 Open Access
Open Access
ARTICLE
Mortality Rates of Ventricular Septal Defect for Children in Kazakhstan: Spatio-Temporal Epidemiological Appraisal
1 Pediatric Cardiac Surgery Department, National Scientific Medical Center, Astana City, Republic of Kazakhstan
2 Department of Surgical Diseases with Courses in Cardiothoracic Surgery and Maxillofacial Surgery, Аstana Medical University, Astana City, Republic of Kazakhstan
3 Faculty of General Medicine, Аstana Medical University, Astana City, Republic of Kazakhstan
* Corresponding Author: Akkerbez Adilbekova. Email:
Congenital Heart Disease 2023, 18(4), 447-459. https://doi.org/10.32604/chd.2023.028742
Received 05 January 2023; Accepted 19 May 2023; Issue published 15 September 2023
Abstract
Objective: The aim is to study the trends in ventricular septal defect (VSD) mortality in children in Kazakhstan. Methods: The retrospective study was done for the period 2011–2020. Descriptive and analytical methods of epidemiology were applied. The universally acknowledged methodology used in sanitary statistics is used to calculate the extensive, crude, and age-specific mortality rates. Results: Kazakhstan is thought to be seeing an increase in mortality from VSDs in children. As a result, this study for the years 2011 to 2020 was conducted to retrospectively assess data from the central registration of the Bureau of National Statistics that was available throughout the nation. Age-standardized mortality data were obtained and compared between age categories. It was shown that 507 children died from this condition throughout the time period under study. The average annual standardized mortality rate was 1.88 per 100,000 population and tended to decrease over time. The peak of mortality was noted at the age of up to 1 year, namely the neonatal period. Cartogram mortality rates were calculated using standardized indicators. Additionally, age-sex variations were taken into account when performing all calculations. Conclusion: In recent years, the death rate from VSD has declined from 1.5 to 0.6 per 100,000 people, with the trend remaining constant (T = 1.4%, R2 = 0.5825). The analysis of mortality trends related to VSD is crucial in both theoretical and practical aspects, as it enables early detection and treatment of VSDs. The findings of this study will be valuable to public health authorities in developing a strategy to treat VSDs effectively.Graphic Abstract
Keywords
Congenital heart defects (CHDs) are the most prevalent type of all major congenital anomalies in the world, influencing millions of newborns every year [1,2]. Moreover, CHDs are the leading cause of birth defects which are related to morbidity, mortality, and expanded healthcare costs [3]. Also, nine out of ten of the world’s babies born with CHD live in locations with little or no care and where mortality remains high [4]. Even though there are a lot of types of CHDs, ventricular septal defects (VSDs) are one-third of the most commonly diagnosed heart defects [5,6]. Moreover, the detection of VSDs has increased due to the improvement of diagnostic methods in the last few decades [7]. The VSDs occur at a rate of 0.5 per 1000 live births and in 4.5 to 7 of 1000 preterm children [8]. Approximately 20% of the VSDs can exist in isolation and, if one includes VSDs in combination with other abnormalities, the VSDs are diagnosed in 50% of all patients with CHDs [9]. Despite the abovementioned combinations, the cause of the VSDs may be associated with chromosomal disorders, which account for about 5% of cases (such as trisomy 21 and 22q11 deletion) [10].
Ventricular septal defects accounted for (36.77%) of all CHDs between 2000 and 2020, according to statistics provided by the European Commission on CHDs [11]. Also, the prevalence of ventricular septal defect in East Africa was found to be 29.92% [12,13]. According to a meta-analysis by Lui et al. published in 2019, including global data on the prevalence of CHDs from 260 studies (130,758,851 births), it was found that VSDs took the leading position, in particular, rising whole the study period, reflecting progressed detection [14,15].
Based on statistics provided by the Centers for Disease Control and Prevention (CDC) from Atlanta appraisal that 42 of every 10,000 births had a ventricular septal defect [16]. This indicates that 16,800 infants are born in the United States (US) each year with a ventricular septal defect [17]. Alternatively stated, around 1 in every 240 infants born in the US every year have a ventricular septal defect [18]. In the Republic of Kazakhstan, the frequency of congenital heart defects among live births is 8–10 per 1000, and the total number of children born with congenital heart defects is about 3000 per year [19,20].
Currently, treatment options for ventricular septal defects may include regular check-ups, medication, and surgical therapy. Approximately 75% of small VSDs do not require surgery and close spontaneously in the first year of life. The rate of spontaneous closure for medium to large VSDs is between 5% and 10% and in other cases, surgical closure is required. If the VSD hasn’t closed by the age of 10, spontaneous closure is likely not to occur at all [21,22]. There are three surgical methods for ventricular septal defect: the traditional method with a heart-lung machine, the interventional method, and the hybrid method without the heart-lung machine [23–26]. Based on most studies that have long-term outcomes in people operated on for surgical closure of the VSDs in infancy, they showed good results. However, if the operation is not done on time, as a result of the persistent left-to-right shunt will occur in pulmonary hypertension and Eisenmenger syndrome [27,28].
The study’s objective is to assess the temporal and spatial trends of VSD mortality in Kazakhstan while accounting for administrative-territorial division.
According to data from the Bureau of National Statistics (BNS) of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan, ventricular septal defect (VSD) was recorded as the primary cause of mortality from 2011 to 2020. Beginning in 2011, records of mortality were kept electronically. Causes of mortality were classified using the International Classification of Diseases (ICD): Q-21.0 in ICD-10. The data of mortalities were collected by year of death along with details on birth year, age at death, and sex. The inclusion criteria for the sample were as follows: patients with isolated VSD (perimembranous, muscular, atrioventricular conal type, and subarterial) from birth to 18 years, as well as those with infectious complications. Exclusion criteria included: the combination of VSD with other CHD. Data were distributed into five age groups—under 1 year (infants), 1 to 2 years (toddlers), 3 to 6 years (preschool), 7 to 11 years (school age), and 12 to 17 years (adolescent) —to prevent disclosure.
The main method used in the study of mortality was a retrospective study using descriptive and analytical methods of epidemiology. Age-standardized mortality rates (ASRs) were calculated for five different age groups (0–1, 1–2, 3–6, 7–11, and 12–17) and ten calendar periods from 2011 to 2020 (1-year intervals). ASRs standardized to the world population proposed by World Health Organization [29] were calculated for each analyzed year.
The infant mortality rate (IMR) was estimated for 10 years period (2011–2020) for each year. The percentage of death distribution under 1 year was defined by age on a daily basis.
According to the commonly used methodology in sanitary statistics, the extensive, crude rate (CR) and age-specific mortality rates (ASMR) were calculated. Calculations were made using the annual averages (M, P), mean error (m), 95% confidence interval (95% CI), and average annual upward/downward rates (T%) [30]. The least squares method was used for determining the dynamics of mortality rates for 10 years. The average annual growth/decline rates (T%) were calculated using a geometric mean.
When creating cartograms, crude rates, and ASRs were used for 10 years (2011–2020). The process for creating a cartogram, first proposed by S.I. Igisinov in 1974, was employed, and it was based on calculating the standard deviation (σ) from the average (x). The formula used to calculate the scale of steps is as follows: using as an interval, the maximum and minimum mortality levels were calculated using the formula: x ± 1.5 σ, with the minimum indicator being equal to x − 1.5 σ and the highest being equal to x + 1.5 σ.
Viewing and analyzing the materials were received using the Microsoft 365 software package, in addition, online statistical calculators were used (https://medstatistic.ru/calculators/calcdynamic.html).
An ethics review board’s examination and approval were not necessary because this study only involved the analysis of publicly accessible administrative data and did not involve contacting any participants.
During the 10 years (2011–2020), 507 children from birth to 17 years old inclusive died from VSD, of which 251 (49.5%) were boys and 256 (50.5%) were girls. The majority of VSDs observed in the study had a large diameter, but only 95 patients were operated while the remaining children did not receive any surgical treatment. However, all patients received medications for heart failure (including diuretics and ACE inhibitors). In Kazakhstan, a significant share of deaths from VSD was identified for infants and children aged from 1 to 2 years for both sexes −82.6% and 11.5% of all childhood deaths, respectively.
In most cases, a fatal outcome results from a viral or bacterial infection acquired at home, with the possible development of sepsis. Also, accession of nosocomial infection in a hospital due to various reasons, such as prolonged artificial ventilation of the lungs due to severe pulmonary and heart failure. As a result of the already-developed sepsis and multiple organ failure, the patient could not be transferred to a specialized cardiac surgery clinic for surgical treatment. In addition, genetic diseases are rarely diagnosed due to the high cost of research, and the lack of a clear algorithm for compensating the financial costs of additional laboratory tests outside the nosology with which the patient was admitted to the clinic further complicates matters.
The most numerous numbers of deaths from VSD according to the distribution by age was in the group of children under 1 year–419 deaths, or 82.6% of all children deaths (Table 1). The proportion of deaths from VSD by age group among boys and girls was similar within the population.
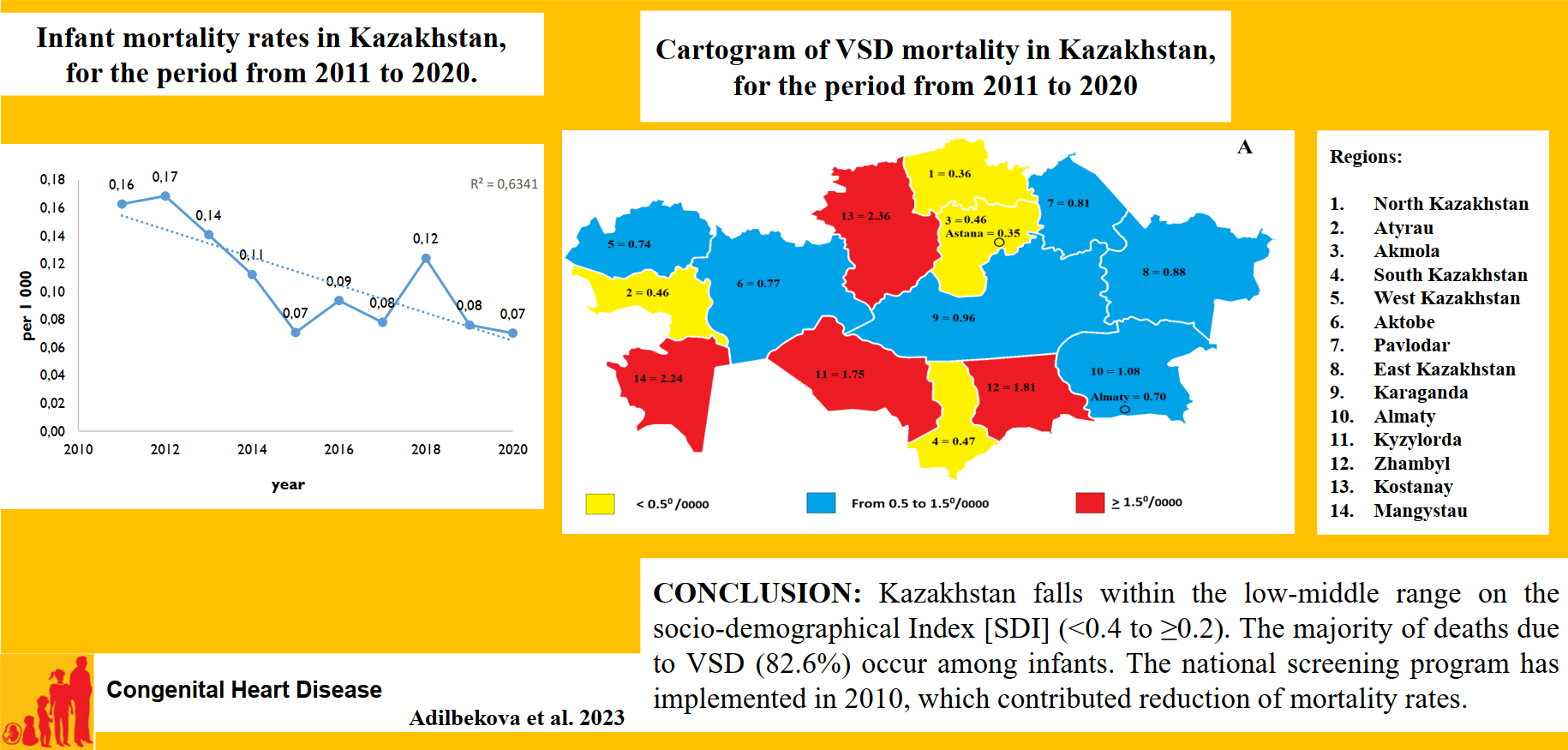
Infant mortality rate (IMR) was the highest mortality rate for ten years from 2011 to 2020. Within the period IMR of VSD reduced by 56% from 0.16 per 1000 in 2011 to 0.07 per 1000 in 2020 (Fig. 1).
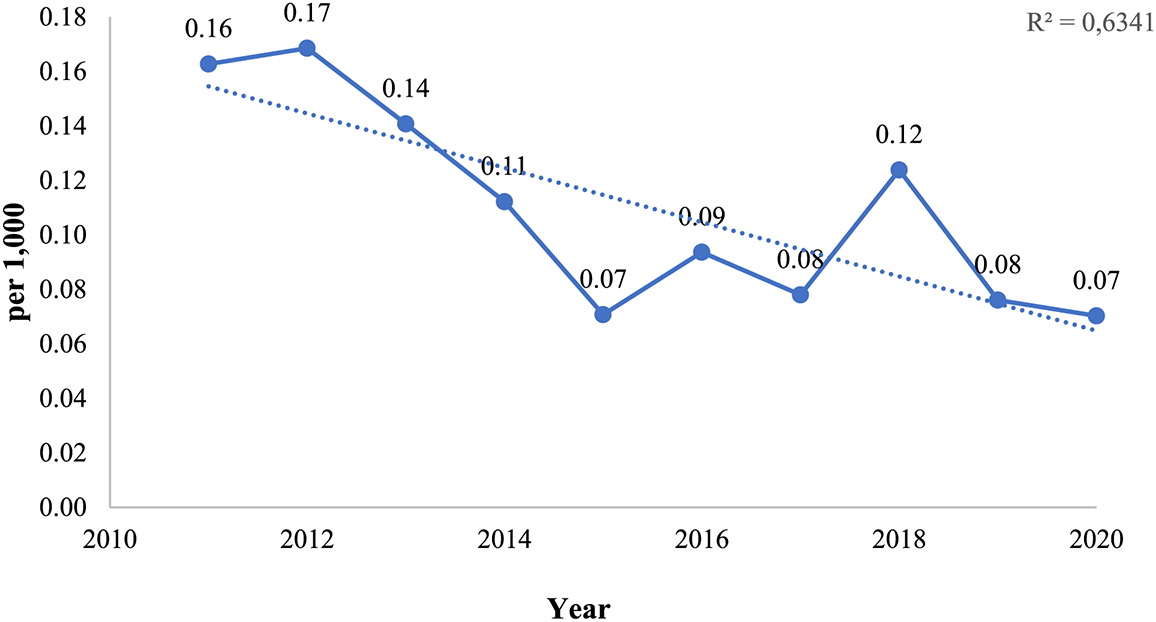
Figure 1: Infant mortality rates in Kazakhstan, for the period from 2011 to 2020
As shown in Fig. 2 the highest mortality was observed in the neonatal period which was the riskiest for children. For the analysed period of ten years 151 deaths, or 36% of overall 419 children’s deaths, occurred in the neonatal period.
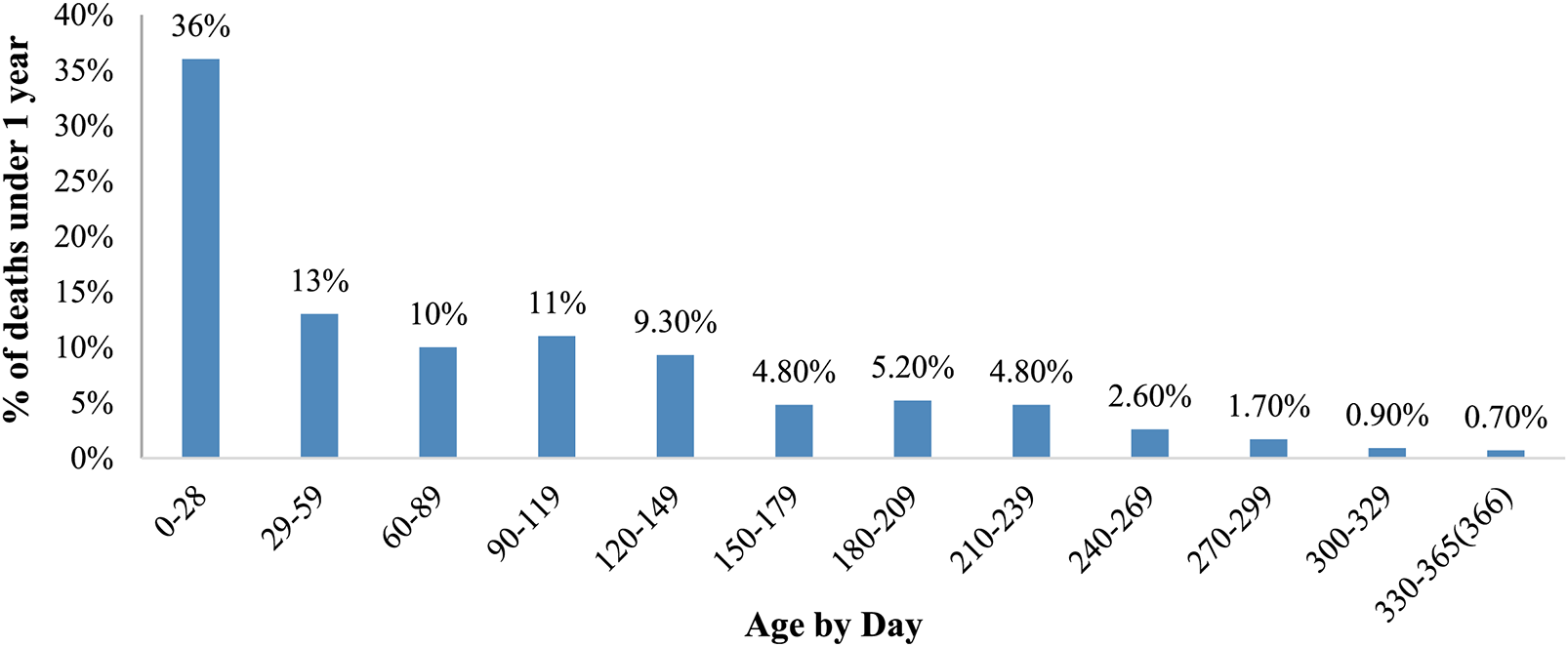
Figure 2: Age-by-day distribution of VSD deaths under 1 year in Kazakhstan, for the period from 2011 to 2020
Table 2 indicates a sharp decrease in infants’ deaths after 29 days from birth. Thus, more than 150 deaths were in the neonatal period and around 55 deaths for 29–59 days of children. The downward trend persists during the postneonatal period, and the number of deaths decreases from 54 deaths, or 13% of all infants’ death, for 29–50 days infants to 3 deaths, or 0.7% of all infants’ death, for 330–365 days infants.
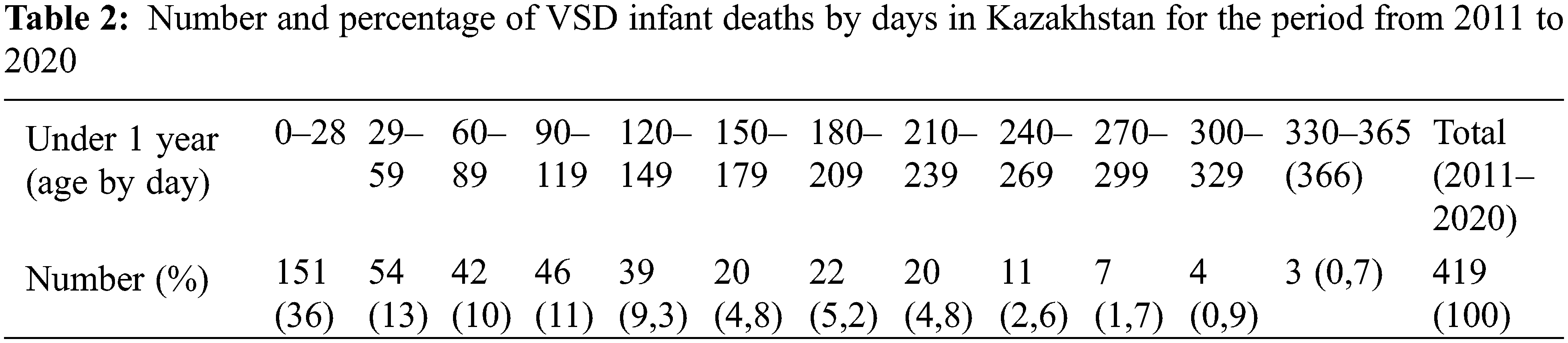
Fig. 3 provides data about children’s mortality by age distribution. The mortality rate decreases steadily from birth to 17 years, from the highest mortality of 10.96 per 100,000 population for the age group under 1 year to 0.09 per 100,000 population for 12 years children.
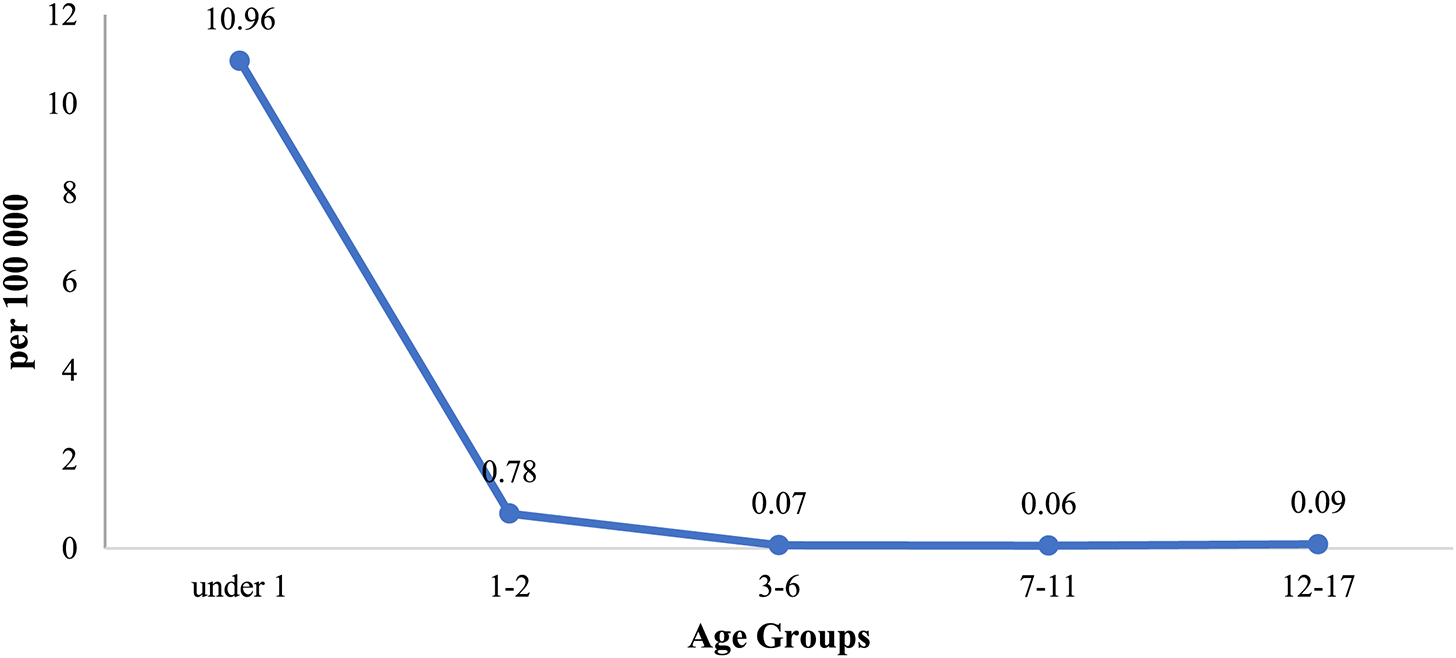
Figure 3: Age distribution of VSD mortality in Kazakhstan, for the period from 2011 to 2020
From 2011 to 2022 the standardized children mortality rate from VSD was 1.88 per 100,000 population in Kazakhstan. For the period the average annual growth rate was T = −2% (R2 = 0.9971).
According to Fig. 4, crude rates of VSD mortality for children decreased from 1.55 ± 0.2 (95% CI = 1.5–1.6) in 2011 to 0.61 ± 0.2 (95% CI = 0.56–0.66) in 2020 per 100,000, with the average being 0.96 ± 0.22 (95% CI = 0.91–1.0).
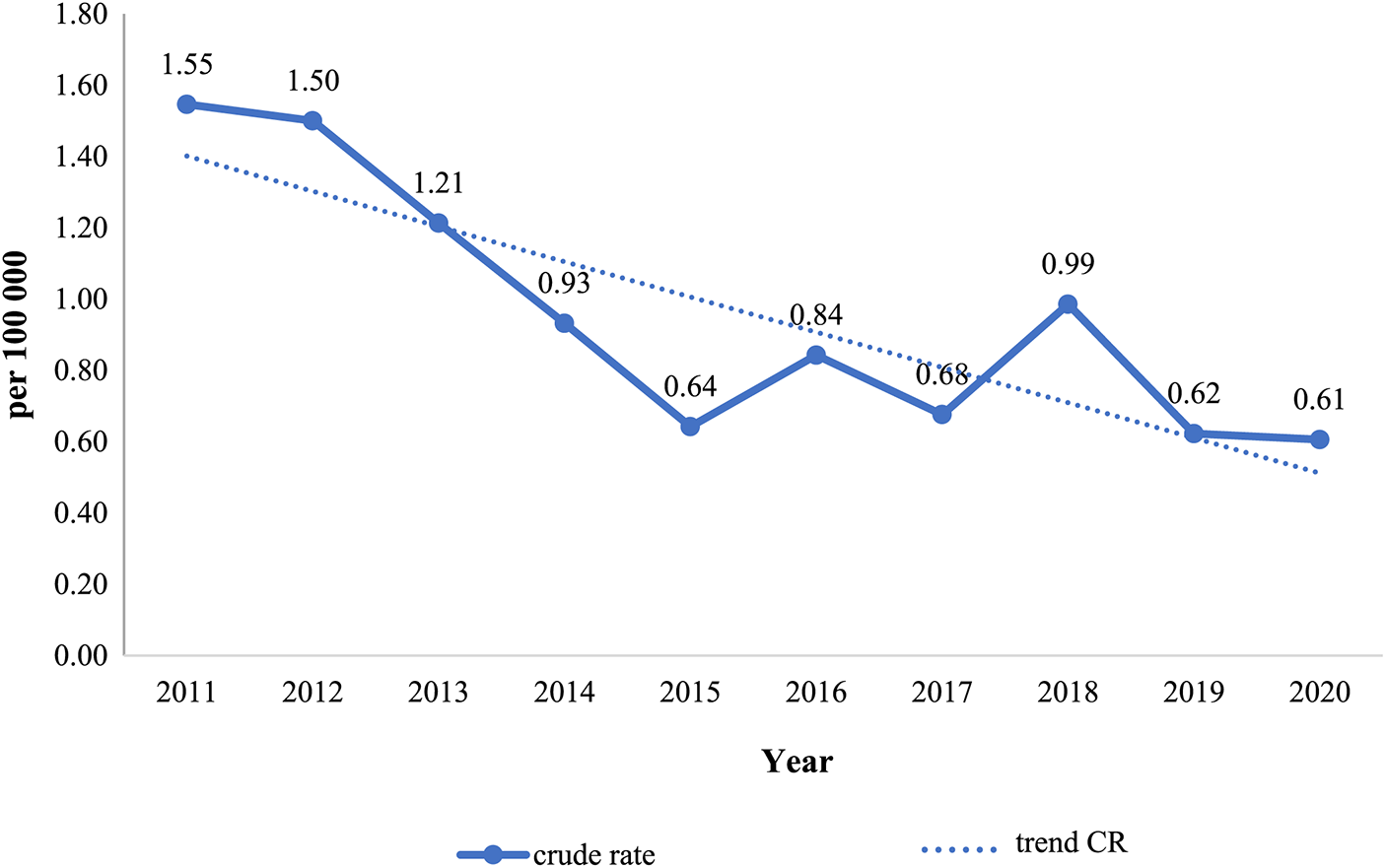
Figure 4: Dynamics of VSD mortality in Kazakhstan, for the period from 2011 to 2020
The 95% CI of the indicators do not overlap with one another, suggesting that these indicators are influenced by different factors, and the average annual rate of mortality decline by VSD was significant (T = −7%), demonstrating a real decline in mortality from this type of congenital heart defect in Kazakhstan. Additionally, the moderate degree of approximation reliability (R2 = 0.5825) supports this conclusion.
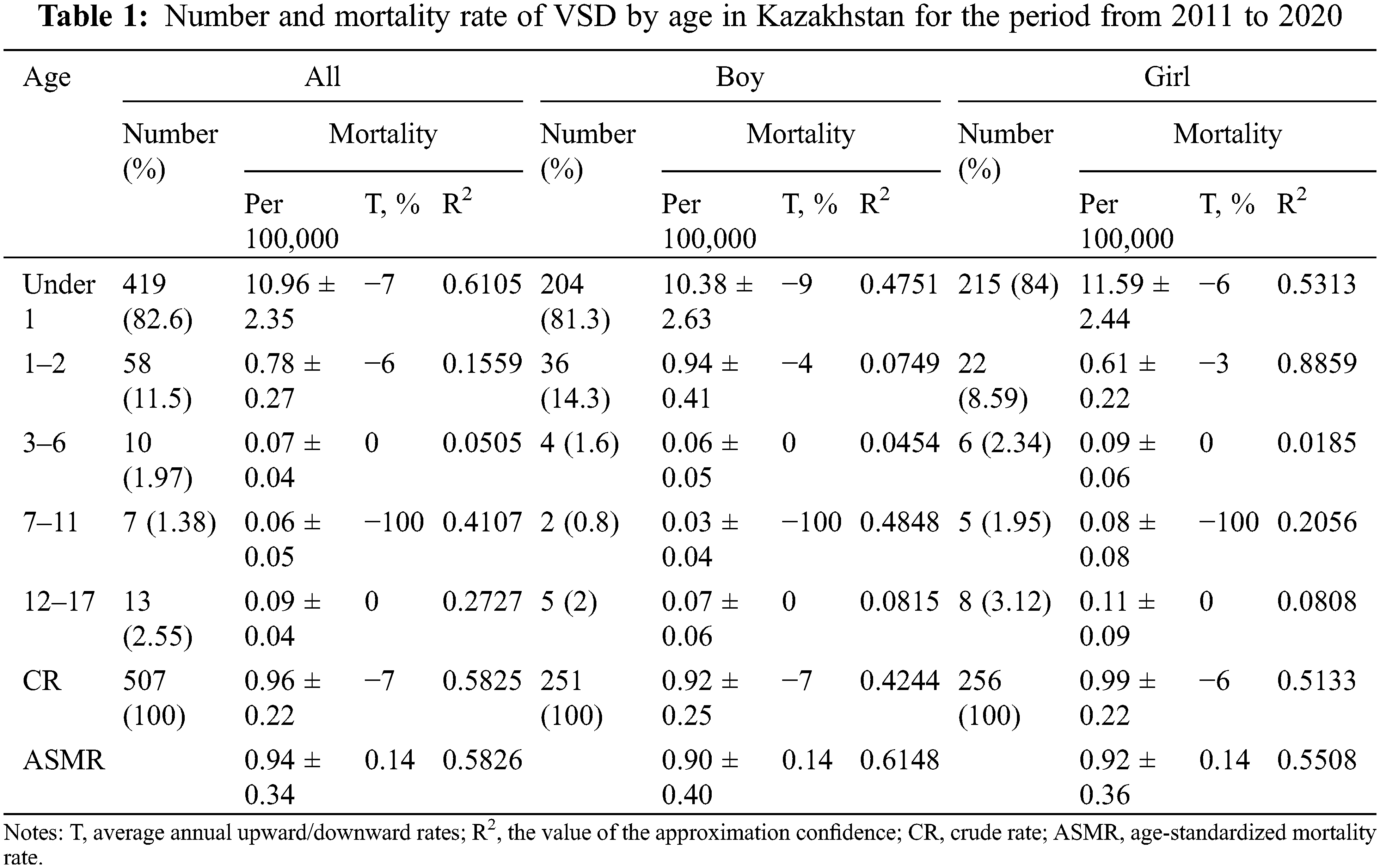
This, as reported by Fig. 5A, spatial assessment of mortality from VSD in the entire population shows that the following regions, including North Kazakhstan, Akmola, Astana City (capital city), Atyrau, and South Kazakhstan were among the regions with the lowest mortality rate. This applies to both boys and girls, as can be seen in Figs. 5B and 5C, respectively. For boys regions (Fig. 5B) with the lowest mortality rate also include Aktobe and Pavlodar, for the Atyrau region mortality rate for boys is moderate. Overall, the lowest mortality rate for boys was in North Kazakhstan. For girls (Fig. 5C), the lowest mortality rate was in Astana. According to the cartogram from VSD mortality in Fig. 5A the southern regions (Kyzylorda, Zhambyl), Kostanay, and Mangystau had the highest mortality rates for the entire population. The highest mortality rate for boys (Fig. 5B) was in Kostanay, and for girls (Fig. 5C) in Mangystau. In the remaining regions, including Almaty, West Kazakhstan, Aktobe, Pavlodar, East Kazakhstan, and Karaganda the mortality rate from VSD was moderate.
Figure 5: Cartogram of VSD mortality in Kazakhstan, for the period from 2011 to 2020 (A-both sexes, B-boys, C-girls)
Regions: 1. North Kazakhstan, 2. Atyrau, 3. Akmola, 4. South Kazakhstan, 5. West Kazakhstan, 6. Aktobe, 7. Pavlodar, 8. East Kazakhstan, 9. Karaganda, 10. Almaty, 11. Kyzylorda, 12. Zhambyl, 13. Kostanay, 14. Mangystau
More detailed information about geographical variation in VSD mortality in Kazakhstan is provided in Table 3.
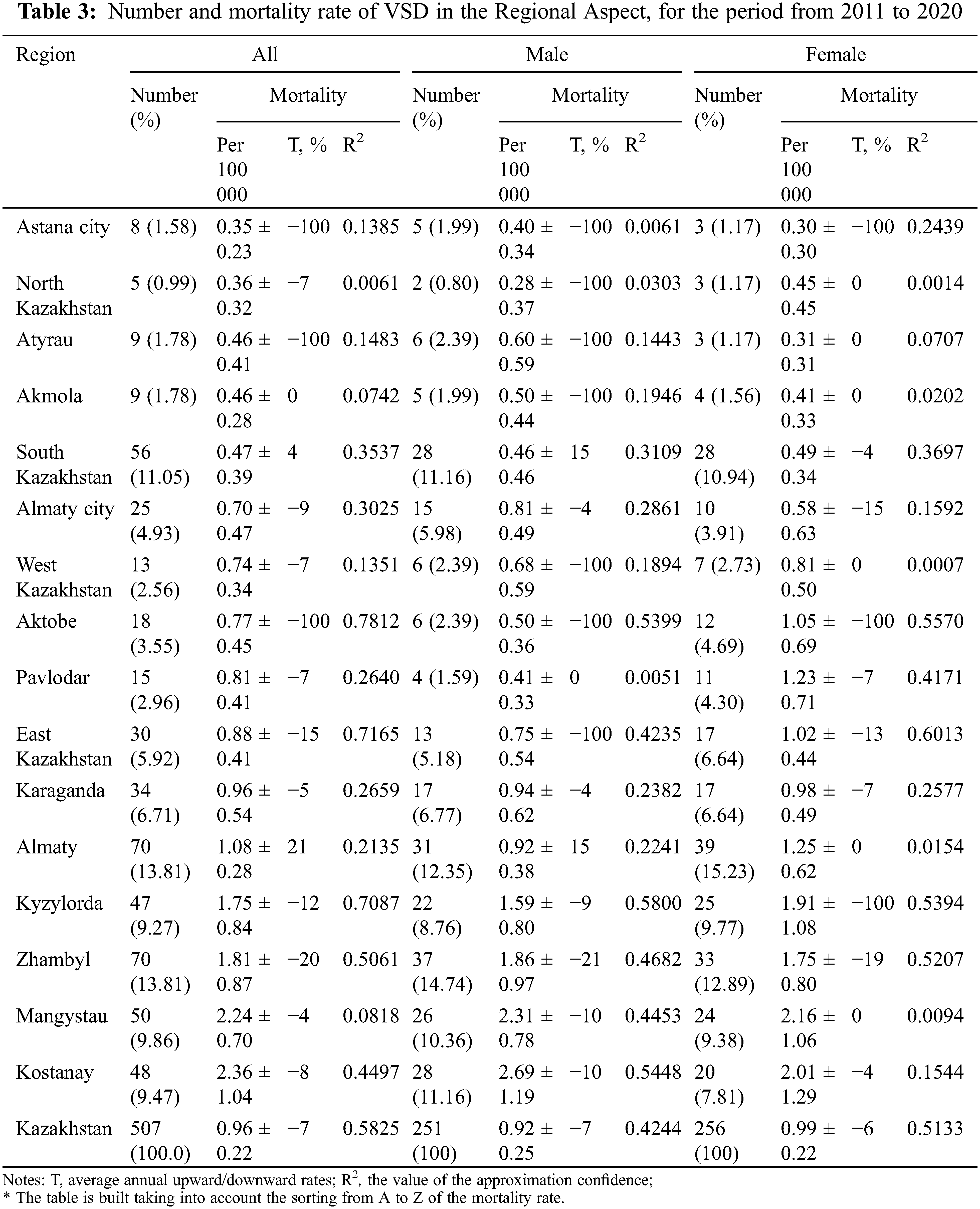
For all regions, the minimum indicator of average annual growth rates was in North Kazakhstan (T = −7.0%; R2 = 0.0061), and the maximum in Aktobe (T = −100%; R2 = 0.7812). By a level of approximation, the highest was East Kazakhstan (R2 = 0.7165) and Kyzylorda (R2 = 0.7087) regions.
As a result of analysis of the boys’ population average annual growth rate of the standardized indicator by region, the highest was Kyzylorda region (T = −9%; R2 = 0.7087), the lowest was the City of Astana (T = +2.2%; R2 = 0.1359). And the girls’ indicators were similar to the overall population.
CHD is the leading cause of death from non-communicable diseases (NCDs) in childhood. Global CHD deaths in 2019 were 217,000 (95% uncertainty interval 177,000–262,000) [31,32]. There were 129 countries with at least 50,000 deaths [33]. Moreover, there has been an increase in the prevalence of CHD over the past decade with improvements in CHD screening and pediatric care [34,35]. Some studies have found evidence that neonatal screening by echocardiography incidences of VSD increased [36]. Also, increased preterm delivery and environmental factors may be responsible for the increase in the reported incidence of VSD [37]. There are a large number of epidemiological studies showing that maternal exposure to air pollution during pregnancy, especially in the first trimester, is associated with a higher chance of CHD in children [38]. For instance, particulate matter <10 microns and the density of traffic may influence the occurrence of ventricular septal defects and pulmonary valve stenosis [39]. In Kazakhstan, the decision to terminate a pregnancy due to a diagnosis of CHD in the fetus is made exclusively by the parents [40].
Several studies have shown from 2017, the incidence rate of CHD was 17.9/1000 worldwide, with 19.1/1000 for males and 16.6/1000 for females [41]. Especially, ventricular septal defect and atrial septal defect were the most common subtype of CHD with an incidence of 5.29/1000 and accounted for about 29.6% of all cases of CHD, of the VSDs, 20% are isolated lesions [42]. According to past statistical analysis, the incidence of isolated VSDs in neonates was 1.5–2.5/1000 [43]. As previously noted, surgical intervention is the typical treatment for the majority of medium and large ventricular septal defects (VSDs). If left untreated over time, VSDs can cause long-term damage that increases the risk of complications such as heart failure, pulmonary hypertension, arrhythmia, or stroke. Furthermore, in cases where Eisenmenger’s syndrome develops, which leads to the disability of patients, the addition of infection can be fatal. In conclusion, managing VSDs incurs significant expenses and places a heavy burden on healthcare resources [44,45].
Kazakhstan’s mortality rates mirror the general trend. The study’s findings demonstrate that, despite a decline in VSD-related deaths in Kazakhstan (Fig. 4), Kazakhstan still falls into the category of countries with high mortality rates. As proof study results depict, the standardized mortality rate of VSD was 1.88 per 100,000 people. According to the Sustainable Development Index, Kazakhstan is low-middle on the socio-demographical Index [SDI] (<0.4 to ≥0.2) [32]. One of the major findings is the highest mortality rates equal to, 39.7% [34] of deaths globally are seen, in the middle SDI (<0.6 to ≥0.4) [32] countries, such as India, China, Pakistan, and Nigeria by studies from 2019 Global Burden of Disease (GBD).
Based on research on GBD about the incidence rate between 1990 and 2017, all SDI regions saw a decrease in the incidence rate of CHD, but the increase in the frequency of VSD during this time period has largely contributed to the increase in the period of CHD in regions with high SDI (from 12.4/1000 to 12.6/1000) [34]. Also, the lowest incidence rates mainly were found in developed countries, such as Qatar (6.2/1000), Portugal (6.7/1000), and France (8.6/1000) [40].
Worldwide, the age-standardized mortality rate (ASMR) of CHD declined by about 38.1%, from 6.3 per 100.000 population in 1990 to 3.9 per 100.000 population in 2017 [40]. Also in Kazakhstan, the ASMR of VSD declined from 1.5 per 100.000 population in 2011 to 0.6 per 100,000 population in 2020 (Table 1; Fig. 4). Another interesting fact, the infant mortality rate (IMR) of VSD decreased (Fig. 1), accompanied by a steady decline in deaths across the board for children, which may be a sign that neonatal therapies now have a higher ability to extend survival for those with CHDs for the period from 2011 to 2020. The ongoing decline in mortality rates may be due to the growing popularity of primary newborn surgical repair. Kazakhstan is the first nation in the post-Soviet region to have a national screening program, which was established in 2010 [46]. It has been demonstrated that the introduction of screening causes a short-term increase in VSD incidence due to the greater detection of prevalent cases, which is often followed by a long-term decrease in incidence and mortality from VSD [47]. For instance, this may explain why VSD mortality rates have declined in the majority of high-SDI nations like South Korea, the United States, and Japan as well as middle-SDI nations like Brazil, where CHD screening programs have been in place for two to three decades [48]. The implementation of screening measures in Kazakhstan has led to a 20% reduction in mortality rates from VSDs, with the number of deaths decreasing from 2 per 100,000 in 2011 to 1.6 per 100,000 in 2020. Additionally, the prenatal detection of CHD has increased to 70% [49]. Moreover, the treatment of VSDs has been significantly advanced through the use of improved surgical methods, in combination with medical therapy. As a result, 60% of specialized cardiac surgery clinics in the Republic of Kazakhstan now offer surgical treatment to newborns with large VSDs [50].
Geographic variation in mortality rates was also present in Kazakhstan. The northern regions had the lowest indicators, whereas the southwest and Kostanay regions had the highest. This is brought on by changes in the age structure, the population’s ethnic makeup, and people’s dietary and behavioral habits.
There are limitations to our study. The Republic of Kazakhstan observed very strictly accounting and registration procedures for deaths, and our study examined secondary data derived from administrative data. It is crucial to distinguish mortality from other reasons and from VSD during the course of a patient’s life because mortality in VSD can also result from other causes, such as congestive heart failure, complications of infection, and concurrent chronic conditions.
On the other hand, we performed a detailed standardized mortality assessment to identify age and geographic characteristics. The analysis of mortality trends related to VSD is crucial in both theoretical and practical aspects, as it enables early detection and treatment of VSDs. The findings of this study will be valuable to public health authorities in developing a strategy to treat VSDs effectively.
Acknowledgement: The Ministry of Health of the Republic of Kazakhstan provided data for this study, which the authors appreciate. This study was conducted as a part of an independent research work, the findings of which will be used to defend dissertations. As the study involved the analysis of administrative data that was readily accessible to the public and did not involve any contact with people, it complies with Republic of Kazakhstan law and was not subject to review or approval by the ethics commission.
Funding Statement: This study was funded via the National Scientific Medical Center, Astana City, the Republic of Kazakhstan for International Publication. The funding agencies did not have any role in the registry design, analysis, and interpretation of data.
Author Contributions: AA, ShM, BN, SK, AB-Data collection and preparation, main material processing, and their verification. AA, ShM, BN, SK, AB-Writing the article’s text and statistically processing and analyzing the data (material and methods, results). AA, ShM, BN, SK, and AB-Composing the article’s text (introduction, discussion). Conceptualization, design, and oversight of the research; approval of the article’s final draft by AA, ShM, BN, and SK. The final draft of the work was approved by all authors.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Bravo-valenzuela, N. J., Peixoto, A. B., Araujo Júnior, E. (2018). Prenatal diagnosis of congenital heart disease: A review of current knowledge. Indian Heart Journal, 70(1), 150–164. https://doi.org/10.1016/j.ihj.2017.12.005 [Google Scholar] [PubMed] [CrossRef]
2. Dolk, H., Loane, M., Garne, E. (2011). Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation, 123(8), 841–849. https://doi.org/10.1161/CIRCULATIONAHA.110.958405 [Google Scholar] [PubMed] [CrossRef]
3. Bernier, P. L., Stefanescu, A., Samoukovic, G., Tchervenkov, C. I. (2010). The challenge of congenital heart disease worldwide: Epidemiologic and demographic facts. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 13(1), 26–34. https://doi.org/10.1053/j.pcsu.2010.02.005 [Google Scholar] [PubMed] [CrossRef]
4. Khairy, P., Ionescu-Ittu, R., MacKie, A. S., Abrahamowicz, M., Pilote, L. et al. (2010). Changing mortality in congenital heart disease. Journal of the American College of Cardiology, 56(14), 1149–1157. https://doi.org/10.1016/j.jacc.2010.03.085 [Google Scholar] [PubMed] [CrossRef]
5. Moons, P., Sluysmans, T., de Wolf, D., Massin, M., Suys, B. et al. (2009). Congenital heart disease in 111 225 births in Belgium: Birth prevalence, treatment and survival in the 21st century. Acta Paediatrica, 98(3), 472–477. https://doi.org/10.1111/j.1651-2227.2008.01152.x [Google Scholar] [PubMed] [CrossRef]
6. Eleyan, L., Zaidi, M., Ashry, A., Dhannapuneni, R., Harky, A. (2021). Ventricular septal defect: Diagnosis and treatments in the neonates: A systematic review. Cardiology in the Young, 31(5), 756–761. https://doi.org/10.1017/S1047951120004576 [Google Scholar] [PubMed] [CrossRef]
7. Roberts, W. C. (1984). The 2 most common congenital heart diseases. The American Journal of Cardiology, 53(8), 1198. https://doi.org/10.1016/0002-9149(84)90662-3 [Google Scholar] [PubMed] [CrossRef]
8. David, L. S., Brandi, B. S., Charles, D. F. (2014). Congenital cardiac surgery. In: Ventricular septal defect. USA: McGraw Hill Medical. [Google Scholar]
9. Tuuli, M. G., Dicke, J. M., Stamilio, D. M., Gray, D. L., Macones, G. A. et al. (2009). Prevalence and likelihood ratios for aneuploidy in fetuses diagnosed prenatally with isolated congenital cardiac defects. American Journal of Obstetrics and Gynecology, 201(4), 390.e1–390.e5. https://doi.org/10.1016/j.ajog.2009.06.035 [Google Scholar] [PubMed] [CrossRef]
10. Edgar, L. J., Anderson, R. H., Stickley, J., Crucean, A. (2020). Borders as opposed to so-called geography: Which should be used to classify isolated ventricular septal defects? European Journal of Cardio-Thoracic Surgery, 58(4), 801–808. https://doi.org/10.1093/ejcts/ezaa081 [Google Scholar] [PubMed] [CrossRef]
11. European Commission, EUROCAT (2020). Prevalence charts and tables. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en [Google Scholar]
12. Zikarg, Y. T., Yirdaw, C. T., Aragie, T. G. (2021). Prevalence of congenital septal defects among congenital heart defect patients in East Africa: A systematic review and meta-analysis. PLoS One, 16(4), e0250006. https://doi.org/10.1371/journal.pone.0250006 [Google Scholar] [PubMed] [CrossRef]
13. Awori, M. N., Ogendo, S. (2013). The Spectrum of Paediatric congenital heart disease at the Kenyatta National Hospital: Implications for surgical care. Annals of African Surgery, 10(1), 8–10. [Google Scholar]
14. Liu, Y., Chen, S., Zühlke, L., Black, G. C., Choy, M. K. et al. (2019). Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology, 48(2), 455–463. https://doi.org/10.1093/ije/dyz009 [Google Scholar] [PubMed] [CrossRef]
15. Egbe, A., Uppu, S., Lee, S., Ho, D., Srivastava, S. (2014). Changing prevalence of severe congenital heart disease: A population-based study. Pediatric Cardiology, 35(7), 1232–1238. https://doi.org/10.1007/s00246-014-0921-7 [Google Scholar] [PubMed] [CrossRef]
16. Reller, M. D., Strickland, M. J., Riehle-Colarusso, T., Mahle, W. T., Correa, A. (2008). Prevalence of congenital heart defects in Metropolitan Atlanta, 1998–2005. Journal of Pediatrics, 153(6), 807–813. https://doi.org/10.1016/j.jpeds.2008.05.059 [Google Scholar] [PubMed] [CrossRef]
17. Reefhuis, J., Honein, M. A. (2004). Maternal age and non-chromosomal birth defects, Atlanta–1968–2000: Teenager or thirty-something, who is at risk? Birth Defects Research Part A–Clinical and Molecular Teratology, 70(9), 572–579. https://doi.org/10.1002/bdra.20065 [Google Scholar] [PubMed] [CrossRef]
18. Yang, Q., Chen, H., Correa, A., Devine, O., Mathews, T. J. et al. (2006). Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Research Part A–Clinical and Molecular Teratology, 76(10), 706–713. https://doi.org/10.1002/(ISSN)1542-0760 [Google Scholar] [CrossRef]
19. Mukasheva, G., Abenova, M., Shaltynov, A., Tsigengage, O., Mussabekova, Z. et al. (2022). Incidence and mortality of cardiovascular disease in the Republic of Kazakhstan: 2004–2017. Iranian Journal of Public Health, 51(4), 821–830. https://doi.org/10.18502/ijph.v51i4.9243 [Google Scholar] [PubMed] [CrossRef]
20. Davletov, K. K., Berkinbaev, S. F. (2014). Analysis of standardized mortality from diseases of the circulatory system in 2008–2012 in Kazakhstan. Eurasian Journal of Internal Medicine, 1, 28–34. [Google Scholar]
21. Tayyab, P. (2020). Ventricular septal defects. http://www.cardiopk.com/VentricularSeptalDefects.aspx [Google Scholar]
22. Tao, K., Lin, K., Shi, Y., Song, H., Lui, R. C. et al. (2010). Perventricular device closure of perimembranous ventricular septal defects in 61 young children: Early and midterm follow-up results. Journal of Thoracic and Cardiovascular Surgery, 140(4), 864–870. https://doi.org/10.1016/j.jtcvs.2010.05.013 [Google Scholar] [PubMed] [CrossRef]
23. Yang, J., Yang, L., Yu, S., Liu, J., Zuo, J. et al. (2014). Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: A randomized controlled trial. Journal of the American College of Cardiology, 63(12), 1159–1168. https://doi.org/10.1016/j.jacc.2014.01.008 [Google Scholar] [PubMed] [CrossRef]
24. Gan, C., An, Q., Lin, K., Tang, H., Lui, R. C. et al. (2008). Perventricular device closure of ventricular septal defects: Six months results in 30 young children. Annals of Thoracic Surgery, 86(1), 142–146. https://doi.org/10.1016/j.athoracsur.2008.03.058 [Google Scholar] [PubMed] [CrossRef]
25. Rao, P. S., Harris, A. D. (2018). Recent advances in managing septal defects: Ventricular septal defects and atrioventricular septal defects. F1000Research, 7, F1000 Faculty Rev-498. https://doi.org/10.12688/f1000research [Google Scholar] [CrossRef]
26. Lu, W., Zhang, F., Fan, T., Zhao, T., Han, Y. et al. (2021). Minimally-invasive-perventricular-device-occlusion versus surgical-closure for treating perimembranous-ventricular-septal-defect: 3-year outcomes of a multicenter randomized clinical trial. Journal of Thoracic Disease, 13(4), 2106–2115. https://doi.org/10.21037/jtd-20-3298 [Google Scholar] [PubMed] [CrossRef]
27. Roos-Hesselink, J. W., Meijboom, F. J., Spitaels, S. E. C., van Domburg, R., van Rijen, E. H. M. et al. (2004). Outcome of patients after surgical closure of ventricular septal defect at young age: Longitudinal follow-up of 22–34 years. European Heart Journal, 25(12), 1057–1062. https://doi.org/10.1016/j.ehj.2004.04.012 [Google Scholar] [PubMed] [CrossRef]
28. Roth, T. S., Aboulhosn, J. A. (2016). Pulmonary hypertension and congenital heart disease. Cardiology Clinics, 34(3), 391–400. https://doi.org/10.1016/j.ccl.2016.04.002 [Google Scholar] [PubMed] [CrossRef]
29. Ahmad, O. B., Boschi-pinto, C., Lopez, A. D. (2001). Age standardization of rates: A new WHO standard. GPE Discussion Paper Series, 31, 10–14. [Google Scholar]
30. Wayne, W. D., Chad, L. C. (2013). Biostatistics. In: Descriptive statistics, pp. 20–43. USA: Wiley. [Google Scholar]
31. di Cesare, M., Khang, Y. H., Asaria, P., Blakely, T., Cowan, M. J. et al. (2013). Inequalities in non-communicable diseases and effective responses. The Lancet, 381(9866), 585–597. https://doi.org/10.1016/S0140-6736(12)61851-0 [Google Scholar] [PubMed] [CrossRef]
32. Su, Z., Zou, Z., Hay, S. I., Liu, Y., Li, S. et al. (2022). Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: An age-period-cohort analysis for the Global Burden of Disease 2019 Study. eClinicalMedicine, 43(25), 101249. https://doi.org/10.1016/j.eclinm.2021.101249 [Google Scholar] [PubMed] [CrossRef]
33. Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E. et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. Journal of the American College of Cardiology, 76(25), 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 [Google Scholar] [PubMed] [CrossRef]
34. Zimmerman, M. S., Smith, A. G. C., Sable, C. A., Echko, M. M., Wilner, L. B. et al. (2020). Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet Child and Adolescent Health, 4(3), 185–200. https://doi.org/10.1016/S2352-4642(19)30402-X [Google Scholar] [PubMed] [CrossRef]
35. Roguin, N., Du, Z. D., Barak, M., Nasser, N., Hershkowitz, S. et al. (1995). High prevalence of muscular ventricular septal defect in neonates. Journal of the American College of Cardiology, 26(6), 1545–1548. https://doi.org/10.1016/0735-1097(95)00358-4 [Google Scholar] [PubMed] [CrossRef]
36. Ekici, F., Tutar, E., Atalay, S., Arsan, S., Özçelik, N. (2008). The incidence and follow-up of isolated ventricular septal defect in newborns by echocardiographic screening. Turkish Journal of Pediatrics, 50(3), 223–227. [Google Scholar] [PubMed]
37. Chang, J. K., Jien, W. Y., Chen, H. L., Hsieh, K. S. (2011). Color doppler echocardiographic study on the incidence and natural history of early-infancy muscular ventricular septal defect. Pediatrics and Neonatology, 52(5), 256–260. https://doi.org/10.1016/j.pedneo.2011.06.003 [Google Scholar] [PubMed] [CrossRef]
38. Yang, B. Y., Qu, Y., Guo, Y., Markevych, I., Heinrich, J. et al. (2021). Maternal exposure to ambient air pollution and congenital heart defects in China. Environment International, 153(1), 106548. https://doi.org/10.1016/j.envint.2021.106548 [Google Scholar] [PubMed] [CrossRef]
39. Padula, A. M., Tager, I. B., Carmichael, S. L., Hammond, S. K., Yang, W. et al. (2013). Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatric and Perinatal Epidemiology, 27(4), 329–339. https://doi.org/10.1111/ppe.12055 [Google Scholar] [PubMed] [CrossRef]
40. Wu, W., He, J., Shao, X. (2020). Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine, 99(23), e20593. https://doi.org/10.1097/MD.0000000000020593 [Google Scholar] [PubMed] [CrossRef]
41. Bouma, B. J., Mulder, B. J. M. (2017). Changing landscape of congenital heart disease. Circulation Research, 120(6), 908–922. https://doi.org/10.1161/CIRCRESAHA.116.309302 [Google Scholar] [PubMed] [CrossRef]
42. Adilbekova, A., Marasulov, S., Nurkeyev, B., Kozhakhmetov, S. (2022). Evolution of surgery of ventricular septal defect closure. Journal of Clinical Medicine of Kazakhstan, 19(5), 4–8. https://doi.org/10.23950/jcmk/12505 [Google Scholar] [CrossRef]
43. Riko, M., Toyoshima, K., Shimokaze, T., Kumagai, T., Suzuki, H. (2020). Clinical presentation of preterm infants with ventricular septal defect. Tohoku Journal of Experimental Medicine, 252(4), 281–286. https://doi.org/10.1620/tjem.252.281 [Google Scholar] [PubMed] [CrossRef]
44. Congenital Heart Defects (2022). Facts about ventricular septal defect. https://www.cdc.gov/ncbddd/heartdefects/ventricularseptaldefect.html [Google Scholar]
45. Menting, M. E., Cuypers, J. A. A. E., Opić, P., Utens, E. M. W. J., Witsenburg, M. et al. (2015). The unnatural history of the ventricular septal defect: Outcome up to 40 years after surgical closure. Journal of the American College of Cardiology, 65(18), 1941–1951. https://doi.org/10.1016/j.jacc.2015.02.055 [Google Scholar] [PubMed] [CrossRef]
46. CDFT (2014). «Kazakhstan 2050» Strategy. https://afmrk.gov.kz/en/activity/strategy-and-program/strategy-kazakhstan-2050/ [Google Scholar]
47. Wie, J. H., Han, Y. J., Kim, S. H., Kim, M. Y., Cho, H. Y. et al. (2022). Prenatal diagnosis of congenital heart diseases and associations with serum biomarkers of aneuploidy: A multicenter prospective cohort study. Yonsei Medical Journal, 63(8), 735. https://doi.org/10.3349/ymj.2022.63.8.735 [Google Scholar] [PubMed] [CrossRef]
48. Bai, J., Cui, J., Shi, F., Yu, C. (2023). Global epidemiological patterns in the burden of main non-communicable diseases, 1990–2019: Relationships with socio-demographic index. International Journal of Public Health, 68, 1605502. https://doi.org/10.3389/ijph.2023.1605502 [Google Scholar] [PubMed] [CrossRef]
49. Legal Information System of Regulatory Legal Acts of the Republic of Kazakhstan (2018). On approval of the pediatric care standard in the Republic of Kazakhstan. https://adilet.zan.kz/eng/docs/V1700016279 [Google Scholar]
50. Turkulov, B., Aringazina, A., Pak, V., Kuatbekov, K., Baizhigitov, N. (2021). Organization of cardiosurgical care for children with congenital heart diseases in children under one year with determination of risk factors of surgical treatment. Electronic Journal of General Medicine, 18(5), em315. https://doi.org/10.29333/ejgm/11141 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools