 Open Access
Open Access
ARTICLE
Having a Partner and Having Children: Comparisons of Adults with Congenital Heart Disease and the General Population: A 15-Year Case-Control Study
1 Medical Sociology Unit, Hannover Medical School, Hannover, 30625, Germany
2 Department of Pediatric Cardiology, Intensive Care Medicine and Neonatology, University Medical Center Goettingen, Goettingen, Germany
3 Department of Pediatrics, Western University, London, Ontario, Canada
4 Department of Pediatric Cardiology and Intensive Care Medicine, Hannover Medical School, Hannover, Germany
* Corresponding Author: Siegfried Geyer. Email:
Congenital Heart Disease 2023, 18(3), 337-348. https://doi.org/10.32604/chd.2023.028827
Received 10 January 2023; Accepted 18 April 2023; Issue published 09 June 2023
Abstract
Objectives: To examine whether patients with congenital heart disease (CHD) are less likely to have a partner or children than individuals from the general population. Methods: Longitudinal study with two assessments of the same patients (n = 244) from a hospital population and controls (n = 238) from the German Socio-Economic Panel (GSOEP) using parental education, patients age, and sex as matching criteria. The first patient study was conducted between 5/2003 and 6/2004, the second one between 5/2017 and 4/2019. Controls were drawn from GSOEP-surveys 2004 and 2018. CHD-severity was classified according to type of surgery: curative, reparative, or palliative. Living single was used as outcome measure, for offspring the outcome was having children or not. Results: Among women with CHD the rate of those living single was higher than among controls with the differences depending on disease complexity (curative: OR = 5.5; reparative: OR = 1.9; palliative: OR = 2.7). No statistically significant differences between patients and controls emerged in the male study population. With respect to children a marked difference emerged between women with CHD and controls. Among patients the odds of having children were lower than among controls (curative: OR = 0.3; reparative: OR = 0.3; palliative: OR = 0.2). The rate of patients with children with CHD (women: 5.6%; men: 4.9%) was higher than expected (1%) if compared with the general population. Conclusions: Using partnership and children as outcome criteria, patients with CHD are disadvantaged if compared to subjects from the general population. In female patients the social consequences of the disease turned out as more pervasive than in women.Graphic Abstract
Keywords
An increasing number of women and men born with congenital heart disease (CHD) are now reaching old age [1,2], and this also applies to those with complex congenital malformations. Against the backdrop of advanced medical care and improved chances of survival patients can start a family, pursue occupational careers, and master everyday life. Most studies dealing with social and psychological consequences of CHD have examined quality of life [3–6], while social life and parenthood have rarely been considered [7]. In a longitudinal study from the Netherlands [8] it was reported that the majority of patients with CHD (72%) who underwent surgery between 1968 and 1980 were married or in a stable relationship. Comparison data were drawn from the National Statistics Bureau, but the figures on marriage and partnership did not differ between patients and comparison data. In contrast, a Japanese study reported that the percentage of patients with CHD living with a partner was lower than in the general Japanese population [9].
For individuals with CHD the decision to have children is associated with a number of difficult considerations requiring early counseling [10]. Pregnancy is associated with increasing risks of hemodynamic burden, complications and potential deterioration of cardiac functioning, and these risks grow with increasing disease complexity [11]. A recent study with 1,938 pregnant women with CHD reported that complications had occurred in 16% of all cases, primarily due to arrhythmias and heart failures occurring between the 13th and the 40th week of pregnancy [12]. Even in women with the lowest risk of complications 5% of all pregnancies were affected. An important issue for women and men with CHD is the risk of giving birth to a child with a congenital heart disease [11] as heredity is a possible influencing factor. In a US-based study 233 women with CHD and 482 pregnancies of unaffected women were included, and the proportion of children born with a congenital heart defect was 14.2%. This may underestimate the true rate as a substantial number of pregnancies were interrupted [13]. It is a matter of debate whether the findings of this study are still valid today as it was conducted in 1968. As a methodological issue it is also unclear how many women had been contacted and to what extent selection effects may have affected the findings. In a British study 272 women and men with CHD and 393 children a risk of 4.1% was reported, and in siblings of children with CHD the risk was 2.1% [14]. It was also reported that the risk of giving birth to a child with CHD was higher in affected women than in men. These findings may no longer hold as the data collection started in 1985. In a recent study this information was not reported [15].
Within the framework of a longitudinal observational case-control study we wanted to investigate the topics of partnership/marriage and parenthood in patients with CHD. Data on the long-term course of congenital heart disease patients were available that were compared with a group of matched controls from the general population and observed over the same time period. Specifically, the following research questions were dealt with:
• Do patients with CHD and controls differ with respect to partnership/single status and are sex differences present?
• Do patients with CHD and controls differ with respect to having children and does this differ between women and men?
• How high is the rate of children with a congenital heart defect in women and men with congenital heart disease?
2.1 Study Design and Population
The study was performed using a case-control design. The same patients and controls were assessed twice. The time intervals between the two measurements were about the same what made it unnecessary to include time as confounder.
All patients in the study underwent CHD-surgery in the department of Pediatric Cardiology of the University Medical Center Göttingen (Germany). Patients were invited to participate, and if they accepted, written informed consent was obtained. In Göttingen patients with CHD continue to be treated in the department of pediatric cardiology also after having reached adolescence. The first study was conducted between May 2003 to June 2004, the second one from May 2017 to April 2019. Individuals with syndromes or impaired cognitive abilities were excluded [16]. Patients were interviewed and examined in hospital by means of standardized personal interviews for assessing the social situation. The cardiologic component consisted of taking medical history, medical examinations, laboratory measures, echocardiography, electrocardiography and spiroergometry for obtaining information on cardiac status. Sociodemographic data were assessed by adopting questionnaires from the German Socio-Economic Panel. These instruments can be downloaded from https://www.diw.de/de/diw_01.c.32045.de/frageb_ouml_gen.html. Data on the structure of respondents and non-respondents were published in a methodological paper derived from the first study [16].
Controls were drawn from the German Socio-Economic Panel (GSOEP) [17,18], a national survey project that started in 1984 with annual follow-ups. In order to establish representativity and to keep it with respect to defined population characteristics, loss to follow-up is continuously being compensated by drawing new subjects in refresher samplings. Analogous to the years when patients were examined, controls were drawn from SOEP-respondents who participated in 2004 as well as in 2018 for depicting long-term courses of careers. The selection of controls took place by using parental education, age and sex of respondents as matching criteria and by using the characteristics of patients as point of departure. Applying this procedure should make sure that the social backgrounds of cases and controls were comparable.
Type of surgery: The severity of congenital cardiac malformations was classified according to the type of surgery [19]:
• Curatively operated patients with no or minor residual defects or symptoms. This applies to atrial and ventricular septal defects and patent ductus arteriosus.
• Reparatively operated patients with an approximately normal heart function with residual symptoms. This group consists of defects not assigned to the curative or palliative group.
• Palliatively treated patients with permanent impairments and continuously perceptible symptoms. This group includes patients with Fontan circulation and transposition of the great arteries after Mustard or Senning procedure.
We decided against a diagnostic grouping as the resulting case numbers would be too small, and the CHD types are not occurring with the same probability. This might lead to estimation problems, especially after stratifying the study population by additional covariates.
Sex: Respondents’ sex was classified based on their information provided during the interview. Sex classifications were also part of the patient documentations in the hospital.
Educational level: was collected for patients/survey respondents and for parents. It was classified into “no formal educational qualification achieved”, “8/9 years of school education (Hauptschulabschluss)”, “10 years of school education (Realschulabschluss)”, and “12/13 years of school education (Abitur)”. For parents, educational level was assigned according to fathers’ school education, and if unavailable, mothers’ education was assigned. This procedure was applied because the number of missing data was lower for fathers’ education. The difference between the two cannot be assumed to biasing our results, because inspecting the available mothers’ and fathers’ degrees led to a high concordance of qualifications what in turn supports the practice described. A possible alternative option might be the combined inclusion of mothers’ and fathers’ educational level. Finally, it was decided against this solution, because the proportion of successful matchings of cases and controls would have become smaller. Educational level was introduced as a possible confounder as the number of children may be dependent on socio-economic position [20].
Children: In all cases data were collected on the number of children with a maximum of five, and on the year of birth. Additional information from patients was obtained whether their children were also born with a congenital heart disease, and whether they intended to have more.
Partnership/single person status: In both surveys, respondents were asked to report their present marital status by using one of the following categories: Married and or living with partner/married, but separated from partner/single/divorced/bereaved. As case numbers became low after differentiating by sex and group, regression analyses were performed with single vs. the remaining categories as our main question was whether an individual had a partner or not. Single status was assigned if an individual reported not to have a partner in the first and in the second interview. Partnership was also used as a confounder for the likelihood of having children.
For all variables descriptive analyses were performed by means of cross-tabulations. Multivariable logistic regressions were performed for estimating effects of the type of surgery on living single or having children. In the latter case the number of children was not considered as low case numbers would have led to estimation problems. In all cases age of respondents and education were used as covariates as they may have effects on outcomes. With increasing educational level a decreasing number of children can be expected [20]. All statistical analyses were performed using STATA 16MP [21]. For drawing and matching cases and controls from the SOEP the supplementary ado-module CCMATCH was used [22].
The matching process started with the 244 patients that remained after the two assessments as shown in Fig. 1. Patients with CHD were matched with SOEP-respondents of the second wave using age, sex and parents’ socio-economic position as indicator of social background. The latter indicator was chosen because parental socio-economic position may determine social starting points for success in life. Against the backdrop of this selection process subjects who had participated also in the first SOEP-survey were sampled.
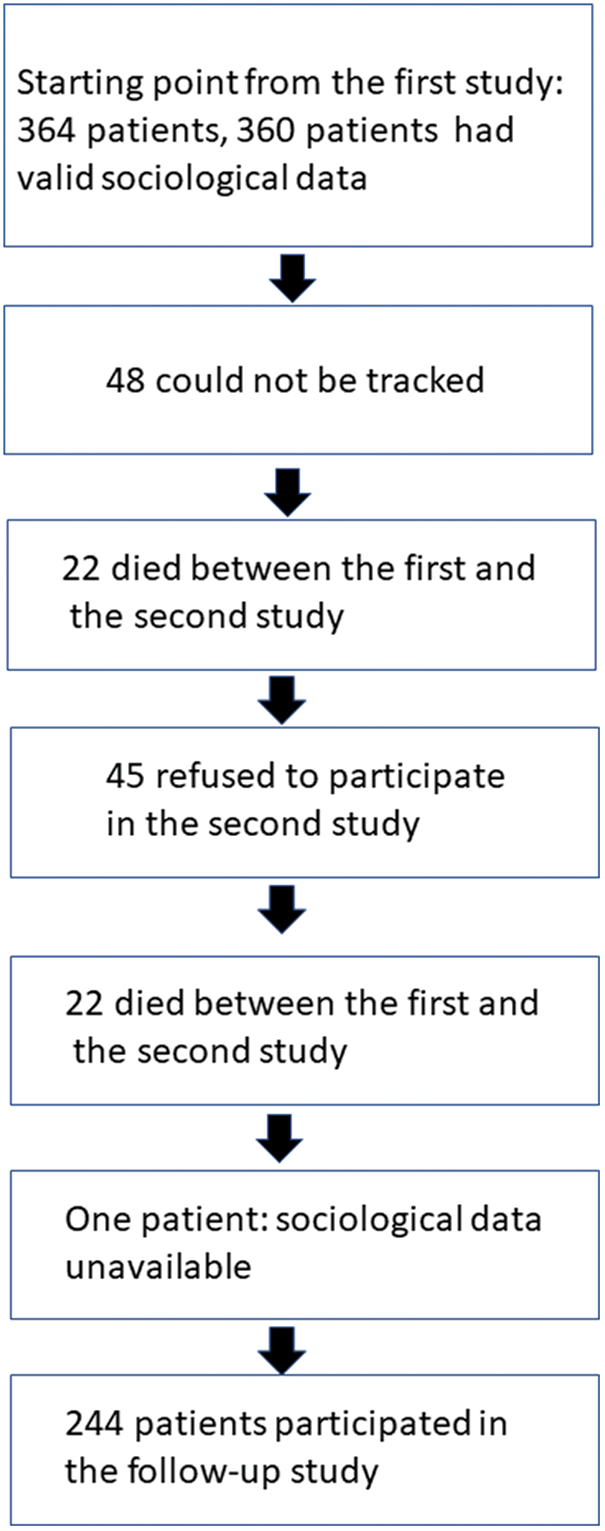
Figure 1: Development of the sample from the first study to follow-up
At the date of the first study a total number of 820 individuals had ever undergone surgery of congenital heart disease at the University Clinic of Göttingen. After having excluded patients with mental retardation and complex disabilities 698 patients remained. Finally, the population of the first study consisted of 364 men (57.9%) and women (42.1%) between 14 and 45 years of age, and complete social data were available for 360 cases. The mean age at first surgery was 7.0 years with a large standard deviation (Sd = 7.2). Testing for systematic loss-to-follow-up from the first and the second study led to the conclusion that age, sex and cardiologic variables did not differ between respondents and non-respondents. More details on this issue can be found in two of our earlier papers [16,23]. Except for the case of parental education, no missing values emerged in the variables used in the regression analyses. Fig. 1 depicts the development of the follow-up sample used in this report from the cases included in the first study. In the second one the mean age was ranging between 31 and 59 (M = 40.4) years.
Finally, n = 244 patients with CHD with complete social data participated in both studies. Drawing parallel controls with several variables turned out as difficult, thus the control group included n = 238 respondents. Table 1 delineates the basic distributions of the variables for analysis.
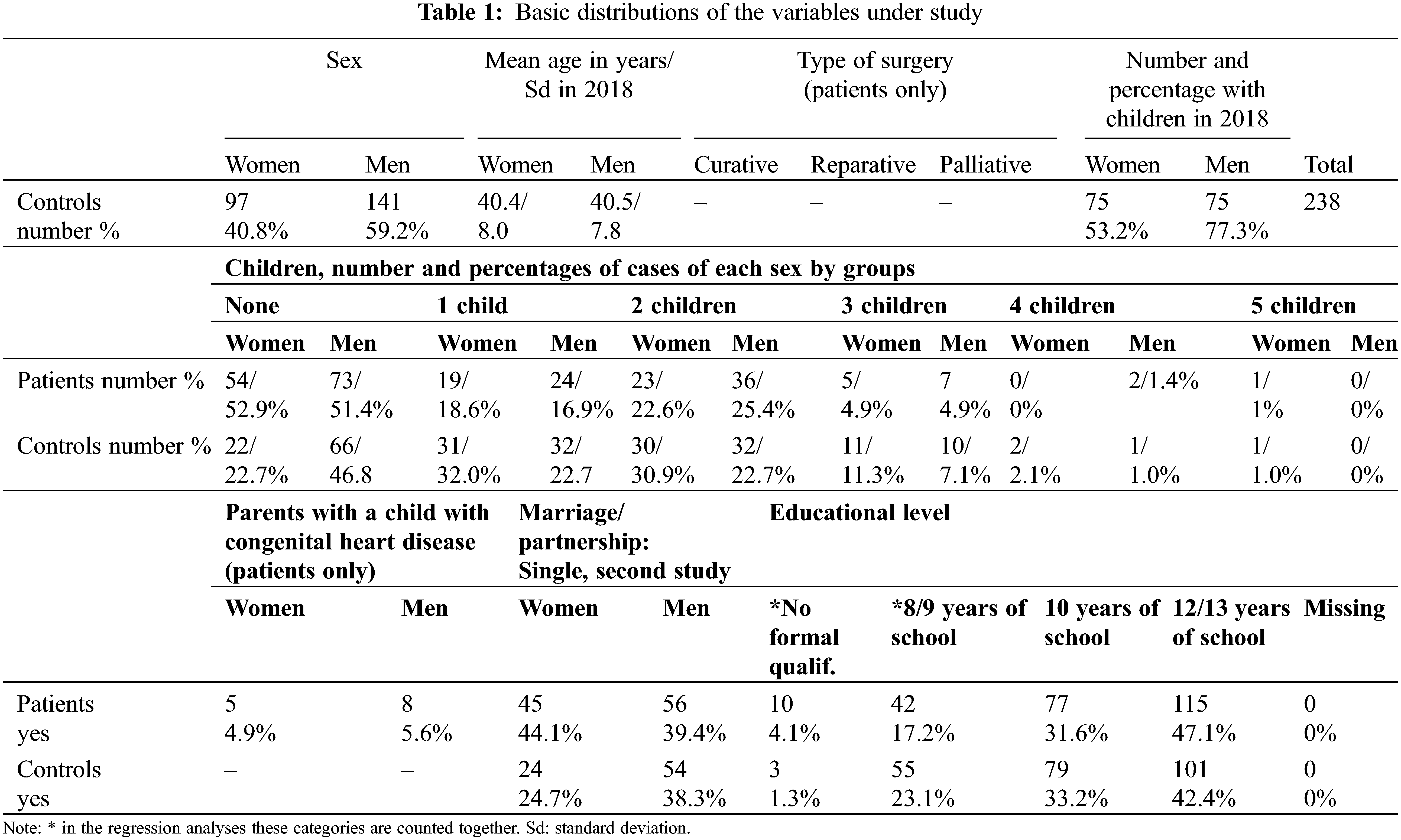
Single person status: In patients the rate of single women (44.1%) was higher than in men (39.4%), in controls also a difference into the same direction emerged. Among controls the proportions of single individuals (men: 38.3%; women: 24.7%) were lower than in patients.
Logistic regression reveals that in men the “chance” of a status as single does not differ significantly between controls from the general population and patients. The respective figures in female respondents were different as CHD-severity was strongly associated with the “chance” of living as a single person. This finding held particularly in curatively treated patients. The odds ratios are indicating that in men the chance of having a partner decreased with age (Table 2).
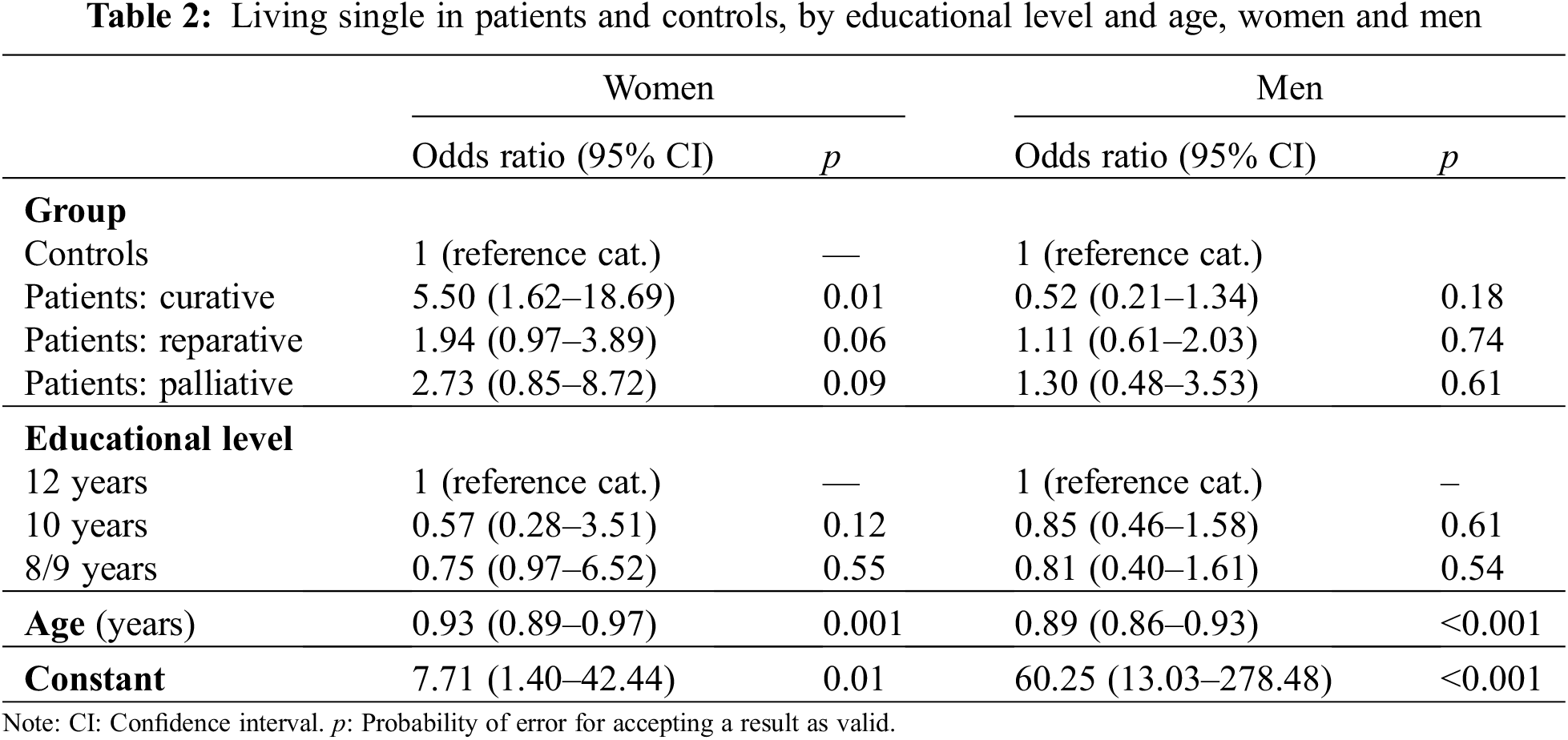
If differences within male and female patients are considered, the OR for women as compared to men was OR = 1.21 (95% CI: 0.71–2.07; p = 0.49), but this difference was not statistically significant in a logistic regression analysis.
Children: In patients the proportion of individuals without children was higher than in controls, but this finding was mainly due to the female patient sample (Table 1). This pattern emerged irrespective of the number of children. The difference between groups was most pronounced if the distribution is dichotomized, as 52.9% of women with CHD did not have children in contrast to 22.7% in women from the general population. For men the difference was lower as 51.4% of male patients did not have children, but 46.8% of those from the general population. In addition, 5.6% of men with CHD had a child with a congenital malformation and 4.9% of women (Table 1). In the patient group 60.6% of men and 50% of women reported that they did not want to have any more children. Having children was used as the target category compared to not having offspring (Table 3).
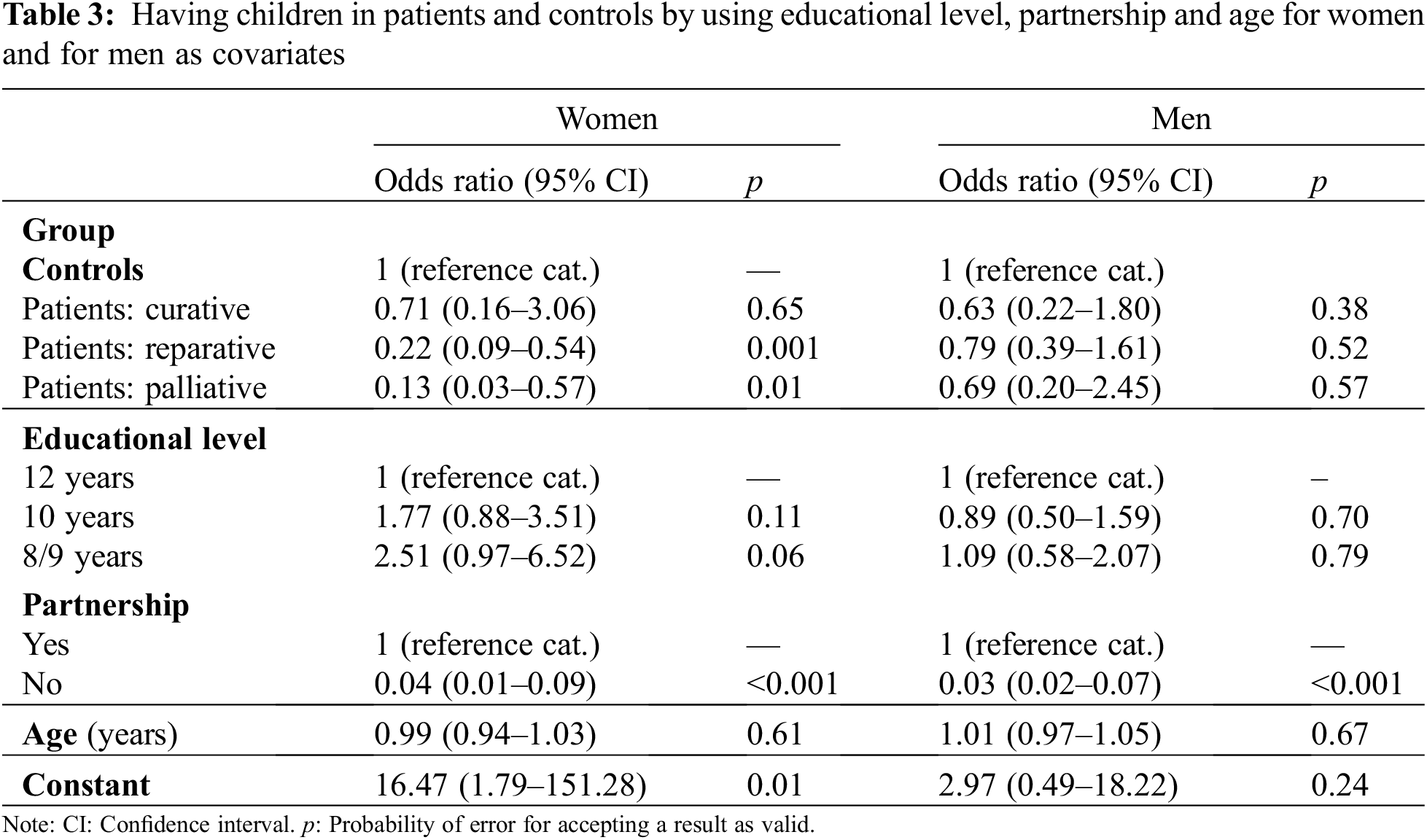
Table 3 shows that in women the odds ratios for having children were decreasing with increasing CHD-severity, but the results were statistically significant only for patients with reparative and palliative surgery. In men the odds ratios for patients were statistically not significant. In no case significant effects of educational level emerged.
As a supplementary analysis effects of sex on the “chance” of having children were estimated by using age and educational level as confounder. The odds ratio for women with CHD was small and also, statistically not significant (OR = 0.95; 95% CI: 0.56–1.59; p = 0.84).
In this paper reports one of the few studies on the social consequences of congenital heart disease, it has used a recent dataset, and an appropriate control group was introduced that used multivariable direct matching.
Our first question asked whether the rates of single individuals among patients differed between patients with CHD and a matched comparison group from the general population. Multivariable analyses revealed that the answer is not straightforward. While among men no statistically significant differences emerged between patients and controls, in patients the rate of single women was higher than among the general population sample. Thus, as far as women are concerned, our findings correspond to the results of a Japanese study [9], but they are not in agreement with a longitudinal study from the Netherlands [8]. The comparability of the studies published so far is however limited as they were published in 2002 and 2003, thus patients were recruited in a time period when mortality rates in patients with CHD were higher than nowadays [2,24]. Both studies used aggregated information for obtaining comparison data from the whole population, and they did not perform separate analyses for women and for men, thus it is not warranted that comparisons between patients and whole population may permit valid conclusions. This is plausible as in our study the proportion of patients with the highest educational level was considerably higher than in the general population, and drawing appropriate controls had to take this into account. The regression analyses on partnership relied on information from the second survey only, but marriages and partnerships may change over time, thus we do not know what may have occurred between the two measurements.
The second question asked for differences in terms of having children. A higher proportion of patients than controls decided against having offspring, and the largest differences again occurred in women. There was a marked division between patients and controls, although in reparatively treated women health risks associated with pregnancy may be relatively low. Among patients the differences for having children were rather small, but in accordance with expectation the odds ratios were lowest in women with palliative surgery. In men the differences between controls and patients of different degrees of CHD-complexity were low and statistically insignificant.
The reasons of women for not having children may be due to associated risks for their cardiovascular health and less to the risk of having an affected child. In our CHD-population the rates of children born with a congenital heart disease (4.9% in women and 5.6% in men) were higher than the expected figure of about 1% of all life births in Germany [25], and this applies both to women and to men with CHD. This points toward heredity of congenital malformations, although the relationship is far from deterministic. The figures found in our study have however to be interpreted with caution as they are marking a lower boundary. A large proportion of patients did not respond to the question whether they had children with CHD. Patients were reported to having decided against parenthood, because mothers are running the risk of heart failure, arrhythmias, bleeding, or thrombosis. The fetus may also be in danger of having low birthweight or stillbirth may occur. The likelihood of complications is dependent on the type of the maternal heart defect [26]. In an unknown number of cases abortions may have taken place, and the rate of abortions of children with CHD was reported to have increased over time [27].
In this paper we reported about partnership and offspring of patients with CHD, but these are only two spheres where life chances of patients may become manifest. Another one is occupation that was examined in a preceding paper. It was reported that once having entered the labor market, patients had the same opportunities for occupational upward mobility than controls from the general population [28]. A higher proportion of patients than controls from the general population had part-time employment what puts this finding again into perspective [29]. These topics will have to be resumed with further analyses of our follow-up data.
Finally, it needs to be discussed whether the reported findings may have limitations. A possible candidate is selectivity as it was not possible to get hold of all patients who participated in the first study, and some had died. Possible candidates are dropouts due to impaired health, e.g., the risk of premature cardiac insufficiency that increases with CHD-complexity [30,31], a broad range of possible comorbidities [32] or death. In our sample a twofold pattern emerged as out of those with the best prognosis, i.e., with curative treatment, 60.3% participated in the follow-up. 56.2% with palliative surgery participated, but 73.5% with reparative surgery, thus there was a tendency towards a higher participation rate in the category indicating moderate disease severity. Thus, the total bias due to non-participation may be limited, but dropouts from the two opposite ends of disease severity may lead to a higher weighting of patients with reparative surgery. Another limitation is due to not having asked patients with CHD why they refrained from having children and to what extent this may be explained by information or lack of information from their physicians. Apprehensions against giving birth to a child with CHD may only partly explain our findings. In two studies it was reported that among patients the knowledge about pregnancy-associated risks was low [33,34]. In the Swedish study only 11% of the respondents with CHD had sufficient knowledge about the likelihood of giving birth to a child with CHD [33]. The decision in favor of a child may rather be higher if more information is available, but in a recent study has shown that information is only one aspect guiding the decision process [35]. Another source of error may be information bias. Our findings may hardly be affected because information on education, children and family status can be remembered well should not be prone to interpretation.
Our interpretations should be taken up with caution as the stratified analyses are leading to small case numbers and thus to lower statistical power. Stratifications hade however been necessary for doing justice to sex differences and different levels of impairments of patients.
Summing up, our project has found evidence for patients with CHD having lower chances of being with a partner, and this holds particularly for women. With respect to children, also a larger proportion of women than men decided against offspring.
Acknowledgement: We acknowledge the work Iris Bolle (Dept. of Pediatric Cardiology, Intensive Care and Pneumology, University Medical Center Göttingen) has done in organizing the study patients, and we acknowledge Monika Zoege for having collected the social data of the first survey.
Funding Statement: This report is based on two research projects. The first one was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft-DFG) under Grant Numbers WE 2670/1-1 and GE1167/2-1 to SG (URL: https://www.dfg.de) The follow-up was funded by Stiftung Kinderherzen, Grant Number WGÖ-014/2016 (URL: https://www.kinderherzen.de) to TP, KN and SG. The funders had not been involved in the study design, in the collection, analysis and interpretation of data, and in writing the manuscript.
Author Contributions: Study conception and design: SG, KN, TP; Data collection: CD, MM; Analyses and interpretation of results: SG, CD, KN; Draft manuscript preparation: SG; Critical revision of the paper for important intellectual content: All authors; Approval of the final version of the manuscript: All authors.
Availability of Data and Materials: The datasets generated and analysed during the current study are not publicly available because the University Medical Center Göttingen does not allow data to be shared with out-of-hospital facilities due to ethical consideration. However, the datasets with the variables used for preparing this paper are available upon request from the first author.
Ethics Approval: Informed consent was obtained from all patients. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines, in the present case: STROBE and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by institutional committees. The first part of the study was reviewed and approved by the Ethics Committee of Hannover Medical School under No. 3710 (date: 04-10-2004) and by the University Clinic of Göttingen under No. 10/2/01 (date: 01-03-2001), the second part was reviewed and approved by the Ethics Committee of the University of Göttingen under number.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Brida, M., Gatzoulis, M. A. (2019). Adult congenital heart disease: Past, present and future. Acta Paediatrica, 108(10), 1757–1764. https://doi.org/10.1111/apa.14921 [Google Scholar] [PubMed] [CrossRef]
2. Spector, L. G., Menk, J. S., Knight, J. H., McCracken, C., Thomas, A. S. et al. (2018). Trends in long-term mortality after congenital heart surgery. Journal of the American College of Cardiology, 71(21), 2434–2446. https://doi.org/10.1016/j.jacc.2018.03.491 [Google Scholar] [PubMed] [CrossRef]
3. Apers, S., Kovacs, A. H., Luyckx, K., Thomet, C., Budts, W. et al. (2016). Quality of life of adults with congenital heart disease in 15 countries: Evaluating country-specific characteristics. Journal of the American College of Cardiology, 67(19), 2237–2245. https://doi.org/10.1016/j.jacc.2016.03.477 [Google Scholar] [PubMed] [CrossRef]
4. Bertoletti, J., Marx, G. C., Hattge, S. P. J., Pellanda, L. C. (2014). Health-related quality of life in adolescents with congenital heart disease. Cardiology in the Young, 25(3), 526–532. https://doi.org/10.1017/S1047951114000304 [Google Scholar] [PubMed] [CrossRef]
5. Apers, S., Luyckx, K., Moons, P. (2013). Quality of life in adult congenital heart disease: What do we already know and what do we still need to know? Current Cardiologic Reports, 15(10), 1–6. https://doi.org/10.1007/s11886-013-0407-x [Google Scholar] [PubMed] [CrossRef]
6. Kamphuis, R. P., Ottenkamp, J., Vliegen, H. W., Vogels, T., Zwinderman, K. H. et al. (2002). Health related quality of life and health status inadult survivors with previously operated complex congenital heart disease. Heart, 87(4), 356–362. https://doi.org/10.1136/heart.87.4.356 [Google Scholar] [PubMed] [CrossRef]
7. Zomer, A. C., Vaartjes, I., Uiterwaal, C. S. P., van der Velde, E. T., Sieswerda, G. J. T. et al. (2012). Social burden and lifestyle in adults with congenital heart disease. The American Journal of Cardiology, 109(11), 1657–1663. https://doi.org/10.1016/j.amjcard.2012.01.397 [Google Scholar] [PubMed] [CrossRef]
8. van Rijen, E. H. M., Utens, E. M., Roos-Hesselink, J. W., Meijboom, F. J., van Domburg, R. T. et al. (2003). Psychosocial functioning of the adult with congenital heart disease: A 20–33 years follow-up. European Heart Journal, 24(7), 673–683. https://doi.org/10.1016/S0195-668X(02)00749-2 [Google Scholar] [PubMed] [CrossRef]
9. Niwa, K., Tateno, S., Tatebe, S., Fujita, K., Sugita, K. et al. (2002). Social concern and independence in adults with congenital heart disease. Journal of Cardiology, 39(5), 259–266. [Google Scholar]
10. Lindley, K. J., Conner, S. N., Cahill, A. G. (2015). Adult congenital heart disease in pregnancy. Obstetrical & Gynecological Survey, 70(6), 397–407. https://doi.org/10.1097/OGX.0000000000000190 [Google Scholar] [PubMed] [CrossRef]
11. Bhatt, A. B., DeFaria Yeh, D. (2015). Pregnancy and adult congenital heart disease. Cardiology Clinics, 33(4), 611–623. https://doi.org/10.1016/j.ccl.2015.07.008 [Google Scholar] [PubMed] [CrossRef]
12. Silversides, C. K., Grewal, J., Mason, J., Sermer, M., Kiess, M. et al. (2018). Pregnancy outcomes in women with heart disease: The CARPREG II study. Journal of the American College of Cardiology, 71(21), 2419–2430. https://doi.org/10.1016/j.jacc.2018.02.076 [Google Scholar] [PubMed] [CrossRef]
13. Whittemore, R., Hobbins, J. C., Engle, M. A. (1982). Pregnancy and its outcome in women with and without surgical treatment of congenital heart disease. The American Journal of Cardiology, 50(3), 641–651. https://doi.org/10.1016/0002-9149(82)90334-4 [Google Scholar] [PubMed] [CrossRef]
14. Burn, J., Brennan, P., Little, J., Holloway, S., Coffey, R. et al. (1998). Recurrence risks in offspring of adults with major heart defects: Results from first cohort of British collaborative study. Lancet, 351(9099), 311–316. https://doi.org/10.1016/S0140-6736(97)06486-6 [Google Scholar] [PubMed] [CrossRef]
15. Arjmandnia, M., Besharati, M., Rezvan, S. (2018). Studying the determinant factors leading to congenital heart disease in newborns. Journal of Education and Health Promotion, 7(1), 53. https://doi.org/10.4103/jehp.jehp_146_17 [Google Scholar] [PubMed] [CrossRef]
16. Geyer, S., Zoege, M., Norozi, K., Kempa, A., Buchhorn, R. et al. (2008). Study participation and nonresponse in a population of adolescents and adults with operated congenital heart disease (GUCH patients). Congenital Heart Disease, 3(1), 26–32. https://doi.org/10.1111/j.1747-0803.2007.00159.x [Google Scholar] [PubMed] [CrossRef]
17. Goebel, J., Grabka, M. M., Liebig, S., Kroh, M., Richter, D. et al. (2019). The german socio-economic panel (SOEP). Jahrbücher für Nationalökonomie und Statistik, 239(2), 345–360. https://doi.org/10.1515/jbnst-2018-0022 [Google Scholar] [CrossRef]
18. Haisken-DeNew, J. P., Frick, R. (2005). DTC-desktop companion to the german socio-economic panel study (SOEPversion 8, pp. 14195. Berlin, Germany: Deutsches Institut für Wirtschaft Berlin, Königin-Luise-Str. 5. [Google Scholar]
19. Perloff, J. K., Child, J. S. (1998). Congenital heart disease in adults. Philadelphia: Saunders. [Google Scholar]
20. Destatis, S. B. (2019). Kinderlosigkeit, Geburten und Familien. Ergebnisse des Mikrozensus 2018 [Childlessness, births and families. results of the microcensus 2018]. Wiesbaden: Statistisches Bundesamt. [Google Scholar]
21. Stata, S. C. (2019). STATA statistical software: Release 16.1. College Station, TX: S. Corporation. [Google Scholar]
22. Cook, D. (2015). CCMATCH: Stata module to match cases and controls using specified variables. https://EconPapers.repec.org/RePEc:boc:bocode:s457372 [Google Scholar]
23. Geyer, S., Fleig, K., Norozi, K., Röbbel, L., Paul, T. et al. (2021). Life chances after surgery of congenital heart disease: A case-control-study of inter- and intragenerational social mobility over 15 years. PLoS One, 16(2), e0246169. https://doi.org/10.1371/journal.pone.0246169 [Google Scholar] [PubMed] [CrossRef]
24. Best, K. E., Rankin, J. (2016). Long-term survival of individuals born with congenital heart disease: A systematic review and mETA-ANalysis. Journal of the American Heart Association, 5(6), e002846. https://doi.org/10.1161/JAHA.115.002846 [Google Scholar] [PubMed] [CrossRef]
25. Lindinger, A., Schwedler, G., Hense, H. W. (2010). Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN study (July 2006 to June 2007). Klinische Pädiatrie, 70(6), 397–407. https://doi.org/10.1097/OGX.0000000000000190 [Google Scholar] [CrossRef]
26. Niwa, K. (2018). Adult congenital heart disease with pregnancy. Korean Circation Journal, 48(4), 251–276. https://doi.org/10.4070/kcj.2018.0070 [Google Scholar] [PubMed] [CrossRef]
27. Lytzen, R., Vejlstrup, N., Bjerre, J., Petersen, O. B., Leenskjold, S. et al. (2018). Live-born major congenital heart disease in Denmark: Incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiology, 3(9), 829–837. https://doi.org/10.1001/jamacardio.2018.2009 [Google Scholar] [PubMed] [CrossRef]
28. Geyer, S., Norozi, K., Zoege, M., Buchhorn, R., Wessel, A. (2007). Life chances after surgery of congenital heart disease: The influence of cardiac surgery on intergenerational social mobility. A comparison between patients and general population data. Journal of Cardiovascular Prevention and Rehabilitation, 14(1), 128–134. https://doi.org/10.1097/01.hjr.0000238398.27471.39 [Google Scholar] [PubMed] [CrossRef]
29. Geyer, S., Norozi, K., Buchhorn, R., Wessel, A. (2009). Chances of employment in women and men after surgery of congenital heart disease: Comparisons between patients and the general population. Congenital Heart Disease, 4(1), 25–33. https://doi.org/10.1111/j.1747-0803.2008.00239.x [Google Scholar] [PubMed] [CrossRef]
30. Norozi, K., Wessel, A., Alpers, V., Arnhold, J. O., Geyer, S. et al. (2006). Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. American Journal of Cardiology, 97(8), 1238–1243. https://doi.org/10.1016/j.amjcard.2005.10.065 [Google Scholar] [PubMed] [CrossRef]
31. Faccini, A., Micheletti, A., Negura, D. G., Giugno, L., Butera, G. et al. (2018). Heart failure in grown-up congenital heart disease. Minerva Cardioangiologica, 66(3), 329–336. [Google Scholar] [PubMed]
32. Raissadati, A., Haukka, J., Pätilä, T., Nieminen, H., Jokinen, E. (2020). Chronic disease burden after congenital heart surgery: A 47-year population-based study with 99% follow-up. Journal of the American Heart Association, 9(9), e015354. https://doi.org/10.1161/JAHA.119.015354 [Google Scholar] [PubMed] [CrossRef]
33. Burström, Å., Acuña Mora, M., Sparud-Lundin, C., Moons, P., Bratt, E. L. (2022). Adolescents with congenital heart disease: What do they know about reproductive health and risks? Journal of Cardiovascular Nursing, 37(6), E172–E180. https://doi.org/10.1097/JCN.0000000000000838 [Google Scholar] [PubMed] [CrossRef]
34. Janssens, A., Goossens, E., Luyckx, K., Budts, W., Gewillig, M. et al. (2016). Exploring the relationship between disease-related knowledge and health risk behaviours in young people with congenital heart disease. European Journal of Cardiovascular Nursing, 15(4), 231–240. https://doi.org/10.1177/1474515114565214 [Google Scholar] [PubMed] [CrossRef]
35. Delaney, R. K., Pinto, N. M., Ozanne, E. M., Brown, H., Stark, L. A. et al. (2022). Parents’ decision-making for their foetus or neonate with a severe congenital heart defect. Cardiology in the Young, 32(6), 896–903. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools