 Open Access
Open Access
ARTICLE
Quantitative Parameters Analysis for Prenatally Echocardiographic Diagnosis of Atrioventricular Septal Defects
1 Maternal-Fetal Consultation Center of Congenital Heart Disease, Department of Echocardiography, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
2 State Key Laboratory of Software Development Environment, Beihang University, Beijing, China
* Corresponding Author: Yihua He. Email:
Congenital Heart Disease 2023, 18(3), 387-397. https://doi.org/10.32604/chd.2023.029060
Received 29 January 2023; Accepted 01 March 2023; Issue published 09 June 2023
Abstract
Background: Atrioventricular septal defects (AVSDs) are screened and diagnosed usually rely on the imaging characteristics of fetal echocardiography (FE). However, diagnosis on images is heavily depended on sonographers’ experience and the quantitative data are rarely studied. Objective: This study aimed to realize the prenatal diagnosis of AVSDs by analyzing the quantitative data on FE. Methods: One hundred and thirteen cardiac quantitative data was analyzed in 370 normal and 49 AVSDs fetuses retrospectively. The top six with the highest diagnostic accuracy rate were acquired according to the area under the curve (AUC), and the diagnostic value of six variables was analyzed. Results: Six parameters obtained on the four-chamber view (4CHV), including the atrial to ventricular length ratio in end-diastole (AVLR-ED), AVLR-ED combined with the atrial to ventricular length ratio in end-systole (AVLR-ES), quantile score (Q score) of AVLR-ED, Q score of AVLR-ES, Q score of ventricle length in end-diastole (VL-ED), and AVLR-ES, were the top six with the highest diagnostic value, and the AUC was 0.99 (95%CI 0.99–1.00), 0.99 (95%CI 0.99–1.00), 0.99 (95%CI 0.98–1.00), 0.95 (95%CI 0.91–0.99), 0.93 (95%CI 0.87–0.99), and 0.91 (95%CI 0.83–1.00), respectively. And within the 20% false positive rate, the diagnostic sensitivity was greater than 100%, 100%, 100%, 90%, 90%, and 88%, respectively. Conclusions: Six variables could be used for prenatal diagnosis of AVSDs. Among them, AVLR-ED and Q score of AVLR-ED, obtained on the 4CHV, were more convenient to acquire and had higher diagnostic accuracy.Keywords
Abbreviations
| AVSD | Atrioventricular septal defect |
| CHD | Congenital heart disease |
| FE | Fetal echocardiography |
| AVLR | Atrial to ventricular length ratio |
| GA | Gestational age |
| VSD | Ventricular septal defects |
| HLHS | Hypoplastic left heart syndrome |
| HRHS | Hypoplastic right heart syndrome |
| M | Median |
| IQR | Interquartile range |
| HL | Heart length |
| VL | Ventricle length |
| AL | Atrium length |
| 4CHV | Four-chamber view |
| ED | End diastole |
| ES | End systole |
| Q score | Quantile score |
| AUC | Area under the curve |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| PLR | Positive likelihood ratio |
| NLR | Negative likelihood ratio |
| N | Normal |
| 95%CI | 95% confidence intervals |
The spectrum of atrioventricular septal defects (AVSDs) ranges from simple to critical in congenital heart diseases (CHDs) [1]. The formation of AVSDs is due to the abnormally embryonic development of the crisscross structure [2,3]. Several syndromes, especially Down syndrome and heterotaxia syndrome usually exist together with AVSDs [4–6]. Additionally, other intracardiac malformations are often encountered [7,8]. For complete AVSDs, the repair is challenging, and the ventricular development affects the choice of surgery as well as the survival rates of patients [9–11]. Therefore, timely and accurate detection of AVSDs is crucial for prenatal counseling and perinatal management.
Careful examination of some marks, including complete or partial crisscross defect, linear insertion of the atrioventricular valves, and the number of atrioventricular annuli on fetal echocardiography (FE) is contributed to checking AVSDs prenatally [12–14]. But these are imaging data, and the detection of these lesions depends heavily on the operators’ experience. van Nisselrooij et al. [15] investigated the prenatal screening of 114 fetuses with severe CHDs (contained AVSDs) in the northwest of the Netherlands in 2020 and found more than half were missed diagnoses due to the standard views could not be obtained or the lesions could not be accurately identified. In 2023, a national cohort study in the UK showed the prenatal screening rate of AVSDs was 69.2% although was higher than 50% which was expected by the National Health Service Fetal Anomaly Screening Program [16].
Atrial to ventricular length ratio (AVLR), firstly proposed by Machlitt [17] in 2004, was a quantitative data to diagnose AVSDs prenatally and with a diagnostic rate of more than 85%. In the big data area, artificial intelligence software could provide new diagnostic methods and may help to improve diagnostic accuracy. Q score, a more reliable range system of normal FE quantitative data, was established by Gu et al. [18] in 2019, and it has not been applied in AVSDs fetuses so far. Therefore, the purpose of this study was to realize the antenatal diagnosis of AVSDs by analyzing the quantitative data on FE and evaluate their diagnostic efficacy.
Three hundred and seventy normal fetuses, forty-nine AVSDs fetuses, and forty-three cases with other CHDs were selected retrospectively and were named as normal group, AVSDs group, and the cohort 3 which was used to make the further differential diagnosis. According to the last menstruation, the gestational age (GA) for normal and AVSDs fetuses was 18.3–36.2 and 21.1–35.3 weeks respectively when performing FE. Cohort 3 included fifteen ventricular septal defects (VSD), sixteen hypoplastic left heart syndrome (HLHS), and twelve hypoplastic right heart syndrome (HRHS) fetuses, and GA in this group ranged from 21.4 to 35 weeks.
All cases were from the database of the Maternal-Fetal Consultation Center of Fetal Congenital Cardiac Disease in Beijing Anzhen Hospital between January 2019 and February 2022. And pictures and cine loops of each fetus were stored in the database. The median maternal age was 29.6 years old, and the interquartile range (IQR) was 22.0 to 36.3. The study was approved by the ethics committee of Beijing Anzhen Hospital (2020016X).
2.2 Inclusion and Exclusion Criteria
The inclusion criteria of the normal group were (1) singleton pregnancy with normal growth and development, (2) second or third-trimester pregnancy; and the exclusion criteria were (1) twins or multiple pregnancies, (2) first-trimester pregnancy, (3) combined with maternal diseases that could affect the fetal heart, (4) combined with intracardiac, extracardiac structural abnormalities, cardiomyopathy, or genetic anomaly, and (5) cardiac arrhythmia. In AVSDs and cohort 3, fetuses with discordance connection in atriums and ventricles, ventricles and great arteries, and the cardiac tumor were excluded. All FE image quality was evaluated by one sonographer with senior title and images with poor quality were excluded.
2.3 Fetal Echocardiography Examination
All FE images were performed by Voluson E10-C1-5-D machine equipped with a 2- to 4-MHz transducer (GE Healthcare, Milwaukee, Wisconsin), and Aloka 10-UST-9130 equipped with a 3–6 MHz transducer (Aloka, Tokyo, Japan). FE images were obtained and analyzed according to the segmental analysis method [19] published by the American Heart Association in 2004. The final diagnosis was confirmed by the postnatal imaging examination, the autopsy, or two doctors with more than 8 years of FE experience.
Measurements of conventional parameters, including 2-dimensional fetal cardiac structural values, the Doppler parameters, and the umbilical and cerebral Doppler measurements, a total of 48 measurements were obtained based on the guidelines published by the American Society of Echocardiography [20]. The special parameters, including heart length (HL), ventricle length (VL), atrium length (AL), and the AVLR (AL/VL ratio), a total of 8 variables were acquired on the four-chamber view (4CHV) at the end diastole (ED) and end systole (ES). All special parameters were calculated with an image review. ED was a frame just prior to the start of the systolic period. At this time, both the atrioventricular valve and the semilunar valve were closed, and the ventricle was the largest. ES was a frame before the start of the diastolic period. And both the atrioventricular valve and the semilunar valve were also closed at this moment, but the ventricle was the smallest. In the ED, measuring methods of HL and VL were consistent with Machlitt’s [17]. But AL in the normal group and cohort 3 was the measurement from the top of the atrium to the attachment point of the mitral valve at the interventricular septum (Fig. 1A); and in AVSDs, AL was the measurement from the roof of the atrium to a closed common atrioventricular valve (Fig. 1B). In the ES, the measurement rule was consistent with that in the ED. All values were measured twice by the same operator who had 5 years of FE experience, and the average value was taken.
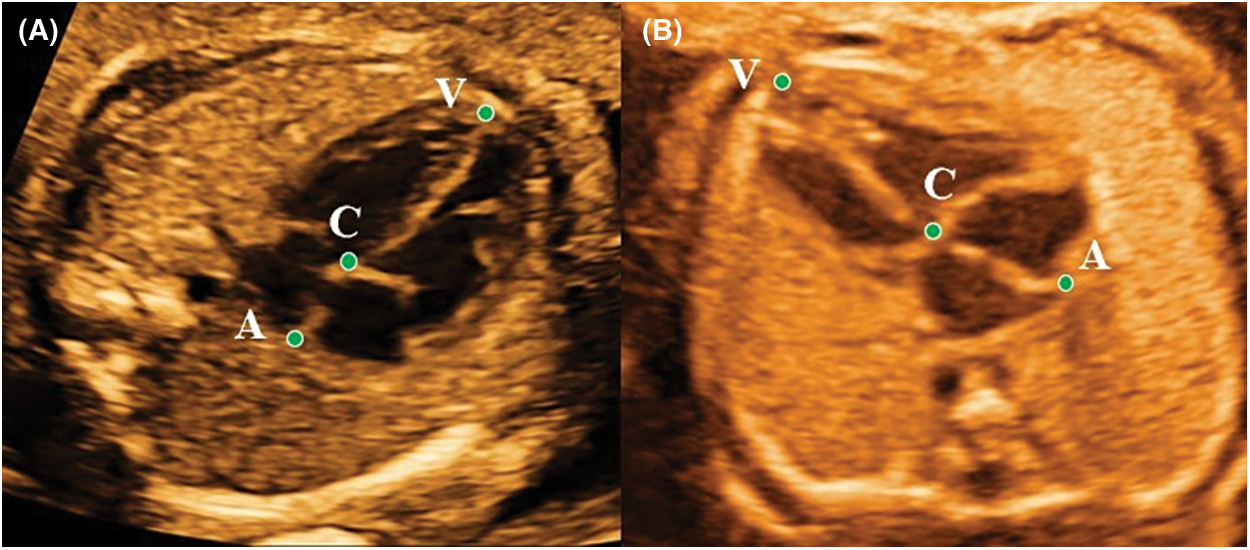
Figure 1: The VL (point V to C), AL (point C to A), and HL (point V to A) measured in end-diastolic period on the apical four-chamber view in the normal fetus at 24.3 gestational weeks (A) and in the AVSDs fetus at 25.1 gestational weeks (B). AVLR = AL/VL
The quantile score (Q score) of 48 conventional as well as 8 special parameters were acquired. Additionally, a combination of AVLR-ED and AVLR-ES was added to observe the diagnostic value. Finally, 48 conventional variables, 8 special parameters, Q score of 48 conventional variables, Q score of 8 special parameters, and one combination of AVLR-ED and AVLR-ES, a total of 113 variables were included in this study. Among these variables, the critical diagnostic variables of AVSDs would be identified and the diagnostic value would be evaluated.
Statistical analysis of all variables was performed by using the Medical (19.0 version) and IBM SPSS (23.0 version) software. Normal distribution tests, including the Kolmogorov-Smirnov and the Shapiro-Wilk test, were performed for echocardiographic variables at each gestational week in normal fetuses. In 113 variables, the top six parameters were selected according to the area under the curve (AUC). In this study, the quantitative parameters were expressed in median and IQR. Kruskal-Wallis statistical test was used to compare the statistical differences among different groups. All statistical methods were two-sided with p-values less than 0.05 considered to have a statistical difference. The sensitivity, specificity, Youden index, cut-off value, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR) as well as the 95% confidence intervals (95%CI) were also calculated.
3.1 Types and Combinations in AVSDs
In AVSDs group, the number of complete, partial, and transitional fetuses was 32, 10, and 7, respectively. And in those 49 AVSDs fetuses, there were 8 cases combined with right ventricular outflow tract obstruction, 11 cases with left ventricular outflow tract obstruction, and 30 were isolated.
Of the 113 variables, 6 parameters including AVLR-ED, AVLR-ES, Q score of VL-ED, Q score of AVLR-ED, Q score of AVLR-ES, and AVLR-ED combined with AVLR-ES, showed the highest diagnostic value, and all the AUC were more than 0.90 (Fig. 2). Medians and IQRs of the six parameters were presented in Table 1. Within the 20% false positive rate (FPR), a range acceptable to doctors, the diagnostic sensitivity for AVSDs was greater than 88%, 90%, 90%, 100%, 100%, and 100% in AVLR-ES, Q score of VL-ED, Q score of AVLR-ES, AVLR-ED, Q score of AVLR-ED, and AVLR-ED combined with AVLR-ES, respectively.
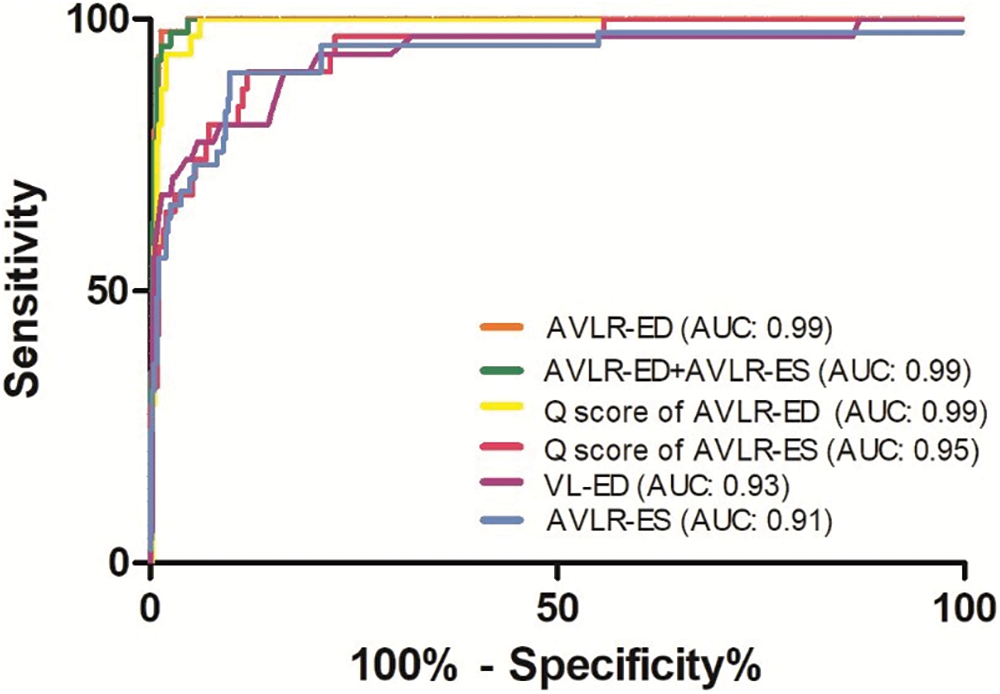
Figure 2: Receiver operating characteristic curves of detecting atrioventricular septal defects in six parameters
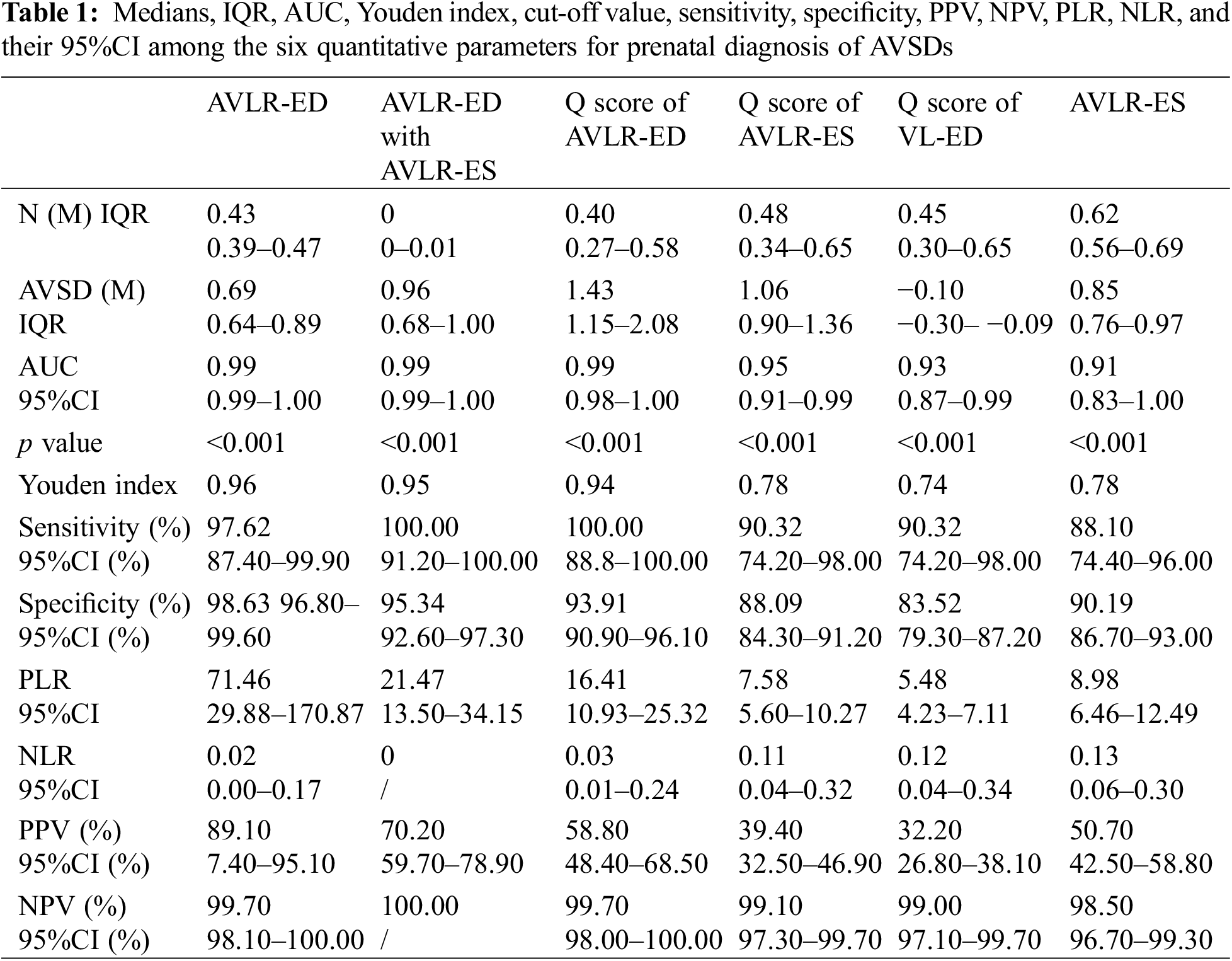
The AUC of AVLR-ED was 0.99 (95%CI 0.99–1.00), and the diagnostic accuracy could reach 96.30% with a sensitivity of 97.62% and a specificity of 98.63%. When a 0.60 was selected, 97.0% of AVSDs could be detected within the PFR of 3.0%; and when 0.56 was selected, all AVSDs and 5.0% of normal fetuses could be included (Fig. 3A). The AUC of AVLR-ED combined with AVLR-ES was 0.99 (95%CI 0.99–1.00) and the diagnostic accuracy was 0.95 with a sensitivity of 100% and a specificity of 95.34%. When 0.49 was selected, 90.0% AVSDs could be screened within the range of 1.0% FPR (Fig. 3B). The AUC of the Q score of AVLR-ED was 0.99 (95%CI 0.98–1.00). When the smallest data (0.85) was chosen, all AVSDs and 7.0% normal fetuses were included (Fig. 3C). Compared with the first three parameters, the Q score of AVLR-ES, the Q score of VL-ED, and AVLR-ES had relatively poor diagnostic effectiveness; AUC and the 95%CI of three parameters were showed in Table 1. Diagnostic accuracy with Q score of AVLR-ES was 0.78, and when 0.785 was selected, more than 90.0% of AVSDs fetuses could be detected with a PFR of 12.0% (Fig. 3D). The accuracy of the Q score of VL-ED was 0.74. Selecting 0.21 as a cutoff value, 90.0% AVSDs and 17.0% normal fetuses could be included (Fig. 3E). The accuracy of AVLR-ES was 0.78. Selecting 0.748 as the cutoff value, more than 88.0% AVSDs and less than 10.0% normal fetuses were included (Fig. 3F). The PPV, NPV, PLR, NLR, and the relevant 95%CI were shown in Table 1.
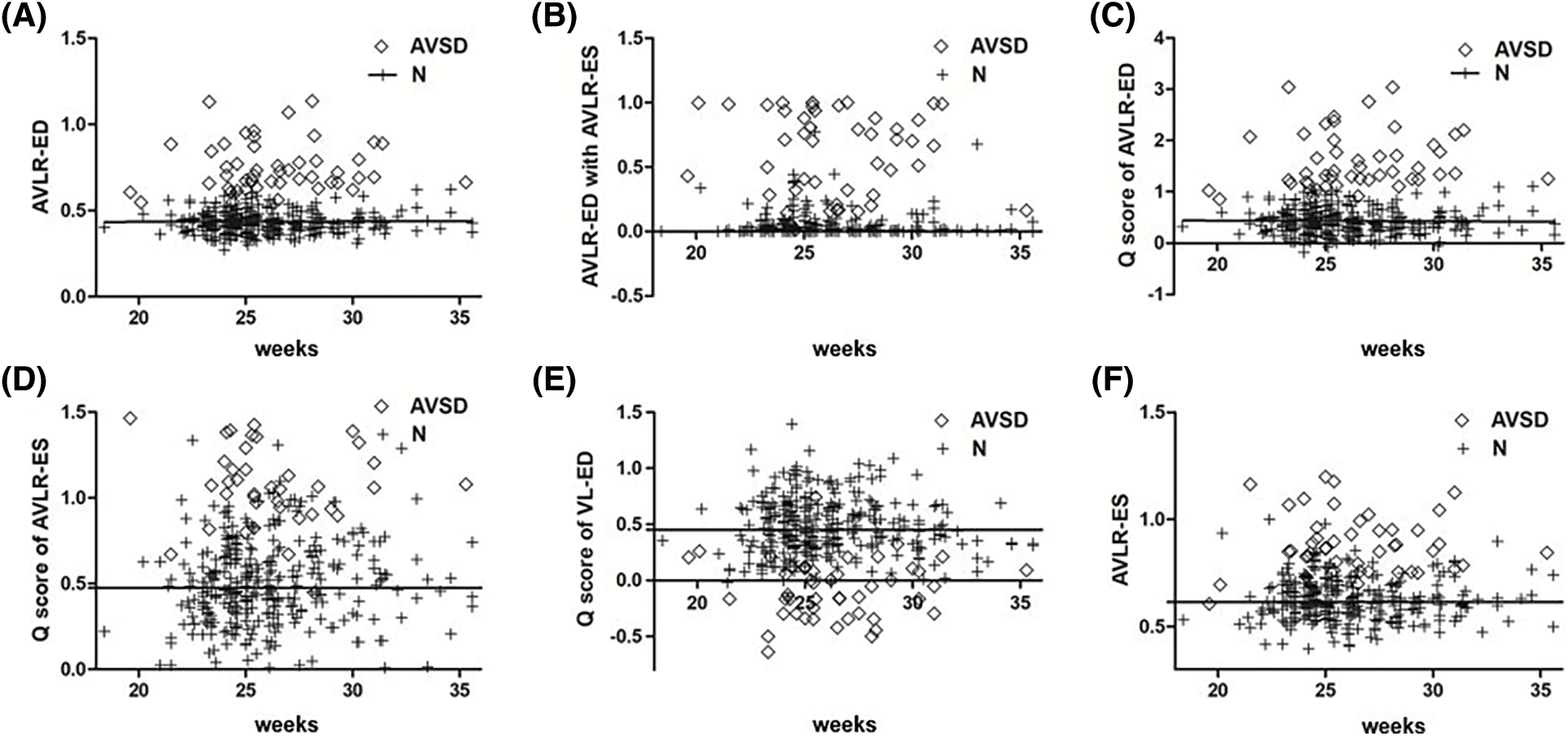
Figure 3: Scatter plot of AVLR-ED (A), AVLR-ED combine with AVLR-ES (B), Q score of AVLR-ED (C), Q score of AVLR-ES (D), Q score of VL-ED (E), and AVLR-ES (F) in normal group and AVSDs group
3.3 Verification of Key Parameters in Other CHDs
To further verify the value of these six indicators, cohort 3 was used to make the differential diagnosis. The results showed except Q score of VL-ED, all variables in the AVSDs were larger than other CHDs; And Q score of VL-ED was smaller than other groups (p < 0.05). Details of medians and IQR of the three differential groups were shown in Table 2. For parameters of AVLR-ED, AVLR-ES, Q score of AVLR-ED, Q score of AVLR-ES, and Q score of VL-ED, there were statistical differences between AVSDs and other CHDs (p < 0.05, Figs. 4A–4E), but it was not statistically different among the normal, VSD, HLHS, and HRHS fetuses (p > 0.05, Figs. 4A–4E). For AVLR-ED combined with AVLR-ES, though there was no statistical difference between AVSDs and HRHS fetuses (p > 0.05, Fig. 4F), the variable in AVSDs was greater than in HRHS; And the p value was statistically significant between AVSDs and HLHS, VSD, as well as the normal group (p < 0.05, Fig. 4F).
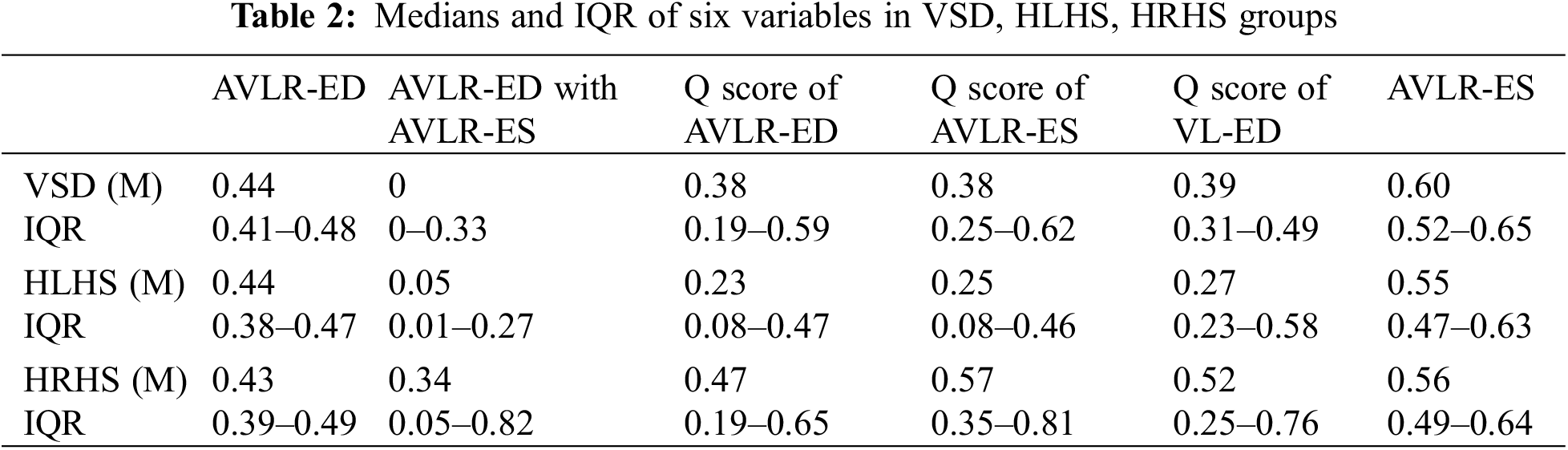
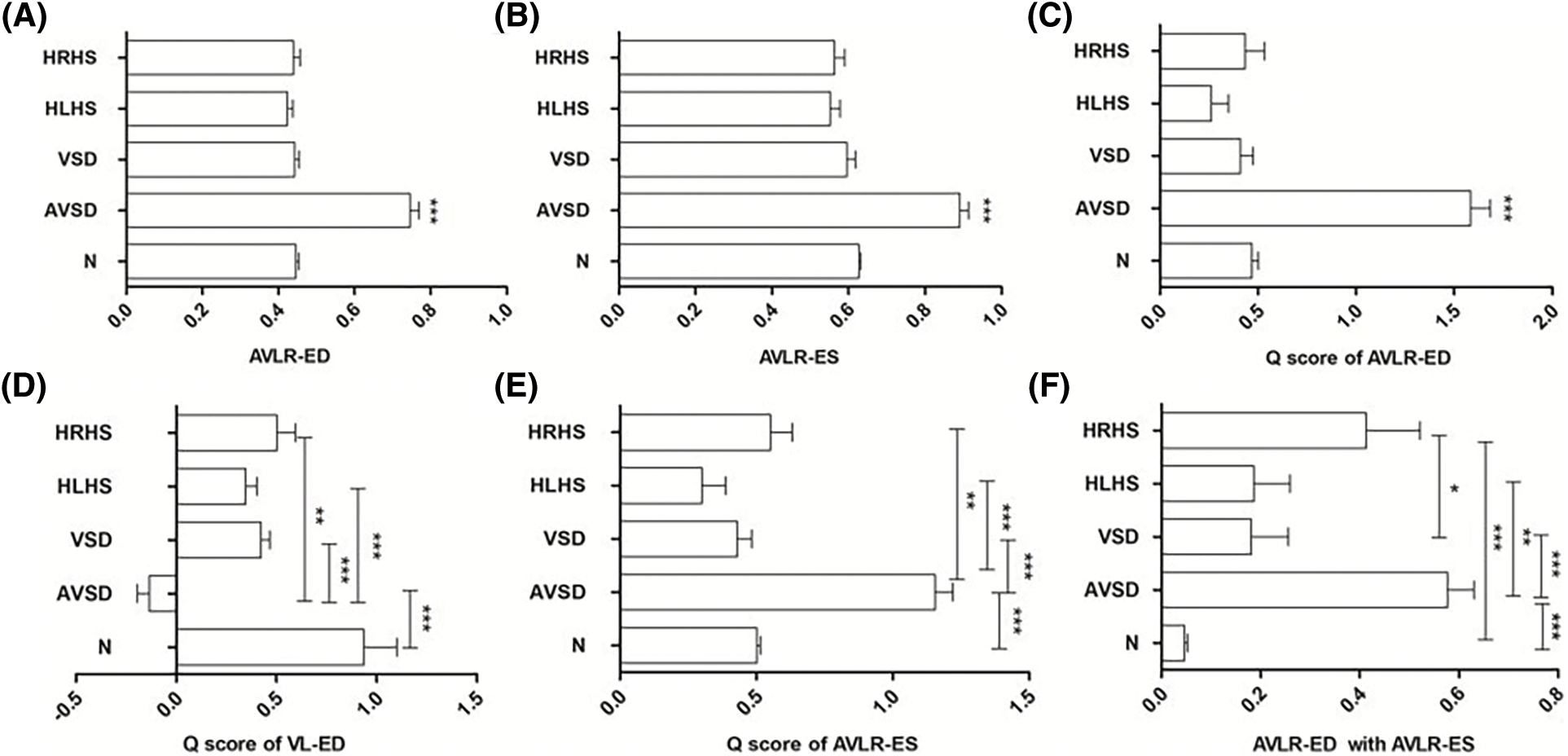
Figure 4: Comparation of AVLR-ED (A), AVLR-ES (B), Q score of AVLR-ED (C), Q score of VL-ED (D), Q score of AVLR-ES (E), and AVLR-ED combined with AVLR-ES (F) among the normal, AVSDs, VSD, HLHS, and HRHS groups. * p < 0.05; ** p < 0.01, *** p < 0.001
In this study, five special parameters and one combination, AVLR-ED, AVLR-ES, Q score of AVLR-ED, Q score of AVLR-ES, Q score of VL-ED, and AVLR-ED with AVLR-ES were the top six in prenatal diagnosis of AVSDs. The reason why the parameters did not contain the conventional parameters was determined by the anatomical characteristics of AVSDs, which were mainly reflected in the absence of crisscross and the resulting shortened inflow tract on 4CHV [21]. In addition, AVSDs are easy to be associated with other intracardiac abnormalities [8], and there exist unbalanced types of AVSDs [9]. Under the above circumstances, conventional parameters could not screen them out accurately.
Among the six parameters, AVSD-ED and Qscore of AVSD-ED had the best diagnostic performance, which included AUC, diagnostic accuracy, sensitivity, specificity, and the differential diagnosis of other CHDs. Though AVLR-ED combined with AVLR-ES had the same efficiency to distinguish AVSD from normal fetuses, it could not distinguish AVSDs from HRHS. In addition, the acquisition of AVLR-ED and AVLR-ES was on both diastolic and systolic periods, it was less convenient compared with the acquisition of AVSD-ED and Qscore of AVSD-ED.
AVLR-ED, based on four-chamber views and proposed by Machlitt [17], was a simple and effective method for prenatal diagnosis of AVSDs, but was merely used to compare with normal fetuses. In this study, we comprehensively verified its effectiveness in the diagnosis of AVSDs by increasing the sample size of the case group and made the differential diagnosis with other CHDs. Additionally, the diagnosis and differential diagnosis of the AVLR in systole was also analyzed in our study and the results showed AVLR-ED has the highest diagnostic efficiency.
Q score, defined as the percentile of each measured value on the quantile line in the quantile regression model corresponding to the given GA and representing the variables’ changes under normal growth and development, is a method to standardize quantitative data of non-normal distribution [18]. Previous studies [22–24] have used percentile to calculate the size of the human body to determine whether the heart was abnormal and the extent of the abnormality; and quantiles methods were used to manage the blood pressure of children in other studies [25,26]. In FE area, Q score has been proven to provide a more accurate reference range for normal fetal cardiac measurements, and has been used to make differential diagnosis between big VSD and Tetralogy of Fallot [27], but has not been used in diagnosing AVSDs so far. Therefore, this was the first time to apply Q score system to the field of prenatal diagnosis of AVSDs. In this study, we established the Q scores of 56 parameters, and the top three with better diagnostic efficacy were selected. The results showed that the comprehensive diagnostic efficiency of the Q score of AVLR-ED was similar to AVLR-ED.
Diagnosis of imaging characteristics of AVSDs mainly relies on the experience of the ultrasound physician and is easily caused missed diagnosis [16]. Quantitative parameters could eliminate the influence of subjective factors as much as possible. This is of great significance for the early, rapid, and effective diagnosis of AVSDs on FE, especially in places without prenatal screening for CHD; and the six parameters which could be used to improve the prenatal detection rate of AVSDs would be helpful for perinatal management and prognosis consultation. In addition, the diagnostic parameters in this study were acquired on the 4CHV only, which reduced the difficulty in obtaining the diagnostic views.
There were some limitations in our study. On the one hand, this study may only could be used as a pilot study in the quantitative diagnosis of AVSDs in the area of big data. Because even though the sample size of the normal group and AVSDs group has been increased, the sample size was still small in the big data field. On the other hand, due to the lack of fetal electrocardiogram as well as the inability to simultaneously measure the cardiac structure when using M-mode for fetal cardiac cycle monitoring, the selection of cardiac cycle was subjective more or less.
Our research demonstrated that prenatal diagnosis of AVSDs and the differential diagnosis with other CHDs could be achieved by using quantitative FE data. Compared with the images, the quantitative parameters were more objective and with high diagnostic accuracy. Among 6 parameters, AVLR-ED and Q score of AVLR-ED could be recommended as the optimal parameters.
Funding Statement: “Dengfeng” Project of Talent Training Plan of Beijing Medical Management Center (Number DFL20220601), Beijing Municipal Administration of Hospitals Incubating Program (Number PX2023023), National Natural Science Foundation of China (Number 82170301), Beijing Municipal Administration of Hospitals Incubating Program (Number PX2022026), and Beijing Natural Science Foundation (Number L222152).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Y. H, T. Y; data collection: Y. Z, Y. R, J. H, X. L, Y. Z, X. G; analysis and interpretation of results: X. Z, T. Y, T. L, H. W; draft manuscript preparation: X. Z. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data will be shared on reasonable request to the corresponding author.
Ethics Approval: The study was approved by the Ethics Committee of Beijing Anzhen Hospital (2020016X).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Taqatqa, A. S., Vettukattil, J. J. (2021). Atrioventricular septal defects: Pathology, imaging, and treatment options. Current Cardiology Reports, 23(8), 93. https://doi.org/10.1007/s11886-021-01523-1 [Google Scholar] [PubMed] [CrossRef]
2. Buratto, E., Konstantinov, I. E. (2021). Atrioventricular valve surgery: Restoration of the fibrous skeleton of the heart. The Journal of Thoracic and Cardiovascular Surgery, 162(2), 360–365. https://doi.org/10.1016/j.jtcvs.2021.03.128 [Google Scholar] [PubMed] [CrossRef]
3. Calkoen, E. E., Hazekamp, M. G., Blom, N. A., Elders, B. B., Gittenberger-de Groot, A. C. et al. (2016). Atrioventricular septal defect: From embryonic development to long-term follow-up. International Journal of Cardiology, 202, 784–795. https://doi.org/10.1016/j.ijcard.2015.09.081 [Google Scholar] [PubMed] [CrossRef]
4. Irving, C. A., Chaudhari, M. P. (2012). Cardiovascular abnormalities in down’s syndrome: Spectrum, management, and survival over 22 years. Archives of Disease in Childhood, 97(4), 326–330. https://doi.org/10.1136/adc.2010.210534 [Google Scholar] [PubMed] [CrossRef]
5. Pradhan, A. K., Pandey, S., Usman, K., Kumar, M., Mishra, R. (2013). Noonan syndrome with complete atrioventricular canal defect with pulmonary stenosis. Journal of the American College of Cardiology, 62(20), 1905. https://doi.org/10.1016/j.jacc.2013.06.062 [Google Scholar] [PubMed] [CrossRef]
6. Huggon, I. C., Cook, A. C., Smeeton, N. C., Magee, A. G., Sharland, G. K. (2000). Atrioventricular septal defects diagnosed in fetal life: Associated cardiac and extra-cardiac abnormalities and outcome. Journal of the American College of Cardiology, 36(2), 593–601. https://doi.org/10.1016/S0735-1097(00)00757-9 [Google Scholar] [PubMed] [CrossRef]
7. Gilljam, T., McCrindle, B. W., Smallhorn, J. F., Williams, W. G., Freedom, R. M. (2000). Outcomes of left atrial isomerism over a 28-year period at a single institution. Journal of the American College of Cardiology, 36(3), 908–916. https://doi.org/10.1016/S0735-1097(00)00812-3 [Google Scholar] [PubMed] [CrossRef]
8. Ramgren, J. J., Zindovic, I., Nozohoor, S., Gustafsson, R., Hakacova, N. et al. (2022). Impact of concomitant complex cardiac anatomy in nonsyndromic patients with complete atrioventricular septal defect. The Journal of Thoracic and Cardiovascular Surgery, 163(4), 1437–1444. https://doi.org/10.1016/j.jtcvs.2021.08.039 [Google Scholar] [PubMed] [CrossRef]
9. Jegatheeswaran, A., Pizarro, C., Caldarone, C. A., Cohen, M. S., Baffa, J. M. et al. (2010). Echocardiographic definition and surgical decision-making in unbalanced atrioventricular septal defect: A Congenital Heart Surgeons’ Society multi-institutional study. Circulation, 122, S209–215. https://doi.org/10.1161/CIRCULATIONAHA.109.925636 [Google Scholar] [PubMed] [CrossRef]
10. King, G., Buratto, E., Celermajer, D. S., Grigg, L., Alphonso, N. et al. (2022). Natural and modified history of atrioventricular valve regurgitation in patients with fontan circulation. Journal of the American College of Cardiology, 79(18), 1832–1845. https://doi.org/10.1016/j.jacc.2022.02.022 [Google Scholar] [PubMed] [CrossRef]
11. Kwon, M. H., Schultz, A. H., Lee, M., Permut, L. C., McMullan, D. M. et al. (2022). Complete atrioventricular septal defect with absent or diminutive primum component: Incidence, anatomic characteristics, and outcomes. The Journal of Thoracic and Cardiovascular Surgery, 163(3), 1156–1162. https://doi.org/10.1016/j.jtcvs.2021.06.041 [Google Scholar] [PubMed] [CrossRef]
12. Adriaanse, B. M., Bartelings, M. M., van Vugt, J. M., Chaoui, R., Gittenberger-de Groot, A. C. et al. (2014). Differential and linear insertion of atrioventricular valves: A useful tool? Ultrasound in Obstetrics & Gynecology, 44(5), 568–574. https://doi.org/10.1002/uog.13326 [Google Scholar] [PubMed] [CrossRef]
13. Minich, L. A., Snider, A. R., Bove, E. L., Lupinetti, F. M., Vermilion, R. P. (1992). Echocardiographic evaluation of atrioventricular orifice anatomy in children with atrioventricular septal defect. Journal of the American College of Cardiology, 19(1), 149–153. https://doi.org/10.1016/0735-1097(92)90066-V [Google Scholar] [PubMed] [CrossRef]
14. Roberson, D. A., Muhiudeen, I. A., Silverman, N. H., Turley, K., Haas, G. S. (1991). Intraoperative transesophageal echocardiography of atrioventricular septal defect. Journal of the American College of Cardiology, 18(2), 537–545. https://doi.org/10.1016/0735-1097(91)90612-D [Google Scholar] [PubMed] [CrossRef]
15. van Nisselrooij, A. E. L., Teunissen, A. K. K., Clur, S. A., Rozendaal, L. et al. (2020). Why are congenital heart defects being missed? Ultrasound in Obstetrics & Gynecology, 55(6), 747–757. https://doi.org/10.1002/uog.20358 [Google Scholar] [PubMed] [CrossRef]
16. Aldridge, N., Pandya, P., Rankin, J., Miller, N., Broughan, J. et al. (2023). Detection rates of a national fetal anomaly screening programme: A national cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 130(1), 51–58. https://doi.org/10.1111/1471-0528.17287 [Google Scholar] [PubMed] [CrossRef]
17. Machlitt, A., Heling, K. S., Chaoui, R. (2004). Increased cardiac atrial-to-ventricular length ratio in the fetal four-chamber view: A new marker for atrioventricular septal defects. Ultrasound in Obstetrics & Gynecology, 24(6), 618–622. https://doi.org/10.1002/(ISSN)1469-0705 [Google Scholar] [CrossRef]
18. Gu, X., Zhu, H., Zhang, Y., Han, J., Zhang, H. et al. (2019). Quantile score: A new reference system for quantitative fetal echocardiography based on a large multicenter study. Journal of the American Society of Echocardiography, 32(2), 296–302.e5. https://doi.org/10.1016/j.echo.2018.09.012 [Google Scholar] [PubMed] [CrossRef]
19. Donofrio, M. T., Moon-Grady, A. J., Hornberger, L. K., Copel, J. A., Sklansky, M. S. et al. (2014). Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation, 129(21), 2183–2242. https://doi.org/10.1161/01.cir.0000437597.44550.5d [Google Scholar] [PubMed] [CrossRef]
20. Rychik, J., Ayres, N., Cuneo, B., Gotteiner, N., Hornberger, L. et al. (2004). American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. Journal of the American Society of Echocardiography, 17(7), 803–810. https://doi.org/10.1016/j.echo.2004.04.011 [Google Scholar] [PubMed] [CrossRef]
21. Yoo, S. J. (2004). What does an increased atrial-to-ventricular length ratio mean in fetuses with atrioventricular septal defect? Ultrasound in Obstetrics & Gynecology, 24(6), 597–598. https://doi.org/10.1002/(ISSN)1469-0705 [Google Scholar] [CrossRef]
22. Hornberger, L. K., Weintraub, R. G., Pesonen, E., Murillo-Olivas, A., Simpson, I. A. et al. (1992). Echocardiographic study of the morphology and growth of the aortic arch in the human fetus. Observations related to the prenatal diagnosis of coarctation. Circulation, 86(3), 741–747. https://doi.org/10.1161/01.CIR.86.3.741 [Google Scholar] [PubMed] [CrossRef]
23. Hornberger, L. K., Sanders, S. P., Rein, A. J., Spevak, P. J., Parness, I. A. et al. (1995). Left heart obstructive lesions and left ventricular growth in the mid-trimester fetus. A longitudinal study. Circulation, 92(6), 1531–1538. https://doi.org/10.1161/01.CIR.92.6.1531 [Google Scholar] [PubMed] [CrossRef]
24. Tan, J., Silverman, N. H., Hoffman, J. I., Villegas, M., Schmidt, K. G. (1992). Cardiac dimensions determined by cross-sectional echocardiography in the normal human fetus from 18 weeks to term. American Journal of Cardiology, 70(18), 1459–1467. https://doi.org/10.1016/0002-9149(92)90300-N [Google Scholar] [PubMed] [CrossRef]
25. Flynn, J. T., Kaelber, D. C., Baker-Smith, C. M., Blowey, D., Carroll, A. E. et al. (2017). Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics, 140(3), e20171904. https://doi.org/10.1542/peds.2017-1904 [Google Scholar] [PubMed] [CrossRef]
26. Rosner, B., Cook, N., Portman, R., Daniels, S., Falkner, B. (2008). Determination of blood pressure percentiles in normal-weight children: Some methodological issues. American Journal of Epidemiology, 167(6), 653–666. https://doi.org/10.1093/aje/kwm348 [Google Scholar] [PubMed] [CrossRef]
27. Lv, J., Yang, T., Gu, X., Zhang, Y., Sun, L. et al. (2020). Differential diagnosis of fetal large ventricular septal defect and tetralogy of Fallot based on big data analysis. Echocardiography, 37(4), 620–624. https://doi.org/10.1111/echo.14642 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools