 Open Access
Open Access
ARTICLE
Ventricular Arrhythmia in the Fontan Circulation: Prevalence, Risk Factors and Clinical Implications
1
Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Camperdown, Australia
2
Sydney Medical School, University of Sydney, Camperdown, Australia
3
Clinical Epidemiology and Biostatistics Unit, Murdoch Children’s Research Institute, Parkville, Australia
4
Department of Cardiology, Royal Prince Alfred Hospital, Camperdown, Australia
5
Department of Paediatric Cardiology, Starship Hospital, Auckland, New Zealand
6
Department of Paediatric Cardiology, The Children’s Hospital at Westmead, Westmead, Australia
7
Department of Cardiology, Westmead Hospital, Westmead, Australia
8
Adult Congenital Heart Disease Unit, The Prince Charles Hospital, Brisbane, Australia
9
Department of Cardiology, The Royal Melbourne Hospital, Melbourne, Australia
10 Heart Research Institute, Camperdown, Australia
11 Department of Cardiac Surgery, The Royal Children’s Hospital Melbourne, Parkville, Australia
12 Department of Paediatrics, The University of Melbourne, Parkville, Australia
* Corresponding Author: Rachael Cordina. Email:
Congenital Heart Disease 2023, 18(5), 507-523. https://doi.org/10.32604/chd.2023.028829
Received 10 January 2023; Accepted 15 May 2023; Issue published 10 November 2023
Abstract
Objective: Sudden cardiac death (SCD) and malignant ventricular arrhythmia (VA) are increasingly recognized as important issues for people living with a Fontan circulation, but data are lacking. We sought to characterize the cohort who had sudden cardiac death, most likely related to VA and/or documented VA in the Australia and New Zealand Fontan Registry including risk factors and clinical outcomes. Methods: A retrospective cohort study was performed. Inclusion criteria were documented non-sustained ventricular tachycardia, sustained ventricular tachycardia, ventricular fibrillation, resuscitated cardiac arrest or SCD > 30 days post-Fontan completion. Results: Of 1611 patients, 20 (1.2%) had VA; 14 (1.0%) had VA without SCD and 6 (<1%) had SCD (6% of all deaths recorded in Registry; 5 of those had documented VA at the time of arrest and 1 was presumed to be VA-associated). The median age at first VA was 20.5 (14–32) years, 10 (50%) were females, and the median age at Fontan operation was 8 (4–17) years. On univariable analysis, hypoplastic left heart syndrome (p = 0.03) and older age Fontan operation (p < 0.001) were associated with VA. Earlier Fontan era (p < 0.003), atriopulmonary Fontan (p < 0.001), pre-Fontan atrioventricular valve repair (p = 0.013) pre- or post-Fontan atrial arrhythmia (p = 0.010) were associated with SCD. Patients with VA had a 3 times higher risk of death or heart transplant (HR 3.27(1.19, 8.98), p = 0.02). Conclusions: A proportion of people living with a Fontan circulation have malignant VA. Routine VA screening in this cohort is essential. More data are needed to aid risk stratification.Keywords
With optimized management and surgical refinement, most children born with complex univentricular cardiac conditions repaired with a Fontan circulation will survive to adulthood. However, life expectancy is reduced [1–5] with sudden cardiac death (SCD) accounting for 5%–13% of deaths [4,6–8]. Malignant ventricular arrhythmia (VA) are a likely contributor to SCD in patients with a Fontan circulation but risk factors, appropriate screening strategies and management strategies are not well-established [4,5,7,9–11].
In this retrospective study, we sought to characterise the cohort of patients followed by the Australian and New Zealand Fontan Registry who had documented VA or SCD most likely related to malignant VA.
Approval for this study was obtained as part of ongoing ethical approval for the Australia and New Zealand Fontan Registry. The Registry, created in 2008 [8], is a bi-national registry that collects the clinical data of all patients who have survived a Fontan procedure. Inclusion criteria were documented non-sustained ventricular tachycardia (NSVT), sustained ventricular tachycardia (VT), ventricular fibrillation (VF), resuscitated cardiac arrest or sudden cardiac death (SCD) >30 days post-Fontan completion. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Participants were included in the VA group if the clinical record documented either non-sustained VT (defined as three or more consecutive ventricular beats, at a rate of more than 100 beats per minute and a duration of less than 30 s that terminates spontaneously) or sustained VT (more than 30 s or requiring termination in less than 30 s due to hemodynamic instability). Holter monitor reports are not routinely collected by the registry so the VA needed to be reported in a clinical letter for inclusion. SCD was defined as the sudden cessation of cardiac activity so that the victim became unresponsive, with no normal breathing or no signs of circulation [3,4]. SCD was presumed to be related to VA if there was documented VT or VF during resuscitation or if there was no other explanation such as a thromboembolic event. Moderate to severe ventricular dysfunction (i.e., moderate or worse) is defined as the ejection fraction of less than 40% documented with cardiac MRI or based on the subjective assessment of the treating cardiologist based on transthoracic echocardiographic images [12].
Data were analysed using R software (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria). Patient characteristics were described using the mean and standard deviation for normally distributed variables, or the median and interquartile range for variables that had skewed distributions. Categorical variables were calculated as frequencies and percentages. Separate subgroup analyses were performed for patients with VA. Time to ventricular arrhythmia was measured from completion of the Fontan operation and was censored at the time last known alive with death and heart transplantation as competing risks. Cumulative incidence curves were used to depict the time to ventricular arrhythmia and time to sudden cardiac death (SCD). Time to death or heart transplantation was measured from completion of the Fontan and depicted using the Kaplan-Meier method. Proportion estimates for the time-to-event endpoints at the landmark times of 5, 10, and 15 years were reported with 95% confidence intervals. Cause-Specific Cox regression models were used to identify univariable predictors of developing ventricular arrhythmia and sudden cardiac death. Ventricular arrhythmia was modelled as a time-varying covariate using a Cox regression model to assess its association with time to SCD and death or heart transplantation. We determined freedom from SCD since the date of the initial recorded VA. Due to the small number of events, the multivariable model for ventricular arrhythmia was limited to one variable and was only performed post-hoc to assess whether there was an interaction between HLHS and pre-Fontan Glenn shunt in the model. Hazard ratios and their corresponding 95% confidence intervals were reported.
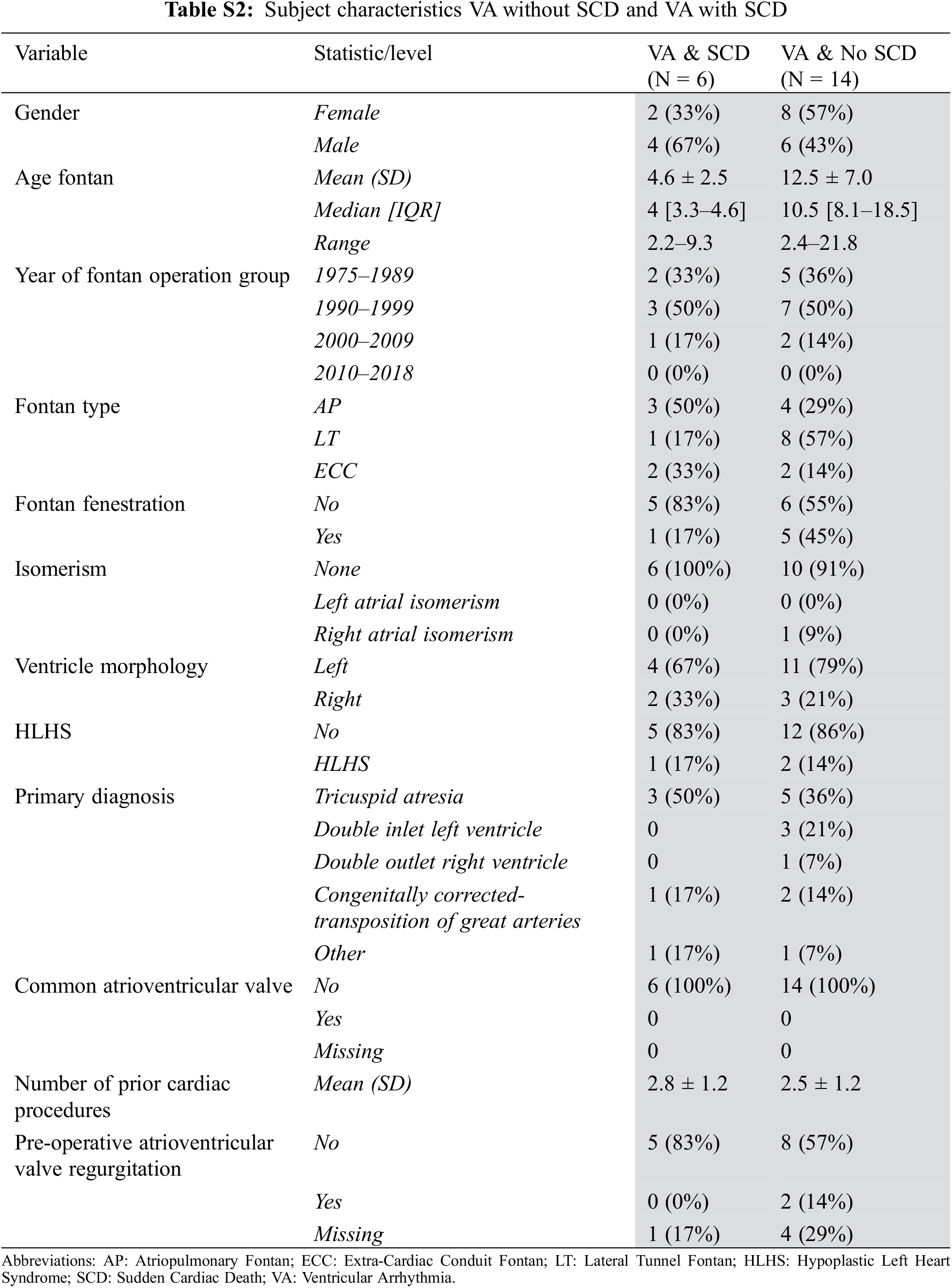
The covariates that were entered into the regression model are shown in Supplementary Tables S1 and S2.
Subject characteristics are demonstrated in Table 1. A total of 1611 patients followed by the ANZ Fontan Registry were included in this study. Of those, 20 (1.2%) had VA recorded (1 case per 1,000 person-years of follow-up). Of the 107 patients who died during follow-up since 2008, 43 (40%) were cardiac-related deaths, 5 (4.7%) of the total deaths were SCD with documented VA and 1 (0.9%) were SCD attributed to VA in the absence of other more likely causes–thus 6 (5.6%; 2.8 deaths per 10,000 person-years of follow-up) of deaths recorded in the Registry were likely related to VA during follow-up.
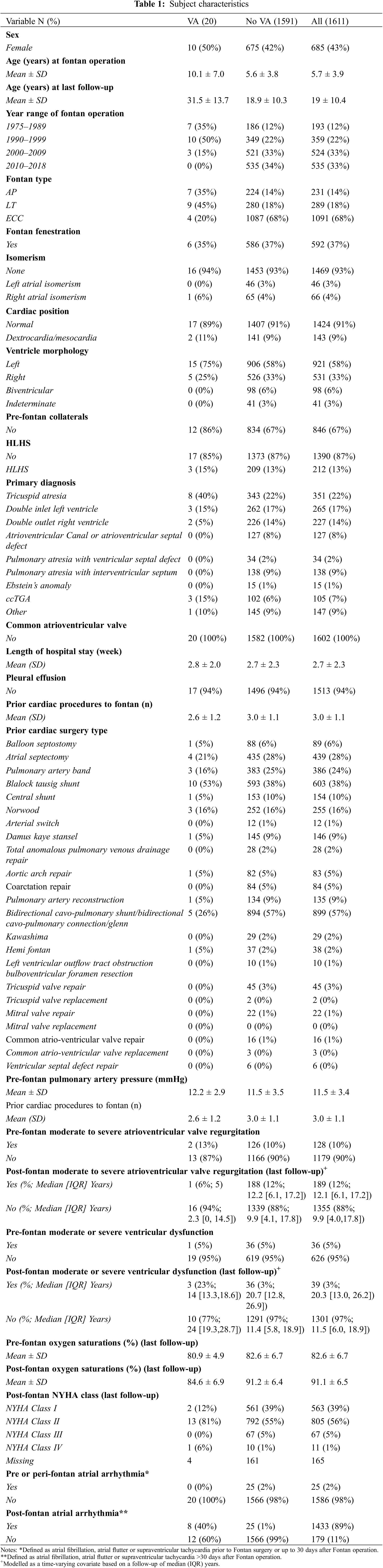
3.2 Ventricular Arrhythmia and Sudden Cardiac Death
In patients with VA, the median follow-up time since Fontan surgery was 23.5 (16–29) years. The VA event occurred at a median age of 20.5 (14–32) years and 14.5 (8.5–22) years post-Fontan repair. The median age at Fontan operation was 8 (4–17) years old and 10 (50%) were females. Subject characteristics are demonstrated in Table 1. Of all Fontan patients followed in the Registry, 43 of 1611 (3%; 2 cases per 1,000 person-years of follow-up) had a cardiac-related death >30 days after their Fontan completion. Of these people, 6 of the 43 deaths (14%) were secondary to VA.
The classification of VA events is demonstrated in Fig. 1. Fourteen of the 20 patients who had VA survived the event and did not suffer from SCD (non-SCD VA). Of the 14 patients with non-SCD VA, arrhythmia was identified via Holter monitoring in 3 patients and exercise stress test in 3 patients, inpatient telemetry for 4 patients (2 had syncopal episodes, 1 had angina, and 1 had palpitations) and the remaining 4 had VA (2 VT, 2 NSVT) noted on their cardiologist’s letter but the mode for identification was unclear. Of the 14 non-SCD VA patients, 3 have died from other causes during follow-up at a median age of 36 (30–40), at ages 24, 36 and 43 years of age, a median time of 19 (16.5–21.5) years from their Fontan repair and a median of 12 (11–13) years from their first VA event. Two deaths were from Fontan circulatory failure and 1 from an unknown cause. It was also observed that if a VA event was noted on other investigations, then an EP study was not performed. In general, most centres will not perform an EP study if there is considered to be a definite sustained ventricular tachycardia or long or frequent episodes of non-sustained ventricular tachycardia documented on monitoring even if there has not been a syncopal episode. However, if a cause for syncope or symptoms is not found, then a diagnostic EP study is usually performed.
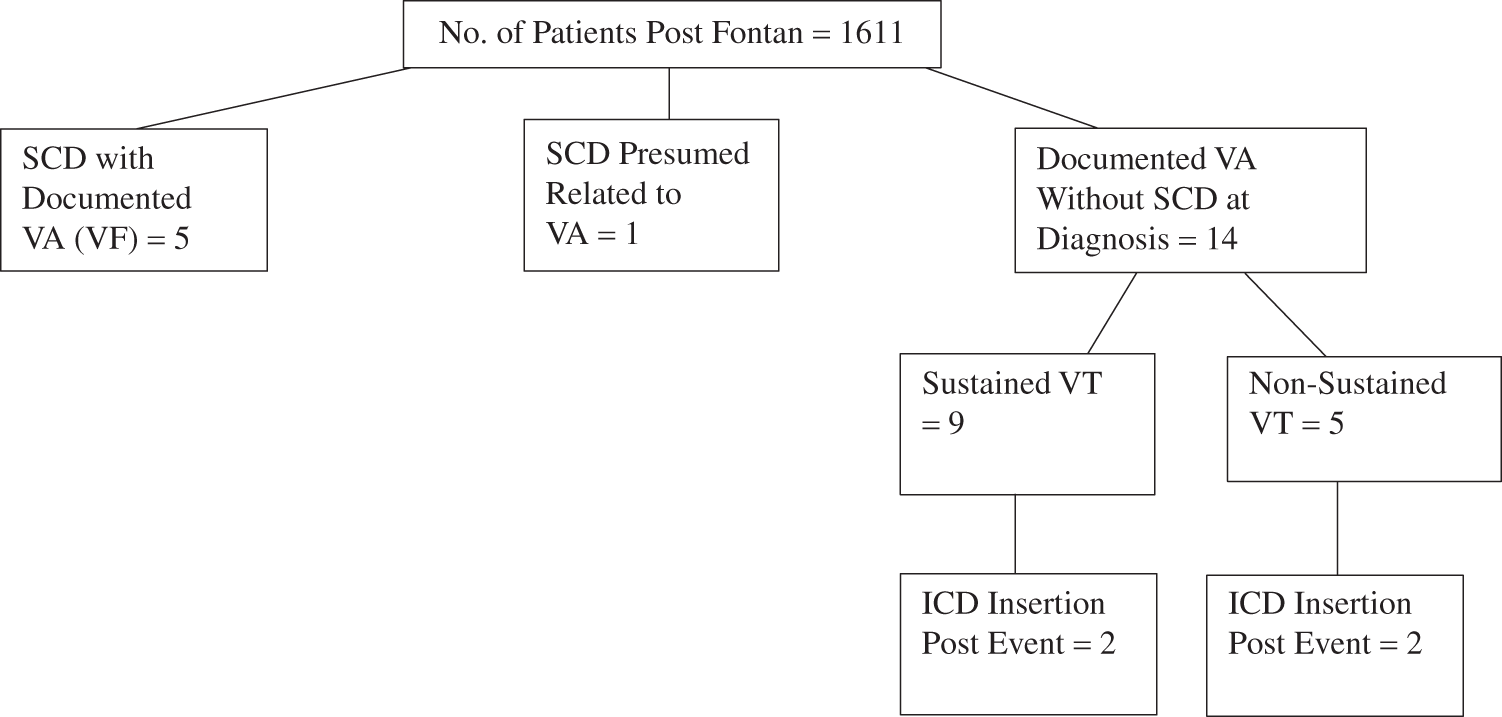
Figure 1: Table describing VA and SCD events
Of the 6 who had SCD due to documented or presumed VA, 5 (83%) were males and 1 (17%) was female. Median age at the time of SCD was 15.5 (14–18) years old and at a median time of 12 (10–14) years from Fontan operation. Four (67%) had dominant left ventricular morphology and the remaining 2 (33%) patients had dominant right ventricular morphology. Three (50%) had an atriopulmonary Fontan, 2 (33%) had an extracardiac conduit and one (17%) had a lateral tunnel Fontan connection. One patient had had an ICD implanted for primary prevention due to severely impaired ventricular function and had SCD from VA in the setting of lead malfunction.
3.3 Management and Cardioverter-Defibrillator Implantation (ICD) Post VA Event
Four of the 14 (29%) patients who survived the VA event had an implantable cardioverter-defibrillator (ICD) inserted after the event. These 4 patients remained alive at a median time of 4.5 (0–27) months from ICD implantation. ICD implantation occurred at a median age of 45 (37–52) years old, 12.5 (8.5–21) days from their VA event. Of the 4 who had an ICD implanted, 2 received an ICD after a documented sustained VT event. The other 2 had received an ICD as they were both symptomatic from their non-sustained VT (NSVT), where one had a syncopal episode and was noted to have frequent NSVT and the other had decreased exercise tolerance and was found to have a high burden of NSVT (multiple episodes up to 12 beats) on exercise stress test. To date, none have received therapy from their ICD.
Of the remaining 10 patients (71%), two were commenced on beta-blockade, one was thought to have had the event due to flecainide (commenced for atrial arrhythmia) which was ceased, one had fenestration closure as the treating team suspected severe cyanosis was the VA trigger, two were referred for cardiac transplantation and in three, uncontrolled rapid atrial arrhythmia was thought to be the trigger (one underwent Fontan conversion, one had an ablation and one was managed medically).
3.4 Hazard and Survival Analysis
Results from the univariable Cause-Specific Cox regression analysis are shown in Tables 2 and 3. Kaplan Meier analysis is shown in Fig. 2 and a competing risks plot is shown in Fig. 3.
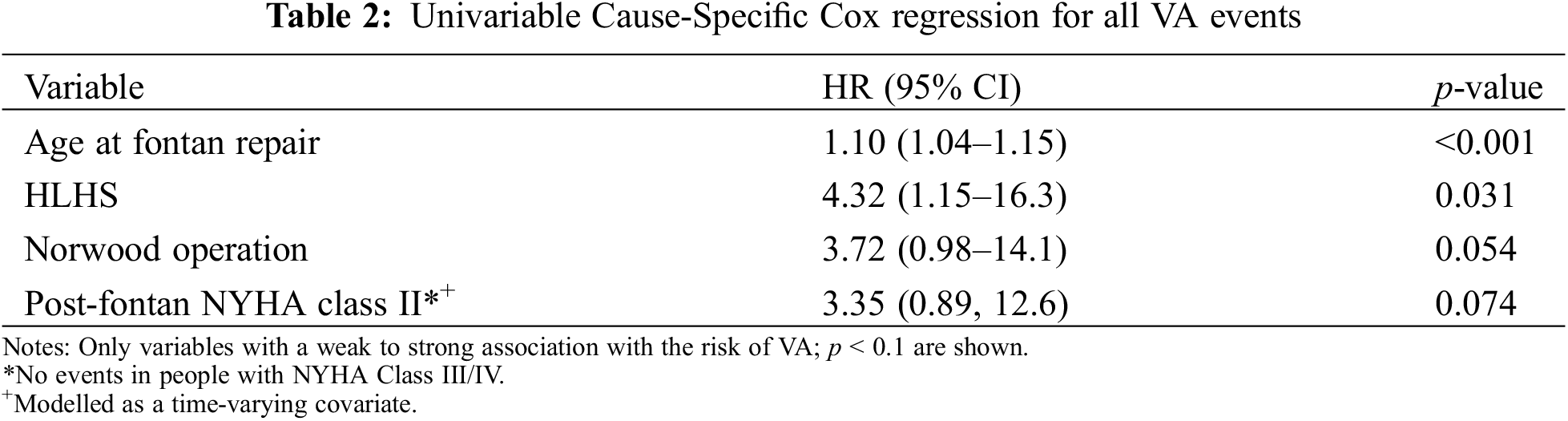
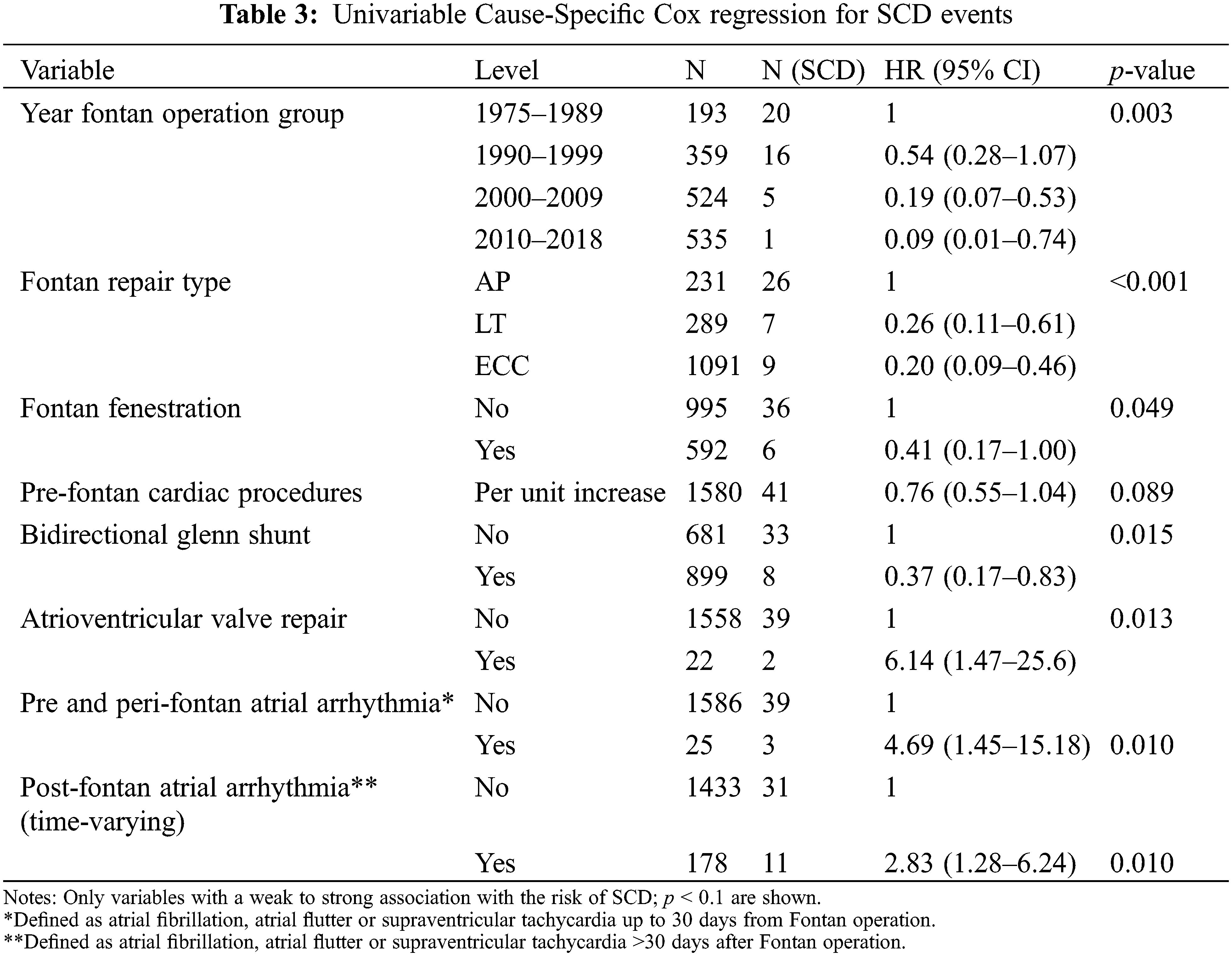
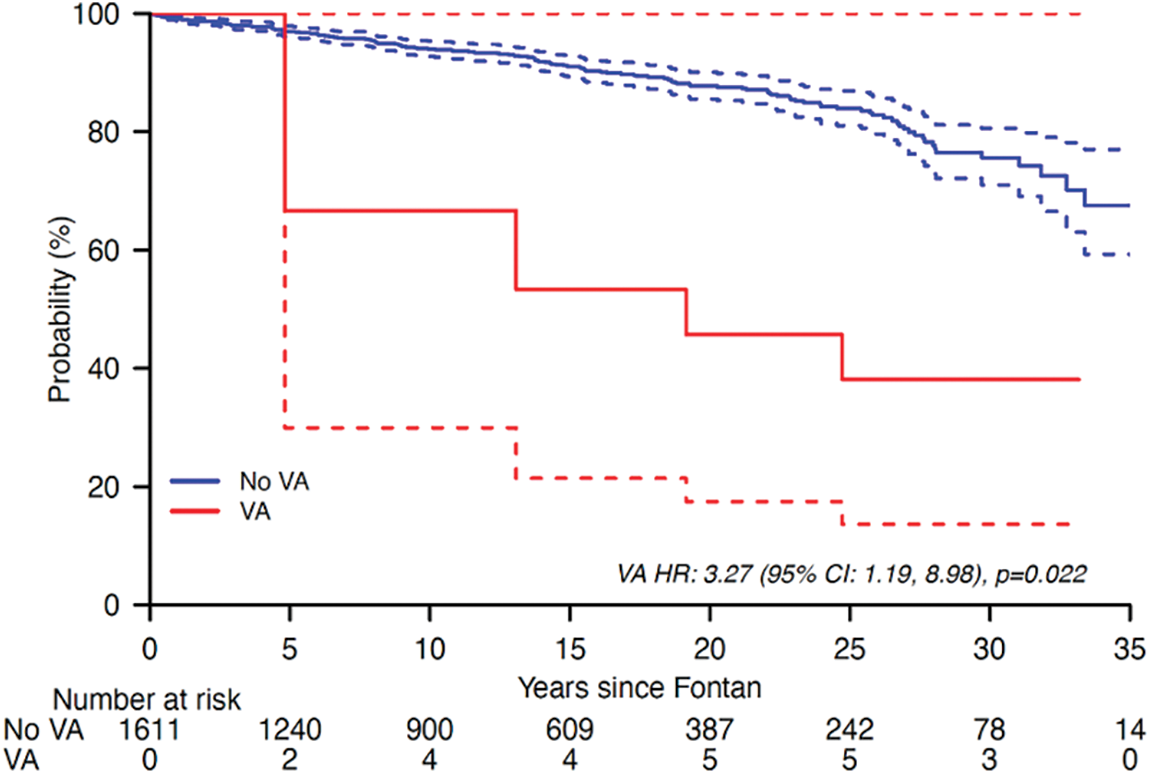
Figure 2: Kaplan-Meier curve demonstrating risk for death or heart transplantation with VA as a time-varying covariate
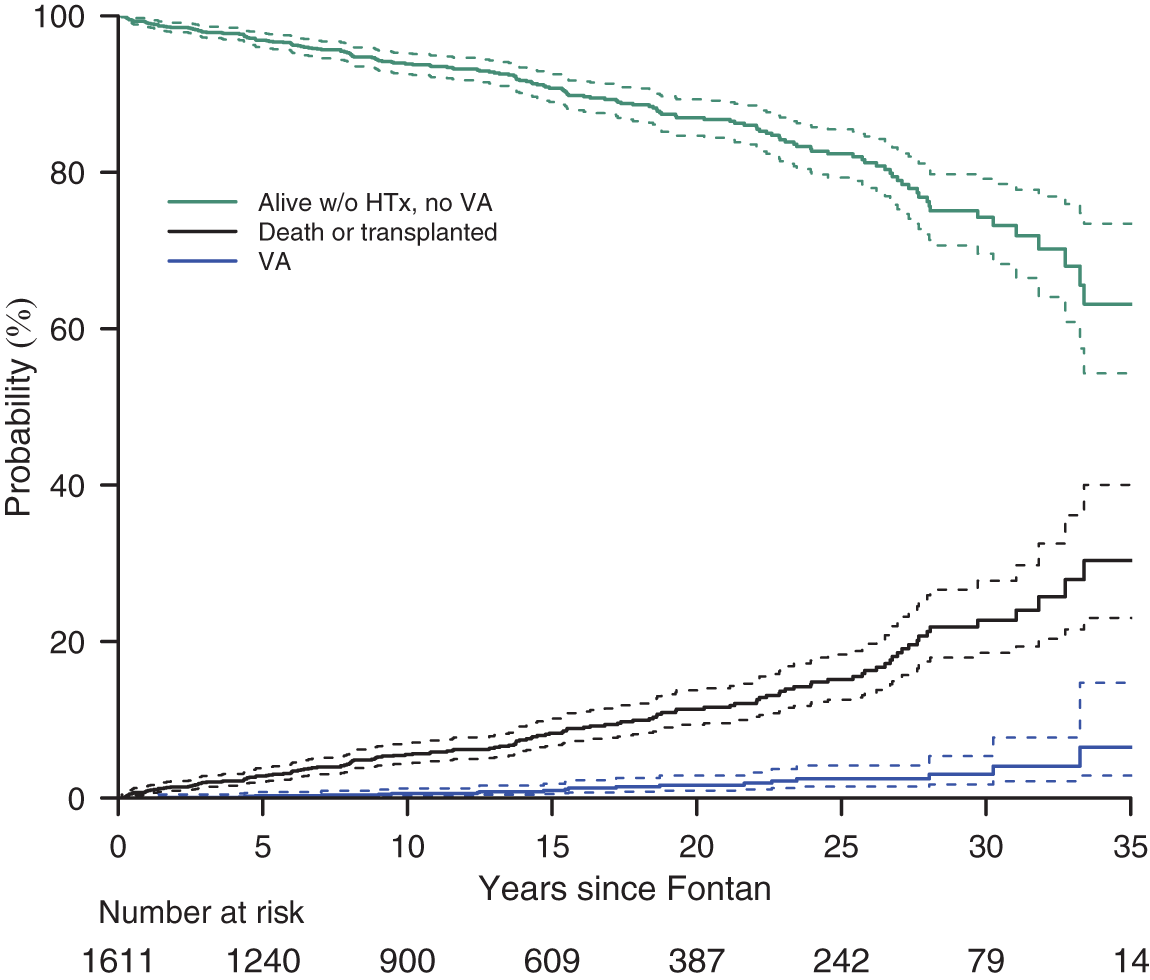
Figure 3: Competing risks plot showing cumulative incidence of VA
Older age at Fontan repair (p < 0.001), having HLHS morphology (p = 0.031), a previous Norwood operation (p = 0.05) and post-Fontan NYHA Class II (p = 0.074) were associated with VA events occurring post–Fontan (Table 2).
Earlier Fontan repair era (p = 0.003), AP Fontan repair (p < 0.001), having a Fontan, pre-Fontan cardiac procedures (p = 0.089), atrioventricular valve repair (p = 0.013), pre and peri-Fontan atrial arrhythmia (p = 0.01) and post-Fontan atrial arrhythmia (p = 0.01) were associated with SCD events post-Fontan repair. Fontan fenestration (p = 0.049) and pre-Fontan Bidirectional Glenn shunt (p = 0.015) were protective against SCD (Table 3).
The Kaplan Meier analysis demonstrates a 3-fold risk of Fontan failure leading to death or heart transplant in those with VA (HR 3.27 (1.19, 8.98), p = 0.02) (Fig. 2).
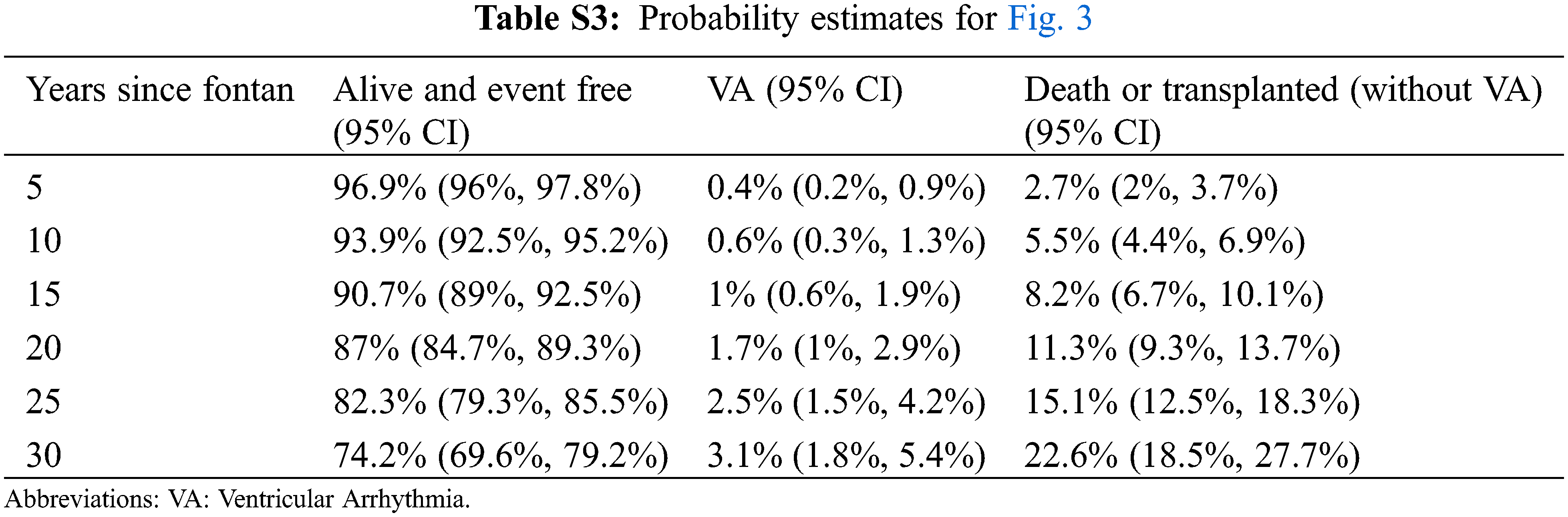
Competing risk analysis demonstrates the cumulative incidence of VA and the contribution of VA to morbidity and mortality (Fig. 3). Probability estimates for Fig. 3 are shown in Supplementary Table S3.
We performed a post hoc multivariable analysis to assess whether HLHS was a confounder with some of the associations observed; we found that the association between pre-Fontan Glenn shunt and VA lost significance when HLHS was added to the model (HLHS–HR 5.49 (1.35,22.3), p = 0.017, pre-Fontan Glenn shunt 0.51 (0.16,1.64), p = 0.26)).
This study, the largest series to date investigating VA in people who have a Fontan circulation, demonstrates that in the current era, ventricular arrhythmia are important clinical issue closely linked to adverse clinical outcomes in the setting of Fontan physiology. However, few patients have an ICD, even in those who have had documented VA, likely reflecting uncertainties relating to risk stratification and challenges with implantation.
Doctor et al. [13] found that patients with adult congenital heart disease (ACHD) other than Tetralogy of Fallot (TOF) and non-sustained ventricular tachycardia (NSVT) were at a higher risk of SCD. Current guidelines recommend an automated implantable cardioverter defibrillator (AICD) for high-risk patients with congenital heart disease with NSVT as a primary prevention for VT/VF or SCD but clinical management has remained cautious [13]. Ventricular arrhythmia have been increasingly recognized as an important late consideration post-Fontan repair [2,4,5,10,11,14] accounting for up to one-quarter of deaths [4,6–8,15]. Despite the relatively low prevalence of VA (1%–8%) in the Fontan population compared with atrial arrhythmia, sustained VA is highly likely to be life-threatening [2], [4,10,11]. One study found that around one fifth of people with a Fontan circulation who had SCD had previously documented VA [4]. A large retrospective study and survey of 996 patients from the Mayo Clinic reported a prevalence of 5% in respondents [4]. In one of the largest series to date from the Pediatric Heart network, VA was identified in 3.5% (n = 520) [10]. Our data demonstrate that just over 1% of the ANZ Fontan have ventricular arrhythmia based on information collected from the clinician letters and 6% of deaths were likely to be VA-associated; our cohort includes current-era patients across the lifespan, the majority of whom have ECC Fontan repair.
Risk factors for VA in the setting of a Fontan circulation are not well-defined. We found that having hypoplastic left heart syndrome and older age at time of Fontan were associated with VA. In our cohort, earlier Fontan operation era, having an atriopulmonary Fontan type repair, a pre-Fontan atrioventricular valve repair and pre- or post-Fontan atrial arrhythmia were associated with SCD. Interestingly having a Fontan fenestration and a pre-Fontan Glenn shunt were protective against SCD which are likely confounded by an earlier era of Fontan operation. While risk factors associated with atrial arrhythmia, death and Fontan circulatory failure are reasonably well characterized, the risk factors for ventricular arrhythmia are not well understood [2,16–18]. The only previously reported clinical risk factors for VA in the setting of Fontan physiology are atrioventricular valve replacement and post-Fontan completion central venous pressures >20 mm Hg; pre-Fontan sinus rhythm was reportedly protective–these data were collected from a people who had Fontan completion from 1973–2012 [4]. The degree of MRI detected myocardial fibrosis has also been shown to correlate with the burden of non-sustained VT after Fontan completion and may be an important underutilized tool to aid risk stratification [19]. It is currently unclear whether the association we and others have observed between atrial arrhythmia and risk for SCD is the result of atrial arrhythmia degenerating into VA, a low output state associated with atrial arrhythmia causing SCD or atrial and ventricular adverse remodeling which predisposes to both VA and atrial arrhythmia. While it is generally accepted that atrial arrhythmia should be aggressively treated in this population, more data are needed to address these uncertainties.
In most large series, HLHS is associated with worse late clinical outcomes including ventricular dysfunction [3] and thus the association we found between HLHS and VA was not unexpected. The association between VA and HLHS was weakened when pre-Fontan Glenn shunt was included in the model suggesting that HLHS combined with pre-Fontan Glenn may be an especially high-risk combination. Delving further into this topic, we noted that patients with Norwood operation had a significantly higher risk of VA and further analysis found that it was likely related to HLHS. Given that all patients who had HLHS had a Norwood operation after.
Of note, none of the patients in our series with HLHS and VA had a Sano (right ventricle-pulmonary artery shunt) procedure which has also been associated with an increased risk for ventricular arrhythmia [20]. One of the most striking negative findings of our study is the lack of association between pre-Fontan ventricular dysfunction and the risk for VA–this may partly be explained by the subjective nature of grading ventricular dysfunction in the setting of univentricular heart–however we have previously reported important associations with other clinical outcomes using these data. Fontan fenestration was originally introduced to create a controlled right-to-left shunt between the systemic venous system and the pulmonary venous atrium to partially offload systemic venous pressures and improve ventricular preload [7,21]. In more recent times, some Australia and New Zealand (ANZ) centers only fenestrate the Fontan circulation in children with suboptimal hemodynamics which may explain why fenestration was protective against SCD in our cohort [7]. The association between (the now obsolete) atriopulmonary connection and SCD likely reflects an era with less effective intraoperative cardiac protection as well as the predisposition to atrial arrhythmia [9,17]. An alternative explanation may be that chronic cyanosis is associated with myocardial dysfunction and fibrosis–emerging data have suggested that chronic cyanosis does negatively impact other organs such as the brain [22] and endothelium [23].
Adequate risk stratification to aid decision making for primary preventative ICD in the setting of Fontan circulation is virtually impossible due to lack of data, reflected in international guidelines [24]. It is also unclear what the ‘tipping point’ should be when short runs of non-sustained VT are identified on routine screening in asymptomatic patients. Data from the Mayo Clinic demonstrated that none of the patients who had suffered SCD in their cohort had an ICD yet all the patients who had an ICD implanted for secondary prevention did not die from SCD reflecting the challenge for clinicians. This therefore reflects the inadequacies of current risk stratifications [4]. Similarly, in our study, none of the patients who had an ICD received appropriate therapy during follow-up–the one patient who did have an ICD and died from VA did not receive appropriate therapy due to lead malfunction. In the absence of data, many centers resort to using severely reduced ejection fraction (<30%–35%) as an indication for primary preventative ICD extrapolating data from acquired heart failure populations [1,25]. However, it is becoming increasingly apparent that using ejection fraction to guide ICD implantation in the setting of complex congenital lesions such as a systemic right ventricle is inadequate for risk stratification. Especially considering the high number of inappropriate shocks that affect this cohort who are prone to atrial arrhythmia [26]. The lack of association we found between ventricular dysfunction and risk of VA further highlights the inadequacies of ejection fraction-determined decisions. There is an urgent need for detailed phenotyping in large multi-center studies designed to develop superior risk stratification tools.
Inadequate risk stratification is not the only barrier to ICD prescription in the Fontan population. Due to the anatomy of the circulation, transvenous implantation is not possible and so historically, surgical epicardial ICD implantation was the only option. The invasiveness of the procedure, perioperative risk and longer post-operative recovery may outweigh the possible benefits in many patients. Secondly, epicardial device-related complications such as lead conductor defects and insulator issues causing the device to lose capture or develop higher thresholds and shorter battery life are also considerations. Lastly, outgrowing of lead length is likely in children. Leadless devices may alleviate many of these issues and have been utilised successfully in the Fontan setting [25–30]. However, many patients are unsuitable for current-generation devices due to baseline ECG abnormalities which would result in poor arrythmia detection [4,25].
This study, although the largest of its kind to date, was limited by small sample size which prevented multivariable analysis. Furthermore, data collection was limited to that collected by the ANZ Fontan registry. We did not have Holter monitor reports–we relied on clinician letters and assumed that clinically significant arrhythmia would have been reported. We did not have sufficient invasive haemodynamic or cardiac MRI data from the cohort to include in our analysis, the latter in particular warrants further investigation.
A proportion of people living with a Fontan circulation have malignant VA which is a common cause for premature death. Routine VA screening in this cohort should be strongly considered as documented VA is not surprisingly a risk factor for SCD. More data are needed to aid risk stratification. International multicentre studies are needed to address the uncertainties in this relatively rare cohort.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Rachael Cordina, Charis Tan; data collection: Charis Tan; analysis and interpretation of results: Diana Zannino, Carley Clendenning; draft manuscript preparation: Charis Tan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data is available via the corresponding author’s email address attached.
Ethics Approval: HREC number 36260.
Conflicts of Interest: The authors declare that they have no conflicts of interests to report regarding the present study.
References
1. Deal, B. J., Jacobs, M. L. (2012). Management of the failing Fontan circulation. Heart, 98(14), 1098–1104. [Google Scholar] [PubMed]
2. Wilson, T. G., Shi, W. Y., Iyengar, A. J., Winlaw, D. S., Cordina, R. L. et al. (2017). Twenty-five year outcomes of the lateral tunnel fontan procedure. Seminars in Thoracic and Cardiovascular Surgery, 29(3), 347–353. [Google Scholar] [PubMed]
3. Iyengar, A. J., Winlaw, D. S., Galati, J. C., Celermajer, D. S., Wheaton, G. R. et al. (2014). Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: The Australia and New Zealand Fontan Registry experience. Journal of Thoracic and Cardiovascular Surgery, 148(2), 566–575. [Google Scholar] [PubMed]
4. Pundi, K. N., Johnson, J. N., Dearani, J. A., Li, Z., Driscoll, D. J. et al. (2017). Sudden cardiac death and late arrhythmias after the Fontan operation. Congenital Heart Disease, 12(1), 17–23. https://doi.org/10.1111/chd.12401 [Google Scholar] [PubMed] [CrossRef]
5. Hakacova, N., Lakomy, M., Kovacikova, L. (2009). Arrhythmias after Fontan operation: Comparison of lateral tunnel and extracardiac conduit. Journal of Electrocardiology, 41(2), 173–177. [Google Scholar]
6. Al-Khatib, S. M., Stevenson, W. G., Ackerman, M. J., Bryant, W. J., Callans, D. J. et al. (2018). 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation, 138(13), e272–e391. https://www.ahajournals.org/doi/epub/10.1161/CIR.0000000000000549 [Google Scholar] [PubMed]
7. Rychik, J., Atz, A. M., Celermajer, D. S., Deal, B. J., Gatzoulis, M. A. et al. (2019). Evaluation and management of the child and adult with fontan circulation: A scientific statement from the american heart association. Circulation, 140(6), e234–e284. https://www.ahajournals.org/doi/epub/10.1161/CIR.0000000000000696 [Google Scholar] [PubMed]
8. Dennis, M., Zannino, D., du Plessis, K., Bullock, A., Disney, P. J. S. et al. (2018). Clinical outcomes in adolescents and adults after the fontan procedure. Journal of the American College of Cardiology, 71(9), 1009–1017. [Google Scholar] [PubMed]
9. Carins, T. A., Shi, W. Y., Iyengar, A. J., Nisbet, A., Forsdick, V. et al. (2016). Long-term outcomes after first-onset arrhythmia in Fontan physiology. Journal of Thoracic and Cardiovascular Surgery, 152(5), 1355–1363.e1. [Google Scholar] [PubMed]
10. Stephenson, E. A., Lu, M., Berul, C. I., Etheridge, S. P., Idriss, S. F. et al. (2010). Arrhythmias in a contemporary Fontan cohort. Journal of the American College of Cardiology, 56(11), 890–896. [Google Scholar] [PubMed]
11. Walsh, E. P. (2002). Arrhythmias in patients with congenital heart disease. Cardiac Electrophysiology Review, 6(4), 422–430. [Google Scholar] [PubMed]
12. Kosaraju, A., Goyal, A., Grigorova, Y., Makaryus, A. N. (2023). Left ventricular ejection fraction. StatPearls Publication. https://www.ncbi.nlm.nih.gov/books/NBK459131/?report=classic [Google Scholar]
13. Doctor, P., Aggarwal, S., Lawrence, D. K., Gupta, P., Singh, G. K. et al. (2022). Device-detected nonsustained ventricular tachycardia in adult congenital heart disease without tetralogy of fallot. Pacing and Clinical Electrophysiology, 45(3), 302–313. [Google Scholar] [PubMed]
14. Peters, N. S., Somerville, J. (1992). Arrhythmias after the Fontan procedure. British Heart Journal, 68(8), 199–204. [Google Scholar] [PubMed]
15. Ohuchi, H., Inai, K., Nakamura, M., Park, I. S., Watanabe, M. et al. (2019). Mode of death and predictors of mortality in adult Fontan survivors: A Japanese multicenter observational study. International Journal of Cardiology, 276, 74–80. [Google Scholar] [PubMed]
16. Nürnberg, J. H., Ovroutski, S., Alexi-Meskishvili, V., Ewert, P., Hetzer, R. et al. (2004). New Onset arrhythmias after the extracardiac conduit fontan operation compared with the intraatrial lateral tunnel procedure: Early and midterm results. The Annals of Thoracic Surgery, 78(6), 1979–1988. [Google Scholar]
17. d’Udekem, Y., Xu, M. Y., Galati, J. C., Lu, S., Iyengar, A. J. et al. (2012). Predictors of survival after single-ventricle palliation. Journal of the American College of Cardiology, 59(13), 1178–1185. [Google Scholar] [PubMed]
18. d’Udekem, Y., Iyengar, A. J., Cochrane, A. D., Grigg, L. E., Ramsay, J. M. et al. (2007). The Fontan procedure: Contemporary techniques have improved long-term outcomes. Circulation, 116(11_supplement). https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.106.676445 [Google Scholar]
19. Rathod, R. H., Prakash, A., Powell, A. J., Geva, T. (2010). Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after fontan operation. Journal of the American College of Cardiology, 55(16), 1721–1728. [Google Scholar] [PubMed]
20. Hall, E. J., Smith, A. H., Fish, F. A., Bichell, D. P., Mettler, B. A. et al. (2018). Association of shunt type with arrhythmias after norwood procedure. The Annals of Thoracic Surgery, 105(2), 629–636. [Google Scholar] [PubMed]
21. Lemler, M. S., Scott, W. A., Leonard, S. R., Stromberg, D., Ramaciotti, C. (2002). Fenestration improves clinical outcome of the fontan procedure: A prospective, randomized study. Circulation, 105(2), 207–212. [Google Scholar] [PubMed]
22. Verrall, C. E., Yang, J. Y. M., Chen, J., Schembri, A., d’Udekem, Y. et al. (2021). Neurocognitive dysfunction and smaller brain volumes in adolescents and adults with a fontan circulation. Circulation, 143(9), 878–891. [Google Scholar] [PubMed]
23. Cordina, R. L., Celermajer, D. S. (2010). Chronic cyanosis and vascular function: Implications for patients with cyanotic congenital heart disease. Cardiology in the Young, 20(3), 242–253. [Google Scholar] [PubMed]
24. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2021). 2020 ESC Guidelines for the management of adult congenital heart disease. European Heart Journal, 42(6), 563–645. [Google Scholar] [PubMed]
25. Silka, M. J., Bar-Cohen, Y. (2008). Should patients with congenital heart disease and a systemic ventricular ejection fraction less than 30% undergo prophylactic implantation of an ICD?: Patients with congenital heart disease and a systemic ventricular ejection fraction less than 30% should undergo prophylactic implantation of an implantable cardioverter defibrillator. Circulation: Arrhythmia and Electrophysiology, 1(4), 298–306. [Google Scholar] [PubMed]
26. Backhoff, D., Kerst, G., Peters, A., Lüdemann, M., Frische, C. et al. (2016). Internal cardioverter defibrillator indications and therapies after atrial baffle procedure for d-transposition of the great arteries: A multicenter analysis: ICD therapy in individuals after atrial baffle procedure for d-TGA. Pacing and Clinical Electrophysiology, 39(10), 1070–1076. [Google Scholar] [PubMed]
27. Clarke, T. S. O., Zaidi, A. M., Clarke, B. (2017). Leadless pacemakers: Practice and promise in congenital heart disease. Journal of Congenital Cardiology, 1(1), 4. [Google Scholar]
28. Al-Ghamdi, B. (2019). Right-sided subcutaneous implantable cardioverter defibrillator system implantation in a patient with complex congenital heart disease and dextrocardia: A case report and literature review. Case Reports in Cardiology, 2019, 1–6. [Google Scholar]
29. Chubb, H., O’Neill, M., Rosenthal, E. (2016). Pacing and defibrillators in complex congenital heart disease. Arrhythmia and Electrophysiol Review, 5(1), 57–64. [Google Scholar]
30. Berul, C. I., Moak, J. P. (2011). Implantable cardioverter-defibrillators in children: Innovation to design a pediatric implantable cardioverter-defibrillator. Journal of Innovations in Cardiac Rhythm Management, 5. [Google Scholar]
Supplementary Materials

Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools