 Open Access
Open Access
ARTICLE
Long-Term Outcomes of Systemic-to-Pulmonary Artery Shunt in Patients with Functional Single Ventricle and Heterotaxy Syndrome
1 Department of Cardiovascular Surgery, Osaka Women’s and Children’s Hospital, Izumi, Osaka, Japan
2 Department of Pediatric Cardiology, Osaka Women’s and Children’s Hospital, Izumi, Osaka, Japan
* Corresponding Author: Takashi Kido. Email:
Congenital Heart Disease 2023, 18(4), 399-411. https://doi.org/10.32604/chd.2023.042243
Received 24 May 2023; Accepted 21 August 2023; Issue published 15 September 2023
Abstract
Objectives: We sought to determine the long-term outcomes and mortality-associated factors after systemic-to-pulmonary artery shunt (SPS) in patients with heterotaxy syndrome. Methods: We retrospectively analyzed all patients with a functional single ventricle and heterotaxy syndrome who underwent SPS at our center from January 2001 to April 2022. Results: This study involved 84 patients. Restrictive pulmonary blood flow requiring early modulation was presented in 34 (40%) patients. Compared with patients without restrictive pulmonary blood flow (N = 50 [60%]), the postnatal survival of these 34 patients was significantly lower at 10 years (log rank: p = 0.04), but the statistical significance disappeared at 20 years (log rank: p = 0.18). Among 31 patients who underwent SPS, 11 (35%) had extracardiac total anomalous pulmonary venous connection (TAPVC). The survival rate after SPS was 80% at 10 years. Cox regression analysis showed that extracardiac TAPVC (hazard ratio 6.44, 95% confidence interval 1.23–33.7, p = 0.03) and pulmonary venous obstruction (PVO) at TAPVC repair (hazard ratio 11.2, 95% confidence interval 2.13–58.5, p = 0.004) were significantly associated with death. In 25 patients who underwent bidirectional cavopulmonary shunt (BCPS), surgical interventions on the pulmonary artery (PA) were performed after SPS in 7 of 9 patients with PA coarctation, 3 of 4 with non-confluent PAs, and 4 of 12 with normal PAs. At SPS, primary central PA plasty was performed in three patients with PA coarctation and 2 with non-confluent PAs. There was no significant difference in the PA index before BCPS between patients with and without primary central PA plasty (p = 0.49). Among 20 patients who underwent total cavopulmonary connection (TCPC), adverse events occurred in 7 (35%) patients, including death in 1 (5%), intervention for pulmonary arteriovenous malformation (PAVM) in 3 (15%), and surgical intervention for PVO in 3 (15%). The B-type natriuretic peptide concentration was significantly higher in patients with than without adverse events (p = 0.02). The adverse event-free survival rate after TCPC was 69% at 10 years. Conclusion: Extracardiac TAPVC and PVO at TAPVC repair were significantly associated with death after SPS in patients who had heterotaxy syndrome with a single ventricle. Surgical interventions on the PA were frequently required after SPS in patients with PA coarctation or non-confluent PAs. Although satisfactory survival was achievable after TCPC, late-onset PAVM and PVO remain concerns.Keywords
Surgical management of patients with heterotaxy syndrome and single ventricular physiology is challenging. Despite advances in operative and perioperative care, recent studies have shown that the inter-stage mortality rate before a Fontan operation is approximately 30% [1–3]. The factors associated with mortality in this patient population have been extensively investigated and include pulmonary venous malformation, atrioventricular valve (AVV) regurgitation, restrictive pulmonary blood flow, and arrhythmia [1–3]. These factors are usually present in the same patient, making their surgical management more complex.
Patients with heterotaxy syndrome and a functional single ventricle frequently present with restrictive pulmonary blood flow, and systemic-to-pulmonary artery shunt (SPS) is commonly performed as surgical palliation. One study showed that surgical mortality after SPS was worse in patients with than without heterotaxy syndrome [4]. A recent report from Germany stated that restrictive pulmonary blood flow requiring early modulation was an independent risk factor for mortality in patients who had heterotaxy syndrome with a single ventricle [1]. The unfavorable outcomes after SPS in this patient population may be attributed to other concomitant cardiac malformations, particularly pulmonary venous malformation and AVV regurgitation. The impacts of these associated cardiac lesions on the outcomes of SPS have not been thoroughly investigated. Additionally, the long-term outcomes after total cavopulmonary connection (TCPC) following SPS in patients with heterotaxy syndrome remain unclear.
Pulmonary artery (PA) stenosis at the site of a patent ductus arteriosus, termed PA coarctation, is a frequent complication of heterotaxy syndrome and can compromise the growth of the PAs and the success of the Fontan operation. In patients with PA coarctation, whether primary central PA reconstruction with ductus tissue resection improves the outcomes has not been determined.
In the present study, we review our 20-year experience in a cohort of patients who had heterotaxy syndrome with a single ventricle and underwent SPS. We examine the factors associated with death after SPS and the impact of primary central PA plasty on PA coarctation. Finally, we will determine the adverse outcomes after TCPC in this patient cohort.
We reviewed all patients with a functional single ventricle and heterotaxy syndrome who underwent SPS at our center from January 2001 to April 2022. Patients who underwent SPS as a part of the Damus–Kaye–Stansel procedure or Norwood procedure were excluded.
The SPSs were constructed with an interposition of a polytetrafluoroethylene vascular graft (Gore-Tex graft; W.L. Gore & Associates, Flagstaff, AZ, USA). The surgeon chose the of shunt type and size based on the patient’s weight and cardiac and vascular anatomy. The SPS procedure was performed through a median sternotomy or lateral thoracotomy. Cardiopulmonary bypass was required in patients who needed concomitant procedures or experienced hemodynamic instabilities. Central PA plasty with resection of the ductal tissue was performed at the time of SPS in selected patients with PA coarctation or non-confluent PAs. To achieve the confluent and non-stenotic PAs, we performed full mobilization of both the right and left PAs beyond the upper branches and complete resection of the ductal tissue. Continuity of the posterior walls of the central PA was established by direct anastomosis. An autologous fresh pericardial patch was used for the anterior aspect to augment the stenotic site. After SPS without central PA plasty, the infusion of prostaglandin E1 was discontinued. The juxtaductal PA stenosis was carefully observed and an additional SPS was constructed for the contralateral PA if necessary. After SPS, all patients were administered aspirin and dipyridamole to prevent thrombosis.
Surgical repair of total anomalous venous connection (TAPVC) was performed with anastomosis of the common pulmonary venous chamber to the atrium under cardiac arrest. Primary sutureless repair has been used in our center since 2012. A staged Fontan palliation consisting of bidierctional cavopulmonary shunt (BCPS) followed by TCPC has been our standard strategy for repair of a functional single ventricle. TAPVC repair was performed in the neonatal period or early infancy if pulmonary venous obstruction (PVO) was present. Otherwise we performed TAPVC repair at the time of BCPS.
PA coarctation was defined as discrete narrowing of the PA at the ductal insertion site in patients with pulmonary atresia. PVO was defined as a Doppler echocardiographic velocity of >1.2 m/s, pressure gradient of >4 mmHg, minimal diameter of the draining vein of <1.5 mm, or pulmonary congestion due to narrowing of the draining veins requiring mechanical ventilation.
We reviewed the patients’ medical records, including their in-hospital and outpatient notes and data regarding echocardiography, computed tomography, and cardiac catheterization. The patients have been maintained outpatient follow-up with both pediatric cardiologists and cardiac surgeons. The follow-up data from the time of SPS until the last known record of the patients were regularly tracked using our institutional database. The follow-up period was defined as the time from SPS to the last follow-up visit. In a subgroup of patients who underwent BCPS, the impact of primary central PA reconstruction was assessed in terms of the requirements for re-interventions involving the PA and the growth of the PAs. In patients who underwent TCPC, the adverse events after TCPC were examined.
Categorical variables are presented as absolute number and percentage. Continuous variables are expressed as median with interquartile range (IQR). Student’s t-test was used to compare continuous variables. For survival analysis, the date of SPS was taken as zero time. The Kaplan–Meier survival estimates with 95% confidence intervals (CIs) were determined. A Cox proportional hazards model was used to examine the effects of anatomic and procedural variables on survival. The impact of a history of AVV surgery on mortality was determined using a Cox proportional hazard model with time-dependent covariates. Hazard ratios (HRs) and 95% CIs were estimated. Statistical significance was defined as p < 0.05. Analyses were performed with SPSS version 28.0 for Windows (IBM Corp, Armonk, NY, USA).
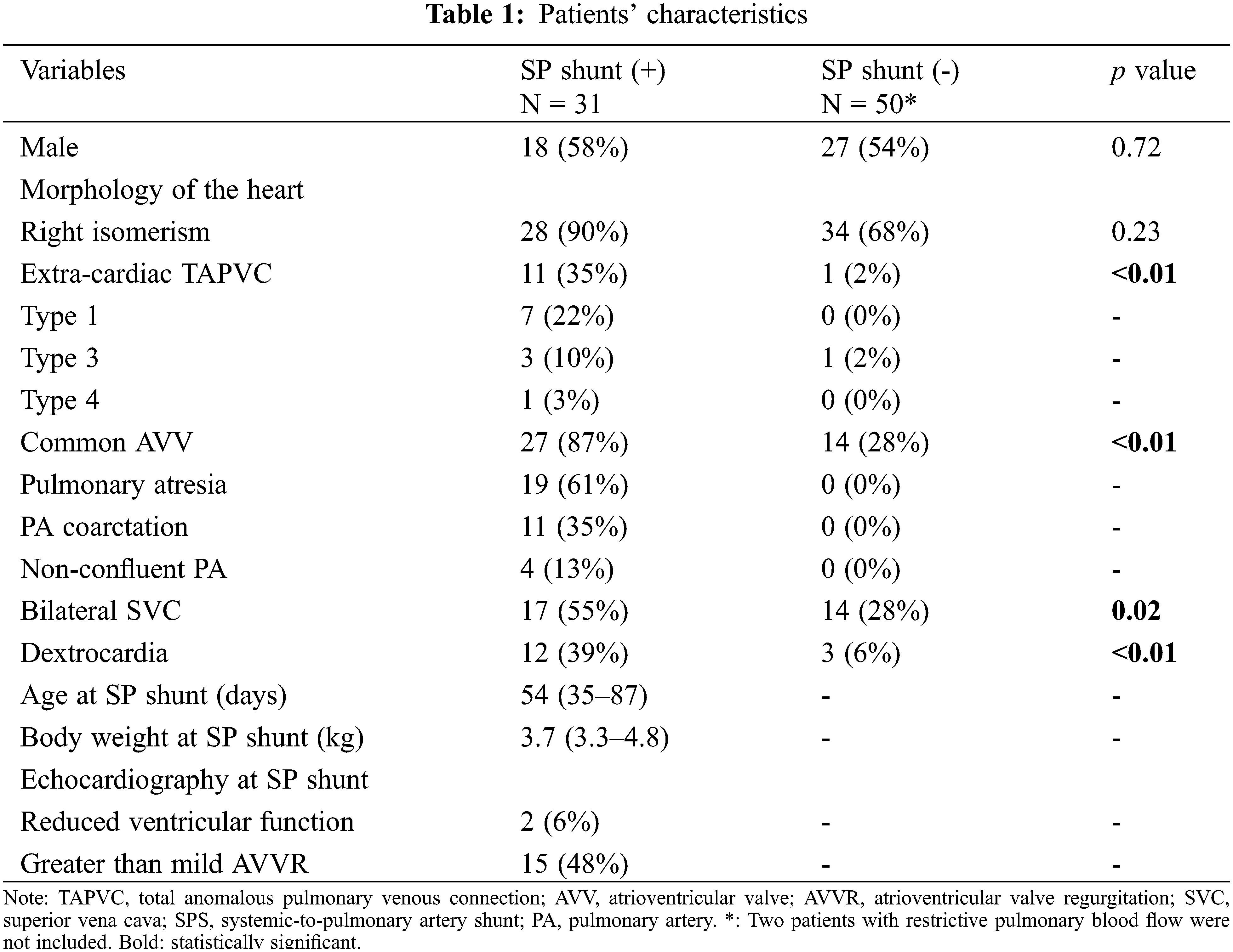
During the study period, a total of 84 patients with heterotaxy syndrome and functional single ventricle who underwent surgical treatments at our center. Among them, 31 patients underwent SPS at a median age of 54 days (IQR: 36–87 days). Before SPS, the ductus arteriosus was kept open with prostaglandin E1 infusion. Twenty-eight patients had right isomerism and 3 had left isomerism. The SPS was constructed on the left side in 15 (48%) patients and on the right side in 16 (52%). The inflow vessel of the shunt was the brachiocephalic artery in 6 (19%) patients, the common carotid artery in 4 (13%), and the subclavian artery in 21 (68%). The size of the shunt was 3.0 mm in 2 (6%) patients, 3.5 mm in 18 (58%), and 4 mm in 11 (35%). On echocardiography, 15 (48%) patients showed greater than mild AVV regurgitation and 2 showed reduced ventricular function before SPS. Eleven (35%) patients had extracardiac TAPVC and underwent TAPVC repair at a median age of 91 days (IQR 35–129 days). Two patients had intracardiac TAPVC and did not require TAPVC repair. The extracardiac TAPVC was the supra-cardiac type in seven patients, infra-cardiac type in three, and mixed type in one. Preoperative computed tomography revealed PA coarctation in 11 (35%) patients and non-confluent PAs in 4 (13%). The patients’ characteristics are shown in Table 1. Extracardiac TAPVC, common AVV, bilateral SVC, and dextrocardia were more frequent in patients with than without SP shunt.
3.2 Impact of Restrictive Pulmonary Blood Flow on Survival
Restrictive pulmonary blood flow requiring early modulation was present in 34 patients (31 patients with SPS, 2 with continuous prostaglandin E1 infusion, and 1 with right ventricle-to-PA shunt). Compared with patients without restrictive pulmonary blood flow (N = 50), the postnatal survival of these 34 patients was significantly lower at 10 years (log rank: p = 0.04), however, the statistical significance disappeared at 20 years (log rank: p = 0.18) (Fig. 1).
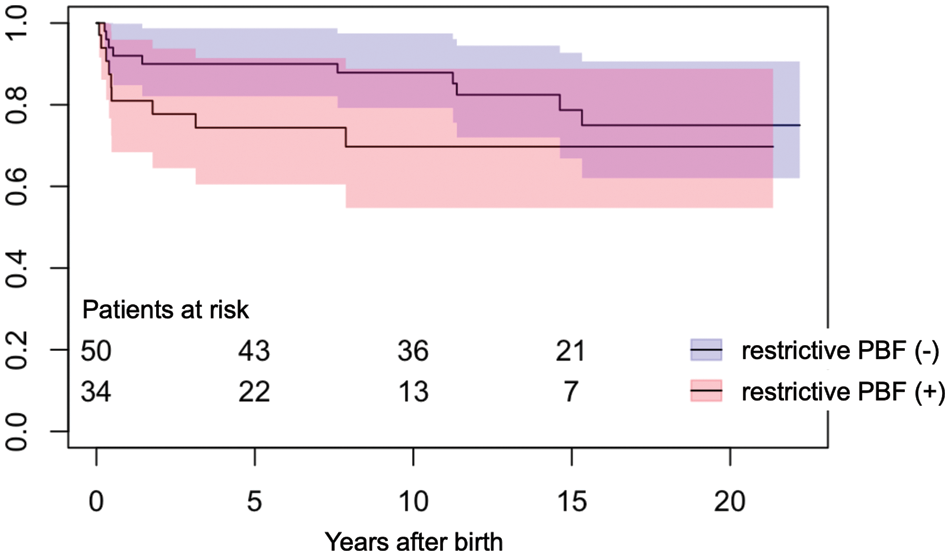
Figure 1: Postnatal survival in patients with and without restrictive pulmonary blood flow. PBF, pulmonary blood flow
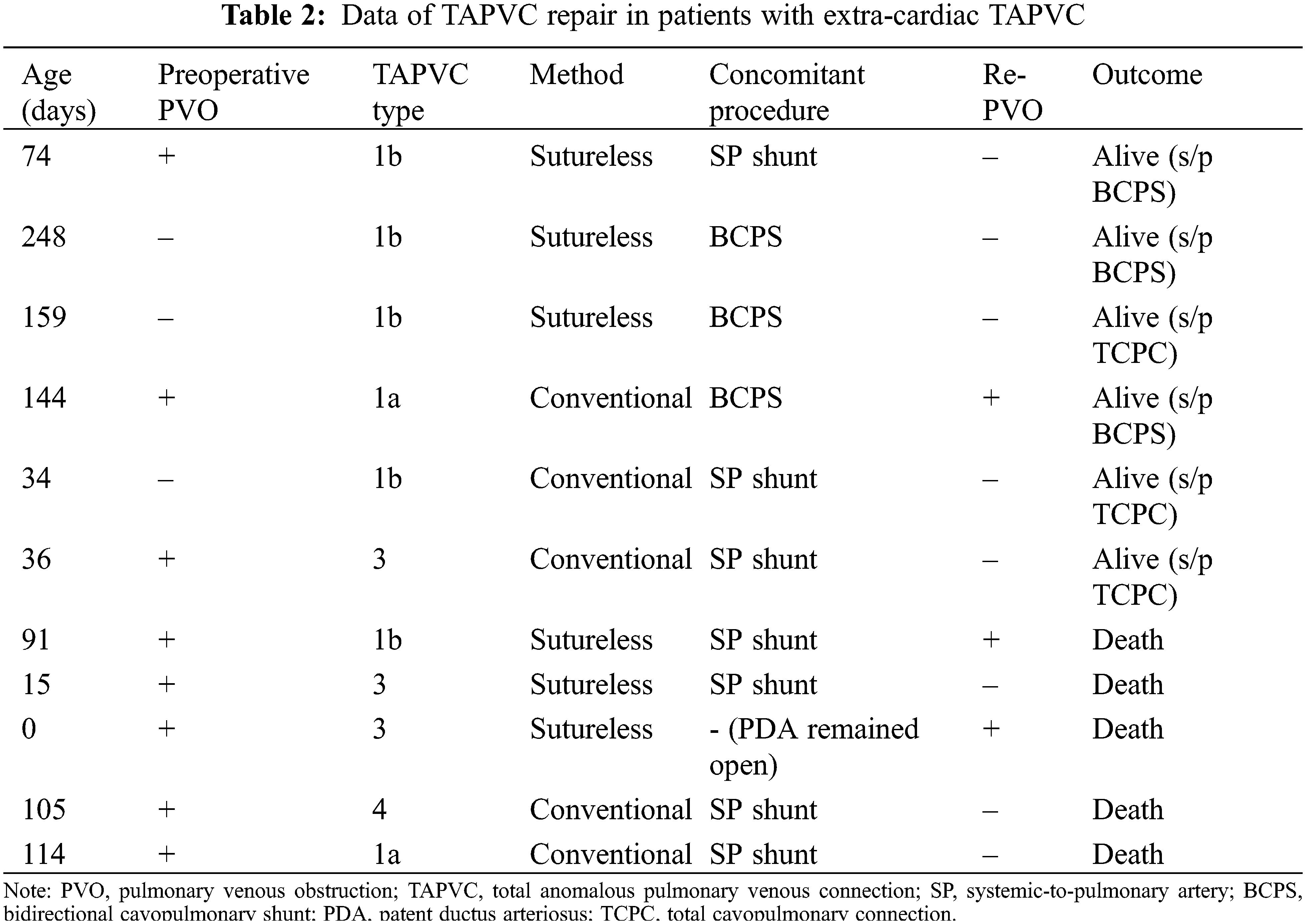
Follow-up was completed in all 31 patients and median follow-up period after SPS was 7.6 years (IQR, 2.3–12.0 years). Among 11 patients with extracardiac TAPVC, 8 patients had preoperative PVO. The TAPVC repair was performed before SPS in one patient, at the time of SPS in seven, between SPS and BCPS in one, and at the time of BCPS in two. The outcomes after SPS in individual patients are shown in Fig. 2. One patient in whom TAPVC repair was performed before SPS died of PVO and severe infection 2 months after the SPS. Among seven patients who underwent TAPVC repair at the time of SPS, one patient was unable to be weaned from cardiopulmonary bypass and died on postoperative day 17. Another patient developed acute cyanosis and died in the hospital on postoperative day 58. Another patient who had Goldenhar syndrome and duodenal atresia developed PVO and died 2 years after the surgery. Another patient underwent fenestrated TCPC at the age of 4 years. This patient had right PVO and severe right PA stenosis. Cardiac catheterization performed at 1 year after TCPC showed a mean PA pressure of 16 mmHg and PA index of 73 mm2/m2. The patient died suddenly at home 3 years after the TCPC. The data regarding TAPVC repair are summarized in Table 2. Five (45%) patients died after SPS. All of these patients presented with PVO at the time of TAPVC repair. The sutureless technique was used for TAPVC repair in six patients. One patient who underwent TAPVC repair between SPS and BCPS developed PVO after BCPS and dropped out of the Fontan track. Among 20 patients without extra-cardiac TAPVC, 2 (10%) died of severe infection at 4 months and 1.6 years after SPS, respectively.
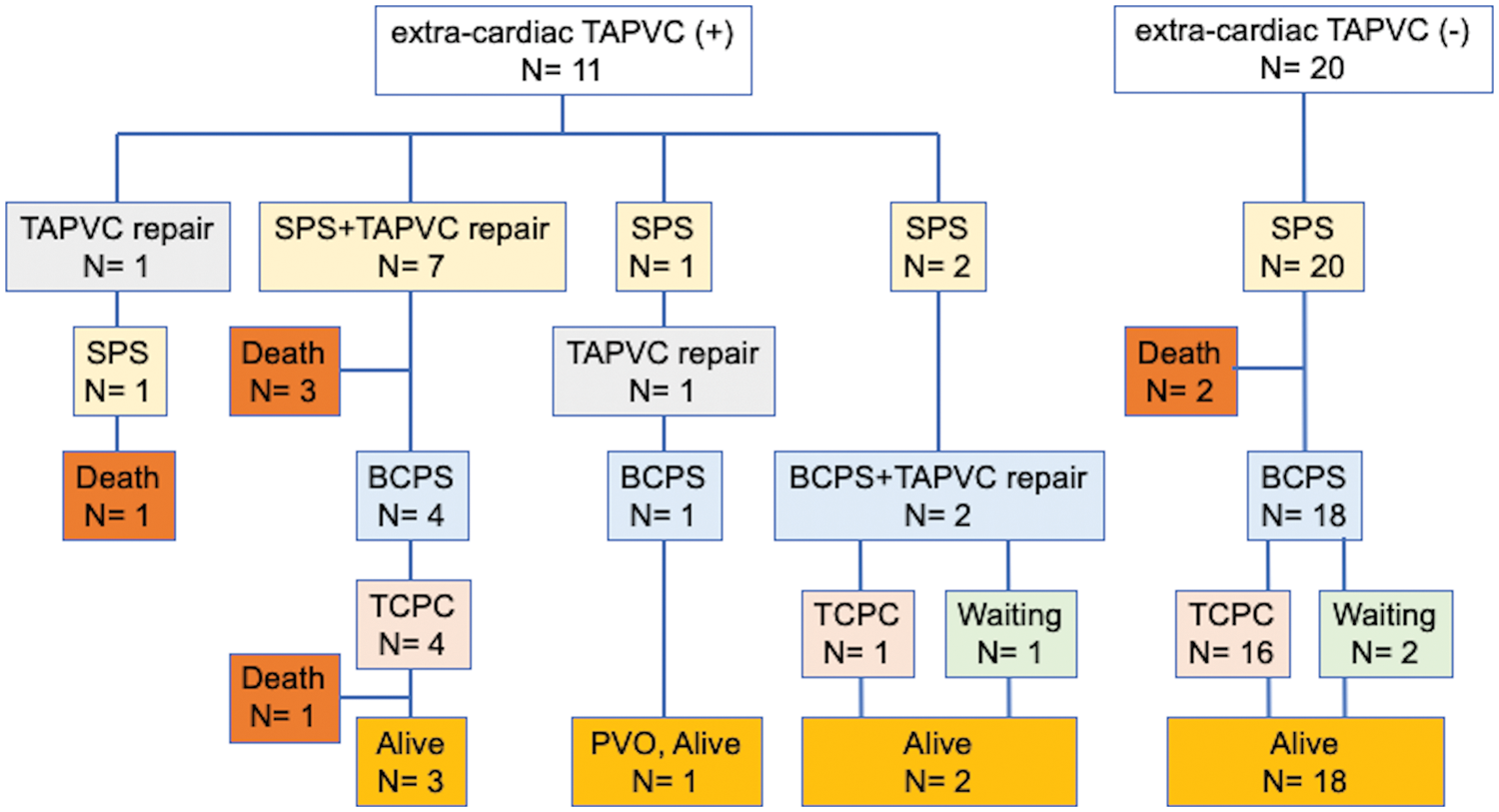
Figure 2: Outcomes after systemic-to-pulmonary shunt in individual patients. TAPVC, total anomalous pulmonary venous connection; SPS, systemic-to-pulmonary artery shunt; BCPS, bidierectional cavopulmonary shunt; TCPC, total cavopulmonary connection; PVO, pulmonary venous obstruction
3.4 Factors Associated with Mortality after SPS
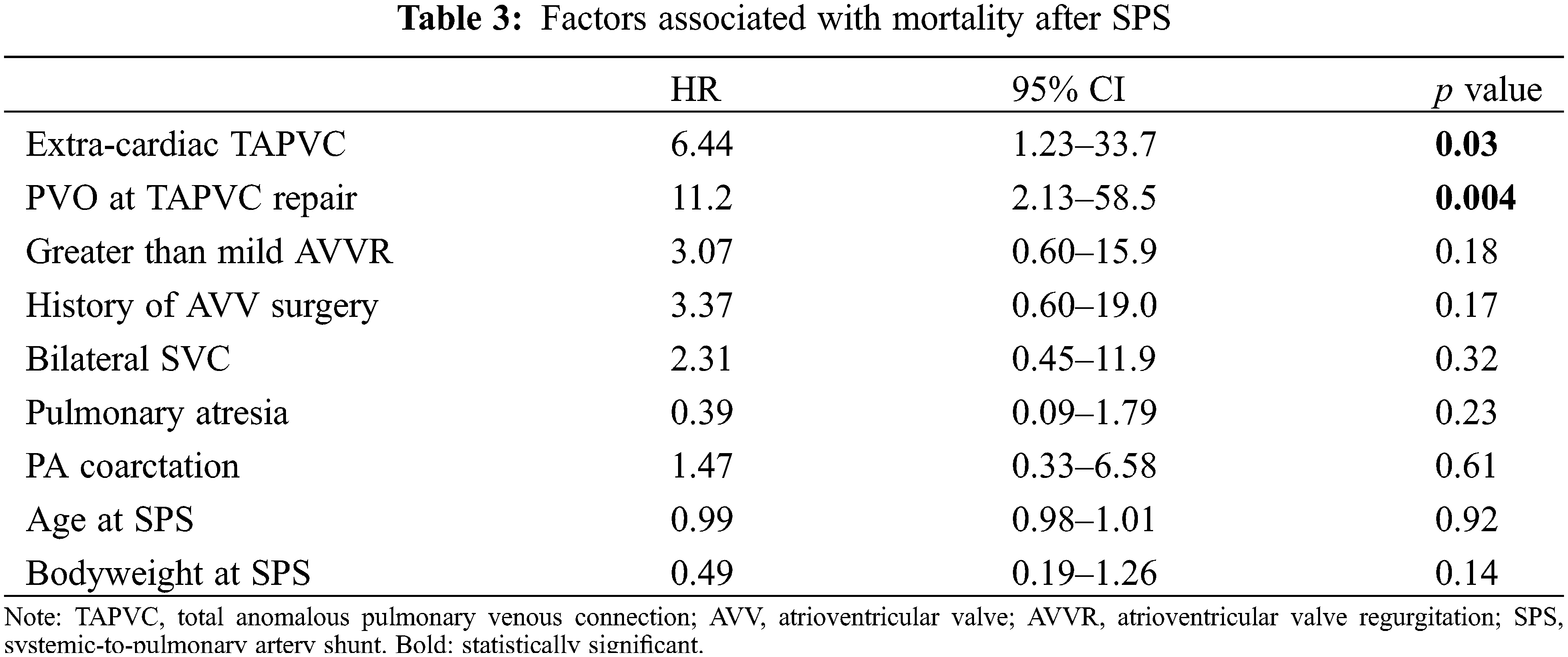
The survival rate after SPS was 83.0% at 5 years and 80.0% at 10 years (Fig. 3). Cox regression analysis showed that extra-cardiac TAPVC (HR, 6.44, 95% CI, 1.23–33.7, p = 0.03) and PVO at the time of TAPVC repair (HR, 11.2, 95% CI, 2.13–58.5, p = 0.004) were significantly associated with mortality, while greater than mild AVV regurgitation and a history of AVV plasty were not (Table 3).
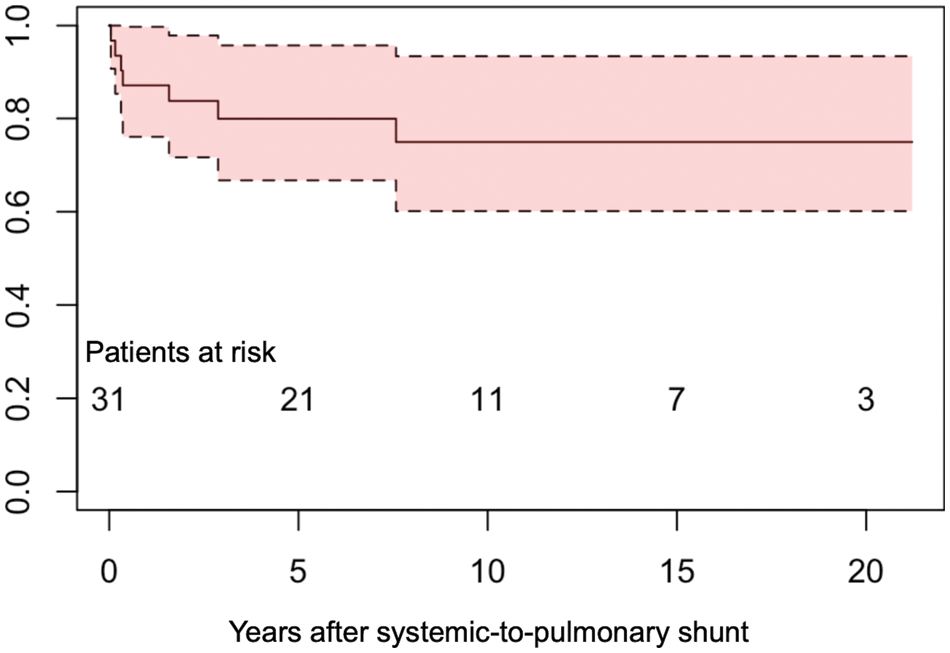
Figure 3: Survival after systemic-to-pulmonary artery shunt
3.5 PA Re-Intervention and PA Growth after SPS
The rate of PA re-interventions and growth of the PAs were assessed in 25 patients who underwent BCPS following SPS. Among the 25 patients, 9 had PA coarctation, 4 had non-confluent PAs, and 12 had normal PAs. Central PA plasty was performed at the time of SPS in three patients with PA coarctation and two patients with non-confluent PAs. The catheter and surgical interventions on the PA after SPS are summarized in Fig. 4. In patients with PA coarctation, a total of 10 surgical interventions on the PA were performed in 2 patients with central PA plasty and 5 without central PA plasty. In patients with non-confluent PAs, six surgical interventions on the PA were performed in one patient with central PA plasty and two without central PA plasty. In patients with normal PAs, four surgical interventions on the PA were performed in four patients. Catheter and surgical PA interventions were frequently needed after SPS in patients with PA coarctaion or non-confluent PAs, either with or without primary central PA plasty. Fig. 5 shows the representative serial angiographs of a patient with PA coarctation and a patient with non-confluent PAs who underwent central PA plasty at the time of SPS. No patients with PA coarctation developed complete PA discontinuation after the cessation of prostaglandin E1 infusion. Fig. 6 shows the PA index before SPS and before BCPS. There was no significant difference in growth of the PAs between the patient groups.
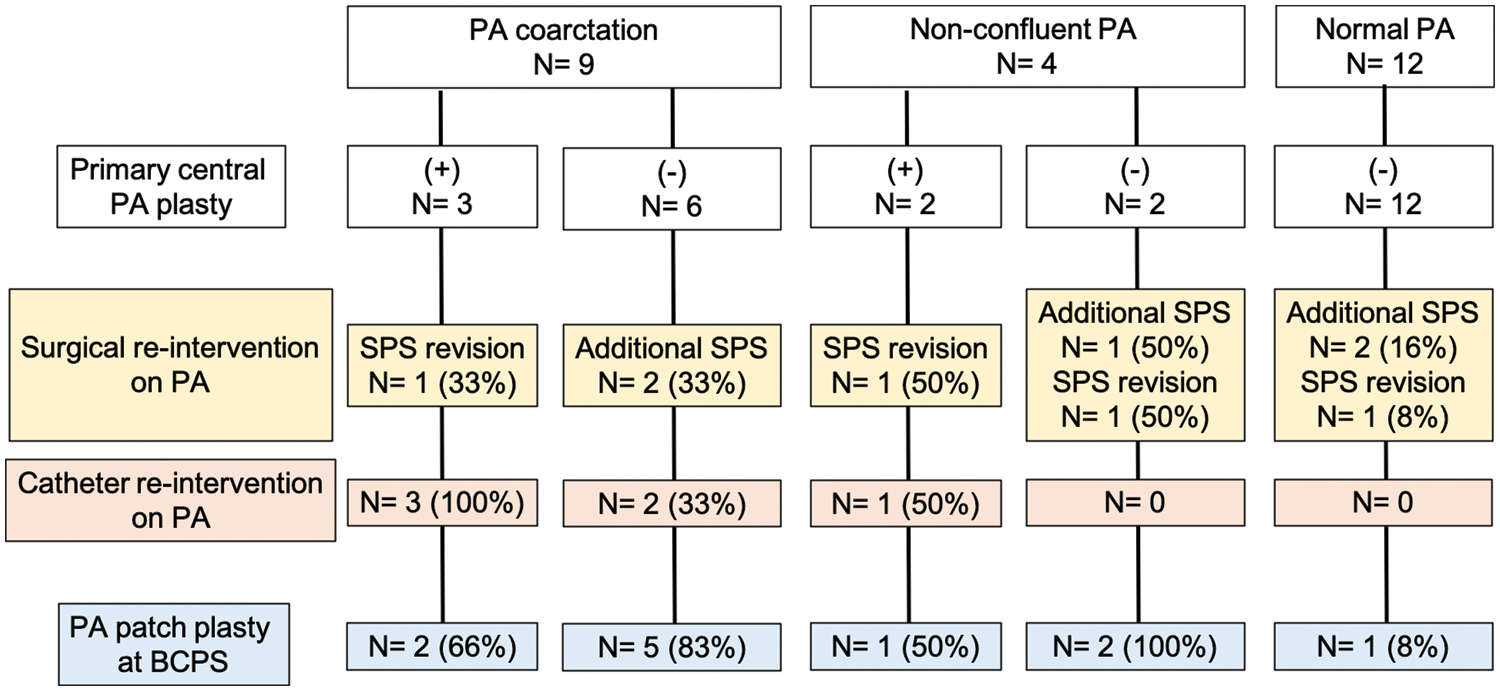
Figure 4: Catheter and surgical interventions on PA after SPS. PA, pulmonary artery; SPS, systemic-to-pulmonary artery shunt; BCPS, bidirectional cavopulmonary shunt
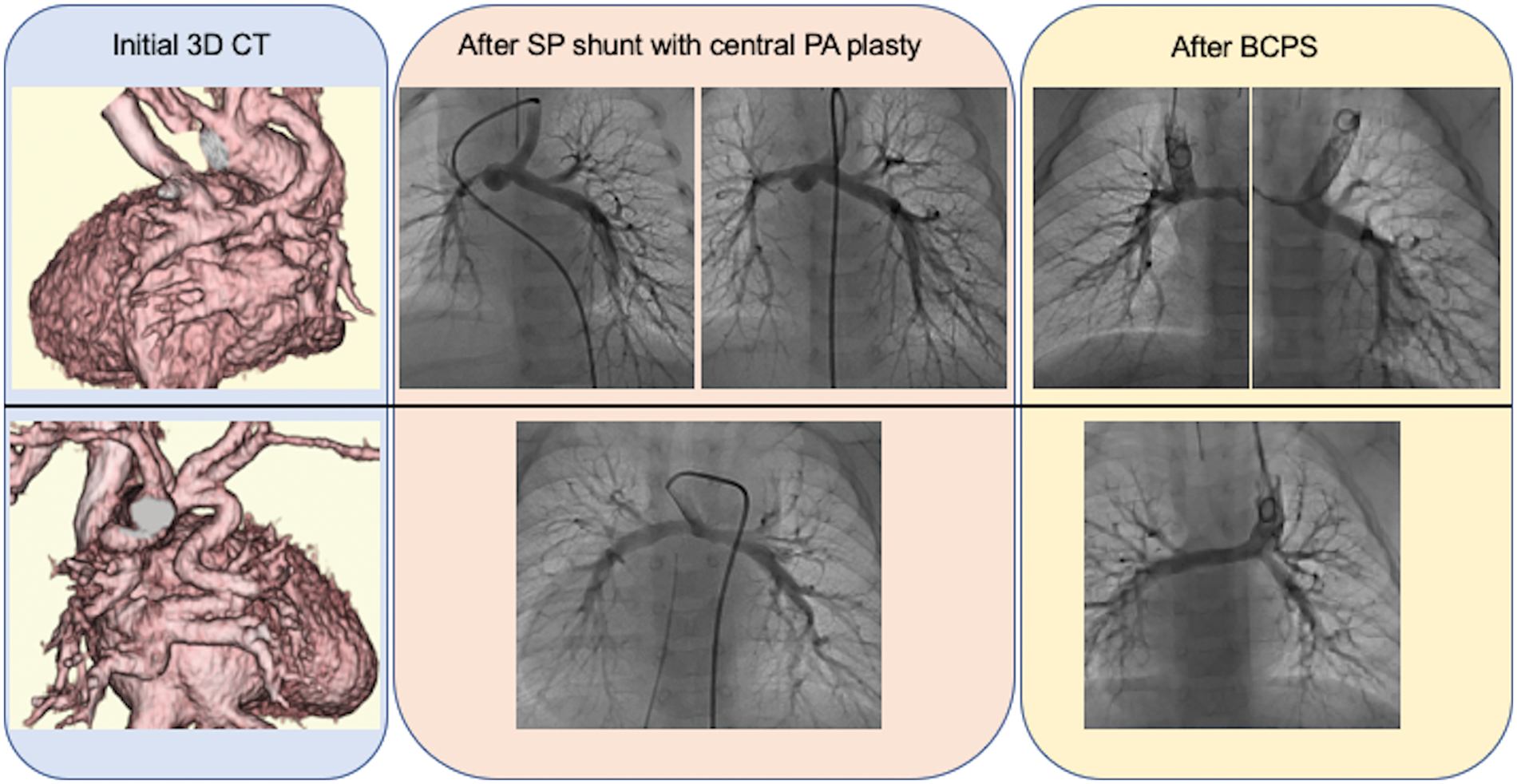
Figure 5: Computed tomography and PA angiography in a patient with PA coarctation (upper case) and a patient with non-confluent PA (lower case) who underwent primary central PA plasty. In the patient with PA coarctation, balloon angioplasty was performed after SPS for right PA stenosis. SPS, systemic-to-pulmonary artery shunt; PA, pulmonary artery; BCPS, bidirectional cavopulmonary shunt
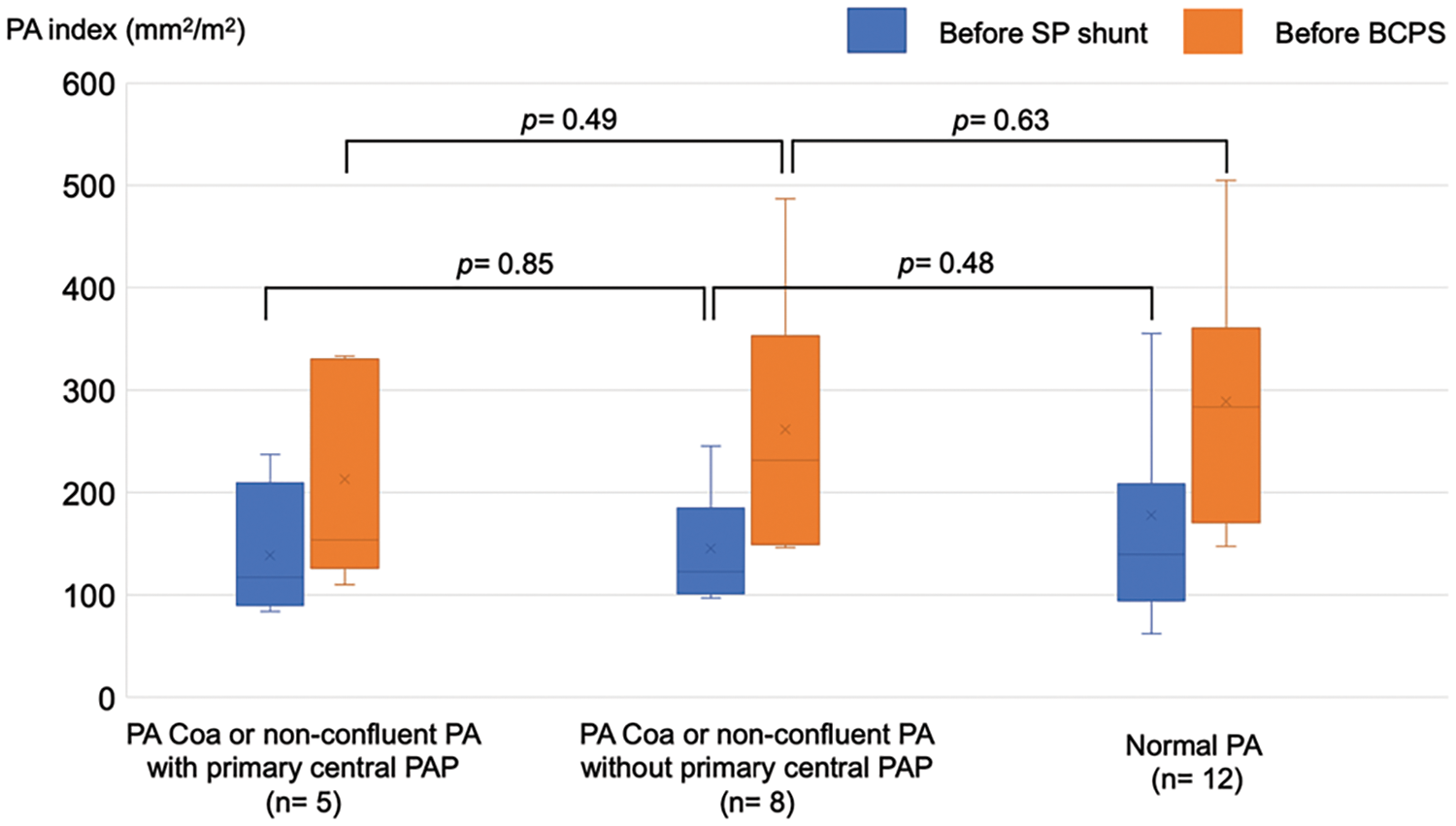
Figure 6: The PA index before SPS and BCPS stratified by PA abnormalities and history of primary central PA plasty. PA Coa, pulmonary artery coarctation; PA, pulmonary artery; PAP, pulmonary artery plasty; SPS, systemic-to-pulmonary artery shunt; BCPS, bidirectional cavopulmonary shunt
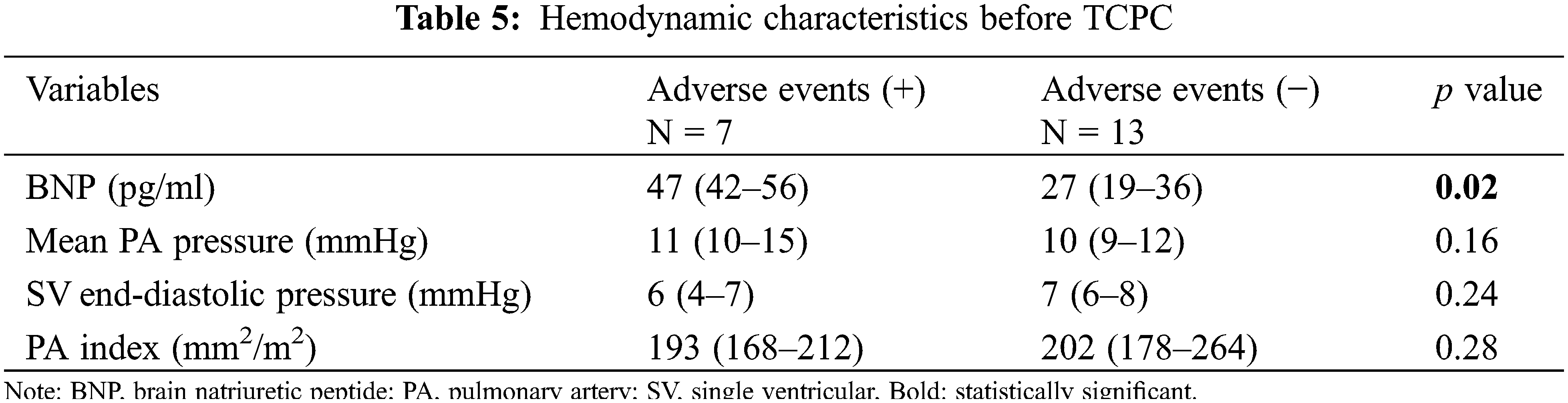
TCPC was performed in 20 patients. The median follow-up period after TCPC was 8.1 years. Adverse events occurred in seven patients, including death in one, intervention for pulmonary arteriovenous malformation (PAVM) in three (coil embolization in two and hepatic vein redirection in one), and surgical intervention for the development of PVO in three. The adverse event-free survival rate after TCPC was 80% at 5 years and 69% at 10 years. The detailed data of patients’ data who developed adverse events after TCPC are shown in Table 4. Patient 1 had right PVO and died suddenly at home 3 years after TCPC. Patients 2 and 3 developed severe hypoxia due to PAVM after TCPC and underwent coil embolization. Both of these patients had right isomerism without inferior vena cava (IVC) interruption. In Patient 2, angiography after TCPC showed right-dominant IVC blood flow and bilateral PAVM. In Patient 3, angiography showed left-dominant IVC blood flow and left-sided PAVM (Fig. 7). Patients 4 and 5 developed PVO due to compression of the extracardiac conduit. They underwent conduit replacement 3 and 5 months after TCPC, respectively and the PVO was relieved. Patient 6 had intracardiac TAPVC and PVO that developed secondary due to membranous obstruction of the pulmonary venous orifice. The patient underwent a surgical resection of the membrane 1 year after TCPC, and the PVO was successfully relieved. Patient 7 had left isomerism with IVC interruption. He developed severe hypoxia due to PAVM and redirection of the hepatic vein to the azygous vein was performed 14 years after TCPC. The hemodynamic characteristics before TCPC were compared between patients with and without adverse events after TCPC (Table 5). The B-type natriuretic peptide (BNP) concentration was significantly higher in patients with than without adverse events (p = 0.02).
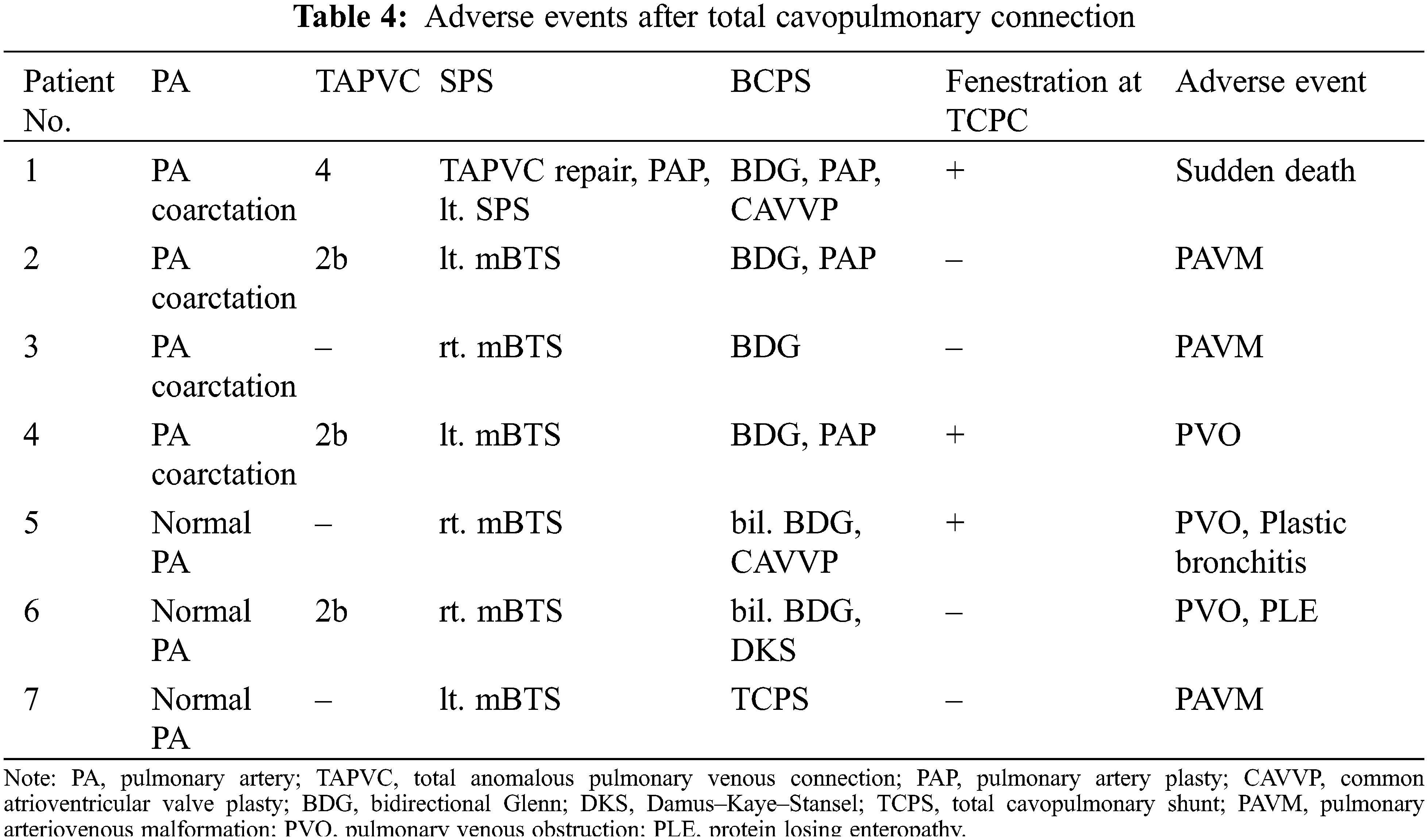
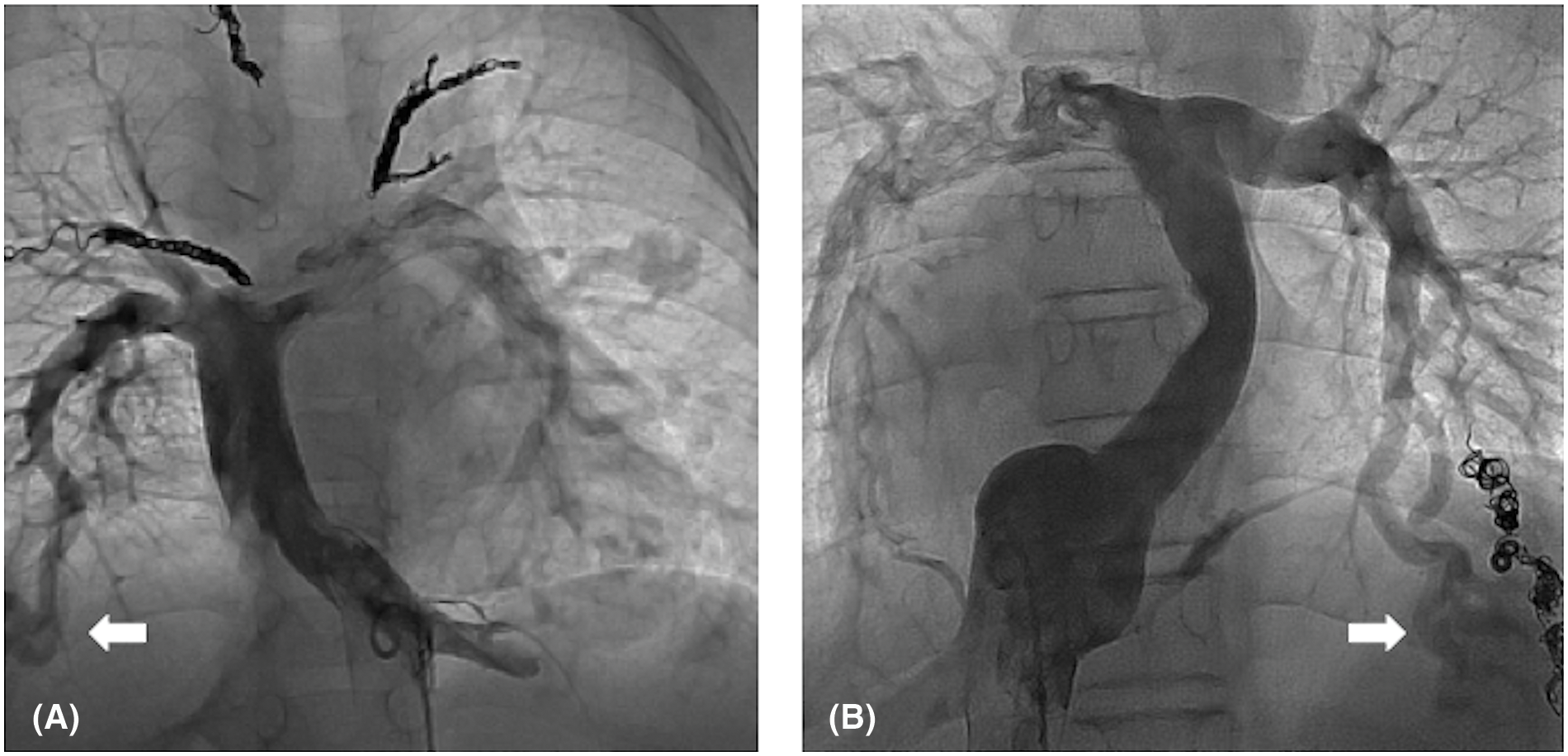
Figure 7: IVC angiography in patients who developed PAVM after TCPC. (A) Bilateral PAVM in a patient with right-dominant IVC blood flow. (B) Left PAVM in a patient with left-dominant IVC blood flow. White arrows indicate PAVM
The present study demonstrated that PVO at the time of TAPVC repair for extracardiac TAPVC was significantly associated with mortality after SPS in patients who had heterotaxy syndrome with a single ventricle. Catheter and surgical PA interventions were frequently needed after SPS in patients with PA coarctation or non-confluent PAs, with or without primary central PA plasty. In our 20-year experience, 65% of patients achieved TCPC, and the adverse event-free survival rate after TCPC was 69% at 10 years. The development of PVO and PAVM were the major issues after TCPC.
Outcomes of SPS for a single ventricular heart in patients with extracardiac TAPVC were recently reported by a research group from Fukuoka, Japan. According to their report, the mortality rate before BCPS was 54% and the main causes of death were PVO and infection. The factors associated with death were preoperative PVO and simultaneous SPS and TAPVC repair [5]. Difficulty regulating pulmonary blood flow results in worse outcomes of SPS for patients who have a single ventricle with extracardiac TAPVC, especially in the setting of simultaneous SPS and TAPVC repair. Commonly, these patients have preoperative PVO and suboptimal lung conditions. Moreover, a histologic examination during autopsy in patients with TAPVC revealed that the pulmonary veins were markedly dilated with increased wall thickness even in the absence of clinical evidence of PVO [6]. The sutureless technique, originally designed as a surgical strategy for PVO after TAPVC repair, has been reported to be effective as a primary procedure to prevent recurrence of PVO and improve survival [7,8]. Although we began using the sutureless technique for primary TAPVC repair in 2012, preoperative PVO remains a significant risk factor for mortality. Delayed TAPVC repair using primary draining vein stenting has been proposed to improve outcomes of patients with obstructive TAPVC and a functional single ventricle, which should be considered when treating this group of patients [9,10].
PA coarctation may cause hypoplasia or even atresia of the branch PA, resulting in unbalanced PA growth. Several authors have described the utility of primary central PA reconstruction with SPS to avoid an unbalanced PA bed [11–15]. Sakamoto et al. [15] emphasized the importance of complete resection of ductal tissue during SPS. Although it is ideal to achieve balanced PA growth by performing primary central PA plasty, the drawbacks of this technique are undesirable effect of cardiopulmonary bypass on the change in pulmonary vascular resistance and the technical difficulty of PA plasty in early infancy. A recent study showed that primary central PA plasty and complete resection of ductal tissue did not contribute to catch-up growth of the PA [16]. Our study showed that the surgical outcomes were comparable in patients who underwent SPS with and without central PA plasty. Primary central PA plasty seems to be ideal for obtaining balanced PA growth after SPS; however, further investigation is warranted to determine the optimal surgical management strategy to deal with PA coarctation and non-confluent PAs in patients with functional single ventricle and heterotaxy syndrome.
The mechanism underlying development of PAVM has yet to be fully elucidated. The development of PAVM is presumed to be related to diversion of hepatic venous flow away from the pulmonary circulation. However, Kwon et al. [17] reported the development of PAVM after TCPC in patients with a normal distribution of hepatic effluent flow, as observed in two of our patients, suggesting the involvement of non-hepatic factors. The multiple forms of pulmonary venous connections in patients with heterotaxy syndrome can cause PVO after TCPC secondary to compression of the conduit. Our group previously described 12 patients with right isomerism who developed PVO after TCPC secondary to compression of the conduit [18]. In patients with ipsilateral locations of the conduit and pulmonary venous orifice, late development of PVO should be carefully followed up.
The present study revealed that BNP was the sole factor associated with adverse events after TCPC.
Previous studies have demonstrated the utility of the preoperative BNP levels as a predictor of postoperative outcomes in patients undergoing TCPC [19,20]. Radman et al. [19] reported that a preoperative BNP concentration >40 pg/mL was highly associated with the need for TCPC takedown. In Taiwan, Lin et al. [20] recently reported that the preoperative log10NT-proBNP level was an independent predictor of post-TCPC adverse events, including Fontan failure, rehospitalization for heart failure, and tachyarrhythmias [20]. Our results are consistent with these previous reports. Patients with heterotaxy who have a high preoperative BNP levels should be closely followed up for the development of serious complications after TCPC.
The present study is limited by its retrospective nature and small number of patients with heterogeneous characteristics over a long study period. Thus, the statistical analysis may not have sufficient power to draw firm conclusions. Although a significant impact of extracardiac TAPVC on survival was shown in the present study, the impacts of AVV regurgitation and arrhythmia in this patient population should be determined using a larger cohort. Because of the small sample size, we were unable to elucidate the cause of adverse events after TCPC.
PVO at TAPVC repair for extracardiac TAPVC was significantly associated with death after SPS in patients who had heterotaxy syndrome with a single ventricle. Based on our findings, new therapeutic strategies such as delayed TAPVC repair using primary draining vein stenting should be considered and further studies are warranted to determine the efficacy of this strategy. Surgical interventions on the PA were frequently required after SPS in patients with PA coarctation or non-confluent PAs, with or without primary central PA plasty. Although satisfactory survival rates were achieved after TCPC, late-onset PAVM and PVO remain concerns.
Acknowledgement: We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding Statement: The authors received no specific funding for the study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Takashi Kido; data collection: Takashi Kido, Shota Kawai, Tomomitsu Kanaya, Koji Miwa, Yuta Teguri, Yoichiro Ishi, Hisaaki Aoki, Futoshi Kayatani, Sanae Tsumura; analysis and interpretation of results:Takashi Kido; draft manuscript preparation: Shota Kawai, Takashi Kido. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the finding of this study are available from the corresponding author upon reasonable request.
Ethics Approval: The Institutional Review Board of the Osaka Women’s and Children’s Hospital approved the study (approval number: 1594) and waived the need for informed consent from the patients who were retrospectively analyzed in the study.
Conflicts of Interest: All authors declare that they have no conflict of interests to report regarding the present study.
References
1. Vodiskar, J., Kido, T., Strbad, M., Cleuziou, J., Hager, A. et al. (2021). Outcomes of single ventricle palliation in infants with heterotaxy syndrome. European Journal of Cardio-Thoracic Surgery, 60(3), 554–561. [Google Scholar] [PubMed]
2. Alsoufi, B., McCracken, C., Schlosser, B., Sachdeva, R., Well, A. et al. (2016). Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. The Journal of Thoracic and Cardiovascular Surgery, 151(5), 1369–1377. [Google Scholar] [PubMed]
3. Bhaskar, J., Galati, J. C., Brooks, P., Oppido, G., Konstantinov, I. E. et al. (2015). Survival into adulthood of patients with atrial isomerism undergoing cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery, 149(6), 1509–1514. [Google Scholar] [PubMed]
4. Jacobs, J. P., Pasquali, S. K., Morales, D. L., Jacobs, M. L., Mavroudis, C. et al. (2011). Heterotaxy: Lessons learned about patterns of practice and outcomes from the congenital heart surgery database of the society of thoracic surgeons. World Journal for Pediatric and Congenital Heart Surgery, 2(2), 278–286. [Google Scholar] [PubMed]
5. Okamoto, T., Nakano, T., Goda, M., Oda, S., Kado, H. (2021). Outcomes of systemic-to-pulmonary artery shunt for single ventricular heart with extracardiac total anomalous pulmonary venous connection. General Thoracic and Cardiovascular Surgery, 69, 646–653. [Google Scholar] [PubMed]
6. Gaynor, J. W., Collins, M. H., Rychik, J., Gaughan, J. P., Spray, T. L. (1999). Long-term outcome of infants with single ventricle and total anomalous pulmonary venous connection. The Journal of Thoracic and Cardiovascular Surgery, 117(3), 506–514. [Google Scholar] [PubMed]
7. Yoshimura, N., Oshima, Y., Henaine, R., Matsuhisa, H. (2010). Sutureless pericardial repair of total anomalous pulmonary venous connection in patients with right isomerism. Interactive Cardiovascular and Thoracic Surgery, 10(5), 675–678. [Google Scholar] [PubMed]
8. Honjo, O., Atlin, C. R., Hamilton, B. C., Al-Radi, O., Viola, N. et al. (2010). Primary sutureless rapair for infants with mixed total anomalous pulmonary venous drainage. The Annals of Thoracic Surgery, 90, 862–868. [Google Scholar] [PubMed]
9. Hoashi, T., Kagisaki, K., Oda, T., Kitano, M., Kurosaki, K. et al. (2013). Long-term results of treatments for functional single ventricle associated with extracardiac type total anomalous pulmonary venous connection. European Journal of Cardio-Thoracic Surgery, 43(5), 965–970. [Google Scholar] [PubMed]
10. Kisamori, E., Kotani, Y., Raja, F. K., Kobayashi, J., Kuroko, Y. et al. (2022). Strategy of delayed repair of total anomalous pulmonary venous connection in right atrial isomerism and functional single ventricle. JTCVS Open, 10, 308–319. [Google Scholar] [PubMed]
11. Brink, J., MacIver, R., Lee, M. G., Konstantinov, I. E., Cheung, M. et al. (2015). Neonatal pulmonary artery reconstruction during shunting to treat and prevent juxtaductal coarctation. The Annals of Thoracic Surgery, 99(2), 641–647. [Google Scholar] [PubMed]
12. Shinkawa, T., Yamagishi, M., Shuntoh, K., Miyazaki, T., Hisaoka, T. et al. (2007). Pulmonary arterial reconstruction for pulmonary coarctation in early infancy. The Annals of Thoracic Surgery, 83(1), 188–192. [Google Scholar] [PubMed]
13. Kim, H. K., Kim, W. H., Kim, S. C., Lim, C., Lee, C. H. et al. (2006). Surgical strategy for pulmonary coarctation in the univentricular heart. European Journal of Cardio-Thoracic Surgery, 29(1), 100–104. [Google Scholar] [PubMed]
14. Brink, J., MacIver, R., Lee, M. G., Konstantinov, I. E., Cheung, M. et al. (2015). Neonatal pulmonary artery reconstruction during shunting to treat and prevent juxtaductal coarctation. The Annals of Thoracic Surgery, 99(2), 641–647. [Google Scholar] [PubMed]
15. Sakamoto, K., Ota, N., Fujimoto, Y., Murata, M., Ide, Y. et al. (2014). Primary central pulmonary artery plasty for single ventricle with ductal-associated pulmonary artery coarctation. The Annals of Thoracic Surgery, 98(3), 919–926. [Google Scholar] [PubMed]
16. Kotani, Y., Kobayashi, Y., Kadowaki, S., Kisamori, E., Kobayashi, J. et al. (2022). Impact of pulmonary artery coarctation on pulmonary artery growth and definitive repair following modified Blalock-Taussig shunt. The Journal of Thoracic and Cardiovascular Surgery, 163(5), 1618–1626. [Google Scholar] [PubMed]
17. Kwon, B. S., Bae, E. J., Kim, G. B., Noh, C. I., Choi, J. Y. et al. (2009). Development of bilateral diffuse pulmonary arteriovenous fistula after fontan procedure: Is there nonhepatic factor? The Annals of Thoracic Surgery, 88(2), 677–680. [Google Scholar] [PubMed]
18. Kugo, Y., Iwai, S., Ishimaru, K., Yamauchi, S., Hasegawa, M. et al. (2020). Pulmonary venous obstruction after extracardiac total cavopulmonary connection in right atrial isomerism. General Thoracic and Cardiovascular Surgery, 68(9), 969–974. [Google Scholar] [PubMed]
19. Radman, M., Keller, R. L., Oishi, P., Datar, S. A., Wellnitz, K. et al. (2014). Preoperative B-type natriuretic peptide levels are associated with outcome after total cavopulmonary connection (Fontan). The Journal of Thoracic and Cardiovascular Surgery, 148(1), 212–219. [Google Scholar] [PubMed]
20. Lin, H. C., Huang, S. C., Wu, M. H., Wang, J. K., Lin, M. T. et al. (2022). Preoperative N-terminal pro-brain natriuretic peptide is associated with Fontan outcomes. The Journal of Thoracic and Cardiovascular Surgery, 164(3), 770–780. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools