 Open Access
Open Access
ARTICLE
The Effect of Atrial Septal Defect Closure on Cardiac Volumetric Changes in Adults, Transcatheter Versus Surgical Closure, a Pilot Cardiac Magnetic Resonance Study
1 Cardiology Department, Congenital and Structural Heart Disease Unit, Ain Shams University Hospitals, Faculty of Medicine, Ain Shams University, Cairo, Egypt
2 Cardiology Department, Faculty of Medicine, Assuit University, Assuit, Egypt
3 Cardiothoracic Surgery Department, Faculty of Medicine, Assuit University, Assuit, Egypt
4 Anesthesia Department, Faculty of Medicine, Assuit University, Assuit, Egypt
* Corresponding Author: Noha M. Gamal. Email:
Congenital Heart Disease 2023, 18(6), 679-691. https://doi.org/10.32604/chd.2023.020028
Received 30 October 2021; Accepted 25 May 2022; Issue published 19 January 2024
Abstract
Background: Closure of an atrial septal defect (ASD) reduces right-side heart volumes by abolishing shunting with simultaneous improvement of the left ventricle (LV) filling and functions due to ventricular interdependence, thereby improving symptoms. Furthermore, studies conducted on atrial volume changes after ASD closure are limited. Cardiac magnetic resonance (CMR) is considered as the gold standard method for measuring cardiac volume and mass. Objective: We aimed to study the effect of transcatheter and surgical closure of secundum ASD on cardiac volumes and systolic functions as well as the fate of tricuspid regurgitation (TR), using CMR analysis. Methods: We prospectively enrolled 30 adult patients with isolated secundum ASD who were referred to ASD closure. CMR evaluation of cardiac chambers indexed volumes, systolic function, myocardial mass index, and tricuspid regurgitant fraction were done at before and 6 months after closure. Results: RV volumes decreased in both groups when compared to baseline (p-value 0.001), the device group had more reduction in volumes and more improvement in RV function after closure (p-value 0.001) when compared to the surgical arm. The changes in the RV mass index were insignificant between both groups (p-value 0.31). Functional TR improved to the same extent in both groups. Left ventricular end diastolic volume index (LVEDVI) and LV mass index increased significantly in both groups when compared to baseline in both groups but with no difference between groups p-value 0.01), left ventricular end systolic volume index (LVESVI) changes were insignificant. LV systolic function improved in patients who underwent device closure only (63.53 ± 3.85 vs. 67.13 ± 4.34, p-value 0.01). There was a significant reduction in right atrial (RA) volumes and an insignificant decrease in left atrial (LA) volumes, with no difference between groups. Conclusion: Transcatheter and surgical secundum ASD closure resulted in volumetric changes in some cardiac chambers with better improvement in bi-ventricular systolic function in the transcatheter arm and no difference in the TR reduction between the two groups at 6 months follow-up by CMR.Keywords
Abbreviations
| ASD | Atrial septal defect |
| ECG | Electrocardiogram |
| CMR | Cardiac magnetic resonance |
| RA | Right atrium |
| LV | Left ventricle |
| RV | Right ventricle |
| LA | Left atrial |
| EDV | End diastolic volume, end systolic volume (RVESV) |
| TR | Tricuspid regurgitation |
| PAH | Pulmonary arterial hypertension |
| PVR | Pulmonary vascular resistance |
| mPAP | Mean pulmonary artery pressure |
| RVSP | Right ventricular systolic pressure |
| BSA | Body surface area |
| TEE | Transesophageal echocardiography |
| SSFP | Steady-state precession |
| TTE | Transthoracic echocardiography |
| MPA | Main pulmonary artery |
| CO | Cardiac output |
| PC | Phase contrast |
| RVol | Tricuspid regurgitant volume |
| SV | Stroke volume |
| TAD | Tricuspid annular diameter |
| NYHA | New York Heart Association |
| ASO | Amplatzer septal occluder |
ASD is considered the most common congenital heart disease diagnosed in adults [1]. Consequently longstanding left to right shunt causes chronic right-side heart volume overload [2]. After ASD closure and shunt prevention cardiac reverse remodeling occurs [3]. Percutaneous transcatheter ASD closure has been shown to be a cost-effective alternative to surgical closure in case of favorable anatomical features [4]. Functional TR secondary to tricuspid vlave (TV) annular dilatation which occurs as a result of prolonged volume overload is a common but frequently overlooked in clinical practice [5,6]. CMR is the recommended imaging modality for precise shunt, cardiac chamber volumes, and function quantification owing to its multiplanar imaging capabilities and absence of any geometric assumptions [7].
The main benefits of transcatheter over surgical closure are avoidance of surgical wounds and cardiopulmonary bypass machines in addition to the fact of being less invasive with significantly shorter hospital stays. However, the presence of other functional benefits over surgical closure on the heart chamber volumes, functions, and functional TR resulting from avoidance of surgical pericardial opening, cardioplegia, and cardiopulmonary bypass machine is an important issue that needs to be thoroughly investigated.
Our local institutional ethical committee approval was obtained, an informed written consent from all of the participants was obtained. All commers with isolated secundum ASD who were referred to our center were enrolled. Patients with isolated secundum ASD with significant shunt fraction (QP/QS more than 1.5) were prospectively included.
Patients with associated ischemic heart disease, other significant congenital lesions, increased pulmonary vascular resistance (PVR) > 5 woods units, and with anomalous pulmonary venous return were excluded.
Patients’ evaluation.
2.1 Transthoracic (TTE) or Transesophageal (TEE) Echocardiography
TTE and TEE were initially used to thorough evaluation of the ASD size, rims, and to rule out any exclusion criteria [8]. The mean pulmonary artery pressure (mPAP) and right ventricular systolic pressure (RVSP) were calculated using the modified Bernoulli equation [9].
2.2 Cardiac Magnetic Resonance Assessment
All CMR examinations were done in supine position and head first. End expiratory, ECG gated scans with breath-holding using a 1.5 Tesla MRI scanner (Ingenia Philips). Retrospective Steady-State Precession (SSFP) sequences were obtained (image matrix 256 × 150, field of view 380 mm, repetition time 52.05 ms, echo time 1.74 ms, and flip angle 70°) [10].
The ventricular image set consists of a stack of cine SSFP images acquired in short axis view from the level of mitral valve annulus to the left ventricular apex with 8 to 12 slice thickness [11]. The ventricular volumes were examined on a slice-by-slice basis, with manual endocardial and epicardial borders tracing. Simpson’s method was used to calculate volumes [12]. The ventricular trabeculations and papillary muscles were included. All measurements were indexed to the patient’s body surface area (BSA) [13].
2.2.2 Area of the Main Pulmonary Artery (MPA)
An oblique cine acquisition immediately distal to the Sino-tubular junction was located in order to obtain a cross-section area of the MPA [14].
The biplane area-length method was used to calculate the maximum RA volume in the 4- and 2-chamber SSFP image views during ventricular systole (last cine image before opening of the tricuspid valve). The RA appendage was included and the cava veins were excluded [15].
The maximal volume was measured as the RA using bi-plane area-length method with exclusion of the LA appendage and pulmonary veins at the same part of the cardiac cycle as the RA [15].
2.2.4 Calculation of Shunt Fraction (QP/QS)
Applying the phase contrast (PC) MRI sequences to calculate cardiac output (CO) in the PA trunk and aortic root using two orthogonal locating planes for adequate cut plane planning [16].
The TR regurgitant fraction was calculated by dividing the tricuspid regurgitant volume (RVol) by the RV stroke volume (RV SV) and multiplying the result by 100 [17]. RVol was calculated as total RV SV minus forward SV across the pulmonary valve, with forward SV measured by pulmonic valve PC imaging (in the absence of significant PR) [18]. TR severity was graded as (mild; regurgitant fraction ≤15%, moderate; 16% to 25%, moderately severe; 26% to 48% and severe; >48%) [18].
Tricuspid annulus diameter (TAD) was calculated as the maximum diameter of the TV annulus in four chamber cine gradient echo sequences [19].
Hemodynamic Study:
Pulmonary artery pressure and pulmonary vascular resistance assessment were routinely done before transcatheter and surgical closure when pulmonary hypertension was found in echocardiography.
2.3 Atrial Septal Defect Closure
All patients were evaluated by a blinded Adult congenital heart disease specialized echocardiographer to determine the suitability for transcatheter closure. Patients with defects that were not suitable for transcatheter closure were referred to surgical closure using the patch technique.
Suitable candidates had percutaneous transcatheter ASD closure under general anesthesia with fluoroscopic and TEE guidance. The stop flow diameter of the defect was measured with a 24- or 34-mm sizing balloon (AGA Medical Corp.). The Amplatzer septal occluder (ASO) device was used for the procedure, anticoagulation was made by 100 IU/Kg heparin.
Surgical ASD closure was performed with cooling the patients to 32 degrees Celsius. Cold crystalloid antegrade cardioplegia was used for myocardial protection. After full cardiac arrest, the right atrium was opened and an autologous pericardial patch was used.
CMR study was performed prior to and 6 months after closure in all patients.
SPSS was used to collect and analyze data (Statistical Package for Social Science, version 25, IBM, and Armonk, New York, USA). Continuous data was expressed as mean, whereas nominal data was expressed as frequency and percentage.
The Chi square test was used to compare nominal data from different groups, and the Mann Whitney test was used to compare continuous data from both groups. The Wilcoxon test was used to compare baseline and follow-up data from the same group. The percentage of change between baseline and follow up data was calculated using the following equation: percentage of change = ((follow up-baseline data)/(baseline 142 data)) * 100. The level of confidence was kept at 95%, and thus the p-value was considered significant if <0.05.
Two thirds of the patients (66.7%) were young females less than 40 years old. The mean age of the transcatheter group was 33.73 ± 13.06 years while mean age of the surgical group was 35.33 ± 15.18 years without significant difference between both groups (p-value 0.75) (Table 1).
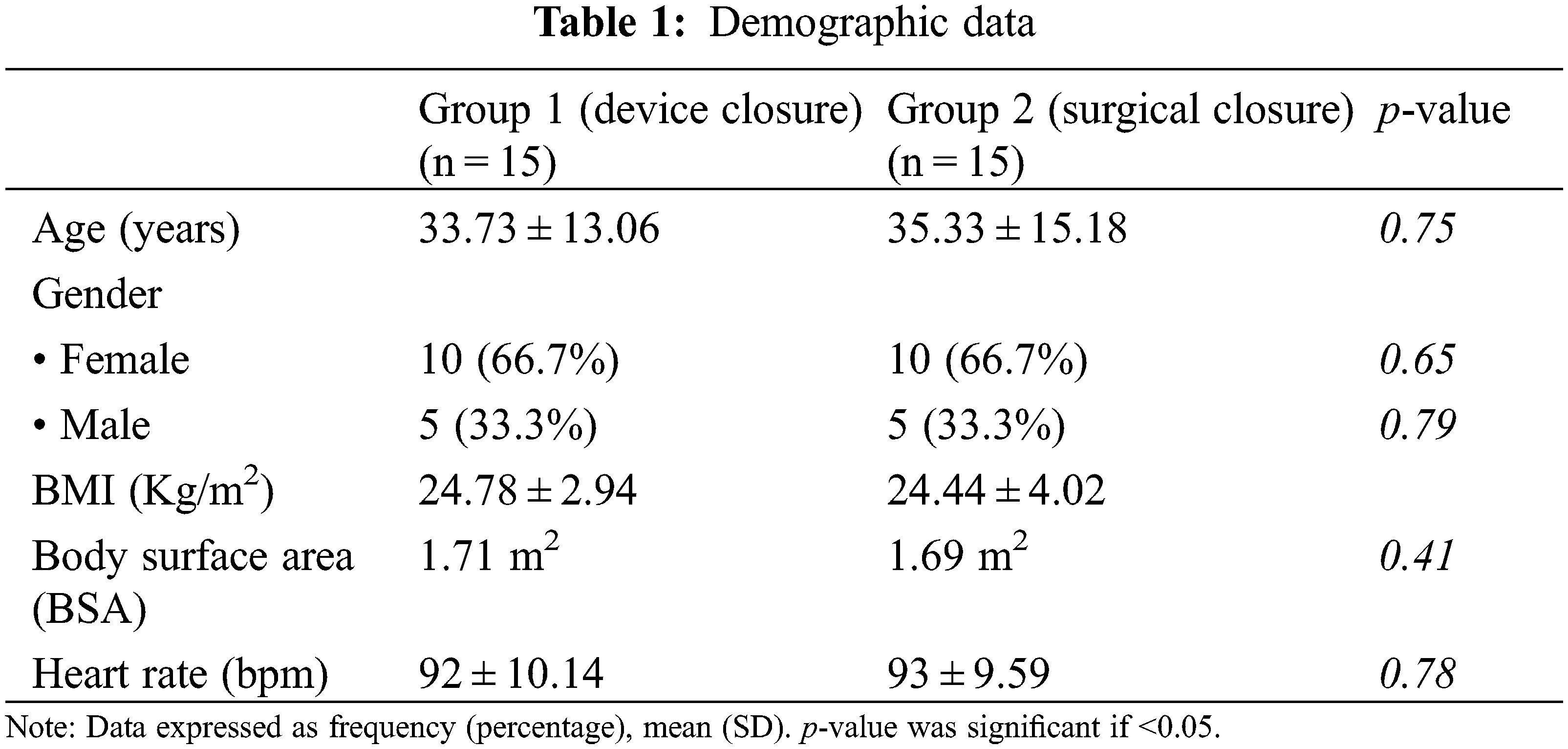
Thirteen patients (86.7%) and twelve patients (80%) had baseline New York Heart Association (NYHA) class II prior to device and surgical closure, respectively. Most of the patient had their NYHA class improved to class I after closure. There were no significant differences in heart rate values (HR) or body mass indices (BMI) between both groups.
There was a significant reduction in the mPAP values after ASD closure in both groups when compared to the baseline values.
The transcatheter group showed more absolute reduction in the mPAP than the surgical group on follow up (18.13 ± 7.21 vs. 23.93 ± 6.63 (mmHg)); however, the p-value was insignificant p-value 0.03 (Table 2).
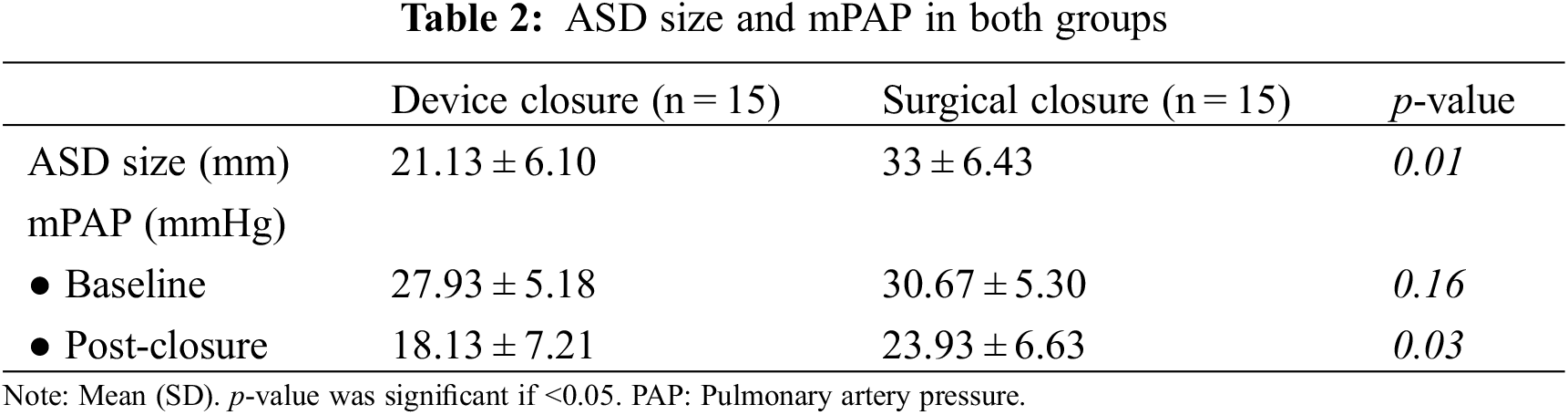
Although the mean ASD size was larger in the surgical arm (Table 2), we did not find a significant difference in the baseline shunt fraction Qp/Qs between the two groups by CMR (Table 3).
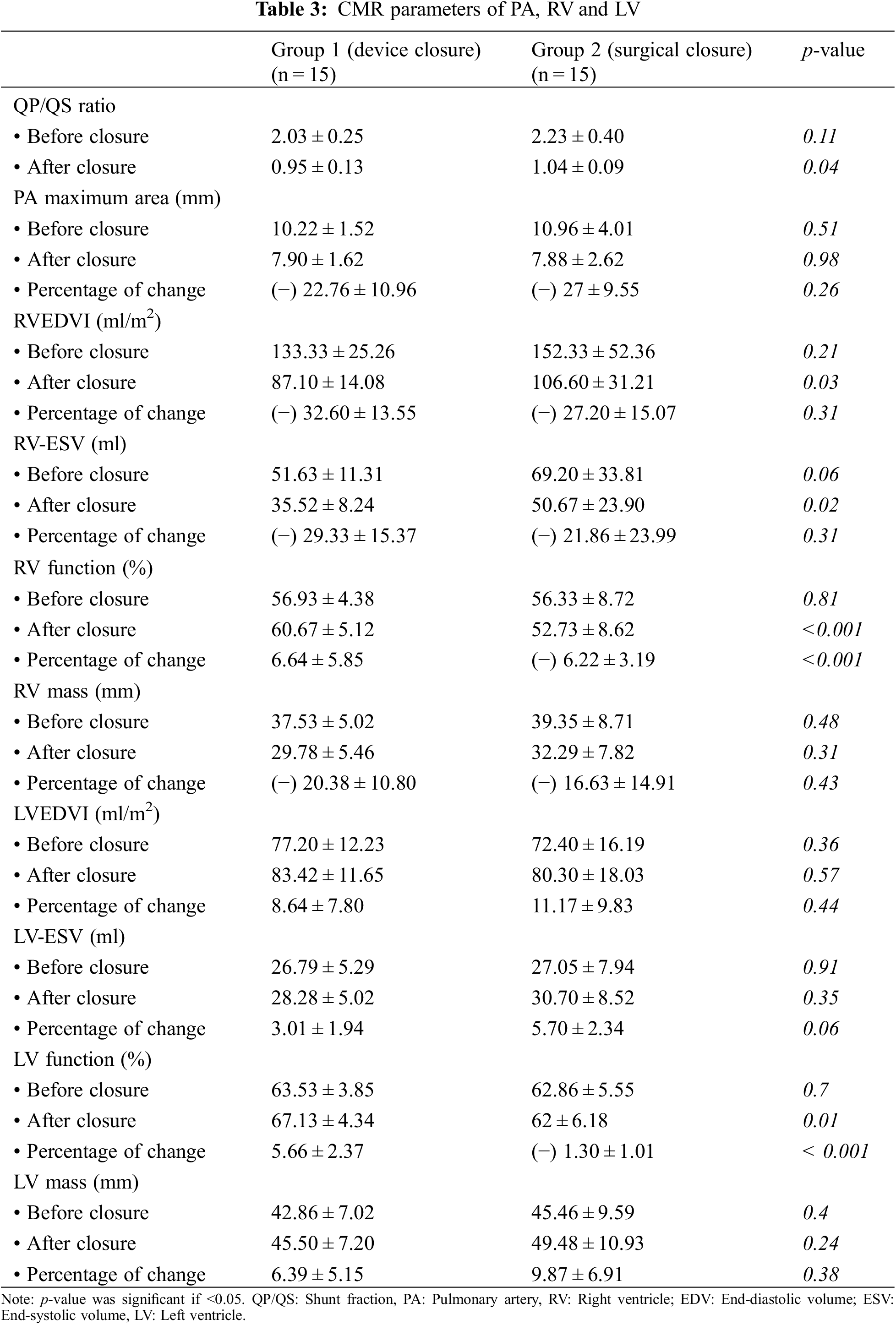
Reverse remodeling of the RV indexed volumes was noticed in both groups after ASD closure.
The transcatheter arm had more reverse remodeling with significant difference with the surgical arm (p-value 0.03 for indexed RVEDV and 0.02 for indexed RVESV) (Table 3, Figs. 1 and 2).
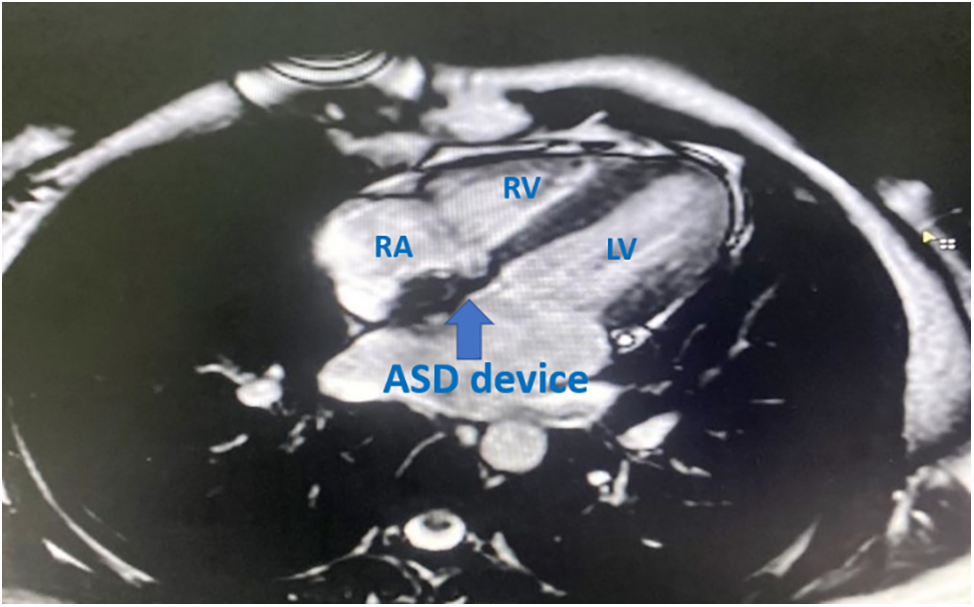
Figure 1: SSFP 4 chamber MRI view in transcatheter closed case by ASO
Note: RA: right atrium, RV: right ventricle, LV: left ventricle.
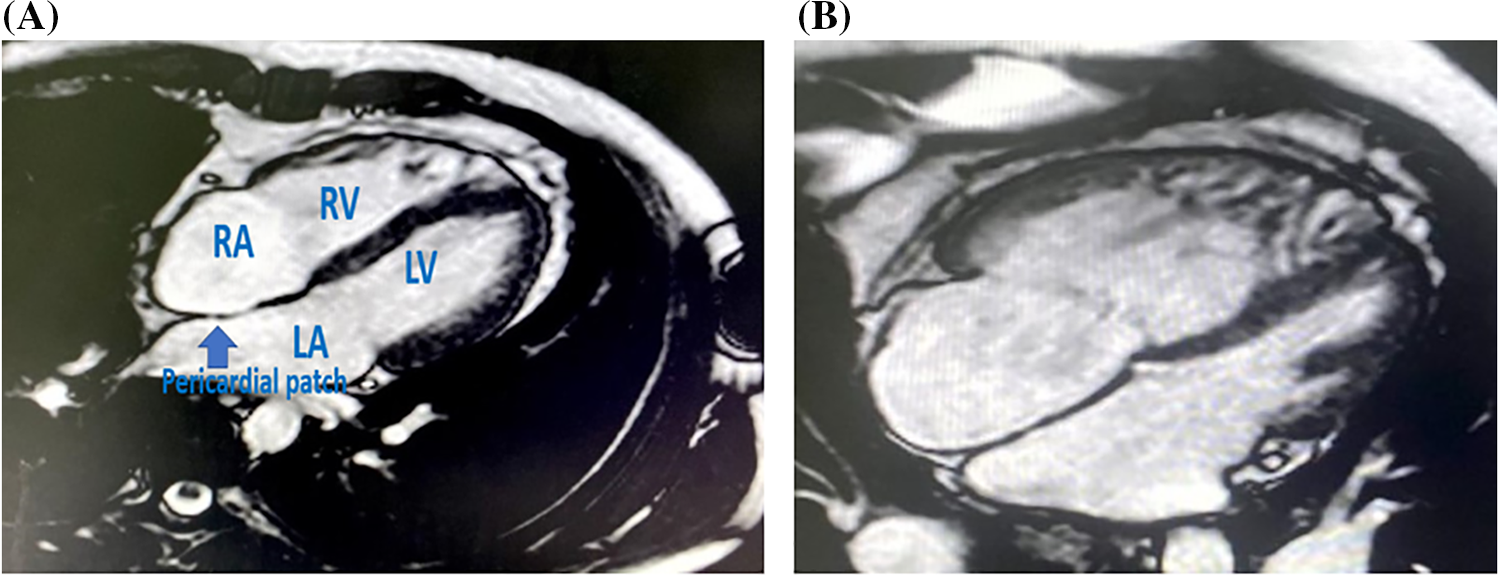
Figure 2: (A) SSFP 4 chamber MRI view in surgically closed case by conventional method with reverse remodelling of RA and RV volumes. (B) SSFP 4 chamber MRI view in surgically closed case by conventional method with persistently dilated of RA and RV volumes
Note: RA: right atrium, RV: right ventricle, LA: left atrium, LV: left ventricle.
The absolute values of the RV mass index decreased significantly from the baseline values in both groups, however there was no significant difference between them (Table 3).
The RV systolic function values showed no significantly difference before closure in both groups, however we found significant improvement in the RV systolic function in the follow up CMR studies in the transcatheter arm (Table 3).
LVEDVI, LVESV and LV mass index increased after ASD closure when compared to the baseline measurements, with no significant difference between both groups at baseline and at follow up (Table 3).
LV systolic function improved only in the device group after closure when compared to the preprocedural values (Table 3).
In our cohort, there was a reduction in pulmonary artery (PA) maximal area after ASD closure, with a statistically insignificant difference between groups (Table 3).
Both groups experienced a significant reduction in RA volumes 6 months after ASD closure, and insignificant changes in the LA volumes, with no difference between the two groups (Table 4).
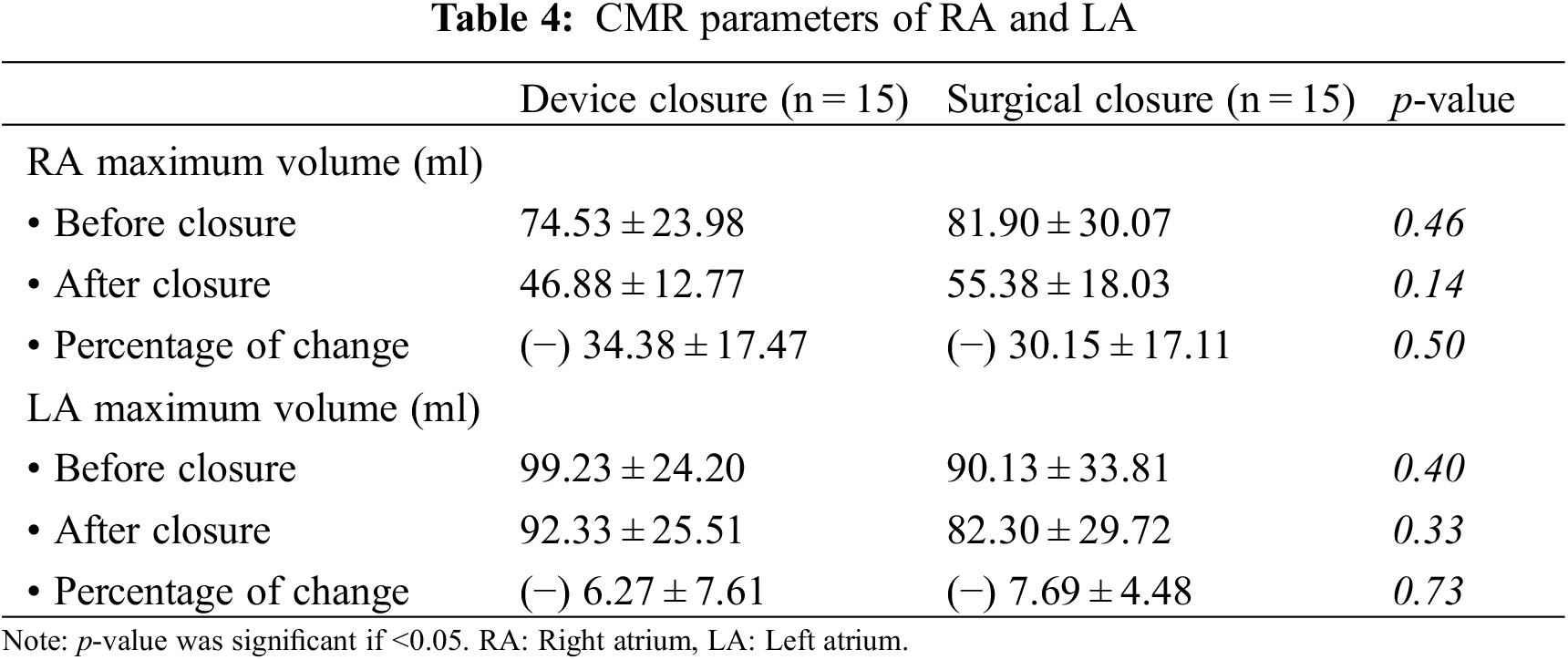
Functional TR was reduced after percutaneous and surgical ASD closure, with no statistically significant difference between groups, but the percentage of change was greater in the device group than the surgical group ((−) 19.18 ± 9.28 in device vs. (−) 18.89 ± 11.01 in surgery).
Persistence TR (moderate grade or more) was discovered in 40% of the ASD-device group and 53.4% of the ASD-surgical group in the follow up (Table 5).
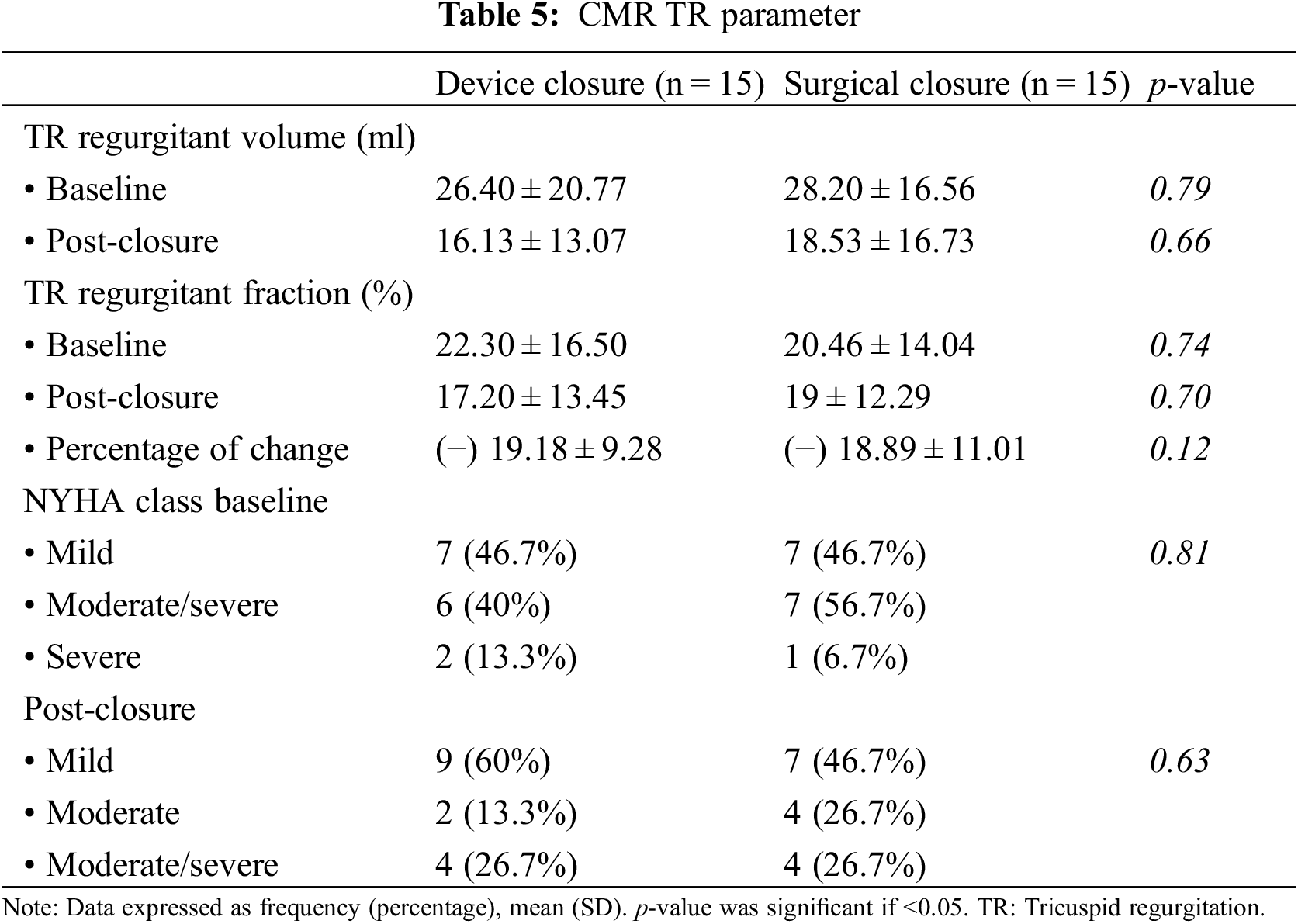
Thirty adult patients with isolated secundum ASD were included in our study. They were studied based on the method of closure (surgical vs. transcatheter arm). The patients were age, gender, and BSA matched.
We studied the 6 months changes in the CMR parameters, as well as the fate of TR.
The mean PAP values decreased in all patients after ASD closure when compared to the baseline values. There was more reduction in patients who underwent device closure with lower final absolute mean PAP. These results concur with the findings reported by Humenberger et al. [20] who found that ASD closure results in a reduction in the pulmonary artery pressure in all his patients, which was mainly secondary to a reduction in the transpulmonary flow and abolishment of the left to right shunt.
In our study we used CMR to measure the volumetric changes and TR severity because the current guidelines and recommendations consider this modality as the gold standard investigation for this function, CMR has consistently proven to be both accurate and reproducible in terms of cardiac chambers volume quantification and valvular regurgitation grading with minimal inter- and intra-observer differences [21]. CMR also has the advantage of being radiation-free, and safe in patients with implantable cardiac devices [22].
Patients who underwent transcatheter ASD closure showed more reverse remodeling and significantly lower RV volumes on follow-up when compared to the surgical arm.
These results confirm the finding reported by Pascotto et al. [23] who found that patients who had surgical ASD repair failed to show complete reverse right chambers volume reduction at 6 months follow up.
However, Castaldi et al. [24] showed that both surgical and percutaneous closure of ASD have a similar long-term efficacy regarding the reduction in the right-sided cardiac chamber volume changes. This contradiction can be explained by the difference in the duration of follow-up in our study, the study performed by Pascotto et al., and the study performed by Castaldi et al.
Castaldi et al. followed their patients for long-term results (8.3 vs. 6.3 years in both groups), while in our study and Pascotto et al. study, we focused on the early changes -6 months- after closure.
These findings may suggest that both device and surgical ASD closure can lead to comparable long-term changes in the right-side volumes on the long-term follow-up, but the transcatheter closure showed better early reverse remodeling when compared to the surgical closure, and the patients who had surgical closure catch up in the long term follow up.
This can be attributed in our opinion to the difference between both closure modalities, with the functional anomalies related to cardiopulmonary bypass, cardioplegia, and cardiac geometric changes caused by pericardial opening.
This might explain the initial superiority of transcatheter closure and the initial lagging of surgical closure to demonstrate equivalent reverse remodeling. However, according to the results of the study performed by Castaldi et al., this superiority appears to fade away in the long term [24].
Our cohort showed a significant reduction in the indexed RV mass values when compared to the baseline values in both groups without significant difference between the surgical and transcatheter arms. Our finding disagrees with the results published by Schoen et al. [25]. They found that the RV mass regression after 6 months of follow-up was not significantly different when compared to the baseline MRI measurements. The difference between Schoen et al. study and our study can be explained by the difference in the methods used for RV mass calculation in the CMR images.
We included the moderator band and the RV trabeculation in the RV mass calculations and excluded them in the RV volume calculation [26] contrary to the other study which excluded them in the RV mass calculations [25].
We could not find any previous study that has reported the effect of surgical ASD closure in RV mass by CMR to compare its results with our results.
Regarding the changes in the LV mass index, Teo et al. [27] found similar findings to our study, with insignificantly difference with the age-matched controls before and after ASD device closure.
After 6 months our transcatheter ASD closure cohort showed a statistically significant improvement in RV and LV systolic functions. These results disagree with the finding of Berger et al. [28] who demonstrated similar improvement of RVEF in both surgically and percutaneously closed ASD. This discrepancy can be explained by the different modalities used in both studies to assess the RV and LV functions. While Berger et al. used echocardiography, we used the gold standard CMR, which is more accurate and reliable than echocardiography in the evaluation of ventricular volumes, especially the RV volumes [29].
Our finding is confirmed by the study conducted by Salehian et al. [30], they found an increase in LVEDV with no changes in LVESV, this resulted in an increase in LV stroke volume and systolic function after ASD device closure.
ASD closure led to a significant reduction in the indexed RA volumes from the preprocedural measurements in both groups, however, there was no significant difference between groups.
We found that RA volume overload had a strong positive correlation (p-value 0.0001, r = 0.7) with the preclosure shunt fraction, and a weak correlation with RA reverse remodeling magnitude. These results agree with Fang et al. [31] who showed that patients with persistent RA enlargement had excessive preclosure RA dilation, he used Fick’s method to calculate shunt fraction and the 2D echocardiography to calculate the RV volumes.
Despite the common notion that the LA volumes increase after ASD closure, we found that the LA volumes decreased in comparison to baseline measurements with insignificant difference to the baseline measurements and similar changes in both groups.
We did not find a significant correlation between the functional TR severity and the TV annulus diameter after ASD closure. This was consistent with the findings of Toyono et al. [32] who discovered that the only factor associated with TR jet area after ASD closure was the baseline pulmonary artery systolic pressure prior to ASD closure.
6 Limitations and Recommendations
• This is a single-center pilot study with a relatively small number of patients.
• We focused mainly on the intermediate-term follow-up results.
• Longer-term follow-up can give us more data on the TR and cardiac chamber volume changes in this kind of patient.
• We recommend further studies from multiple centers including larger numbers of patients.
• Both surgical and transcatheter closure of secundum are well-established and validated modalities for the treatment of patients with isolated secundum ASD.
• Transcatheter ASD closure has the clear advantage of being a less invasive, highly effective procedure with shorter hospital stays, avoidance of surgical scars, and bypass machine.
• However; whether transcatheter ASD closure had other functional benefits needed further studies, we found that transcatheter ASD closure led to better biventricular systolic function improvement and better RV reverse remodeling with volume regression with equivalent effect on the other CMR measured parameters after 6 months follow up.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The following authors affirm their contributions to the paper: Amr Mansour, Noha M. Gamal: data collection; analysis and interpretation of results; all authors: draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: An informed written consent was obtained from all the participant’s legal guardians. The study has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). This study was approved by the Ethics Committee of Assiut University; Institutional Review Board (IRB), Independent Ethics Committee (IEC); Project Approval Number: CA-19-9635.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Oster, M., Bhatt, A. B., Zaragoza-Macias, E., Dendukuri, N., Marelli, A. (2019). Interventional therapy versus medical therapy for secundum atrial septal defect: A systematic review (Part 2) for the 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Journal of the American College of Cardiology, 73(12), 2363–2595. https://doi.org/10.1016/j.jacc.2018.08.1032 [Google Scholar] [PubMed] [CrossRef]
2. Kort, H. W., Balzer, D. T., Johnson, M. C. (2001). Resolution of right heart enlargement after closure of secundum atrial septal defect with transcatheter technique. Journal of the American College of Cardiology, 38(5), 1528–1532. https://doi.org/10.1016/S0735-1097(01)01547-9 [Google Scholar] [PubMed] [CrossRef]
3. Butera, G., Biondi-Zoccai, G., Sangiorgi, G., Abella, R., Giamberti, A. et al. (2011). Percutaneous versus surgical closure of secundum atrial septal defects: A systematic review and meta-analysis of currently available clinical evidence. EuroIntervention, 7(3), 377–385. https://doi.org/10.4244/EIJV7I3A63 [Google Scholar] [PubMed] [CrossRef]
4. Mylotte, D., Quenneville, S. P., Kotowycz, M. A., Xie, X., Brophy, J. M. et al. (2014). Long-term cost-effectiveness of transcatheter versus surgical closure of secundum atrial septal defect in adults. International Journal of Cardiology, 172(1), 109–114. https://doi.org/10.1016/j.ijcard.2013.12.144 [Google Scholar] [PubMed] [CrossRef]
5. Taramasso, M., Vanermen, H., Maisano, F., Guidotti, A., La Canna, G. et al. (2012). The growing clinical importance of secondary tricuspid regurgitation. Journal of the American College of Cardiology, 59(8), 703–710. https://doi.org/10.1016/j.jacc.2011.09.069 [Google Scholar] [PubMed] [CrossRef]
6. Fang, F., Wang, J., Yip, G. W., Lam, Y. Y. (2015). Predictors of mid-term functional tricuspid regurgitation after device closure of atrial septal defect in adults: Impact of pre-operative tricuspid valve remodeling. International Journal of Cardiology, 187(119), 447–452. https://doi.org/10.1016/j.ijcard.2015.03.332 [Google Scholar] [PubMed] [CrossRef]
7. Petersen, S. E., Aung, N., Sanghvi, M. M., Zemrak, F., Fung, K. et al. (2017). Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. Journal of Cardiovascular Magnetic Resonance, 19(1), 18. https://doi.org/10.1186/s12968-017-0327-9 [Google Scholar] [PubMed] [CrossRef]
8. Parasuraman, S., Walker, S., Loudon, B. L., Gollop, N. D., Wilson, A. M. et al. (2016). Assessment of pulmonary artery pressure by echocardiography—A comprehensive review. IJC Heart & Vasculature, 12(24), 45–51. https://doi.org/10.1016/j.ijcha.2016.05.011 [Google Scholar] [PubMed] [CrossRef]
9. Kaya, M. G., Baykan, A., Dogan, A., Inanc, T., Gunebakmaz, O. et al. (2010). Intermediate-term effects of transcatheter secundum atrial septal defect closure on cardiac remodeling in children and adults. Pediatric Cardiology, 31(4), 474–482. https://doi.org/10.1007/s00246-009-9623-y [Google Scholar] [PubMed] [CrossRef]
10. Rominger, M. B., Bachmann, G. F., Pabst, W., Rau, W. S. (1999). Right ventricular volumes and ejection fraction with fast cine MR imaging in breath-hold technique: Applicability, normal values from 52 volunteers, and evaluation of 325 adult cardiac patients. Journal of Magnetic Resonance Imaging, 10(6), 908–918. https://doi.org/10.1002/(sici)1522-2586(199912)10:6<908::aid-jmri2>3.0.co;2-2 [Google Scholar] [CrossRef]
11. Alfakih, K., Plein, S., Thiele, H., Jones, T., Ridgway, J. P. et al. (2003). Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. Journal of Magnetic Resonance Imaging, 17(3), 323–329. https://doi.org/10.1002/jmri.10262 [Google Scholar] [PubMed] [CrossRef]
12. Reiter, G., Reiter, U., Rienmüller, R., Gagarina, N., Ryabikin, A. (2004). On the value of geometry-based models for left ventricular volumetry in magnetic resonance imaging and electron beam tomography: A Bland-Altman analysis. European Journal of Radiology, 52(2), 110–118. https://doi.org/10.1016/j.ejrad.2003.10.003 [Google Scholar] [PubMed] [CrossRef]
13. Hudsmith, L. E., Petersen, S. E., Francis, J. M., Robson, M. D., Neubauer, S. (2005). Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance, 7(5), 775–782. https://doi.org/10.1080/10976640500295516 [Google Scholar] [PubMed] [CrossRef]
14. Burman, E. D., Keegan, J., Kilner, P. J. (2016). Pulmonary artery diameters, cross sectional areas and area changes measured by cine cardiovascular magnetic resonance in healthy volunteers. Journal of Cardiovascular Magnetic Resonance, 18(1), 12. https://doi.org/10.1186/s12968-016-0230-9 [Google Scholar] [PubMed] [CrossRef]
15. Maceira, A. M., Cosín-Sales, J., Roughton, M., Prasad, S. K., Pennell, D. J. (2013). Reference right atrial dimensions and volume estimation by steady state free precession cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 15(1), 29. https://doi.org/10.1186/1532-429X-15-29 [Google Scholar] [PubMed] [CrossRef]
16. Fogel, M. A., Pawlowski, T. W., Whitehead, K. K., Harris, M. A., Keller, M. S. et al. (2012). Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to Fontan: A comparison of 3 groups: Pre-Fontan CMR versus cath evaluation. Journal of the American College of Cardiology, 60(12), 1094–1102. https://doi.org/10.1016/j.jacc.2012.06.021 [Google Scholar] [PubMed] [CrossRef]
17. Leeson, P., Augustine, D., Mitchell, A. R. J., Becher, H. (2012). Echocardiography. Oxford, UK: University Press. https://doi.org/10.1093/med/9780198804161.001.0001 [Google Scholar] [CrossRef]
18. Medvedofsky, D., León Jiménez, J., Addetia, K., Singh, A., Lang, R. M. et al. (2017). Multi-parametric quantification of tricuspid regurgitation using cardiovascular magnetic resonance: A comparison to echocardiography. European Journal of Radiology, 86(10), 213–220. https://doi.org/10.1016/j.ejrad.2016.11.025 [Google Scholar] [PubMed] [CrossRef]
19. Scharhag, J., Schneider, G., Urhausen, A., Rochette, V., Kramann, B. et al. (2002). Athlete’s heart: Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. Journal of the American College of Cardiology, 40(10), 1856–1863. https://doi.org/10.1016/S0735-1097(02)02478-6 [Google Scholar] [PubMed] [CrossRef]
20. Humenberger, M., Rosenhek, R., Gabriel, H., Rader, F., Heger, M. et al. (2011). Benefit of atrial septal defect closure in adults: Impact of age. European Heart Journal, 32(5), 553–560. https://doi.org/10.1093/eurheartj/ehq352 [Google Scholar] [PubMed] [CrossRef]
21. Moon, J. C., Lorenz, C. H., Francis, J. M., Smith, G. C., Pennell, D. J. (2002). Breath-hold FLASH and FISP cardiovascular MR imaging: Left ventricular volume differences and reproducibility. Radiology, 223(3), 789–797. https://doi.org/10.1148/radiol.2233011181 [Google Scholar] [PubMed] [CrossRef]
22. Prasad, S. K., Pennell, D. J. (2004). Safety of cardiovascular magnetic resonance in patients with cardiovascular implants and devices. Heart, 90(11), 1241–1244. https://doi.org/10.1136/hrt.2003.021154 [Google Scholar] [PubMed] [CrossRef]
23. Pascotto, M., Santoro, G., Cerrato, F., Caputo, S., Bigazzi, M. C. et al. (2006). Time-course of cardiac remodeling following transcatheter closure of atrial septal defect. International Journal of Cardiology, 112(3), 348–352. https://doi.org/10.1016/j.ijcard.2005.10.008 [Google Scholar] [PubMed] [CrossRef]
24. Castaldi, B., Vida, V. L., Argiolas, A., Maschietto, N., Cerutti, A. et al. (2015). Late electrical and mechanical remodeling after atrial septal defect closure in children: Surgical versus percutaneous approach. The Annals of Thoracic Surgery, 100(1), 181–186. https://doi.org/10.1016/j.athoracsur.2015.03.017 [Google Scholar] [PubMed] [CrossRef]
25. Schoen, S. P., Kittner, T., Bohl, S., Braun, M. U., Simonis, G. et al. (2006). Transcatheter closure of atrial septal defects improves right ventricular volume, mass, function, pulmonary pressure, and functional class: A magnetic resonance imaging study. Heart, 92(6), 821–826. https://doi.org/10.1136/hrt.2005.070060 [Google Scholar] [PubMed] [CrossRef]
26. Schulz-Menger, J., Bluemke, D. A., Bremerich, J., Flamm, S. D., Fogel, M. A. et al. (2020). Standardized image interpretation and post-processing in cardiovascular magnetic resonance-2020 update: Society for cardiovascular magnetic resonance (SCMRBoard of trustees task force on standardized post-processing. Journal of Cardiovascular Magnetic Resonance, 22(1), 19. https://doi.org/10.1186/s12968-020-00610-6 [Google Scholar] [PubMed] [CrossRef]
27. Teo, K. S., Dundon, B. K., Molaee, P., Williams, K. F., Carbone, A. et al. (2008). Percutaneous closure of atrial septal defects leads to normalisation of atrial and ventricular volumes. Journal of Cardiovascular Magnetic Resonance, 10(1), 55. https://doi.org/10.1186/1532-429X-10-55 [Google Scholar] [PubMed] [CrossRef]
28. Berger, F., Jin, Z., Ishihashi, K., Vogel, M., Ewert, P. et al. (1999). Comparison of acute effects on right ventricular haemodynamics of surgical versus interventional closure of atrial septal defects. Cardiology in the Young, 9(5), 484–487. https://doi.org/10.1017/S1047951100005394 [Google Scholar] [PubMed] [CrossRef]
29. Papavassiliou, D. P., Parks, W. J., Hopkins, K. L., Fyfe, D. A. (1998). Three-dimensional echocardiographic measurement of right ventricular volume in children with congenital heart disease validated by magnetic resonance imaging. Journal of the American Society of Echocardiography, 11(8), 770–777. https://doi.org/10.1016/S0894-7317(98)70051-3 [Google Scholar] [PubMed] [CrossRef]
30. Salehian, O., Horlick, E., Schwerzmann, M., Haberer, K., McLaughlin, P. et al. (2005). Improvements in cardiac form and function after transcatheter closure of secundum atrial septal defects. Journal of the American College of Cardiology, 45(4), 499–504. https://doi.org/10.1016/j.jacc.2004.10.052 [Google Scholar] [PubMed] [CrossRef]
31. Fang, F., Yu, C. M., Sanderson, J. E., Luo, X. X., Jiang, X. et al. (2011). Prevalence and determinants of incomplete right atrial reverse remodeling after device closure of atrial septal defects. The American Journal of Cardiology, 108(1), 114–119. https://doi.org/10.1016/j.amjcard.2011.03.007 [Google Scholar] [PubMed] [CrossRef]
32. Toyono, M., Krasuski, R. A., Pettersson, G. B., Matsumura, Y., Yamano, T. et al. (2009). Persistent tricuspid regurgitation and its predictor in adults after percutaneous and isolated surgical closure of secundum atrial septal defect. The American Journal of Cardiology, 104(6), 856–861. https://doi.org/10.1016/j.amjcard.2009.05.017 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools