 Open Access
Open Access
ARTICLE
Intermediate and Long-Term Follow-Up of Transcatheter Closure of Congenital Coronary Cameral Fistulas in Infants and Children: Experience from a Single Center
1 Department of Pediatric Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, 510100, China
2 Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, 510100, China
* Corresponding Author: Zhiwei Zhang. Email:
Congenital Heart Disease 2023, 18(4), 413-430. https://doi.org/10.32604/chd.2023.029848
Received 10 March 2023; Accepted 16 May 2023; Issue published 15 September 2023
Abstract
Background: Limited data are available regarding intermediate and long-term outcomes of transcatheter closure (TCC) of coronary cameral fistulas (CCFs) in the pediatric patients. Methods: All pediatric patients diagnosed with CCFs who were scheduled to undergo TCC between 2005 and 2019 were retrospectively enrolled in the study. Results: A total of 66 patients (median age: 3.93 years, median weight: 15 kg) underwent attempted TCC of CCFs. Immediate successful device implantation was achieved in 62 patients, and immediate complete occlusion was achieved in 44 patients (44/62%, 71.0%). The closure procedure was waived in 2 patients due to anatomical factors. A total of 6 periprocedural complications occurred in 5 patients, including acute myocardial infarction (n = 3), procedure-related death (n = 1), device embolization (n = 1), and rupture of tricuspid chordae tendineae (n = 1). The acute procedural success rate was 89.4% (59/66), while the acute complication rate was 9.1% (6/66). Follow-up data were collected for 58 (93.5%) out of 62 patients at a median of 9.3 years (range: 3.0–15.7 years). 10 adverse events occurred in 9 patients, including 5 follow-up complications (1 aortic valve perforation, 1 coronary thrombosis, 1 progressive aneurysmal dilation after reintervention, and 2 cases of new-onset tricuspid valve prolapse with significant regurgitation), and 5 closure failure with large residual shunts. The intermediate and long-term adverse event rate was 17.2% (10/58). The anatomical features associated with both acute and follow-up adverse events were large CCFs (p = 0.005), and giant coronary artery aneurysms (CAAs) (p = 0.029). Conclusions: TCC of CCFs in infants and children appears to be effective and is associated with a relatively low complication rate. Large CCFs and giant CAAs represent a higher risk of both acute and intermediate and long-term adverse events after closure.Keywords
Congenital coronary artery fistulas (CCAFs) are rare abnormal connections between coronary arteries and either a chamber of the heart or any segment of the systemic or pulmonary circulation without a capillary junction [1]. Fistulas between coronary arteries and cardiac chambers are defined as coronary cameral fistulas (CCFs) [2]. The incidence of CCFs is rare, ranging from 0.1% to 0.2% in patients undergoing diagnostic coronary angiography [3]. The majority of CCFs originate from the right coronary artery (RCA), and >90% of cases drain to the right side of the heart, with the right ventricle (RV) accounting for the most common drainage site [4].
Clinical presentation generally depends on the hemodynamic significance of CCFs. A CCF that drains to the right cardiac chambers creates a left-to-right shunt, which can lead to volume overload and congestive heart failure [5]. A CCF that drains to the left ventricle (LV) produces hemodynamic changes similar to those of aortic regurgitation, and a CCF that drains to the left atrium (LA) results in volume overload similar to that of mitral regurgitation [6]. If left untreated, CCFs may result in clinical complications, such as myocardial ischemia [7], ventricular dysfunction [8], aneurysmal formation [9], and infective endocarditis [10].
Transcatheter intervention has been considered to be an effective treatment strategy for anatomically suitable CCAFs in pediatric and adult patients [11,12]. However, few studies have reported on long-term outcomes of transcatheter closure (TCC) of CCFs, especially in pediatric patients, and current evidence of its potential benefit is still very weak due to the limited cases in the prior study cohorts [13–15]. The present study describes our experience with TCC of CCFs in a large cohort of pediatric patients, focusing on technical aspects of the closure procedure and intermediate and long-term follow-up.
This retrospective study was conducted at the Department of Pediatric Cardiology, Guangdong Provincial People’s Hospital, Guangzhou, China. All children (<18 years old) diagnosed with medium or large size CCFs who underwent attempted TCC between January 2005 and December 2019 were included in the study. Data were collected from medical records and included demographic characteristics, clinical symptoms, indications for procedure, hemodynamic and angiographic findings, procedural details, post-procedure therapy, and follow-up data. Patients with concomitant cardiac lesions requiring surgical treatment were excluded. The study protocol was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of the hospital. Informed consent for the procedure and clinical record review was obtained from all patients or their legal guardians.
A CCF was classified into a proximal or distal type based on its origin. Briefly, a proximal type of a CCF arises near the origin of a major coronary artery without significant coronary branches supplying the myocardium from the fistula itself. A distal type of a CCF originates from the distal end of a main coronary artery, with the conduit coronary artery proximal to the fistula giving rise to normal coronary branches supplying the myocardium [16,17]. A coronary artery aneurysm (CAA) is defined as a coronary dilatation with a diameter that is greater than 1.5 times that of the adjacent normal arterial segment or the largest coronary artery, and a CAA with a diameter of >20 mm is called a giant CAA [18]. The sizes of CCFs were categorized as medium or large based on the descriptions provided in a previous study [19]. Large CCFs were defined as fistulas larger than 2 times the largest diameter of the coronary vessel not feeding the coronary fistulas. Medium size CCFs were defined as fistulas with diameters 1 to 2 times the largest diameter of the coronary vessel not feeding the coronary fistulas [19].
The closure procedure was performed under general anesthesia in all patients. Access was obtained through femoral arterial and venous catheters. Intravenous heparin (100 IU/kg) and prophylactic antibiotics (cefazolin 50 mg/kg) were administered. Diagnostic catheterization was carried out for a hemodynamic evaluation. Ascending aortogram and selective coronary angiography were performed to determine CCF anatomy, type, size, origin, and drainage site. TCC of CCFs was attempted in cases with suitable anatomy. Diagnostic coronary catheters, guidewires, microcatheters, and coronary guidewires were used to access the fistulas and create a transarterial approach, arterio‑venous loop (A‑V loop) or arterio-arterial loop (A-A loop). Devices were deployed via the antegrade venous/arterial or retrograde arterial approach after an A-V loop/A-A loop, and retrograde arterial approach at the optimal occlusion site. The size of the occluder other than coils was at least 50% larger than the diameter of fistula at the occlusion site [19]. As for coils, the first coil deployed was at least 30% larger than the vessel diameter [19].
Twelve‑lead electrocardiography (ECG) was used to monitor the patient for 10–15 min after device deployment as an occlusion test. Repeat selective coronary angiography was performed to evaluate the presence of acute coronary occlusion and residual shunts immediately after device deployment. After implantation, all patients were treated with antiplatelet therapy that included aspirin at a dose of 3–5 mg/kg per day for at least 6 months, as well as clopidogrel at a dose of 1 mg/kg per day for 1–3 months. Anticoagulation with warfarin after closure was recommended in some patients with dilated, tortuous, and aneurysmal conduit coronary arteries.
Occlusion devices used in the study included the following: coils (Cook Medical, Bloomington, IN, USA), Amplatzer duct occluder II (ADO II; Abbott Vascular, Saint Paul, Minnesota, USA), Amplatzer vascular plug/plug II (AVP/AVP II; Abbott Vascular), domestic vascular plug (Starway Medical Technology, Beijing, China), ventricular septal defect occluder (VSD occluder, Lifetech Scientific, Shenzhen, China), atrial septal defect occluder (ASD occluder, Lifetech Scientific), and patent ductus arteriosus occluder (PDA occluder, Lifetech Scientific).
Within 24 h after the procedure, all patients underwent transthoracic echocardiography (TTE) and ECG to confirm the correct device position and exclude device embolization, thrombus formation, residual shunts, valvular regurgitation, pericardial effusion, myocardial ischemia or infarction, and cardiac arrhythmia. The same evaluations were repeated at 1, 3, 6, and 12 months post procedure and annually thereafter. In addition, follow-up coronary angiography and cardiac computed tomography (CT) were performed in selected patients to further evaluate potential coronary occlusion or thromboembolization. Primary outcomes were a composite of acute procedural success, including no/small residual shunts upon immediate post-deployment coronary angiography, and no periprocedural complications. Secondary outcomes included complications, survival, and degree of residual shunts for patients who received regular follow-ups after successful device closure. Residual shunt status was categorized as [20]: (1) trivial, which was defined as a residual shunt with minimal or insignificant amount of residual flow; (2) small, which was defined as a residual shunt that was less than 50% of the original fistula size; (3) large, which was defined as a residual shunt that was larger than 50% of the original fistula size. Closure with either complete occlusion or trivial/small residual shunts is defined as closure success. Adverse events included complications and large residual shunts, both acute and during follow-up.
Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD) and median (minimum, maximum) for those that were not normally distributed. Categorical variables were summarized using frequencies and percentages. For comparison between groups, a student’s t-test for normally distributed continuous data or Wilcoxon rank-sum test for non-normally distributed continuous data were performed. Chi-square test or Fisher’s exact test were used for categorical variables. Statistical significance was claimed if a two-sided test obtained a p value of <0.05. The data were analyzed using GraphPad Prism 9.0 and SPSS 23.0.
A total of 81 patients (42 males, median age: 3.24 years) were enrolled in the study. Overall, 76 patients (93.8%) were diagnosed with isolated CCFs, whereas 5 patients (6.2%) were found to have concomitant congenital heart diseases, including mild pulmonary stenosis (n = 3), PDA (n = 1), mitral valve prolapse and regurgitation (n = 1). At diagnosis, 60 patients (74.0%) were asymptomatic with a continuous cardiac murmur during physical examination, and 15 patients (18.5%) had a recurrent respiratory infection. Clinical manifestations such as congestive heart failure and growth retardation were detected in 5 patients (6.2%). TTE revealed ventricular enlargement in 57 patients (70.4%). Further patient demographics are detailed in Table 1.
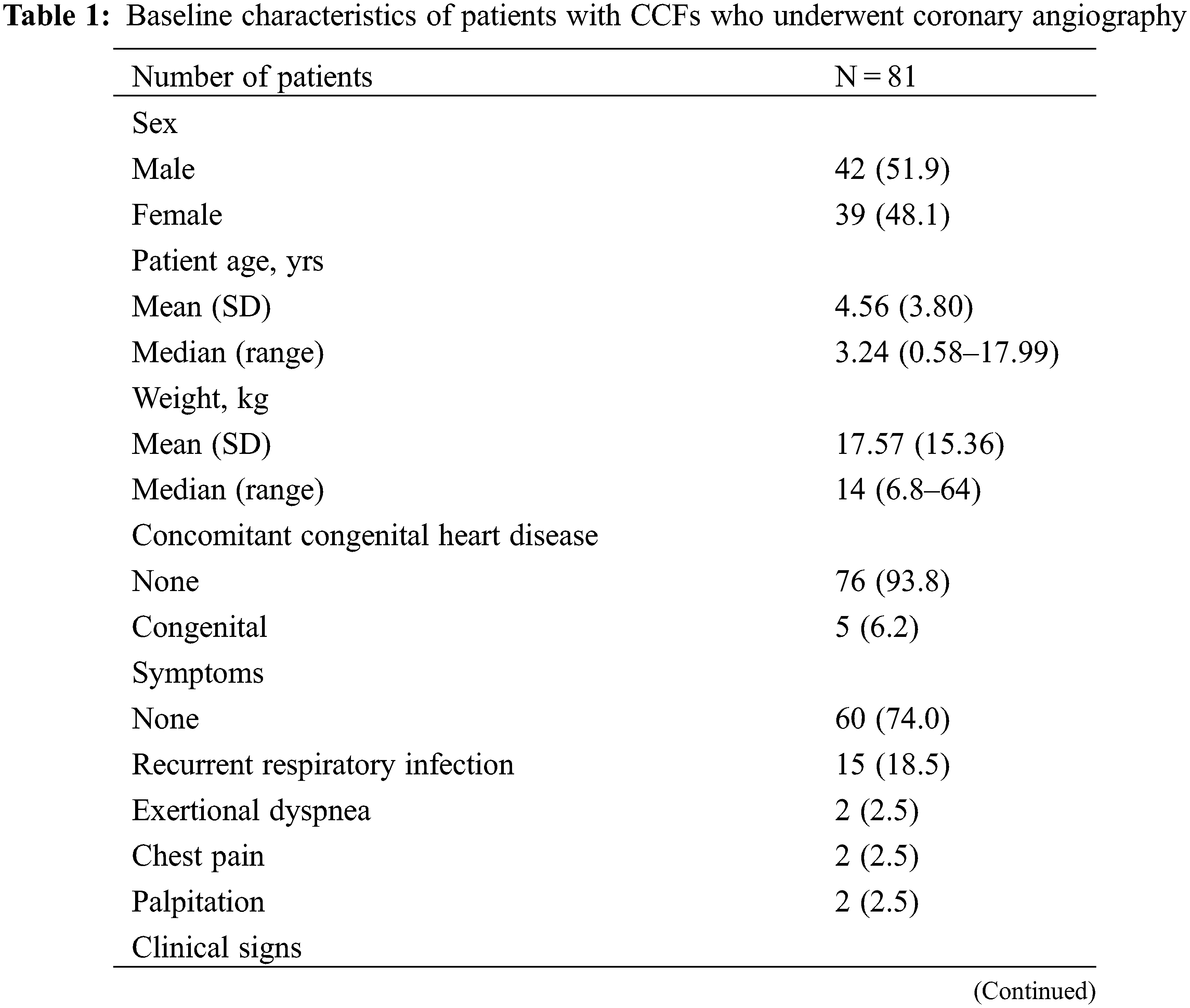
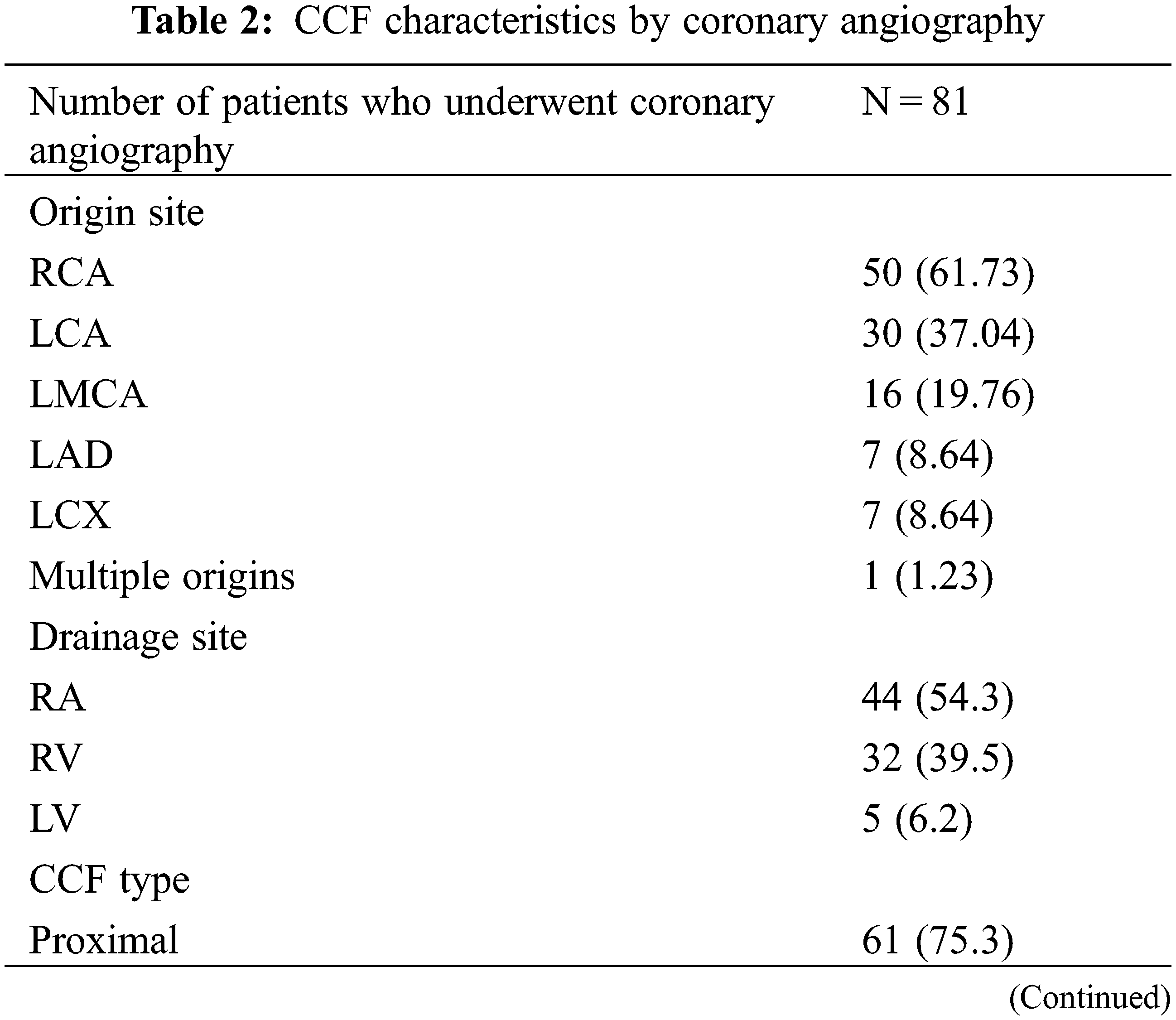
All of the 81 patients underwent diagnostic coronary angiography. The angiographic CCF characteristics are summarized in Table 2. 1 patient had multiple fistulas originating from both coronary arteries and draining into the RV, and the remaining 80 patients presented with a single fistula. Most of the CCFs originated from the RCA (61.73%), and most of them drained into the right atrium (RA) (54.3%). The fistula origin was proximal in 61 patients (75.3%), and distal in 20 patients (24.7%). The CCFs were deemed large in 37 (45.7%) and medium in 44 (54.3%) patients. CAAs were observed in 45 cases (55.5%), 17 of which were identified as giant CAAs.
3.3 Procedure Details and Immediate Outcomes
TCC were attempted in 66 patients and successful device implantation was achieved in 62 patients (Fig. 1). The median patient age at the time of TCC was 3.93 years (range: 0.58–17.99 years), and the median weight was 15 kg (range: 6.8–64 kg). The fistulas were considered not suitable for device closure in the remaining 15 patients. These 15 patients then underwent successful surgical closure. The anatomical differences between patients who underwent TCC and patients who underwent surgical closure are shown in Fig. 2. The majority of patients who underwent TCC had proximal type CCFs that mainly drained into the RA (63.6%, 42/66), while most of the patients who underwent surgical closure had distal type CCFs draining into the RV (60%, 9/15).
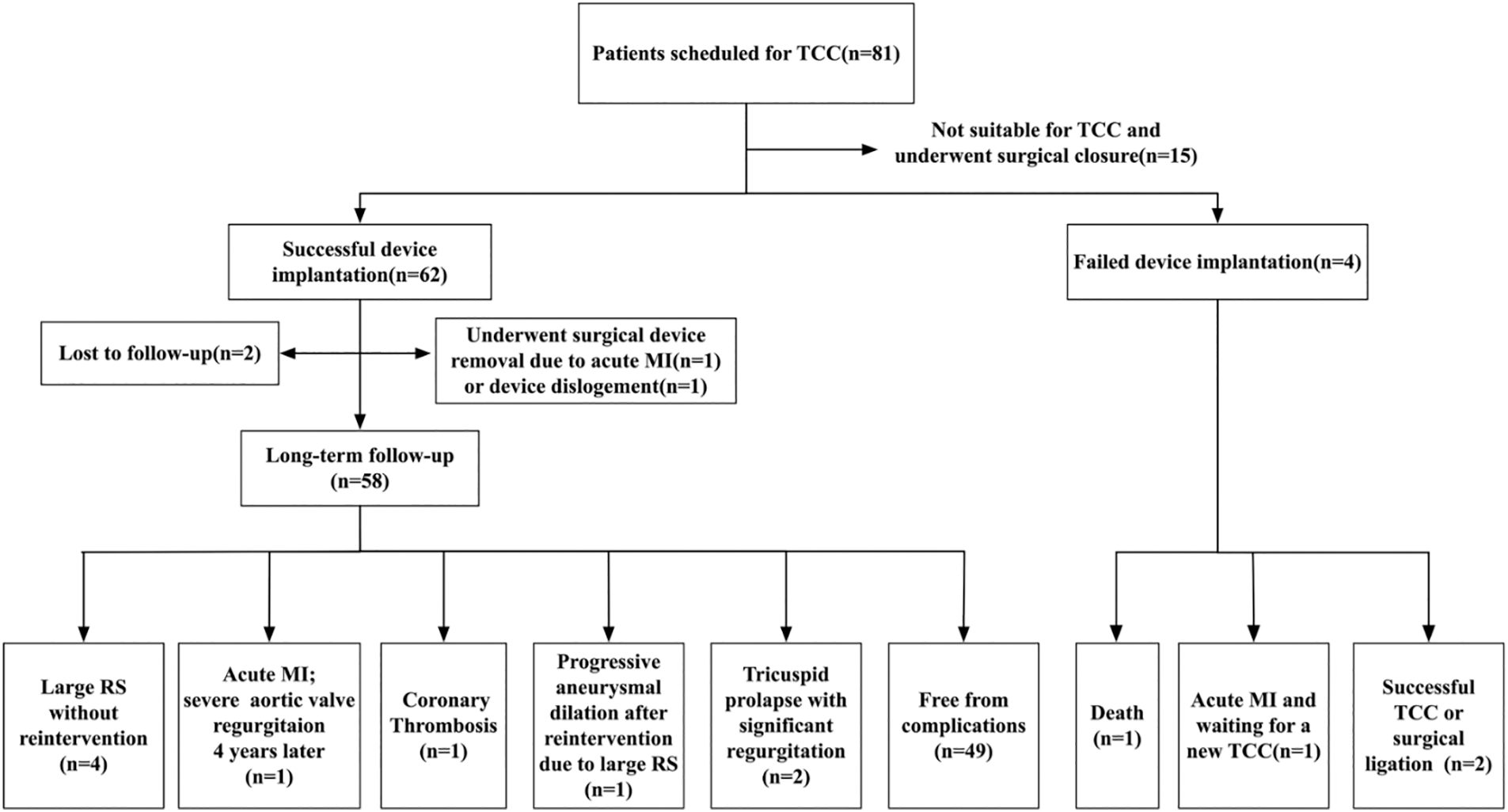
Figure 1: Patient flow chart
Notes: TCC: transcatheter closure; MI: myocardial infarction; RS: residual shunt.
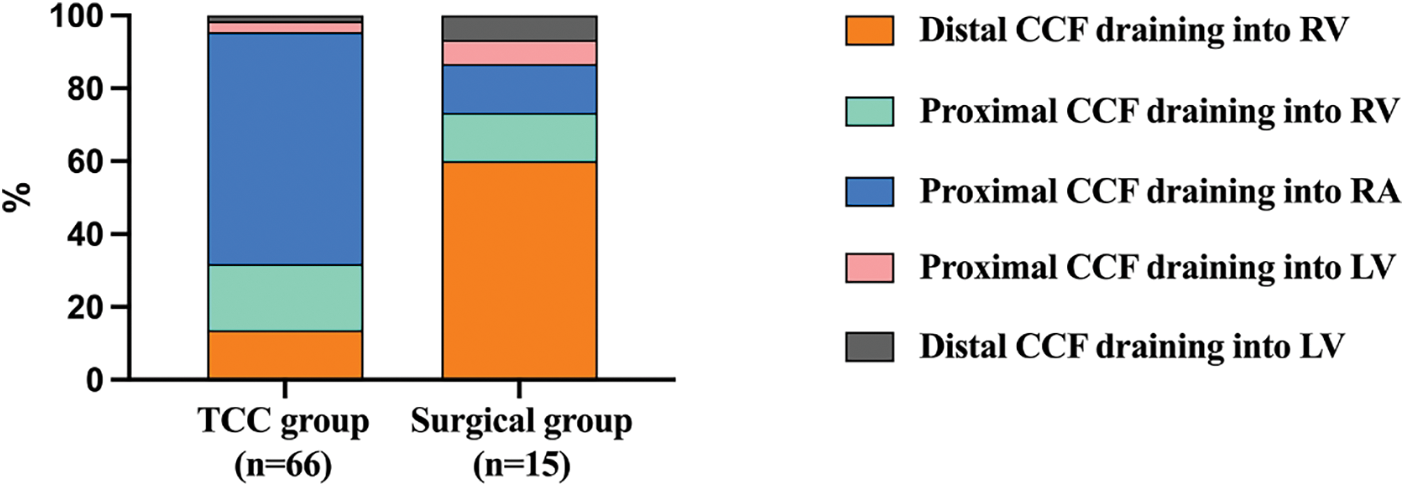
Figure 2: The anatomical differences between patients who underwent TCC and those who underwent surgical closure
Notes: TCC: transcatheter closure; CCF: coronary cameral fistula; RA: right atrium; RV; right ventricle.
Details of TCC are summarized in Table 3. During procedure, antegrade venous/arterial or retrograde arterial approach after the A-V/A-A loops was used in 53 cases (80.3%), and retrograde arterial approach was used in 13 cases (19.7%, Fig. 3). The CCFs were occluded at the proximal site in 22 patients (33.33%), at the distal site in 42 patients (63.64%), and at both sites in 2 patients (3.03%, Fig. 4). Types of devices used in the closure procedure are presented in Table 3. PDA occluder was most commonly used (27.3%), followed by AVP II (22.7%), and VSD occluder (mainly muscular VSD occluder) (16.7%).
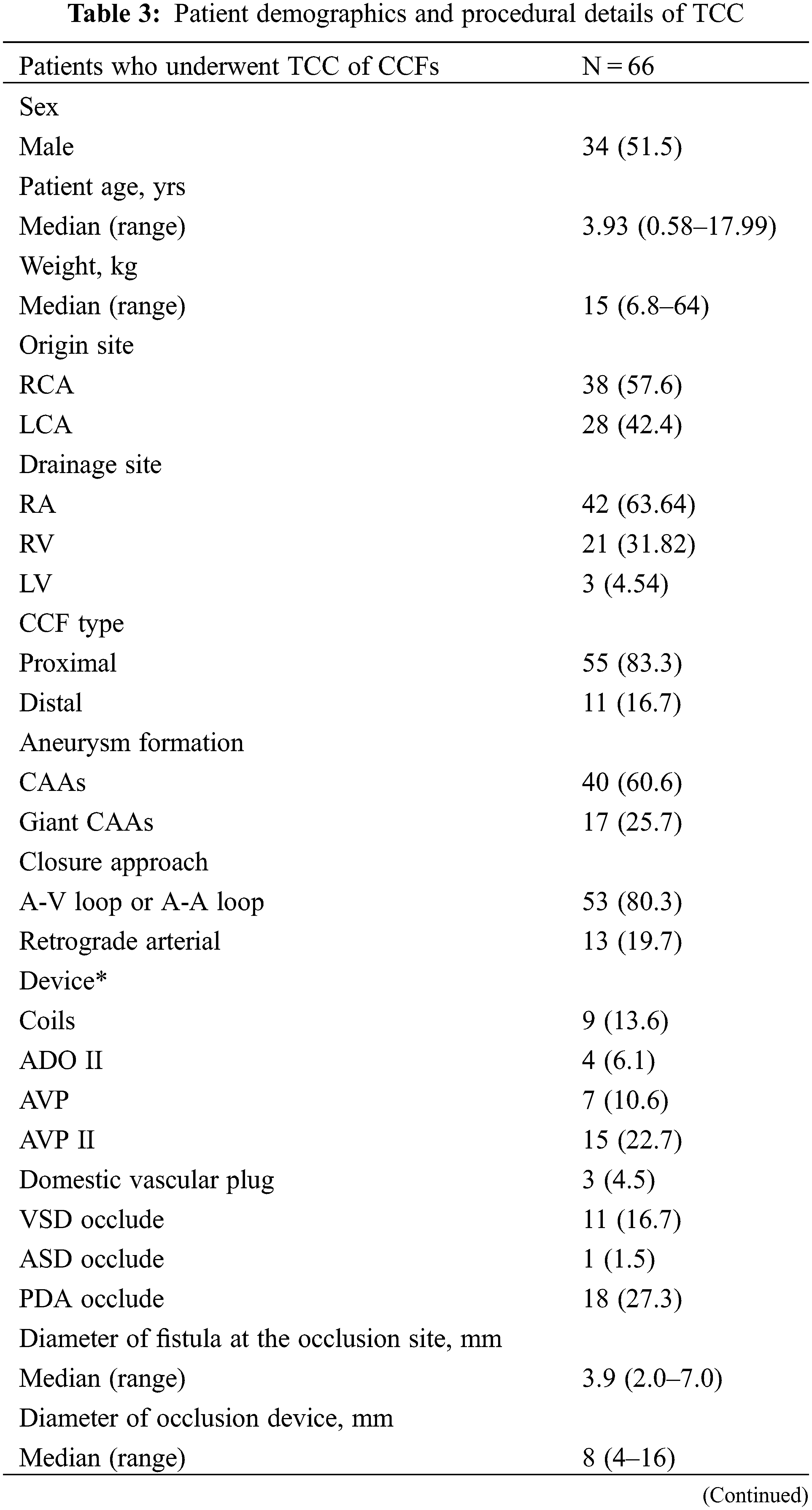
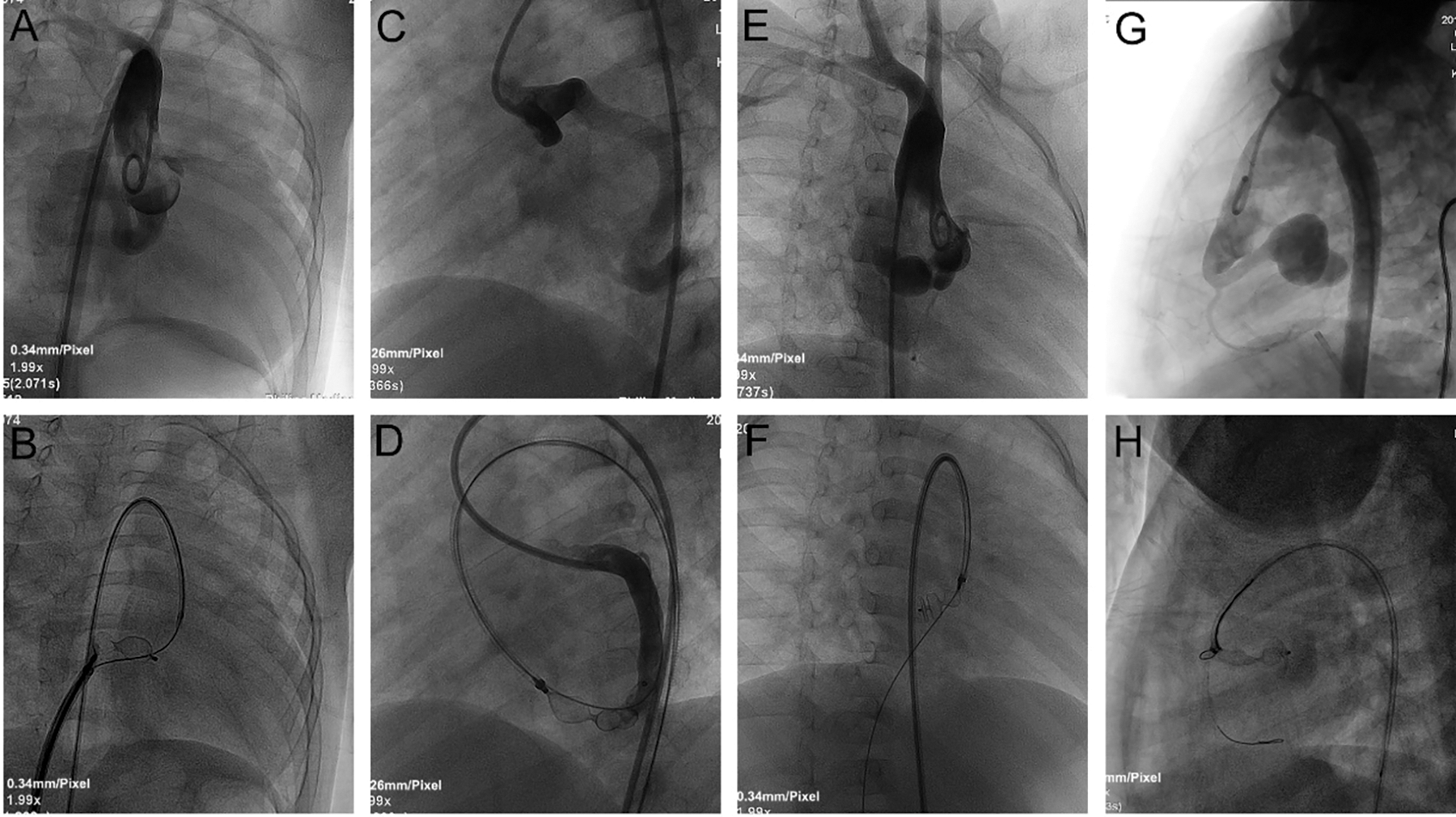
Figure 3: TCC of CCFs using different approaches. A, B: Ascending aortogram in RAO 30° projection demonstrated an RCA-to-RA fistula. An 8 mm VSD occluder was deployed through the antegrade venous approach via an A‑V loop. C, D: Ascending aortogram in LAO 45° projection demonstrated an LCX-to-LV fistula. A 12 mm AVP II was deployed through the antegrade arterial approach via an A-A loop. E, F: Ascending aortogram in RAO 45° projection demonstrated an RCA-to-RA fistula. A 9 mm AVP II was deployed through the retrograde arterial approach via an A-V loop. G, H: Ascending aortogram in LAO 90° projection demonstrated an RCA-to-RA fistula with a giant CAA. A 5/6 mm ADO II was deployed through the retrograde arterial approach
Notes: TCC: transcatheter closure; CCF: coronary cameral fistula; RAO: right anterior oblique; RCA: right coronary artery; RA: right atrium; VSD: ventricular septal defect; A-V loop: arterio-venous loop; LAO: left anterior oblique; LCX: left circumflex coronary artery; LV: left ventricle; A-A loop: arterio-arterial loop; AVP II: Amplatzer vascular plug II; CAA: coronary artery aneurysm; ADO II: Amplatzer duct occluder II
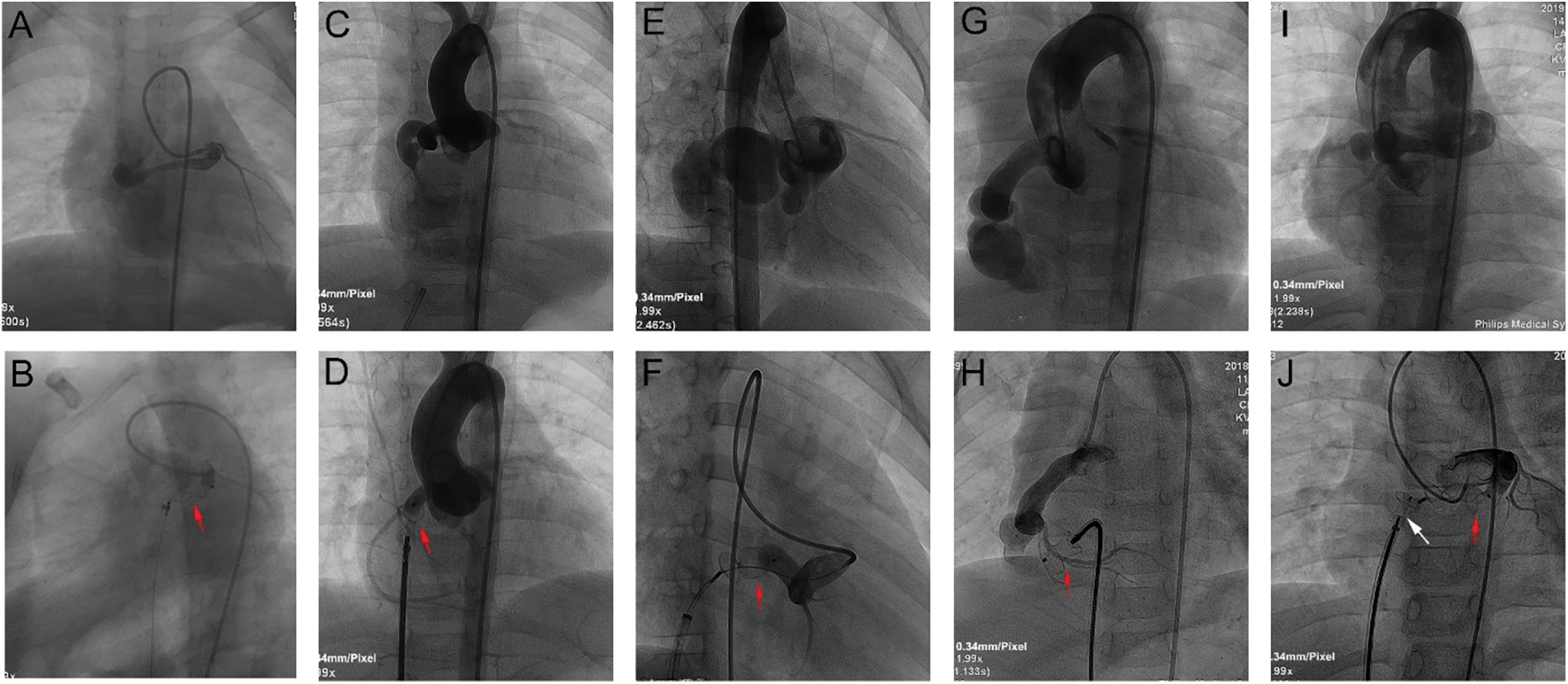
Figure 4: TCC of CCFs with different occlusion sites. A, B: Selective left coronary angiogram in anterior and caudal LAO projections demonstrated an LCA-to-RA fistula. A 10 mm AVP (red arrow) was deployed at the proximal site near the fistula origin. C, D: Ascending aortogram in anterior projection demonstrated an RCA-to-RA fistula. A 6/8 mm PDA occluder (red arrow) was deployed at the fistula orifice (distal site). E, F: Ascending aortogram in RAO 30° projection demonstrated an RCA-to-RA fistula with a giant CAA. A 12 mm domestic vascular plug (red arrow) was deployed at the proximal site near the CAA inlet. G, H: Ascending aortogram in anterior projection demonstrated an RCA-to-RV fistula with a giant CAA. A 10/12 mm PDA occluder (red arrow) was deployed at the distal site near the CAA outlet. I, J: Ascending aortogram in anterior projection demonstrated an LCA-to-RV fistula. A 10/12 mm PDA occluder (white arrow) was deployed at the distal site near the CAA outlet, and a 12 mm domestic vascular plug (red arrow) was deployed at the proximal site near the CAA inlet
Notes: TCC: transcatheter closure; CCF: coronary cameral fistula; LAO: right anterior oblique; LCA: left coronary artery; RA: right atrium; AVP: Amplatzer vascular plug. RCA: right coronary artery; PDA: patent ductus arteriosus; CAA: coronary artery aneurysm; RV: right ventricle.
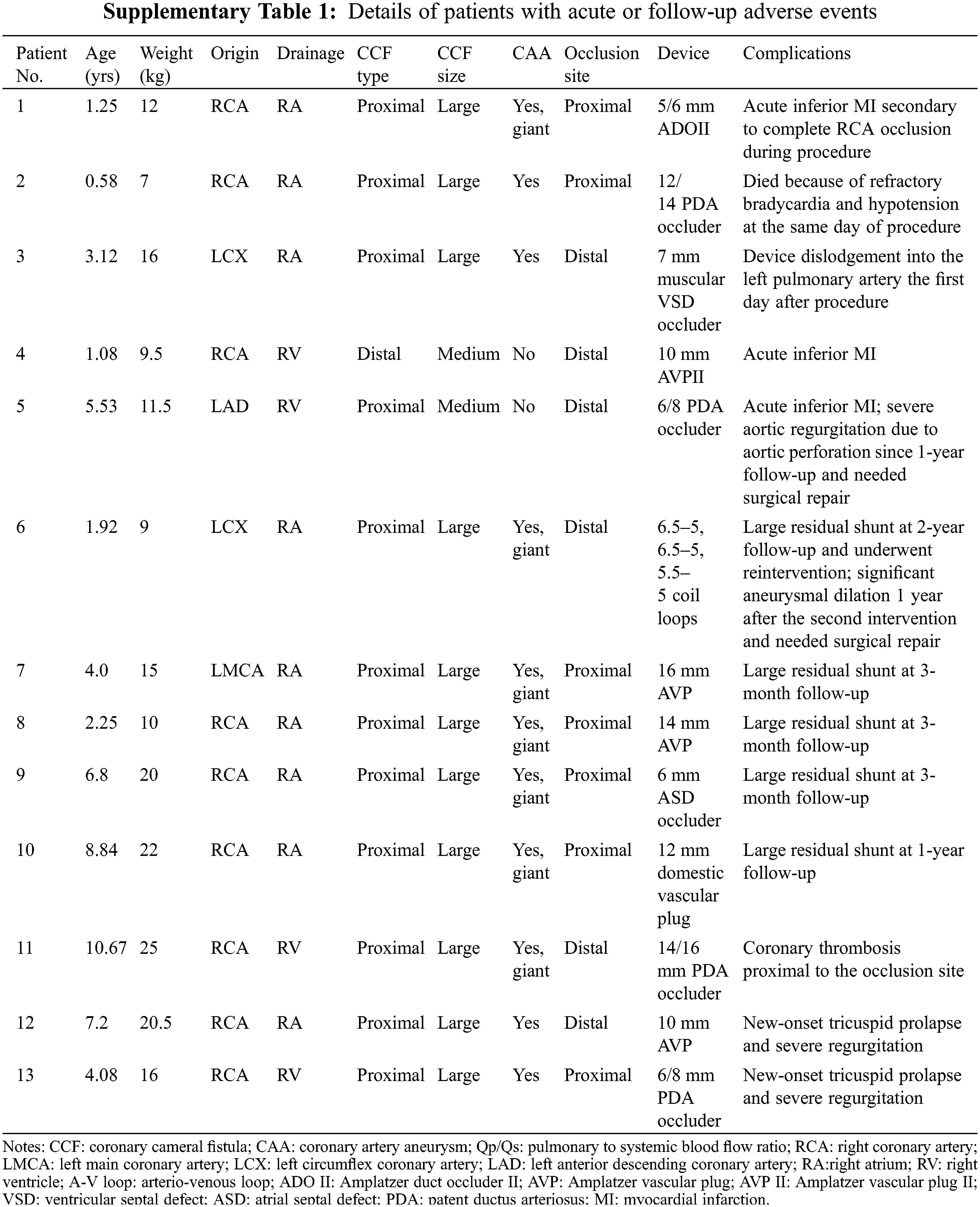
4 procedures were aborted, 2 due to procedural complications (Supplementary Table 1), and 2 because of anatomical factors (the presence of side branches close to fistula orifice, and extreme tortuosity of the fistulas). In the first patient (Patient No. 1) with a large proximal RCA-to-RA fistula, complete RCA occlusion occurred immediately after a 5/6 mm ADO II was released. Thrombus formation was detected within the occluded RCA, and acute inferior myocardial infarction (MI) was confirmed by ECG. The ADO II was then snared and retrieved into an 8-Fr long sheath, and thrombus aspiration of RCA was performed. A repeat coronary angiography demonstrated successful RCA recanalization. The patient was prescribed low molecular weight heparin for 3 days, followed by dual antiplatelet therapy with aspirin and clopidogrel for 1 month. The patient was in good condition and was expecting another TCC. In the second patient (Patient No. 2) with a large proximal RCA-to-RV fistula who suffered from recurrent respiratory infection and heart failure, right heart catheterization revealed severe pulmonary hypertension (pulmonary artery systolic pressure: 63 mmHg, mean pulmonary artery pressure: 47 mmHg). After a 12/14 mm PDA occluder was deployed, the patient suffered third-degree atrioventricular (A-V) block accompanied by hypotension and hypoxemia. Although the occluder was immediately removed, the patient died on the same day due to refractory bradycardia and hypotension.
For the 62 patients with device implantation, immediate post-deployment angiography demonstrated that successful closure was achieved in all patients, including 44 with complete occlusion (71.0%), 15 (24.2%) with trivial residual shunts, and 3 (4.8%) with small residual shunts. ECG during the procedure showed transient third-degree A-V block in 1 patient, and atrioventricular junctional tachycardia in another patient. Both of these cases returned to normal ECG at the end of the procedure.
4 periprocedural complications were observed in 3 patients within 24 h after procedure, including 1 device embolization, 1 rupture of tricuspid chordae tendineae, and 2 acute MI (Supplementary Table 1). 2 patients (Patients No. 3 and No. 4) underwent emergency surgical device removal and fistulas ligation. Patient No. 5, who was diagnosed with acute inferior MI, was managed conservatively with both anticoagulation (warfarin) and antiplatelet (aspirin) therapy. The reason of his MI was thought to be resulted from flow stasis in a large, tortuous LCA after closure with a 6/8 mm PDA occluder at the fistula drainage site.
The remaining 59 patients were free of symptoms and were discharged on the second day after the procedure. Overall, the acute procedural success rate was 89.4% (59/66), and the acute complication rate was 9.1% (6/66). After hospital discharge, medical treatment was based on the choice of referring cardiologist’s recommendations: 30 patients were treated with initial dual antiplatelet therapy with aspirin and clopidogrel, 1 patient was treated with warfarin and aspirin, 24 patients were treated with aspirin alone, and 4 patients were treated with clopidogrel alone.
Of the 62 patients who had immediate successful device implantation, 58 patients were available for follow-up for a median of 9.3 years (range: 3.0–15.7 years). Among them, 7 patients (12.1%) completed 3-year follow-up, 24 patients (41.4%) completed 5-year follow-up, and 27 patients (46.5%) completed 10-year follow-up. 3 patients had coronary angiography, 3 patients had cardiac CT scan, and the rest of patients had TTE to evaluate fistula remodeling and residual shunts during follow-up. Complete closure of CCFs was achieved in 47 patients (81.0%) at the last visit. A total of 11 patients (19.0%) were reported to have residual shunts, and 5 (8.6%) of them had large residual shunts, which was considered as closure failure (Supplementary Table 1). 1 patient (Patient No. 6) with a large proximal LCA-to-RA fistula underwent a second TCC at the same occlusion site 2 years after the initial procedure due to significant recanalization. The remaining 4 patients (Patients No. 7–No. 10) with large residual shunts refused further intervention. For the rest of 6 patients with residual shunts, 2 had trivial residual shunts, and 4 had small residual shunts.
The patients were treated with antiplatelet and/or anticoagulation therapy for a median of 6 months (range: 6–48 months). No special treatment was prescribed for patients with residual shunts during the follow-up visits, and they remained asymptomatic.
During follow-up, 5 complications were reported, including aortic valve perforation (n = 1), coronary thrombosis (n = 1), progressive aneurysmal dilation after reintervention (n = 1), and new-onset tricuspid valve prolapse with significant regurgitation (n = 2; Supplementary Table 1). Patient No. 5, who experienced acute MI and received medical treatment, required surgical aortic valvuloplasty 4 years later because of progressive severe aortic regurgitation secondary to aortic valve perforation. In the patient (Patient No. 11) with a large RCA-to-RV fistula occluded distally with a PDA occluder, TTE at 1-month follow-up revealed thrombus formation within the large aneurysm proximal to the occluder. This patient was treated with aspirin for 2 years and has remained free from myocardial ischemia or infarction. Patient No. 6 experienced significant aneurysmal dilation 1 year after the second TCC and eventually underwent surgical ligation of the fistula and incision of the aneurysm. For the 58 patients who completed follow-up visits, 10 adverse events occurred in 9 patients, including 5 follow-up complications and 5 closure failure. The intermediate and long-term adverse event rate was 17.2% (10/58). In addition, for the 27 patients who completed 10-year follow-up visits, 8 adverse events occurred in 7 patients, whereas the remaining 20 patients were identified to have closure success. The anatomical features associated with both acute and intermediate and long-term adverse events of TCC of CCFs included large fistulas (p = 0.005), and giant CAAs (p = 0.029; Table 4).
The relationship between the closure devices mainly used in the present study and prognosis of TCC of CCFs are depicted in Fig. 5. The adverse event (both acute and follow-up adverse event) rate was 6.7% for the AVP II group, 27.8% for the PDA occluder group, 9.1% for the VSD occluder group, 22.2% for the coil group, and 14.3% for the AVP group, respectively. The complete occlusion rate was 80% for the AVP II group, 61.6% for the PDA occluder group, 72.7% for the VSD occluder group, 66.7% for the coil group, and 42.8% for the AVP group, respectively. The AVP II and VSD occluder (mainly muscular VSD occluder) seem to be more appropriate devices for medium and large size CCFs to achieve successful TCC.
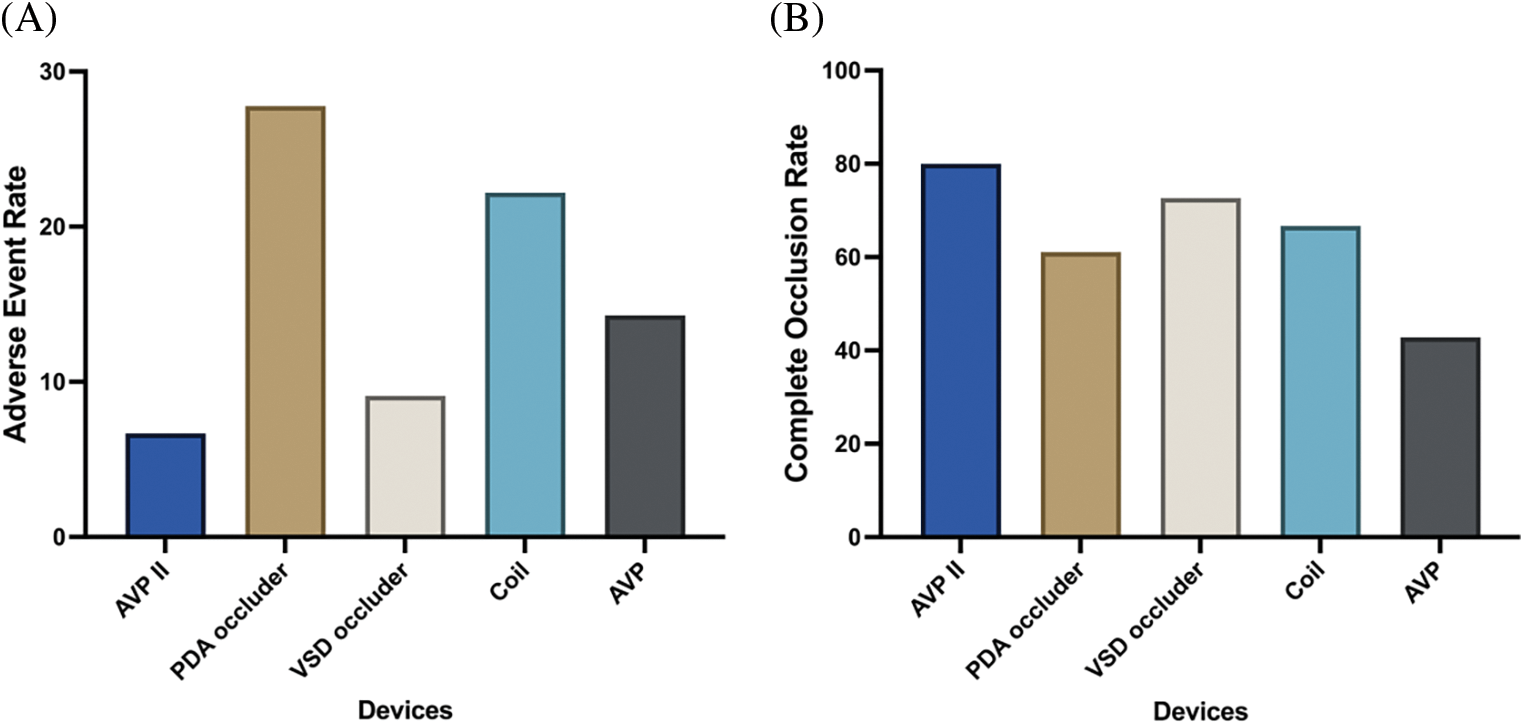
Figure 5: Adverse event rate (A) and complete occlusion rate (B) using different occlusion devices for TCC of CCFs
Notes: AVP II: Amplatzer vascular plug II; PDA: patent ductus arteriosus; VSD: ventricular septal defect; AVP: Amplatzer vascular plug.
TCC of CCAFs is an appropriate alternative therapeutic option for patients with suitable anatomical features and no concomitant need for surgery. Elective closure of CCAFs in children with clinical symptoms (class I indication) or with moderate or large fistulas without clinical symptoms (class IIa indication) has been advocated to prevent late-onset complications [11]. However, due to its rarity and heterogeneity, experience with TCC of CCFs in infants and children is poorly documented, and an ideal treatment approach is not well defined. To the best of our knowledge, the present study is the largest series evaluating intermediate and long-term efficacy and safety of TCC of CCFs in an exclusively selected cohort of pediatric patients. Our results demonstrated favorable intermediate and long-term effectiveness of TCC of CCFs as well as relatively low complication rate. Furthermore, the present study was the first to demonstrate that large CCFs and giant CAAs represented a higher risk of both acute and intermediate and long-term adverse events after TCC in pediatric populations.
The present study only included pediatric patients with medium or large size CCFs who intended to undergo the TCC treatment. Medium or larger size CCFs can enlarge over time and are usually associated with proximal coronary artery dilatation signifying high shunt flow over the years, especially in pediatric patients [19]. Most of the patients (74%) in the present study were asymptomatic. For these patients, TCC was indicated for those with chamber dilation, suggesting significant left-to right shunts, or due to the existence of CAAs or giant CAAs. In some of the cases with CAAs or giant CAAs, a significant left-to-right shunt was not indicated by a small Qp/Qs value and TCC was performed mainly to prevent potential complications such as progressive dilation of conduit coronary artery, aneurysm rupture, thrombosis, coronary steal phenomenon and myocardial ischemia [15,18].
In the present study, complete occlusion was achieved in 81.0% (47/58) of the patients at a median follow-up of 9.3 years, which was concordant with previously reported findings regarding complete closure rate (79%–86%) of transcatheter CCAF closure in adults, children and infants during medium or long-term follow-up [20–23].
The anatomical characteristics of CCFs and the strategy of device selection appeared to have a considerable impact on closure success in the study, as all 5 cases with significant residual shunts had large, tortuous, aneurysmal, proximal fistulas draining into the RA, and most of the fistulas were occluded by coils or AVP. Given the recent development of closure devices and techniques and improvement of clinical experience, more suitable occlusion devices such as PDA occluder, AVP II, and VSD occluder were used in the present study. Furthermore, our study demonstrated that AVP II and VSD occluder (mainly muscular VSD occluder) exhibited a relatively low adverse event rate and high complete occlusion rate compared to other devices. In addition, the favorable complete occlusion rate may have been associated with a relatively large device-to-fistula ratio with a median of 2.0 in our cases, which was similar to the data reported in prior studies [21]. Based upon these observations, we suggested that AVP II and muscular VSD occluder might be more optimal choices for moderate and large CCFs to achieve successful device closure. In addition, the device size should be 50%−100% larger than the diameter of occlusion site.
Only 1 of the patients with residual shunts underwent transcatheter reintervention to achieve complete occlusion. Interestingly, this patient experienced progressive aneurysm dilation after the second TCC, which eventually led to surgical treatment to prevent potential aneurysm rupture. The residual shunt has been demonstrated to not be associated with the risk of late complications after TCC of CCAFs [24]. Some researchers have suggested that the presence of a residual shunt may help reduce the incidence of thrombotic complications due to less blood stagnation [20]. More recently, Ponthier et al. [25] have demonstrated that reintervention of CCAFs occurred later in children, with 89% freedom from reintervention compared to 37.5% in adults at 6-year follow-up. This may help explain the low reintervention rate in the present study cohort, which consisted of infants and children. Although in a previous study by Jama et al. [26], reintervention was performed in all patients with large recanalization of fistulas (22%, 4/18), the present results indicate that the benefit of complete occlusion of residual shunts has yet to be shown. Although coronary angiography and cardiac CT scan are superior to TTE in delineating CCF morphology, unfortunately, most of the study patients only completed a follow-up TTE to define the degree of residual shunts. Since most of our patients were infants and young children, non-invasive and non-radioactive examinations such as TTE were more welcomed during the follow-up. This study limitation led to a lack of rigorous investigations of vessel remodeling and residual shunts after device closure.
In the present study, the acute procedural success rate and the intermediate and long-term adverse event rate were 89.4% and 17.2%, respectively, suggesting a relatively high success rate and low overall morbidity for TCC of CCFs in selected pediatric patients. Notably, both the acute procedural success rate and the follow-up adverse event rate in our study were comparable to the values that have been reported in prior TCC studies with similar patient numbers [22,24]. Similar to prior studies [16–17,20,22], ischemic complications, including MI (n = 3) and coronary thrombosis (n = 1), represented one of the main risks during TCC of CCFs in our study.
Notably, large CCFs and giant CAAs represented a higher risk of acute and follow-up adverse events after TCC in the study cohort. Our results indicated that the success of TCC of CCFs and risk of complications remained dependent upon the CCF anatomy, which was in accordance with previous study findings [20,23]. Shah et al. [23] suggested that a large/giant CAF was one of the “high-risk features of CAF” for thrombotic complications. A possible explanation for this is that the larger of the fistula size, the greater the dilation of the proximal conduit coronary artery tends to have, and the higher the risk of flow stasis and thrombosis after closure [14]. Therefore, for a proximal large CAF, TCC should be performed as close to the coronary origin as possible [17,27], or be performed at both the inlet and outlet of the fistula to exclude the aneurysm in the presence of a CAA [28,29]. However, the CCF occlusion site was not a predictor of adverse events after TCC in the present study. Mechanisms contributing to the development of adverse events in these types of CCFs are still complex, and may be divided into several categories, including anatomical considerations, abrupt cessation of high volume coronary flow occurring after CCF occlusion, and underrecognized differences in procedural strategies of TCC [24].
A CCAF with a giant CAA is an extremely rare pathology, and whether TCC is beneficial in such cases remains uncertain. Although successful TCC cases of this type of fistulas have been reported [29–32], 7 patients with giant CAAs experienced adverse events in the present study cohort, suggesting that the decision to perform TCC for this type of CCFs should be made very cautiously, since CCFs with giant CAAs may be difficult to close using catheter techniques, and device closure could be associated with a relatively high incidence of complications and residual shunts.
Another important factor that affects the prognosis of TCC of CCFs is the optimal antithrombotic therapy post procedure. The antiplatelet/anticoagulation regimens in our series were just empirically administered because no standard antithrombotic therapy has been available, making a definitive recommendation difficult. Previous studies have suggested that long-term warfarin was recommended in patients with significantly dilated conduit coronary artery or residual coronary segment, and that a combination of warfarin and an antiplatelet agent was necessary in patients with a very large residual vascular structure and very high risk of flow stasis [6,17]. For patients without residual coronary dilatation after device closure, antiplatelet therapy for at least 1 year was recommended [17]. Similar to previous studies [20,23], dual antiplatelet therapy or aspirin/clopidogrel monotherapy were mainly used for at least 6 months in our study. After 6 months, in cases with thrombus formation or persistent dilation of the fistulas and the coronary arteries, dual or mono antiplatelet therapy was provided indefinitely. Although no recommendation can be given based on these observations, it makes sense that patients receive at least long-term treatment by an antiplatelet agent after TCC of CCFs.
In conclusion, in CCF cases with complex anatomical features, such as large CCFs or giant CAAs, TCC should be performed after thoroughly weighing the anticipated benefits, procedure risks, and potential adverse outcomes. Further collaborative prospective studies are necessary to assess the benefits after TCC of CCFs in pediatric patients, and long-term periodic evaluation is warranted.
There are several limitations in the present study. First, it was a retrospective study and involved a group of selective pediatric patients from a single center, rendering the results susceptible to certain biases. Second, a follow-up coronary angiography or a cardiac CT scan was not performed in most of the study cases. Therefore, the occurrence of coronary thrombosis and the degree of residual shunts may have been underestimated due to the lack of detailed anatomical and functional evaluations of coronary arteries. Third, standard antithrombotic therapy after TCC of CCFs has not been well established, which may have had adverse effects on the follow‑up results.
TCC of CCFs in infants and children appears to be effective and is associated with a relatively low complication rate. Large CCFs and giant CAAs represent a higher risk of both acute and intermediate and long-term adverse events after TCC. Further multicenter studies are warranted to determine the optimal strategy for CCF closure in pediatric patients.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by Guangdong Provincial Clinical Research Center for Cardiovascular Disease [Grant No. 2020B1111170011], Guangzhou Science and Technology Project [Grant No. 2023A04J0485], and National Key R&D Program of China [Grant No. 2016YFC1100305].
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Zhi-Wei Zhang; administrative support: Zhi-Wei Zhang, Shu-Shui Wang, Yu-Mei Xie; provision of study materials or patients: Zhi-Wei Zhang, Shu-Shui Wang, Yu-Mei Xie, Yi-Fan Li; data collection and assembly of data: Yi-Fan Li, Ze-Wen Chen; Data analysis and interpretation: Yi-Fan Li, Ze-Wen Chen; draft manuscript preparation: Yi-Fan Li, Ze-Wen Chen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: De-identified participant data in this present study will be share upon reasonable request. We may balance the potential benefits and risks for each request and then provide the data that could be shared. Emails could be sent to the address below to obtain the shared data: drzhangzw@sohu.com.
Ethics Approval: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by institutional ethics board of Guangdong Provincial People’s Hospital (No. GDREC2016427A) and individual consent for this retrospective analysis was waived.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Jacobs, M. L., Mavroudis, C. (2010). Anomalies of the coronary arteries: Nomenclature and classification. Cardiology in the Young, 20, 15–19. [Google Scholar] [PubMed]
2. Carminati, M., Giugno, L., Chessa, M., Butera, G., Piazza, L. et al. (2016). Coronary-cameral fistulas: Indications and methods for closure. Eurointervention, 12, X28–X30. [Google Scholar] [PubMed]
3. Mansour, M. K., Nagalli, S. (2023). Coronary cameral fistula. In: StatPearls. Treasure Island (FLStatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK553211/ [Google Scholar]
4. Vaidya, Y. P., Green, G. R. (2019). Coronary artery fistula. Journal of Cardiac Surgery, 34(12), 1608–1616. [Google Scholar] [PubMed]
5. Firouzi, A., Alemzadeh-Ansari, M. J., Mohebbi, B., Khajali, Z., Khalilipur, E. et al. (2022). Diverse transcatheter closure strategies in coronary artery fistulas a state-of-the-art approach. Current Problems in Cardiology, 47(12), 101010. [Google Scholar] [PubMed]
6. Latson, L. A. (2007). Coronary artery fistulas: How to manage them. Catheterization and Cardiovascular Interventions, 70(1), 110–116. [Google Scholar] [PubMed]
7. Shimada, S., Taketazu, M., Sato, M., Nii, M., Shirai, M. (2019). Heart failure and coronary ischemia in a neonate with right coronary artery fistula. Pediatrics International, 61(4), 417–418. [Google Scholar] [PubMed]
8. Sandor, B., Bogats, G., Toth, L., Habon, T. (2020). Surgical treatment of heart failure due to giant coronary artery fistula: A case report. ESC Heart Failure, 7(5), 3203–3207. [Google Scholar] [PubMed]
9. Diao, W., Shi, C., Liu, G., Liu, X. (2021). Coronary artery-left ventricular fistula with giant right coronary aneurysm: A case report and literature review. The Heart Surgery Forum, 24(3), E433–E436. [Google Scholar] [PubMed]
10. Green, T., Crilley, J. (2018). Endocarditis and coronary artery fistula: A case report. European Heart Journal, 2(1), yty023. [Google Scholar] [PubMed]
11. Feltes, T. F., Bacha, E., Beekman, R. R., Cheatham, J. P., Feinstein, J. A. et al. (2011). Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American heart association. Circulation, 123(22), 2607–2652. [Google Scholar] [PubMed]
12. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology, 73(12), e81–e192. [Google Scholar] [PubMed]
13. Bruckheimer, E., Harris, M., Kornowski, R., Dagan, T., Birk, E. (2010). Transcatheter closure of large congenital coronary-cameral fistulae with amplatzer devices. Catheterization and Cardiovascular Interventions, 75(6), 850–854. [Google Scholar] [PubMed]
14. Tang, L., Wang, Z. J., Tang, J. J., Fang, Z. F., Hu, X. Q. et al. (2020). Transcatheter closure of large coronary-cameral fistulas using the patent ductus arteriosus occluder or amplatzer vascular plugs. International Heart Journal, 61(6), 1220–1228. [Google Scholar] [PubMed]
15. Wu, Y. H., Li, T. Y., Lin, Y. J., Fang, C. Y., Huang, C. F. et al. (2021). Mid-term follow-up of transcatheter closure for coronary cameral fistula in pediatrics. Acta Cardiologica Sinica, 37(1), 58–64. [Google Scholar] [PubMed]
16. Gowda, S. T., Forbes, T. J., Singh, H., Kovach, J. A., Prieto, L. et al. (2013). Remodeling and thrombosis following closure of coronary artery fistula with review of management: Large distal coronary artery fistula--to close or not to close? Catheterization and Cardiovascular Interventions, 82(1), 132–142. [Google Scholar] [PubMed]
17. Gowda, S. T., Latson, L. A., Kutty, S., Prieto, L. R. (2011). Intermediate to long-term outcome following congenital coronary artery fistulae closure with focus on thrombus formation. The American Journal of Cardiology, 107(2), 302–308. [Google Scholar] [PubMed]
18. Li, D., Wu, Q., Sun, L., Song, Y., Wang, W. et al. (2005). Surgical treatment of giant coronary artery aneurysm. The Journal of Thoracic and Cardiovascular Surgery, 130(3), 817–821. [Google Scholar] [PubMed]
19. Al-Hijji, M., El Sabbagh, A., El Hajj, S., AlKhouli, M., El Sabawi, B. et al. (2021). Coronary artery fistulas: Indications, techniques, outcomes, and complications of transcatheter fistula closure. JACC: Cardiovascular Interventions, 14(13), 1393–1406. [Google Scholar] [PubMed]
20. El-Sabawi, B., Al-Hijji, M. A., Eleid, M. F., Cabalka, A. K., Ammash, N. M. et al. (2020). Transcatheter closure of coronary artery fistula: A 21-year experience. Catheterization and Cardiovascular Interventions, 96(2), 311–319. [Google Scholar] [PubMed]
21. Armsby, L. R., Keane, J. F., Sherwood, M. C., Forbess, J. M., Perry, S. B. et al. (2002). Management of coronary artery fistulae. Patient selection and results of transcatheter closure. Journal of the American College of Cardiology, 39(6), 1026–1032. [Google Scholar] [PubMed]
22. Mottin, B., Baruteau, A., Boudjemline, Y., Piéchaud, F. J., Godart, F. et al. (2016). Transcatheter closure of coronary artery fistulas in infants and children: A French multicenter study. Catheterization and Cardiovascular Interventions, 87(3), 411–418. [Google Scholar] [PubMed]
23. Shah, A. H., Osten, M., Benson, L., Alnasser, S., Bach, Y. et al. (2020). Long-term outcomes of percutaneous closure of coronary artery fistulae in the adult: A single-center experience. Catheterization and Cardiovascular Interventions, 95(5), 939–948. [Google Scholar] [PubMed]
24. Valente, A. M., Lock, J. E., Gauvreau, K., Rodriguez-Huertas, E., Joyce, C. et al. (2010). Predictors of long-term adverse outcomes in patients with congenital coronary artery fistulae. Circulation: Cardiovascular Interventions, 3(2), 134–139. [Google Scholar] [PubMed]
25. Ponthier, L., Brenot, P., Lambert, V., Petit, J., Riou, J. Y. et al. (2015). Closure of isolated congenital coronary artery fistula: Long-term outcomes and rate of re-intervention. Pediatric Cardiology, 36(8), 1728–1734. [Google Scholar] [PubMed]
26. Jama, A., Barsoum, M., Bjarnason, H., Holmes, D. R., Rihal, C. S. (2011). Percutaneous closure of congenital coronary artery fistulae: Results and angiographic follow-up. JACC: Cardiovascular Interventions, 4(7), 814–821. [Google Scholar] [PubMed]
27. Cao, H., Li, D., Li, Y., Qiu, Y., Liu, J. et al. (2020). Role of occlusion position in coronary artery fistulas with terminal aneurysms: A hemodynamic perspective. Cardiovascular Engineering and Technology, 11(4), 394–404. [Google Scholar] [PubMed]
28. Promphan, W., Prachasilchai, P., Qureshi, S. A. (2015). Progressive aneurysmal dilation of coronary arterial fistula after transcatheter closure: Successfully treated by a second occlusion device. Cardiology in the Young, 25(4), 813–817. [Google Scholar] [PubMed]
29. Freund, J. E., Yuko-Jowi, C., Freund, M. W. (2015). Transcatheter embolization of a large aneurysm in a congenital coronary cameral fistula from the left coronary artery to the right ventricle. Catheterization and Cardiovascular Interventions, 85(3), 435–439. [Google Scholar] [PubMed]
30. Pestana, G., Ribeiro, V., Sousa, C., Cruz, C., Vasconcelos, M. et al. (2018). Percutaneous closure of a fistulous giant coronary aneurysm. Canadian Journal of Cardiology, 34(6), 812–813. [Google Scholar]
31. Li, Y., Chen, Z., Zhuang, J., Zhang, Z. (2022). A rare case of transcatheter closure of both inlet and outlet of a left coronary artery-to-left ventricular fistula with giant coronary artery aneurysm. Congenital Heart Disease, 17(5), 541–549. https://doi.org/10.32604/chd.2022.024907 [Google Scholar] [CrossRef]
32. Shen, Q. S., Li, D. Y., He, Y. F., Li, Y. Y. (2022). Transcatheter closure of a rare giant aneurysm in coronary right ventricle fistula associated with ventricular septal dissection. European Heart Journal, 43(46), 4850. [Google Scholar] [PubMed]
Supplementary Materials
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools