 Open Access
Open Access
ARTICLE
Analysis of Pulmonary Arteries Growth after Initial Shunt Palliation in Neonates and Infants
1 Department of Cardiovascular and Thoracic Surgery, Université catholique de Louvain (UCLouvain)—Cliniques Universitaires Saint-Luc, Brussels, B-1200, Belgium
2 Department of Pediatric Cardiology, Université catholique de Louvain (UCLouvain)—Cliniques Universitaires Saint-Luc, Brussels, B-1200, Belgium
3 Department of Anesthesiology, Université catholique de Louvain (UCLouvain)—Cliniques Universitaires Saint-Luc, Brussels, B-1200, Belgium
4 Department of Pediatric Intensive Care, Université catholique de Louvain (UCLouvain)—Cliniques Universitaires Saint-Luc, Brussels, B-1200, Belgium
* Corresponding Author: Alain J. Poncelet. Email:
# Co-first authors
Congenital Heart Disease 2023, 18(5), 525-537. https://doi.org/10.32604/chd.2023.042341
Received 26 May 2023; Accepted 07 August 2023; Issue published 10 November 2023
Abstract
Objective: Despite increasing enthusiasm for neonatal repair, patients with ductal-dependent circulation (pulmonary/systemic) or restrictive pulmonary blood flow still require initial palliation. Ductal stenting has emerged as an endovascular approach whereas modified-Blalock-Taussig and central shunt remain surgical references. In this study, we analyzed the relationship between pulmonary artery growth, sites of shunt connection, or antegrade pulmonary blood flow in surgically placed shunts. The need for secondary catheter-based interventions or pulmonary arterioplasty was also investigated. Methods: A retrospective single-center study analyzing 175 patients undergoing surgery for a central or modified-Blalock-Taussig shunt. Outcome growth variables were right pulmonary artery/left pulmonary artery diameters/Z scores, the indexed sum area (right pulmonary artery + left pulmonary artery), and the pulmonary symmetry index. Three imaging modalities were used: angiography, computed tomography, and echocardiography. Results: At baseline, pulmonary arteries were larger in patients with antegrade pulmonary blood flow (Nakata index 137 vs. 114, p = 0.047) as well as in patients receiving a modified-Blalock-Taussig shunt (Nakata index 138 vs. 84, p < 0.001). At the time of shunt takedown, both the right pulmonary artery and left pulmonary artery had normalized their diameter. The Nakata index increased from 134 to 233 mm2/m2 (p < 0.001). The pulmonary artery index remained stable (0.86) over time. During the inter-stage period, shunt-related pulmonary artery stenosis and juxta-ductal stenosis were diagnosed in 16 (10%) and 17 patients (11%), respectively. Conclusions: Surgical shunt palliation allows normal pulmonary artery growth. Pulmonary artery stenosis was either shunt-related (10%) or secondary to juxta-ductal stenosis (11%). Close echographic follow-up allows early diagnosis and treatment of juxta-ductal stenosis.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileAbbreviations and Acronyms
| CHD | Congenital heart diseases |
| CPB | Cardiopulmonary bypass |
| CT | Computed tomography |
| DORV | Double outlet right ventricle |
| IQR | Interquartile range |
| LPA | Left pulmonary artery |
| LVOTO | Left ventricular outflow tract obstruction |
| MANOVA | Multivariate analysis of variance |
| MBTS | Modified-blalock-taussig shunt |
| MPA | Main pulmonary artery |
| PA | Pulmonary artery |
| RPA | Right pulmonary artery |
| TGA | Transposition of the great arteries |
| VSD | Ventricular septal defect |
Over the last decades, neonatal repair has emerged as the gold standard for many congenital heart diseases, reducing the scope of early palliation and staged repairs. However specific subgroups of complex congenital heart disease such as those patients with ductal-dependent pulmonary/systemic circulation or restrictive antegrade pulmonary blood flow still require temporary palliation to provide time for pulmonary resistance to decrease and for pulmonary arteries to grow [1].
Surgical palliations include modified-Blalock-Taussig shunt (MBTS) and central shunts, whereas ductal stenting has emerged lately as an attractive alternative endovascular approach [2].
Distortion of the pulmonary arteries, localized stenosis, or asymmetric growth are well-known potential complications of surgical palliation [3–5].
For univentricular patients, optimal pulmonary artery growth and preserved single ventricle systolic and diastolic function are paramount for long-term success. In the hypoplastic left heart syndrome subgroup, emphasis was placed on the comparison of right ventricle-pulmonary artery conduit and MBTS [6] in terms of early/intermediate morbidity and mortality, associated risk factors, and to a lesser extent to PA (pulmonary artery) growth [7,8]. Briefly, MBTS was shown to result in an asymmetrical PA growth with smaller LPA (left pulmonary artery) at the time of stage II palliation. However, previous studies on PA growth after palliation in unselected patients had reported equivocal results, with most studies demonstrating a balanced PA growth or a decreased distal RPA (right pulmonary artery) growth [9–11].
Finally, recent “provocative” studies could renew pediatric cardiologists and surgeons’ interest in early palliation and staged repair for simple congenital heart diseases (CHD) such as tetralogy of Fallot [12,13]. Indeed, analyzing more than 2,300 procedures, Savla et al. reported that early repair was associated with a 51%-increased risk of death at a 2-year follow-up in comparison to a staged strategy. Similarly, Ghimire et al. reported a 2.2-fold increase in in-hospital mortality for those patients repaired in the neonatal period.
In the context of the increasing use of ductal stenting as palliation worldwide, we reviewed our 20-year experience of surgical shunt palliation with a specific interest in the relationship between pulmonary artery growth and shunt origin, site of PA connection, as well as the additive effect of antegrade pulmonary blood flow. In addition, we also analyzed the need for catheter-based pulmonary artery dilation/stenting during the inter-stage period or the need for pulmonary arterioplasty at the time of the second surgery.
This retrospective analysis was approved by our Institutional Ethical Board (Cliniques Universitaires Saint-Luc, Brussels, Belgium, IRB 2015\11-01\ID 327).
2.1 Data Collection and Follow-Up
All data were collected from patient’s medical records, operative notes, and catheterization studies. Among the variables of interest selected for this study (Supplementary Table 1), age and weight a) at the time of the shunt operation and b) at the shunt take-down operation (further palliation or repair), size of the shunt, site of proximal and distal anastomosis, presence of antegrade pulmonary blood flow, and the use of cardiopulmonary bypass.
Echocardiography and cardiac catheterization including pulmonary angiography were systematically performed prior to the second surgical procedure. Angio-computed tomography (CT) or angio-magnetic resonance imaging studies were not routinely obtained during the study period.
For the purpose of this study, pulmonary artery diameter at the time of palliation as well as at the time of subsequent procedure were recorded both in absolute size as well as Z scores which were derived from the nomograms of PA size as previously published [14].
Pulmonary artery size was assessed just prior to definitive surgical repair by any of the following modalities: echocardiography, CT, and/or angiography.
For patients who had pulmonary artery size assessed by different imaging modalities, prioritization was as follows: 1) angiography, 2) CT if available, and 3) echocardiography.
We used two methods to quantify pulmonary artery size: the Nakata index [15], the summed cross-sectional area (assuming a cylindrical vessel) of the right and left pulmonary arteries indexed to the patient’s body surface area, and the symmetry index, a ratio of diameters of the smaller pulmonary artery to the larger pulmonary artery. The symmetry index is always ≤1, with values closer to 1 reflecting more symmetrical vessel size [16].
PA stenosis was either shunt-related or juxta-ductal. A significant stenosis was defined as a luminal diameter constriction greater than 50%.
Continuous data are presented as mean ± standard deviation, or median (interquartile range) for nonparametric data. The normality of the distribution was assessed with the Shapiro–Wilk test. Categorical data are presented as numbers and proportions and compared with the Chi-square test or the Fisher’s exact test, if appropriate. Differences between means or medians were compared using unpaired Student’s t-test or Mann–Whitney U test, according to the distribution. The statistical significance of differences between more than two groups was tested by ANOVA (one-way analysis of variance) with a Bonferroni post hoc test.
All data were recorded at the initial palliation surgery as well as at the second surgical procedure. We first compared outcome variables at baseline: RPA/LPA diameters and RPA/LPA Z score, sum (RPA + LPA) indexed to body surface area and the pulmonary symmetry index.
Secondly, we analyzed the growth differences of these outcome variables between subgroups defined by the presence of antegrade pulmonary flow, the anatomy (uni- vs. bi-ventricular), gender, insertion and origin of the shunt, size of the shunt, the use of cardiopulmonary bypass and the presence of juxta-ductal stenosis.
All statistical analyses were performed using the IBM SPSS Statistic version 26 (IBM Corp., Armonk, New York, USA).
3.1 Demographic and Operative Details
From 1997 to 2019, 175 patients underwent a palliative shunt procedure (MBTS or central shunt) at Cliniques Universitaires Saint-Luc.
Twenty-one patients were excluded from further analysis: in-hospital and inter-stage mortality (19/175 or 10.8%) and lost to follow-up (2/175). Of the remaining 154 patients, 20 patients are awaiting further surgery and 134 completed a second procedure toward single-ventricle palliation (n = 44) or bi-ventricular repair (n = 90). Those 134 patients represent the study cohort for all morphometric analysis (Fig. 1).
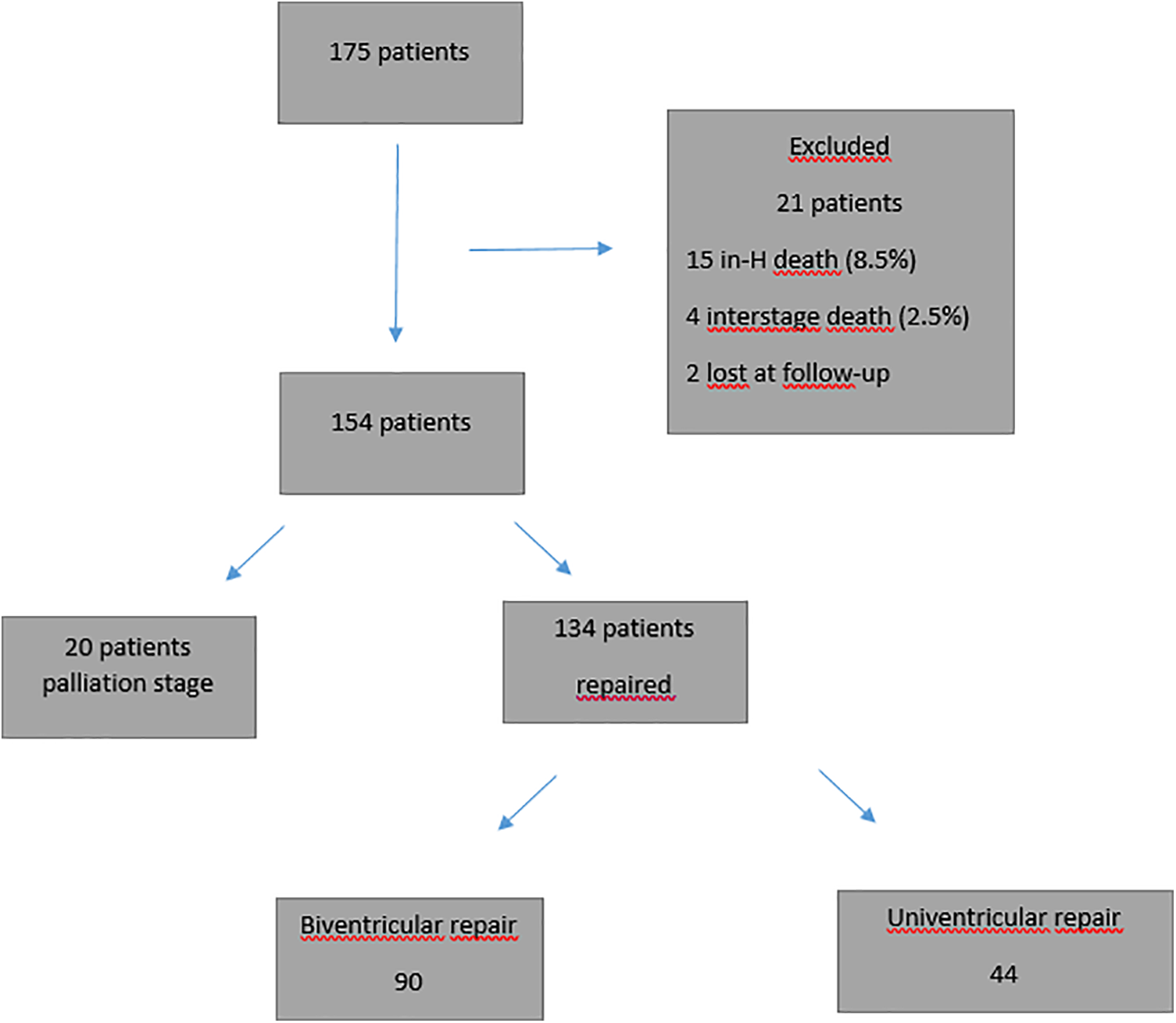
Figure 1: Patient cohort flowchart
The median age and weight at shunt palliation were 24 days (IQR (Interquartile Range) 7–95) and 3.4 Kg (IQR 2.9–4.8).
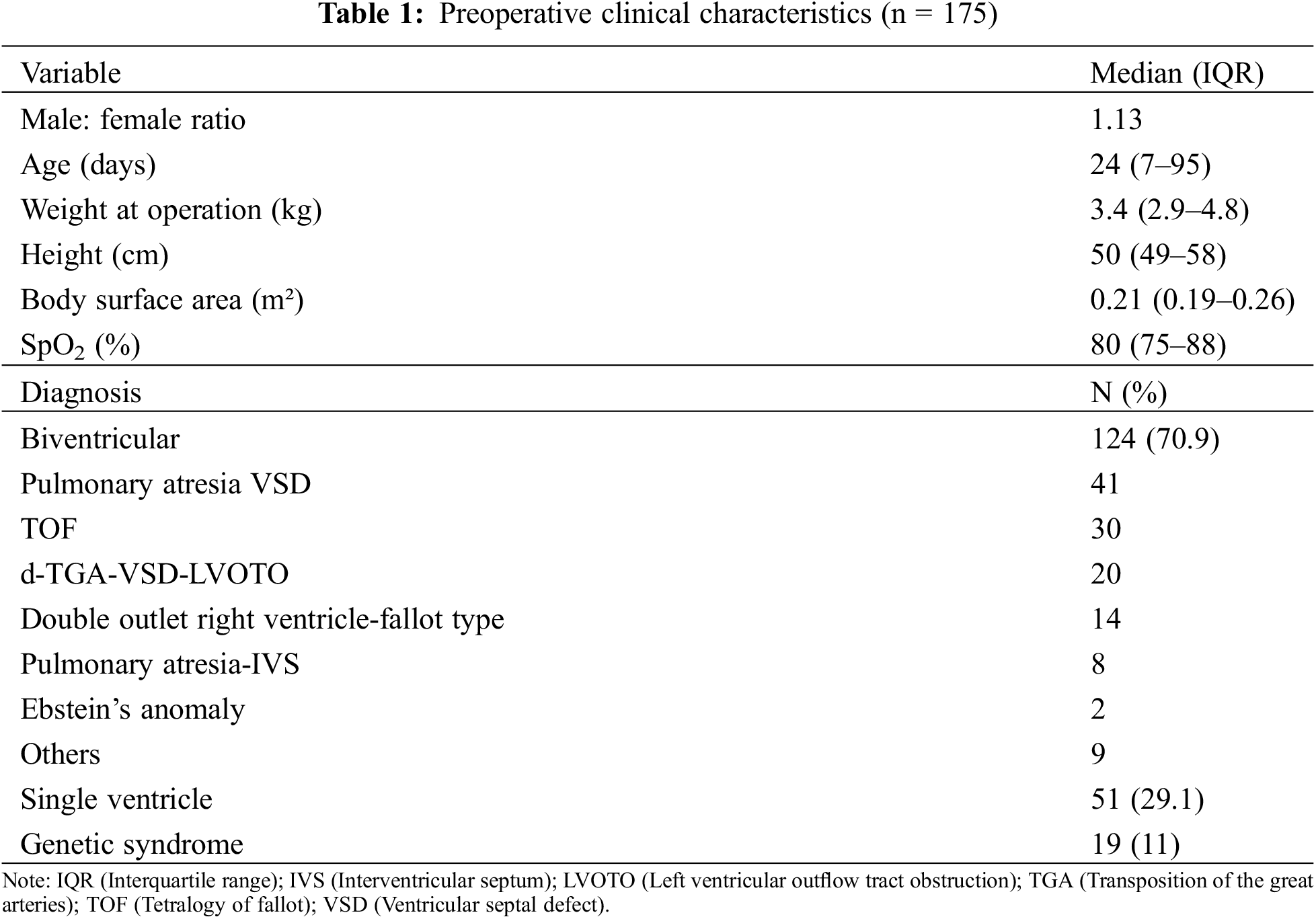
Most of the patients (162/175, 93%) were either neonates or infants. Prior to surgery, median oxygen saturation was 80% (IQR 75%–88%). Antegrade pulmonary blood flow was present in 97 patients (55%). The commonest diagnosis were patients with uni-ventricular heart (n = 51), pulmonary atresia-ventricular septal defect (n = 41), tetralogy of Fallot (n = 30), d-TGA (transposition of the great arteries)-VSD (ventricular septal defect)-LVOTO (left ventricular outflow tract obstruction) (n = 20) and double outlet right ventricle–Fallot type (n = 14) (Table 1).
The operation was performed by thoracotomy or sternotomy in 129 (74%) and 46 (26%) patients respectively. Cardiopulmonary bypass was required in 23 patients (13%).
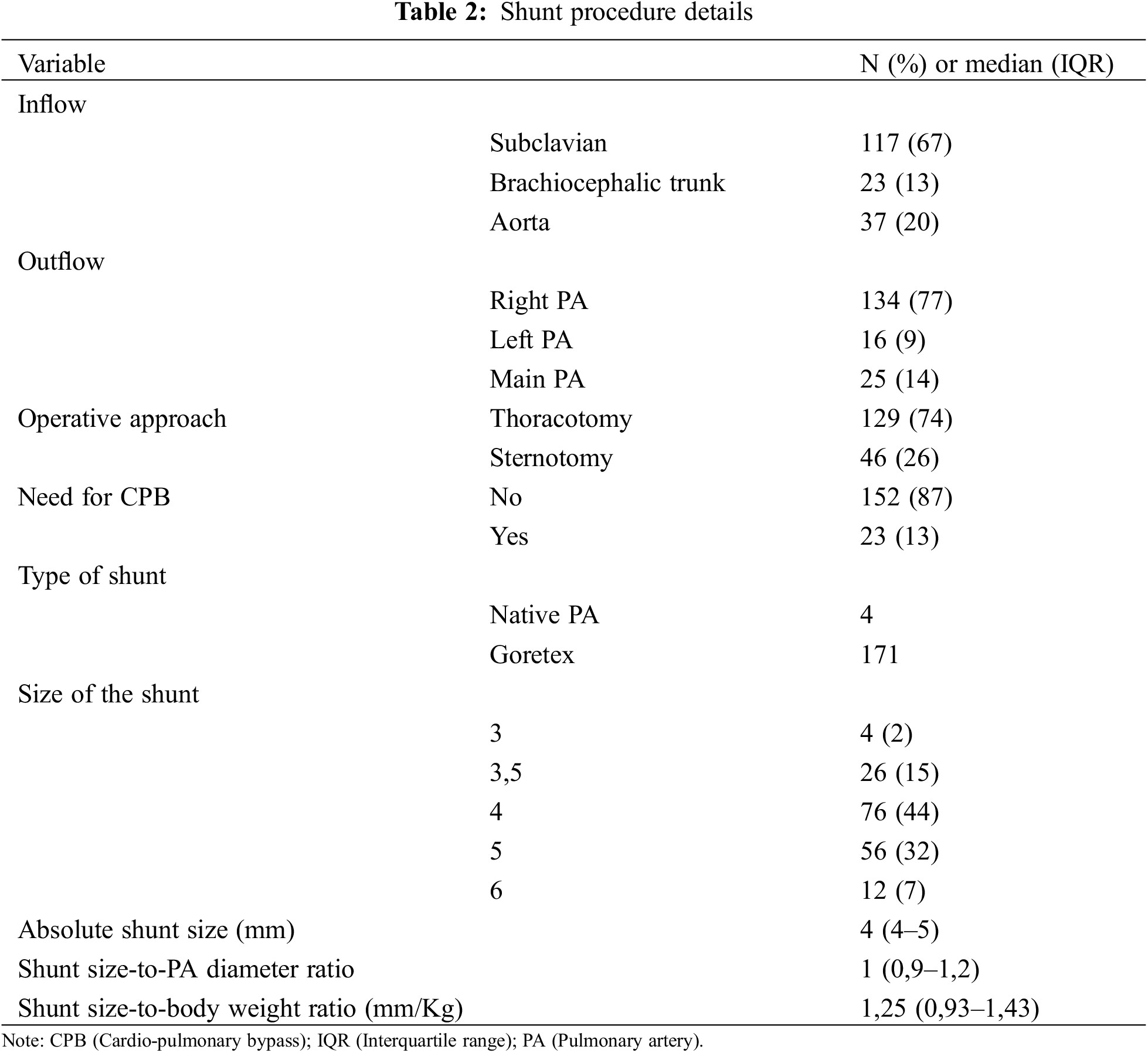
Proximal anastomosis came from the subclavian artery, the brachiocephalic trunk or the ascending aorta in 117, 23 and 35 patients, respectively. Distal anastomosis was performed on the right PA (134 patients, 77%) or left PA (16 patients, 9%), and on the main PA in 25 patients (14%).
Goretex stretch vascular grafts were used in 171 patients, 4 patients had direct MPA (main pulmonary artery) to aorta anastomosis without interposing graft. The most frequent shunt sizes (graft or native PA) were 3.5 mm (n = 26), 4 mm (n = 77) and 5 mm (n = 56) (Table 2). The median shunt-weight ratio was 1.25 (IQR 0.92–1.43) and the median shunt-to-PA diameter was 1 (IQR 0.9–1.2) (Table 2).
Patients were routinely placed under unfractionated heparin on arrival in the pediatric Intensive Care Unit, and started on acetylsalicylic acid during the immediate postoperative period. Overall in-hospital mortality was 8.6% (15/175).
The median interval time from initial palliation to shunt takedown operation was 345 days (IQR 202–755).
3.2 Pulmonary Artery Size and Growth
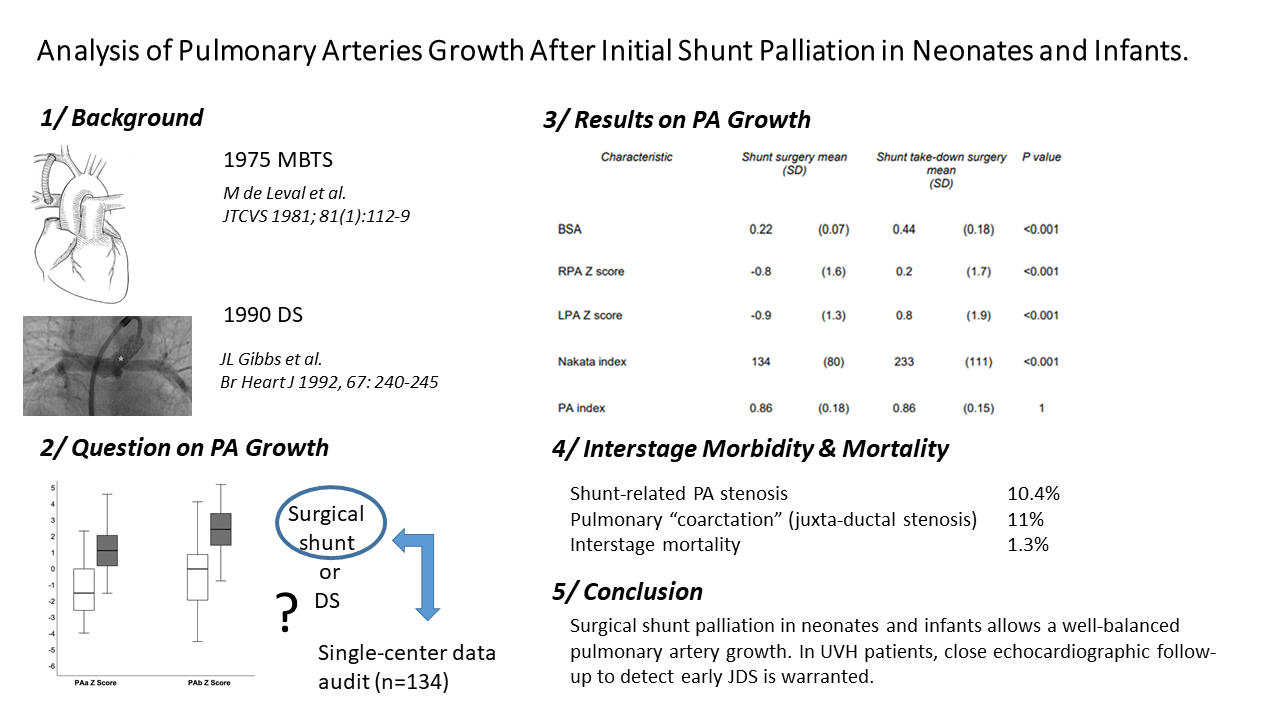
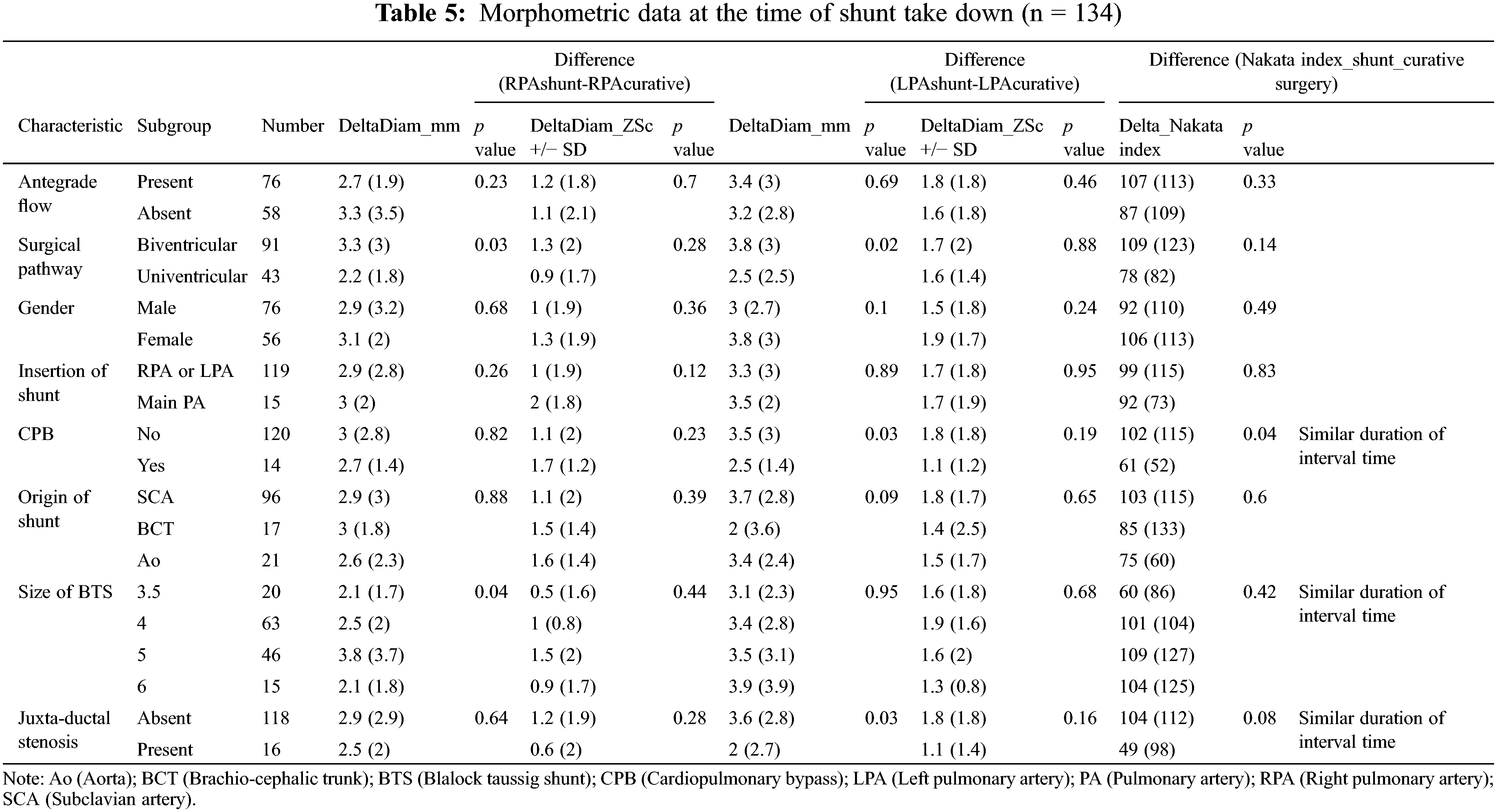
Comparison of morphometric measures at the time of shunt palliation shows that pulmonary arteries were larger in patients with antegrade pulmonary blood flow (Nakata index 137+/−80 vs. 114+/−71 mm2/m2, p = 0.047), in patients in whom the proximal and distal anastomosis were the subclavian artery (Nakata index 138+/−83 vs. 84+/− 49 mm2/m2, p < 0.001) and the right (or left) pulmonary artery (Nakata index 136+/−77 vs. 70+/− 47 mm2/m2, p < 0.001), respectively. Gender, uni- or bi-ventricular anatomy, or the size of the shunt did not correlate to morphometric measures (Table 3).
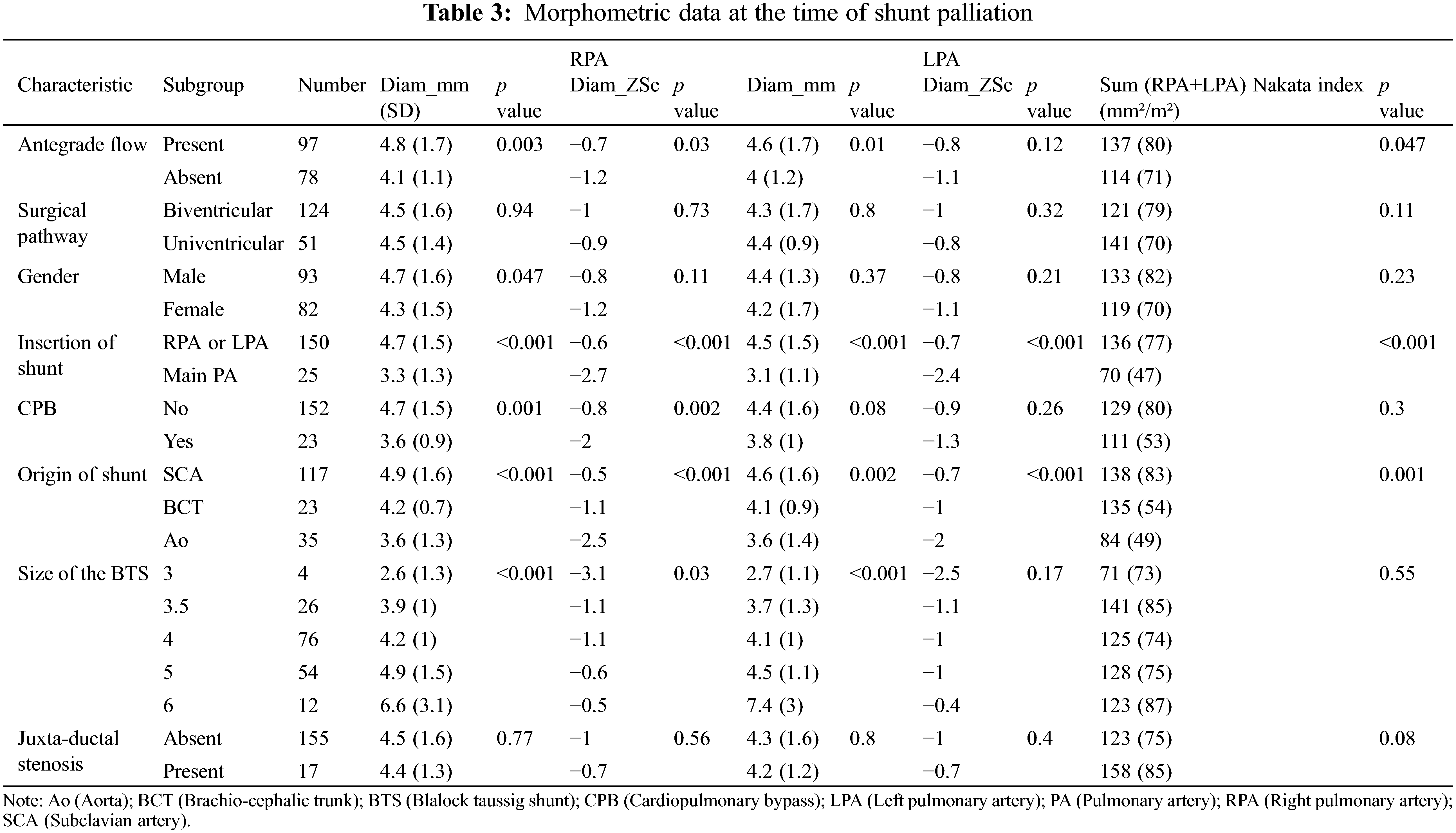
As illustrated in Table 4, all morphometric measures increased significantly from the time of shunt palliation to the second surgical operation. Both RPA and LPA normalized their diameter at the time of shunt takedown (+0.2 and +0.8 ZSc, respectively). The Nakata index increased from 134+/−80 to 233+/−111 mm2/m2 (p < 0.001). Importantly, asymmetric growth was not observed (PA index = 0.86 at each time point), whereas the LPA appeared to grow to a greater extent than the RPA (difference RPA-LPA: −0.07 ZSc at shunt surgery, −0.5 Zsc at shunt take-down, p 0.02).
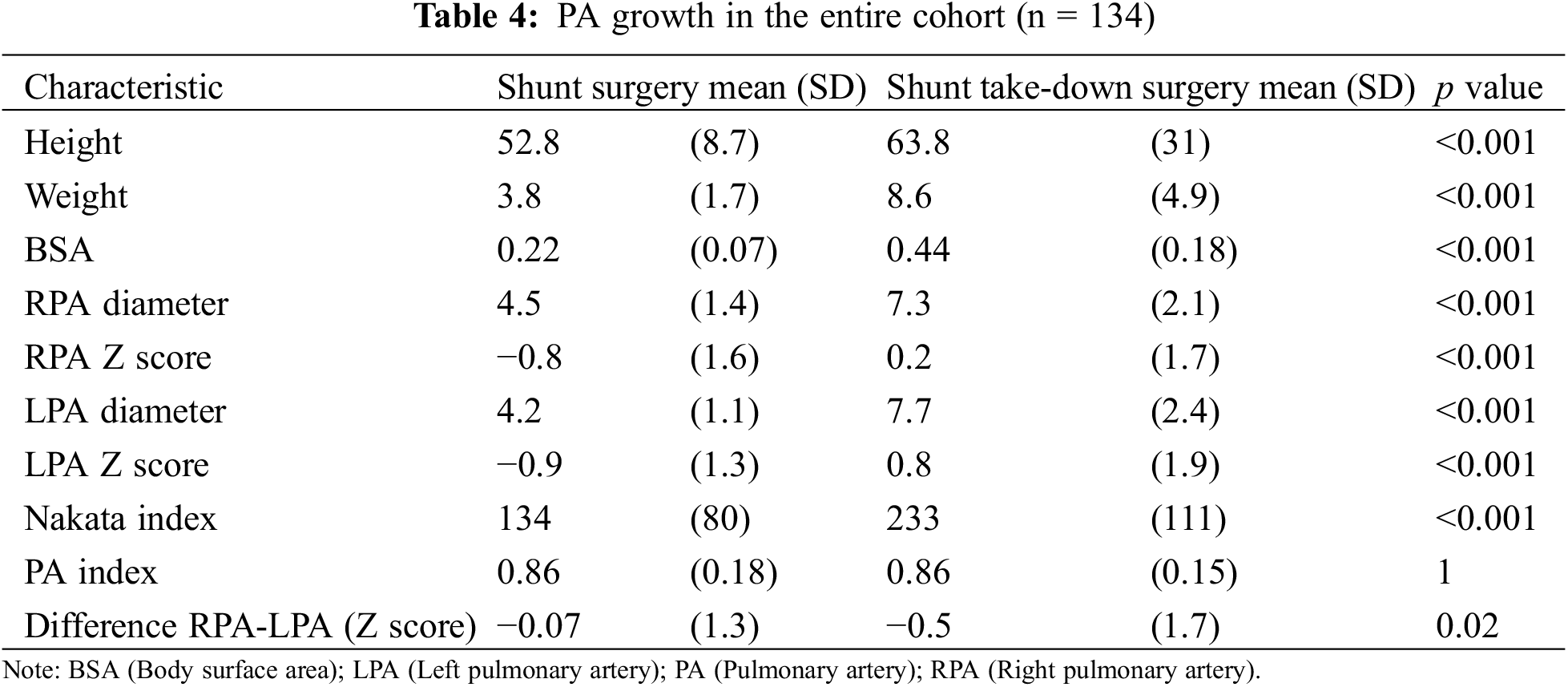
As shown in Table 5, antegrade pulmonary blood flow had no influence on pulmonary artery growth (Delta Nakata index: p = 0.30). The growth of both PA was greater in the biventricular pathway expressed as millimeter gain (Delta RPA diam: 3.3 vs. 2.2 mm, p = 0.03, Delta LPA diam: 3.8 vs. 2.5 mm, p = 0.02) resulting in a greater Nakata index increase (Delta Nakata index: 109 vs. 78 mm2/m2, p = 0.14) but if growth was expressed in Z-scores, the effect was lost. Importantly, the inter-stage period was significantly shorter in the univentricular pathway (median 221 [173–363] vs. 483 [273–1058] days, p < 0.001).
The distal site of shunt anastomosis (MPA vs. Right/(Left) PA) had no influence neither on PA growth (Delta Nakata index: 92 vs. 99 mm2/m2, p = 0.83) nor on the difference between PA arteries (0.3 mm vs. −0.5 mm, p = 0.35).
With similar inter-stage duration (median 316 [159–698] vs. 345 [209–811 days], p = 0.31), the LPA growth was significantly larger in the non-CPB (cardiopulmonary bypass) group (Delta LPA diam: 3.5 vs. 2.5 mm, p = 0.03), resulting in a greater Nakata increase (Delta Nakata index: 102 vs. 61 mm2/m2, p = 0.04).
In contrast, in the presence of juxta-ductal stenosis, LPA growth was significantly smaller (Delta LPA diam: 2 vs. 3.6 mm, p = 0.03), resulting in a smaller Nakata increase (Delta Nakata index: 49 vs. 104 mm2/m2, p = 0.08).
3.3 Inter-Stage Mortality and Shunt-Related Reinterventions
Four patients died during the inter-stage period (2.5%), two were shunt-unrelated (viral pneumonia, n = 1 and recurrence of pulmonary vein stenosis after total anomalous pulmonary venous return repair, n = 1) and two were shunt-related (one sudden death following shunt thrombosis and one death of unknown cause) (1.3%).
Thirty-two patients (20.7%) developed pulmonary artery stenosis (shunt-related and/or juxta-ductal stenosis) during the inter-stage period. Shunt-related pulmonary artery stenosis (>50%) were diagnosed in 16 patients (10.4%) and pulmonary coarctation (juxta-ductal stenosis) were diagnosed in 17 patients (11%), respectively.
Prior to corrective surgery, catheter-based angioplasty or stenting of PAs was required in 19 patients (12.3%). For patients with juxta-ductal stenosis, either balloon angioplasty (n = 2) or contralateral shunt placement (n = 14) was performed. At the time of corrective surgery, for those patients with shunt-related pulmonary artery stenosis, 11 required pulmonary arterioplasty (patch augmentation). In patients with uni-ventricular palliation and shunt-related pulmonary artery stenosis, the bidirectional cavo-pulmonary anastomosis allowed an easy repair without patch augmentation in all patients (n = 5).
Finally, 21 patients (13.6%) underwent elective catheterization for MBTS stenting in order to optimize saturation prior to curative/palliative surgery.
Currently, the most common indications for shunt palliation are neonates and infants with ductal-dependent pulmonary/systemic circulation or restrictive antegrade pulmonary blood flow. Most procedures are performed in uni-ventricular heart patients [17,18]. However, as stated earlier, the “provocative” reports from Slava and Ghimire on short and mid-term outcomes favoring staged repair against early primary repair in tetralogy of Fallot patients could renew pediatric cardiologist and surgeon’s interest in early palliation even for less complex CHDs [12,13].
The field of interventional cardiology has greatly extended over the past years and neonatal ductal stenting has emerged as an alternative to initial surgical palliation [16]. This alternative approach in neonatal care for complex patients has stimulated our group to evaluate its own results with shunt palliation so that it would stand as a benchmark for future comparison.
At baseline, our analysis demonstrates that pulmonary arteries were better developed in patients with residual antegrade pulmonary blood flow (Tetralogy of Fallot, TGA-VSD-LVOTO, DORV (Double outlet right ventricle)-pulmonary stenosis). Our primary analysis also highlights our surgical bias of performing a central shunt when pulmonary arteries were less developed.
Our longitudinal analysis of morphometric data corroborates earlier studies and contradicts the recent multi-centric study comparing ductal stenting and surgical shunt interposition [16].
First, analysis of PA growth in our cohort either separately (RPA or LPA) or together (Nakata index) showed that a significant increase took place in the inter-stage period both in diameter, Z-score, and surface area.
Indeed, our measurements at the time of shunt take-down for the RPA and LPA were 0.73cm and 0.77 cm, respectively. Using previously published nomograms [14], and based on a calculation using the average weight and age of the cohort’s infants (8.6 Kg/64 cm), the respective dimensions of RPA and LPA should have been 0.7 cm (0.5–0.9 cm) and 0.7 cm (0.5–0.9 cm). This underscores that both RPA and LPA diameters and Z-scores had normalized at the time of shunt take-down.
Secondly, our data showed that the overall PA index remained unchanged over time despite shunt interposition, confirming a symmetrical growth of PAs.
In this study, inter-stage mortality was only 2.5% with only two shunt-related deaths (1.3%), which compares favorably to the 5%–6% interstage mortality recently reported [17,19].
The numerous patients with residual antegrade pulmonary blood flow (55%), a lower proportion of univentricular heart diagnosis (51%), and a low threshold to stent the previously placed MBTS when desaturation was noticed could all have contributed to those favorable results.
The shunt-related morbidity during the inter-stage period (20.7% of patients developed pulmonary artery stenosis) was similar to previous studies [8,20]. Importantly, half of those were shunt-unrelated but rather occurred secondary to juxta-ductal stenosis.
In our cohort, the prevalence of severe shunt-related pulmonary artery stenosis (greater than 50%) was only 10.4% (16 out of 154 patients). Those were addressed at the subsequent surgery either by patch augmentation PA plasty or without patch in patients undergoing bi-directional cavopulmonary anastomosis. These findings compare favorably to the previous studies by the Collaborative Cath Int group [16,21] and others [9,22] in which the rate of PA plasty was 48% in the MBTS group. We hypothesize that both surgical technique and center-dependent factors (threshold to surgically augment PAs) could explain such differences.
Altogether, these differences in the prevalence of shunt-induced stenosis could also explain the conflicting results on PA growth between studies.
Another important finding from our study is the 11% incidence of juxta-ductal stenosis which developed after initial shunt surgery. Juxta-ductal stenosis has been recognized worldwide since the mid-1960s [23].
The term “juxtaductal” pulmonary coarctation has been advocated on the basis of histological characteristics observed in pathologic specimens of patients with narrowing at the origin of the left pulmonary artery (PA) in whom ductal tissue was observed [24].
The progression of the stenosis potentially leads to disproportionate PA growth or even to PA discontinuity.
As described earlier [25], juxta-ductal stenosis occurred more frequently in DORV-PA stenosis (21%) and PA-VSD (15%) patients. Patients with juxta-ductal stenosis demonstrated an impaired LPA growth resulting in a lower Nakata index at the time of shunt take-down. While a suboptimal growth of the pulmonary vasculature might not be too detrimental in patients contemplating biventricular repair, this point could be critical in univentricular palliation.
The limited number of patients, the extensive period of recruitment and shunt palliation being performed by two experienced surgeons may not allow to extrapolate our results.
Our longitudinal comparative analysis included only 77% of the initial cohort (134/175) and no sub-group analysis of patients who died during index hospitalization was made. Despite the rate of patient drop-out, each patient serving as its own control in this longitudinal comparative morphometric analysis renders the results highly reliable.
Finally, our definition of pulmonary artery stenosis (>50%) together with the absence of clinical and echocardiographic or angiographic follow-up after the repair (or palliation) surgery did not allow to detect minor and/or progressively developing pulmonary artery stenosis.
Our study demonstrates that shunt palliation in neonates and infants allows well-balanced pulmonary artery growth. Shunt-related pulmonary artery stenosis requiring intervention occurred in about 10%, whereas a higher rate of juxta-ductal stenosis (11%) was found. In univentricular heart patients, a close echographic follow-up is warranted to allow early diagnosis of juxta-ductal stenosis. Finally, in this heterogeneous cohort of patients, shunt-related interstage mortality was low (1.3%).
Acknowledgement: The authors thank Dr D. Castanares Zapatero for his help in statistical reviewing and Mrs P. Segers for her expertise in editorial help.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: FXVV: Conceptualization, data curation, formal analysis, investigation, methodology, writing original draft. KC: Investigation, writing-review & editing. GDB: Investigation, writing-review & editing. SM: Investigation, writing-review & editing. JER: Investigation, writing-review & editing. MM: Investigation, writing-review & editing. LH: Investigation, writing-review & editing. AJP: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, writing original draft, writing-review & editing.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Approval: This retrospective analysis was approved by our Institutional Ethical Board (Cliniques Universitaires Saint-Luc, Brussels, Belgium, IRB 2015\11-01\ID 327). Parental consent was waved by our IEB due to the retrospective nature of the study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2023.042341.
References
1. Holtby, H. M. (2014). Anesthetic considerations for neonates undergoing modified Blalock-Taussig shunt and variations. Paediatric Anaesthesia, 24(1), 114–119. [Google Scholar] [PubMed]
2. Alwi, M., Mood, M. C. (2013). Stenting of lesions in patent ductus arteriosus with duct-dependent pulmonary blood flow: Focus on case selection, techniques and outcome. Interventional Cardiology Clinics, 2(1), 93–113. [Google Scholar] [PubMed]
3. Kirklin, J. W., Bargeron Jr, L. M., Pacifico, A. D. (1977). The enlargement of small pulmonary arteries by preliminary palliative operations. Circulation, 56(4 Pt 1), 612–617. [Google Scholar] [PubMed]
4. Guyton, R. A., Owens, J. E., Waumett, J. D., Dooley, K. J., Hatcher, C. R. et al. (1983). The Blalock-Taussig shunt. Low risk, effective palliation, and pulmonary artery growth. Journal of Thoracic and Cardiovascular Surgery, 85(6), 917–922. [Google Scholar] [PubMed]
5. Godart, F., Qureshi, S. A., Simha, A., Deverall, P. B., Anderson, D. R. et al. (1998). Effects of modified and classic Blalock-Taussig shunts on the pulmonary arterial tree. Annals of Thoracic Surgery, 66(2), 512–517. [Google Scholar] [PubMed]
6. Ohye, R. G., Sleeper, L. A., Mahony, L., Newburger, J. W., Pearson, G. D. et al. (2010). Comparison of shunt types in the Norwood procedure for single-ventricle lesions. New England Journal of Medicine, 362(21), 1980–1992. [Google Scholar] [PubMed]
7. Caspi, J., Pettitt, T. W., Mulder, T., Stopa, A. (2008). Development of the pulmonary arteries after the Norwood procedure: Comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Annals of Thoracic Surgery, 86(4), 1299–1304. [Google Scholar] [PubMed]
8. Pruetz, J. D., Badran, S., Dorey, F., Starnes, V. A., Lewis, A. B. (2009). Differential branch pulmonary artery growth after the Norwood procedure with right ventricle-pulmonary artery conduit versus modified Blalock-Taussig shunt in hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery, 137(6), 1342–1348. [Google Scholar]
9. Gladman, G., McCrindle, B. W., Williams, W. G., Freedom, R. M., Benson, L. N. (1997). The modified Blalock-Taussig shunt: Clinical impact and morbidity in Fallot’s tetralogy in the current era. Journal of Thoracic and Cardiovascular Surgery, 114(1), 25–30. [Google Scholar] [PubMed]
10. Jahangiri, M., Lincoln, C., Shinebourne, E. A. (1999). Does the modified Blalock-Taussig shunt cause growth of the contralateral pulmonary artery? Annals of Thoracic Surgery, 67(5), 1397–1399. [Google Scholar] [PubMed]
11. Batra, A. S., Starnes, V. A., Wells, W. J. (2005). Does the site of insertion of a systemic-pulmonary shunt influence growth of the pulmonary arteries? Annals of Thoracic Surgery, 79(2), 636–640. [Google Scholar] [PubMed]
12. Savla, J. J., Faerber, J. A., Huang, Y. V., Zaoutis, T., Goldmuntz, E. et al. (2019). 2-Year outcomes after complete or staged procedure for tetralogy of fallot in neonates. Journal American College of Cardiology, 74(12), 1570–1579. [Google Scholar]
13. Ghimire, L. V., Chou, F. S., Devoe, C., Moon-Grady, A. (2020). Comparison of in-hospital outcomes when repair of tetralogy of fallot is in the neonatal period versus in the post-neonatal period. American Journal of Cardiology, 125(1), 140–145. [Google Scholar] [PubMed]
14. Sluysmans, T., Colan, S. D. (2005). Theoretical and empirical derivation of cardiovascular allometric relationships in children. Journal of Applied Physiology, 99(2), 445–457. [Google Scholar] [PubMed]
15. Nakata, S., Imai, Y., Takanashi, Y., Kurosawa, H., Tezuka, K. et al. (1984). A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. Journal of Thoracic and Cardiovascular Surgery, 88(4), 610–619. [Google Scholar] [PubMed]
16. Glatz, A. C., Petit, C. J., Goldstein, B. H., Kelleman, M. S., McCracken, C. E. et al. (2018). Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: Insights from the congenital catheterization research collaborative. Circulation, 137(6), 589–601. [Google Scholar] [PubMed]
17. Sasikumar, N., Hermuzi, A., Fan, C. S., Lee, K. J., Chaturvedi, R. et al. (2017). Outcomes of Blalock-Taussig shunts in current era: A single center experience. Congenital Heart Disease, 12(6), 808–814. https://doi.org/10.1111/chd.12516 [Google Scholar] [PubMed] [CrossRef]
18. Dorobantu, D. M., Pandey, R., Sharabiani, M. T., Mahani, A. S., Angelini, G. D. et al. (2016). Indications and results of systemic to pulmonary shunts: Results from a national database. European Journal of Cardiothoracic Surgery, 49(6), 1553–1563. [Google Scholar] [PubMed]
19. Bove, T., Vandekerckhove, K., Panzer, J., De Groote, K., De Wolf, D. et al. (2015). Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World Journal of Pediatric and Congenital Heart Surgery, 6(1), 67–74. [Google Scholar]
20. Dirks, V., Pretre, R., Knirsch, W., Valsangiacomo Buechel, E. R., Seifert, B. et al. (2013). Modified Blalock Taussig shunt: A not-so-simple palliative procedure. European Journal of Cardiothoracic Surgery, 44(6), 1096–1102. [Google Scholar] [PubMed]
21. Meadows, J. J., Qureshi, A. M., Goldstein, B. H., Petit, C. J., McCracken, C. E. et al. (2019). Comparison of outcomes at time of superior cavopulmonary connection between single ventricle patients with ductal-dependent pulmonary blood flow initially palliated with either Blalock-Taussig shunt or ductus arteriosus stent: Results from the congenital catheterization research collaborative. Circulation Cardiovascular Intervention, 12(10), e008110. [Google Scholar] [PubMed]
22. Choi, E. S., Kim, D. H., Kwon, B. S., Park, C. S., Yun, T. J. (2021). Growth of the branch pulmonary arteries after employing ‘Shunt-Only’ strategy for neonates with pulmonary atresia or stenosis. Seminar in Thoracic and Cardiovascular Surgery, 33(4), 1095–1102. [Google Scholar] [PubMed]
23. D’Cruz, I. A., Agustsson, M. H., Bicoff, J. P., Weinberg, M.Jr, Arcilla, R. A. (1964). Stenotic lesions of the pulmonary arteries: Clinical and hemodynamic findings in 84 cases. American Journal of Cardiology, 13, 441–450. [Google Scholar] [PubMed]
24. Elzenga, N. J., Gittenberger-de Groot, A. C. (1986). The ductus arteriosus and stenoses of the pulmonary arteries in pulmonary atresia. International Journal of Cardiology, 11(2), 195–208. [Google Scholar] [PubMed]
25. Elzenga, N. J., von Suylen, R. J., Frohn-Mulder, I., Essed, C. E., Bos, E. et al. (1990). Juxtaductal pulmonary artery coarctation. An underestimated cause of branch pulmonary artery stenosis in patients with pulmonary atresia or stenosis and a ventricular septal defect. Journal of Thoracic and Cardiovascular Surgery, 100(3), 416–424. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools