 Open Access
Open Access
ARTICLE
Safety and Efficacy of Biodegradable Patent Foramen Ovale Occluder in Patients with Migraine: A Clinical Trial
1 Congenital Heart Disease Center, Wuhan Asia Heart Hospital, Wuhan University of Science and Technology, Wuhan, 430022, China
2 Structural Heart Disease Center, Zhongnan Hospital, Wuhan University, Wuhan, 430071, China
3 Department of Cardiothoracic and Vascular Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, China
4 Department of Medical Ultrasound, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, China
* Corresponding Author: Gangcheng Zhang. Email:
# The first two authors contributed equally to this work
Congenital Heart Disease 2023, 18(3), 373-385. https://doi.org/10.32604/chd.2023.028979
Received 21 January 2023; Accepted 21 March 2023; Issue published 09 June 2023 Retracted 15 September 2023
A retraction of this article was approved in:
Retraction: Safety and Efficacy of Biodegradable Patent Foramen Ovale Occluder in Patients with Migraine: A Clinical Trial
Read retraction
Abstract
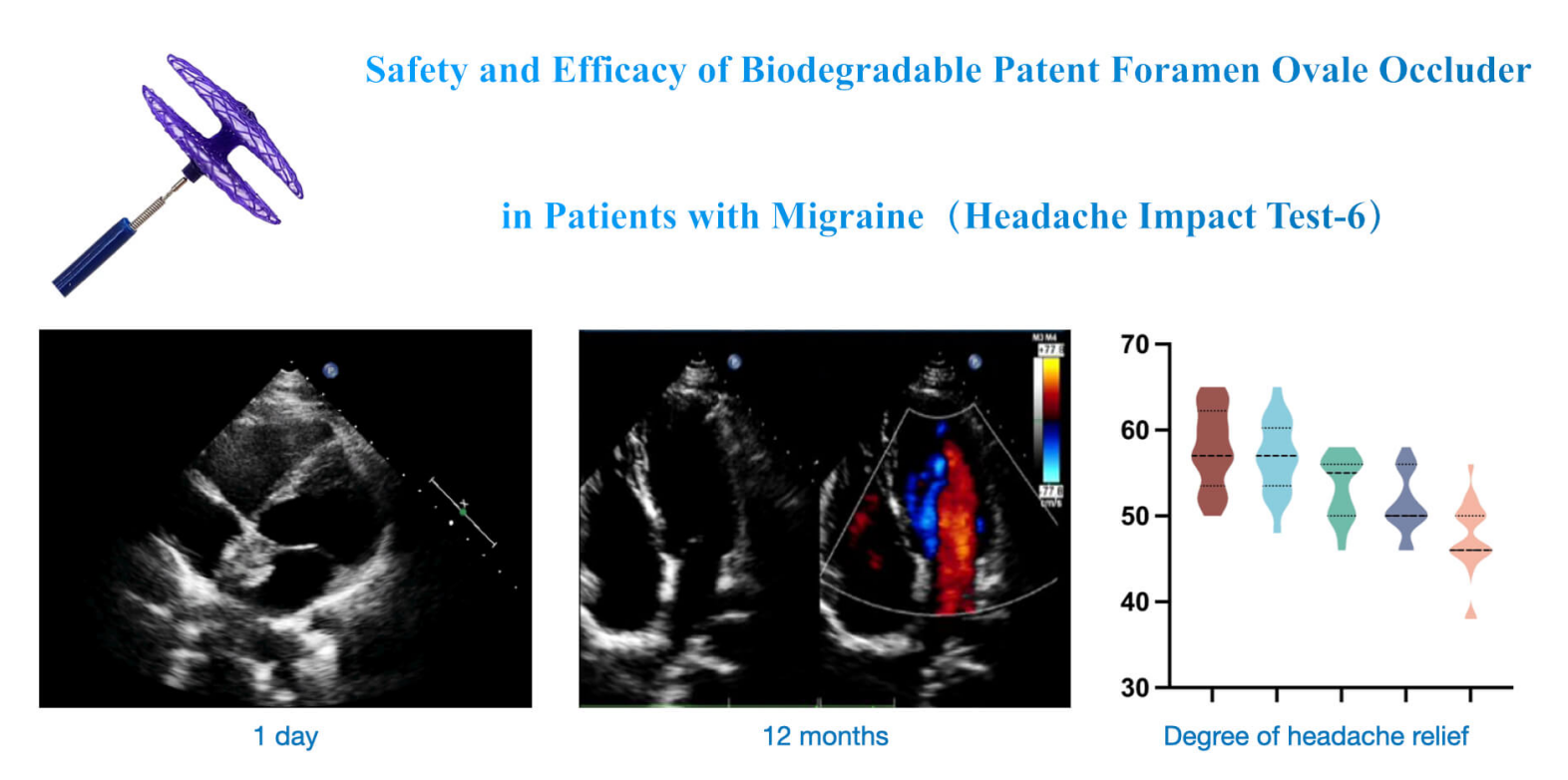
Background: Transcatheter closure of patent foramen ovale (PFO) has been widely accepted as a highly effective way to treat high-risk PFO-related diseases. However, traditional non-degradable occluders made of metal alloys will permanently exist in the body, resulting in thrombosis, valve damage, hemolysis, arrhythmia, or other complications. The biodegradable PFO occluder developed by Shanghai Mallow Medical Instrument Co., Ltd., China can be fully absorbed and degrade into nontoxic ingredients, reducing postoperative complications. Objectives: To study the safety and efficacy of biodegradable PFO occluders in treating PFO. Methods: This single-center clinical trial collected 30 patients treated with a biodegradable PFO occluder. The follow-up period lasted 12 months to analyze the echocardiographic characteristics and headache relief through HIT-6 scores. Results: The immediate success rate was 100%, with no intraoperative severe occlusion-related complications. The contrast transcranial Doppler (cTCD) at 12 months showed that all patients’ right-to-left shunts (RLS) were grade I or 0 with no serious postoperative complications, indicating the overall success rate was 100%. The biodegradable PFO occluder mostly degraded six months after the occlusion. Conclusion: PFO closure with a Mallow biodegradable occluder is safe and effective and has no severe complications.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools