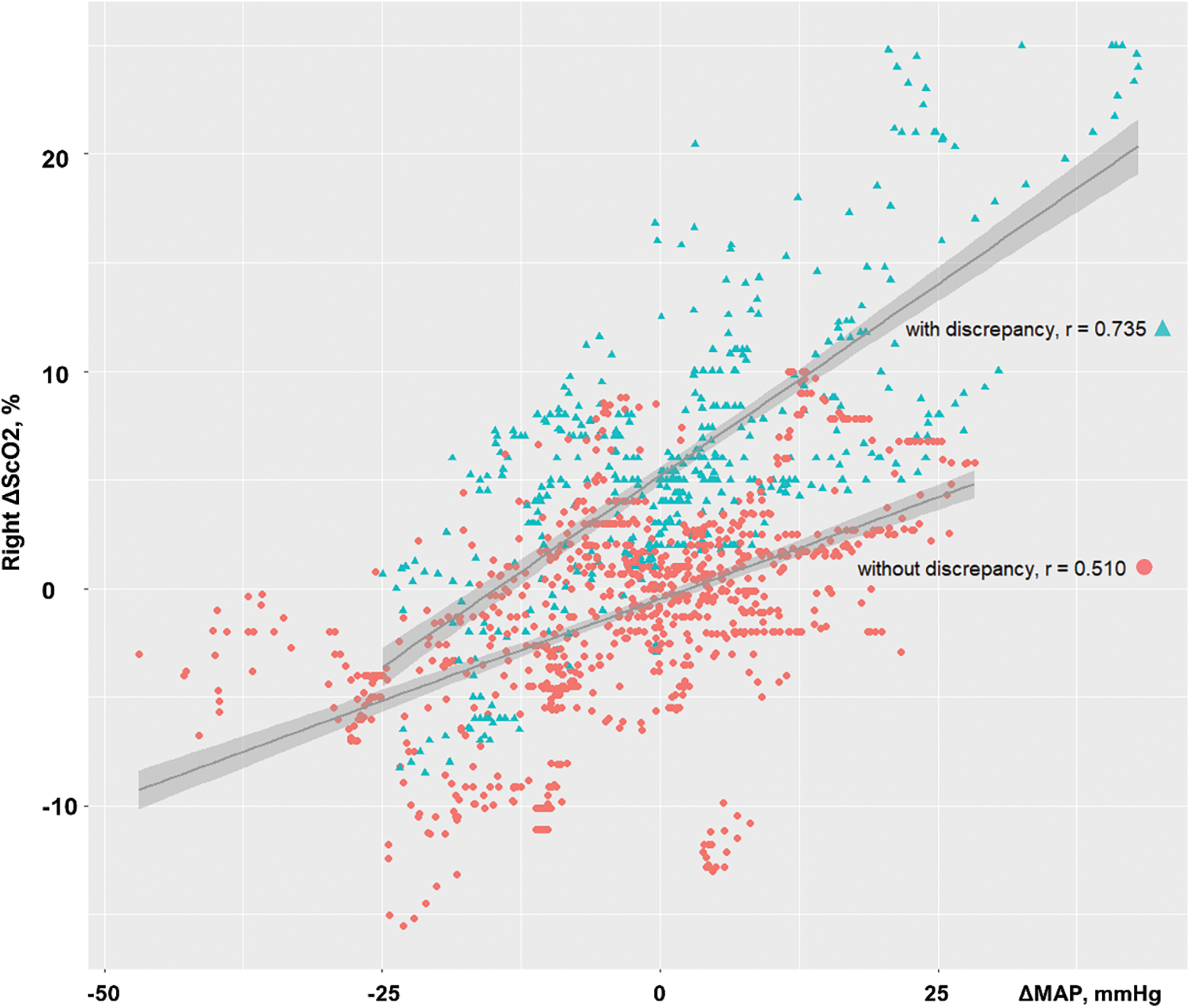
Figure 3: Correlations between changes in cerebral oxygen saturation (ΔScO2) in the right hemisphere and changes in mean arterial pressure (ΔMAP). Patients with a difference in ΔScO2 had a significantly higher correlation coefficient of linear regression than those without discrepancy
This study demonstrates that ScO2 fluctuated during the procedure based on SCP during aortic arch surgery in young infants. Moreover, changes in ScO2 showed the difference between the left and right hemispheres depending on the cessation and resumption of unilateral cerebral perfusion by surgical techniques. 32% of infants exhibited consistently higher right ΔScO2 than left ΔScO2 throughout the SCP period. Interestingly, in these patients with discrepancies in ΔScO2 during SCP, IA clamping for cannulation resulted in a greater decrease in right ScO2 than in left ScO2. We also observed that ΔScO2 in the right hemispheres supplied by direct unilateral SCP, was dependent on the perfusion pressure, especially in patients with discrepancies in ΔScO2.
The SCP is a mainstream procedure during aortic arch surgery in children to improve neurologic morbidity and mortality by ensuring continuous cerebral circulation [1,2,13]. A few studies investigating ScO2 in neonates and young infants undergoing SCP reported that there is no significant difference between left and right ScO2 during SCP, suggesting that SCP can provide efficient cerebral perfusion [9,14]. In this study, ScO2 was also well maintained in both hemispheres throughout the surgery; however, the ScO2 of each side significantly changed depending on the dynamics of the cerebral perfusion technique. During IA clamping for arterial cannulation, a sudden cessation of right cerebral blood flow resulted in decreased ScO2 in the right brain. In contrast, unilateral selective perfusion via the right IA directly increases the right ScO2. Even if sufficient cerebral circulation is achieved by unilateral SCP, we obviously observed that fluctuations in ScO2 differed between the left and right brain.
An imbalance of cerebral circulation between the left and right hemispheres can lead to hyperemia in the perfused hemisphere and hypoperfusion in the contralateral side. In most studies investigating ScO2 during the SCP, cerebral ScO2 increases compared to the pre-SCP value [10,15]. Important factors affecting cerebral circulation are perfusion flow and pressure [16,17]. In this study, the degree of right ΔScO2 was highly correlated with the unilateral perfusion pressure, especially in patients with a discrepancy of ΔScO2 Rt-Lt, which indicates the risk of hyperperfusion in the right side when unilateral perfusion is performed. However, how much flow and pressure are optimal for preventing both hypoperfusion and hyperperfusion in a hypothermic state in neonates remains unresolved. Currently, a flow of 20–150 ml/kg/min at a perfusion pressure of 20–60 mmHg is recommended for regional SCP, which has very wide ranges [1,5,9,10]. Therefore, to avoid significant differences between the hemispheres, unilateral perfusion pressure, and flow rate should be prudently adjusted based on monitoring of bilateral cerebral circulation.
The integrity of bilateral cerebral circulation during the SCP varies based on the completion of the circle of Willis. Unfortunately, the completion rate of the circle of Willis is reported to be 56.1% in the pediatric population, while the young infant population has the lowest completion rate of approximately 40% [4]. The present study showed that the right ScO2 in 32% of patients increased more than the left ScO2 throughout SCP. Regarding the immaturity of cerebral collaterals, this result suggests that SCP may not always ensure uniformity of circulation across both cerebral hemispheres in neonates and young infants. Notably, right ScO2 was significantly decreased compared to left ScO2 during IA clamping in patients with a discrepancy in ΔScO2 Rt-Lt, which was opposite to the change during SCP. We hypothesize that the incompleteness of the circle of Willis may have caused the unevenness of changes in the ScO2 on both sides during ‘left’ unilateral perfusion apart from SCP. We infer that the decrease in right ScO2 following IA clamping may precede the rise in right ScO2 during SCP.
We also found that both ScO2 instantaneously reduced with the transition from SCP to restoration of full CPB. Immediately after declamping the IA, both ScO2 suddenly increased, in contrast to the change observed during SCP. In addition, the degree of the change in ScO2 was greater in the right hemisphere than in the left. Considering that cerebral autoregulation is operational in term infants, a persistent increase in the MAP associated with SCP may induce a rise in cerebrovascular resistance as a protective mechanism against excessive cerebral blood flow [18]. It is believed that cerebral vasoconstriction by static autoregulation during SCP results in a temporal decrease in cerebral blood flow, represented by the change in ScO2 [18]. The reverse could occur with a sudden overshoot of ScO2 immediately after declamping the IA. However, cerebral autoregulation functions below capacity during CPB and hypothermia in infants [19,20]. Besides the immaturity of cerebrovascular collaterals, impaired autoregulation could contribute to hypoperfusion or hyperperfusion when the MAP varies according to the SCP procedure.
As a method of cerebral monitoring during SCP, ScO2 estimates the cerebral blood flow based on the concept of balance between oxygen delivery and consumption in the brain [21]. Measurement of ScO2 using NIRS is limited by being an indirect estimate of cerebral blood flow and cellular metabolism. However, NIRS provides continuous real-time trends in cerebral oxygen delivery given a constant oxygen consumption during hypothermic CPB [7]. The present study is valuable because ScO2 was analyzed in each hemisphere using visualization with dynamic tracking of changes in ScO2 from the baseline value, which was defined as the value of 1-min before IA clamping and SCP. We observed that the distribution of cerebral blood flow can be changed according to an abrupt increase in unilateral cerebral perfusion pressure and sudden interruptions in blood flow during the clamping IA. This highlights the importance of separately measuring left and right ScO2 and the need for vigilant monitoring of changes in relation to surgical steps associated with SCP.
This study has some limitations. First, this study was a retrospective observational study with a small number of patients. Unfortunately, due to the limitations of a retrospective study in our research, a substantial number of cases (124) were excluded from data analysis due to incomplete data collection. Since our center employs SCP as the standard approach for aortic arch surgery in young infants, one of the exclusion criteria, circulatory arrest, was present in only one out of the 150 cases. Therefore, we believe that the 25 cases used for analysis in our study can be considered representative of routine surgical procedures. Second, we could not determine the association between the discrepancy in ΔScO2 Rt-Lt and neurologic complications related to SCP. In addition, the completeness of the circle of Willis was not evaluated to determine its effect on changes in bilateral cerebral circulation. Third, as mentioned above, NIRS reflects, but cannot directly measure, cerebral blood flow and oxygen delivery. Key physiologic variables, including hematocrit, temperature, carbon dioxide tension, and pH also influence cerebral blood flow, however, their effects may not be significant during CPB [16,17]. Moreover, relative changes in ScO2 from the baseline value were used for analysis rather than absolute values in an individual patient.
In conclusion, this study showed that SCP in young infants permits the maintenance of ScO2 indicating adequate bilateral cerebral perfusion. However, differences in cerebral circulation between both hemispheres can be affected by SCP and are related to the technique used, especially in the immature brain with incomplete cerebral collaterals; this may depend on the perfusion pressure. Further studies to elucidate which individual characteristics affect the distribution of cerebral circulation during aortic arch surgery with SCP in young infants are required.
Acknowledgement: None.
Funding Statement: This research was partly supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare of the Republic of Korea (HI18C2383).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: H.Y.J., W.J.S.; data collection: H.Y.J., S.J.B., S.H.K.; analysis and interpretation: H.Y.J., I.K.S., W.J.S.; draft manuscript preparation: H.Y.J., S.J.B., W.J.S.; statistical analysis: H.Y.J., S.J.B., W.J.S.; supervision: W.J.S. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: All study protocols were approved and written informed consent was waived by the Asan Medical Center institutional review board (protocol number, 2022-0964). The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its amendments.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. de Rita, F., Lucchese, G., Barozzi, L., Menon, T., Faggian, G. et al. (2011). Selective cerebro-myocardial perfusion in complex congenital aortic arch pathology: A novel technique. Artificial Organs, 35(11), 1029–1035 [Google Scholar] [PubMed]
2. Kulyabin, Y. Y., Bogachev-Prokophiev, A. V., Soynov, I. A., Omelchenko, A. Y., Zubritskiy, A. V. et al. (2020). Clinical assessment of perfusion techniques during surgical repair of coarctation of aorta with aortic arch hypoplasia in neonates: A pilot prospective randomized study. Seminars in Thoracic and Cardiovascular Surgery, 32(4), 860–871 [Google Scholar] [PubMed]
3. Xie, L., Xu, Y., Huang, G., Ye, M., Hu, X. et al. (2020). MHCA with SACP versus DHCA in pediatric aortic arch surgery: A comparative study. Scientific Reports, 10(1), 4439 [Google Scholar] [PubMed]
4. Solak, S., Ustabasioglu, F. E., Alkan, A., Kula, O., Sut, N. et al. (2021). Anatomical variations of the circle of Willis in children. Pediatric Radiology, 51(13), 2581–2587 [Google Scholar] [PubMed]
5. Amir, G., Ramamoorthy, C., Riemer, R. K., Reddy, V. M., Hanley, F. L. (2005). Neonatal brain protection and deep hypothermic circulatory arrest: Pathophysiology of ischemic neuronal injury and protective strategies. Annals of Thoracic Surgery, 80(5), 1955–1964 [Google Scholar] [PubMed]
6. Simon, B. V., Swartz, M. F., Orie, J. M., Adams, H. R., Seltzer, L. E. et al. (2019). Neurodevelopmental delay after the neonatal repair of coarctation and arch obstruction. Annals of Thoracic Surgery, 108(5), 1416–1422 [Google Scholar] [PubMed]
7. Finucane, E., Jooste, E., Machovec, K. A. (2020). Neuromonitoring modalities in pediatric cardiac anesthesia: A review of the literature. Journal of Cardiothoracic and Vascular Anesthesia, 34(12), 3420–3428 [Google Scholar] [PubMed]
8. Farouk, A., Karimi, M., Henderson, M., Ostrowsky, J., Siwik, E. et al. (2008). Cerebral regional oxygenation during aortic coarctation repair in pediatric population. European Journal of Cardiothoracic Surgery, 34(1), 26–31 [Google Scholar] [PubMed]
9. Kwak, J. G., Kim, W. H., Oh, A. Y., Yoon, T. G., Kim, H. S. et al. (2007). Is unilateral brain regional perfusion neurologically safe during congenital aortic arch surgery? European Journal of Cardiothoracic Surgery, 32(5), 751–755 [Google Scholar] [PubMed]
10. Andropoulos, D. B., Stayer, S. A., McKenzie, E. D., FraserJr, C. D. (2003). Novel cerebral physiologic monitoring to guide low-flow cerebral perfusion during neonatal aortic arch reconstruction. Journal of Thoracic and Cardiovascular Surgery, 125(3), 491–499 [Google Scholar] [PubMed]
11. Lee, H. C., Jung, C. W. (2018). Vital recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Scientific Reports, 8(1), 1527 [Google Scholar] [PubMed]
12. Kim, D. H., Choi, E. S., Kwon, B. S., Yun, T. J., Yang, D. H. et al. (2023). The usefulness of computed tomography in predicting left ventricular outflow tract obstruction after neonatal arch repair. Seminars in Thoracic and Cardiovascular Surgery, 35(1), 127–137 [Google Scholar] [PubMed]
13. Miyaji, K., Miyamoto, T., Kohira, S., Itatani, K., Tomoyasu, T. et al. (2010). Regional high-flow cerebral perfusion improves both cerebral and somatic tissue oxygenation in aortic arch repair. Annals of Thoracic Surgery, 90(2), 593–599 [Google Scholar] [PubMed]
14. Huang, C. H., Wang, Y. C., Chou, H. W., Huang, S. C. (2021). Near-infrared spectroscopy assessment of tissue oxygenation during selective cerebral perfusion for neonatal aortic arch reconstruction. Frontiers in Medicine, 8, 637257 [Google Scholar] [PubMed]
15. Berens, R. J., Stuth, E. A., Robertson, F. A., Jaquiss, R. D., Hoffman, G. M. et al. (2006). Near infrared spectroscopy monitoring during pediatric aortic coarctation repair. Paediatric Anaesthesia, 16(7), 777–781 [Google Scholar] [PubMed]
16. Haydin, S., Onan, B., Onan, I. S., Ozturk, E., Iyigun, M. et al. (2013). Cerebral perfusion during cardiopulmonary bypass in children: Correlations between near-infrared spectroscopy, temperature, lactate, pump flow, and blood pressure. Artificial Organs, 37(1), 87–91 [Google Scholar] [PubMed]
17. Menke, J., Moller, G. (2014). Cerebral near-infrared spectroscopy correlates to vital parameters during cardiopulmonary bypass surgery in children. Pediatric Cardiology, 35(1), 155–163 [Google Scholar] [PubMed]
18. Rhee, C. J., da Costa, C. S., Austin, T., Brady, K. M., Czosnyka, M. et al. (2018). Neonatal cerebrovascular autoregulation. Pediatric Research, 84(5), 602–610 [Google Scholar] [PubMed]
19. Smith, B., Vu, E., Kibler, K., Rusin, C., Easley, R. B. et al. (2017). Does hypothermia impair cerebrovascular autoregulation in neonates during cardiopulmonary bypass? Paediatric Anaesthesia, 27(9), 905–910 [Google Scholar] [PubMed]
20. Votava-Smith, J. K., Statile, C. J., Taylor, M. D., King, E. C., Pratt, J. M. et al. (2017). Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 154(3), 1038–1044 [Google Scholar] [PubMed]
21. Zaleski, K. L., Kussman, B. D. (2020). Near-infrared spectroscopy in pediatric congenital heart disease. Journal of Cardiothoracic and Vascular Anesthesia, 34(2), 489–500 [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools