 Open Access
Open Access
ARTICLE
Role of Surgery on Growth of Tricuspid Valve in Pulmonary Atresia with Intact Ventricular Septum: Mid-Term Results of Modified Right-Ventricular Overhauling Procedure
1 Department of Thoracic and Cardiovascular Surgery, Seoul National University Children’s Hospital, College of Medicine, Seoul National University, Seoul, Korea
2 Department of Thoracic and Cardiovasclar Surgery, Sejong General Hospital, Bucheon, Gyeonggi, Korea
* Corresponding Author: Woong-Han Kim. Email:
Congenital Heart Disease 2023, 18(3), 325-336. https://doi.org/10.32604/chd.2023.027758
Received 14 November 2022; Accepted 04 May 2023; Issue published 09 June 2023
Abstract
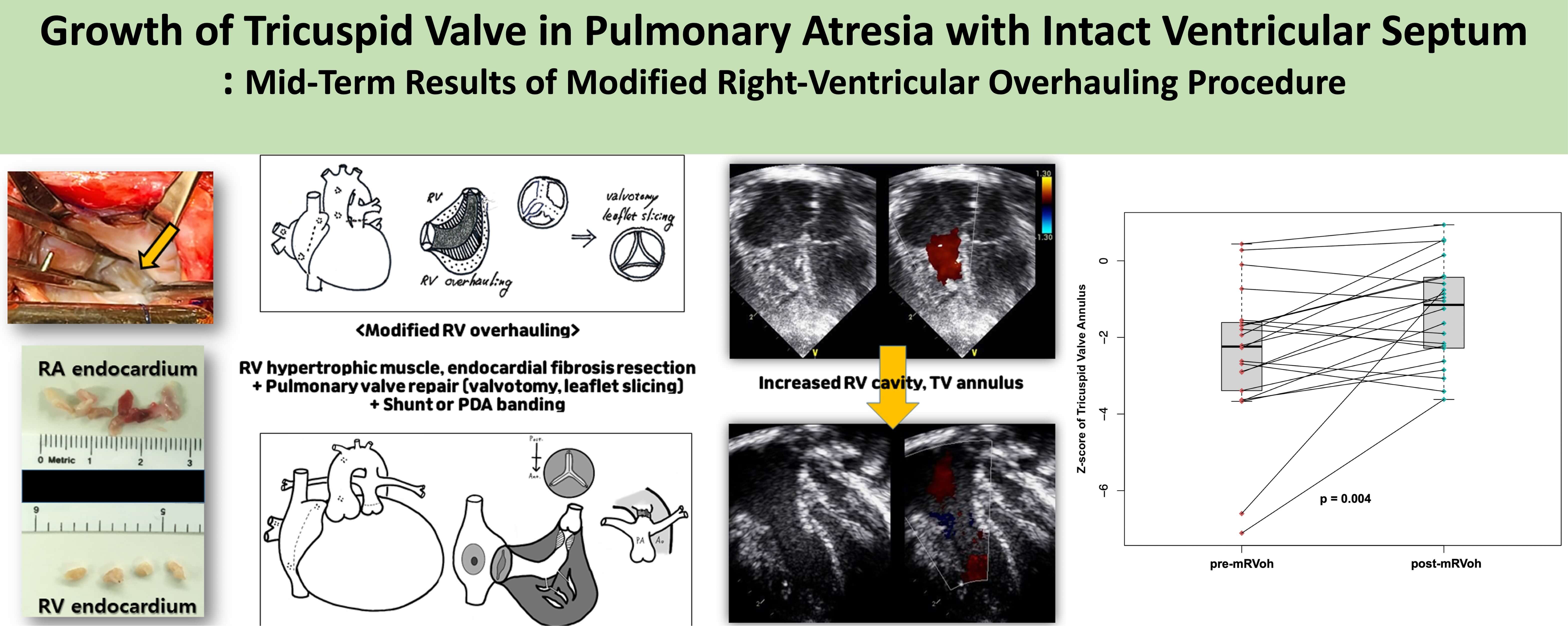
Objectives: To access the effectiveness of our modified right-ventricular overhauling procedure on tricuspid valve (TV) growth in patients with pulmonary atresia with intact ventricular septum (PAIVS). Methods: We retrospectively reviewed 21 patients with PAIVS who underwent modified right ventricular overhauling (mRVoh) between 2008 and 2019 at two institutions. Our mRVoh consisted of wide resection of hypertrophied infundibular and trabecular muscle, peeling off fibrotic endocardial tissue in the right ventricle (RV) cavity, surgical pulmonary valvotomy, and Blalock-Taussig shunt or banding of ductus arteriosus under cardiopulmonary bypass. The TV annulus sizes were measured and analyzed using echocardiography before and after mRVoh. Results: No mortalities were observed during a median follow-up of 3 years (interquartile range: 1.3–4.7 years) of follow-up were noted. mRVoh was performed at a median age of 163.5 days (range: 21–560 days), including seven neonates and two infants (<60 days). During follow-up, the median TV annular z-score increased significantly from −2.24 to −1.15 before and after mRVoh (p = 0.004). In ten patients with a prior history of percutaneous interventions for RV outflow tract (RVOT) widening at least 6 months before mRVoh, the TV annular z-score significantly changed during the period after mRVoh (−2.03 to −1.61, p = 0.028) compared with the period before mRVoh (−2.51 → –2.03, p = 0.575) after percutaneous intervention only. Conclusions: mRVoh in PAIVS patients was positively associated with TV annular growth, and it was more effective than percutaneous RVOT widening interventions without mRVoh.Graphic Abstract
Keywords
Nomenclature
| BSA | Body surface area |
| BT shunt | Blalock-Taussig shunt |
| cMRI | Cardiac magnetic resonance image study |
| IQR | Interquartile range |
| IRB | Institutional review board |
| LV | Left ventricle |
| PAIVS | Pulmonary atresia with intact ventricular septum |
| PDA | Patent ductus arteriosus |
| RV | Right ventricle |
| RVEDVi | Indexed right ventricular end-diastolic volume |
| RVESVi | Indexed right ventricular end-systolic volume |
| mRVoh | Modified right ventricular overhauling procedure |
| RVOT | Right ventricle outflow tract |
| TR | Tricuspid regurgitation |
| TV | Tricuspid valve |
Compared to the Western population, which more frequently presents with left-sided congenital cardiac diseases, right-sided congenital heart diseases are more common in the Eastern population [1]. Pulmonary atresia with intact ventricular septum (PAIVS) has a broad spectrum of treatment strategies ranging from single ventricle palliation to bi-ventricle repair [2]. The relief of right ventricular outflow tract (RVOT) stenosis through percutaneous balloon or surgical pulmonary valvotomy is widely accepted to enable right ventricular (RV) growth in this disease [3–7]. Previous studies have shown that increased antegrade blood flow through the RV leads to RV growth and eventual bi-ventricular circulation in some patients.
Based on our clinical experience, we have occasionally observed fibrotic changes in the RV endocardial layer (Fig. 1A) and hypertrophied muscle bundles in the inlet or trabecular portion, as well as the infundibular area (Fig. 1B), in these patients. We theorized that the endocardial fibrotic tissue and hypertrophied muscle bundles might impede the efficient growth of the RV and tricuspid valve (TV). While we have not yet identified the pathological characteristics of this RV endocardial fibrotic tissue, it reminds us of endocardial fibroelastosis in certain patients with a borderline small left ventricle, a condition known to restrict left ventricular growth [8,9].
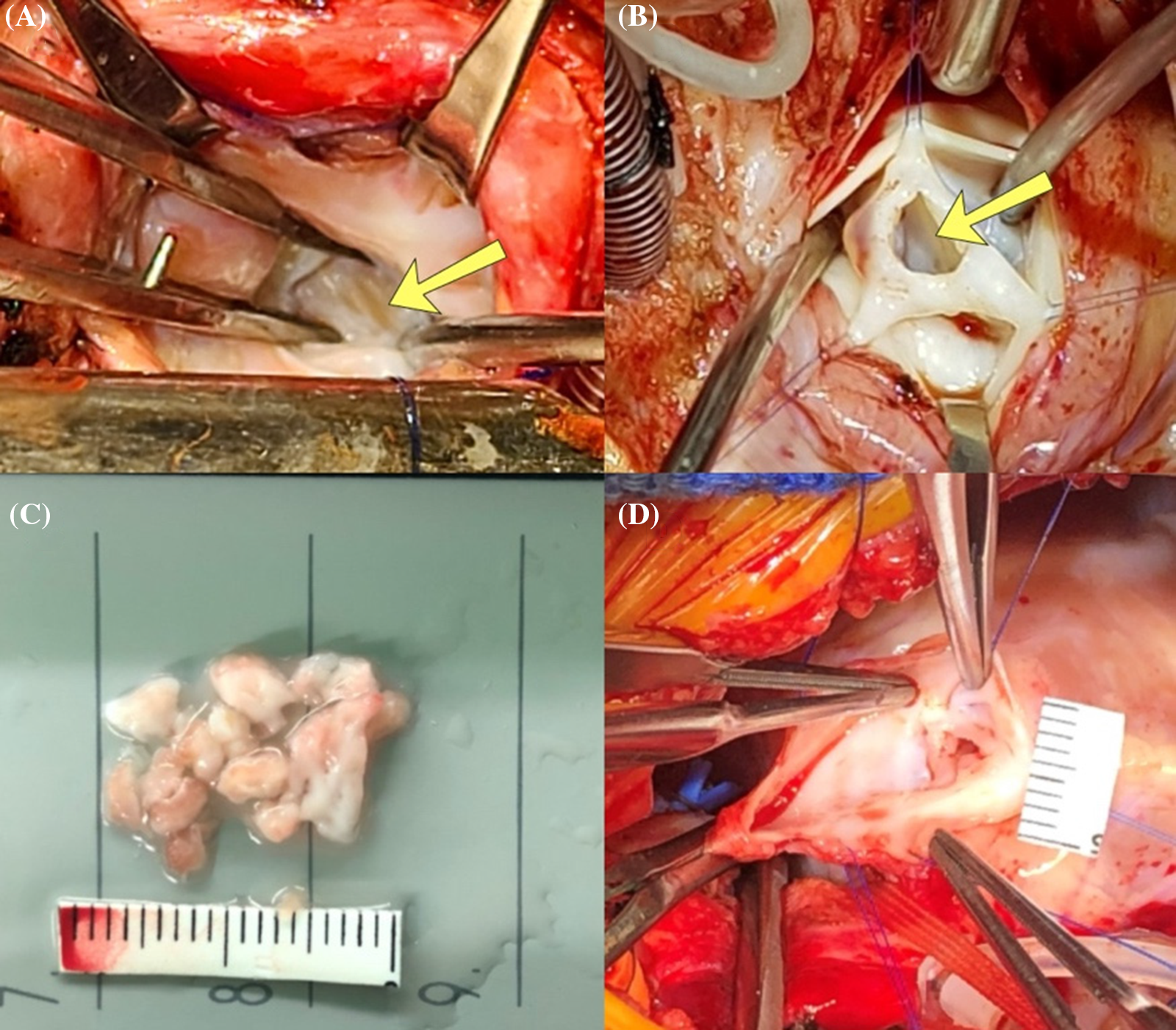
Figure 1: Operative findings of patients with pulmonary atresia with intact ventricular septum. (A) Fibrotic change (yellow arrow) on the right ventricular endocardium, view from tricuspid valve; (B) Fibrotic change in the infundibular area (yellow arrow), view from opened pulmonary valve; (C) Resected out fibrotic endocardium from RV cavity; (D) Atretic pulmonary valve just before surgical valvotomy
As a result, we hypothesized that: (1) hypertrophied trabecular muscle and endocardial fibrosis on the RV endocardium would impede the efficient growth of the RV and TV; (2) removing endocardial fibrotic tissue (Fig. 1C) and dense trabecular muscle in the RV cavity, particularly when performed alongside pulmonary valvotomy—with or without Blalock-Taussig (BT) shunt or patent ductus arteriosus (PDA) stenting—would promote more effective RV growth. To evaluate our hypotheses, we reviewed and analyzed the clinical outcomes of patients who underwent our modified RV overhauling (mRVoh) since 2008.
This study was approved by the Institutional Review Board (IRB) of two institutions [IRB approval number: 1910-037-1068 from Seoul National University Hospital (October 10, 2019); 2020-0488 from Sejong General Hospital (September 02, 2020)].
We conducted a retrospective review of patients with PAIVS (without RV-dependent coronary circulation) who underwent the mRVoh procedure between 2008 and 2019 by three surgeons from two institutions. Some of the patients were referred from other institutions, where they had already undergone single ventricular palliation or one-and-a-half ventricle repair. In one case, we performed the procedure twice when the patient was 17 and 112 days old. We have provided a detailed explanation of our mRVoh procedure in the “operative strategy” section later.
Upon detecting a suspected RVDCC pattern during the initial echocardiographic examination, our cardiologists attempted to perform an angiographic study. If the patient’s condition did not allow for this invasive study, we resorted to computed tomographic angiography to evaluate the coronary pattern, although we were aware that this examination was not ideal for clarifying an RVDCC pattern. We divided these patients into two age groups (1) neonates and infants less than two months old and (2) those older than two months to evaluate the effect of mRVoh according to the age at the time of the operation. Among the 17 patients who underwent balloon pulmonary valvuloplasty once or more prior to the mRVoh procedure, we looked into ten patients who underwent the mRVoh procedure at least six months after the pre-mRVoh interventions to analyze the changes in the tricuspid valve (TV) annulus. This additional analysis aimed to inspect the effectiveness and superiority of the mRVoh procedure compared to interventional treatment alone. Currently all patients are being regularly followed in the outpatient clinic.
All patients underwent pre- and postoperative echocardiography and were completely followed up. The sizes of the TV and pulmonary valve (PV) annulus and opening, as well as valve function, were accessed via echocardiography. The echocardiographic data obtained closest to the time of mRVoh were considered as the preoperative (baseline) data, while the most recent data were selected as the last follow-up data to evaluate changes in TV annular size. To obtain z-scores for cardiac structures, we used previously recorded studies by Pettersen et al. [10], which were based on the patient’s height, weight, and body surface area (BSA).
In cases where the patients had undergone prior surgery or interventions, we performed wide resection of the RV muscle and endocardial fibrotic tissue if observed in the RV cavity. If required, we also conducted valvuloplasty for the PV or TV if needed. In neonates, cases of primary percutaneous intervention failure for right ventricular outflow tract (RVOT) widening were an absolute indication for a surgical approach. Recently, we have been primarily resecting the RV infundibular hypertrophied muscle and fibrotic endocardium in conjunction with surgical pulmonary valvotomy (Figs. 1B, 1D, and 2A) and TV repair, even in the neonatal period. This approach is taken, especially when the patient’s atretic pulmonary valvular portion is less likely to open effectively by ballooning due to the atretic portion’s characteristics (less membranous or much muscular). Another indication for this approach is when effective antegrade RVOT flow is not anticipated by balloon valvuloplasty because of severe hypertrophic infundibular muscle.
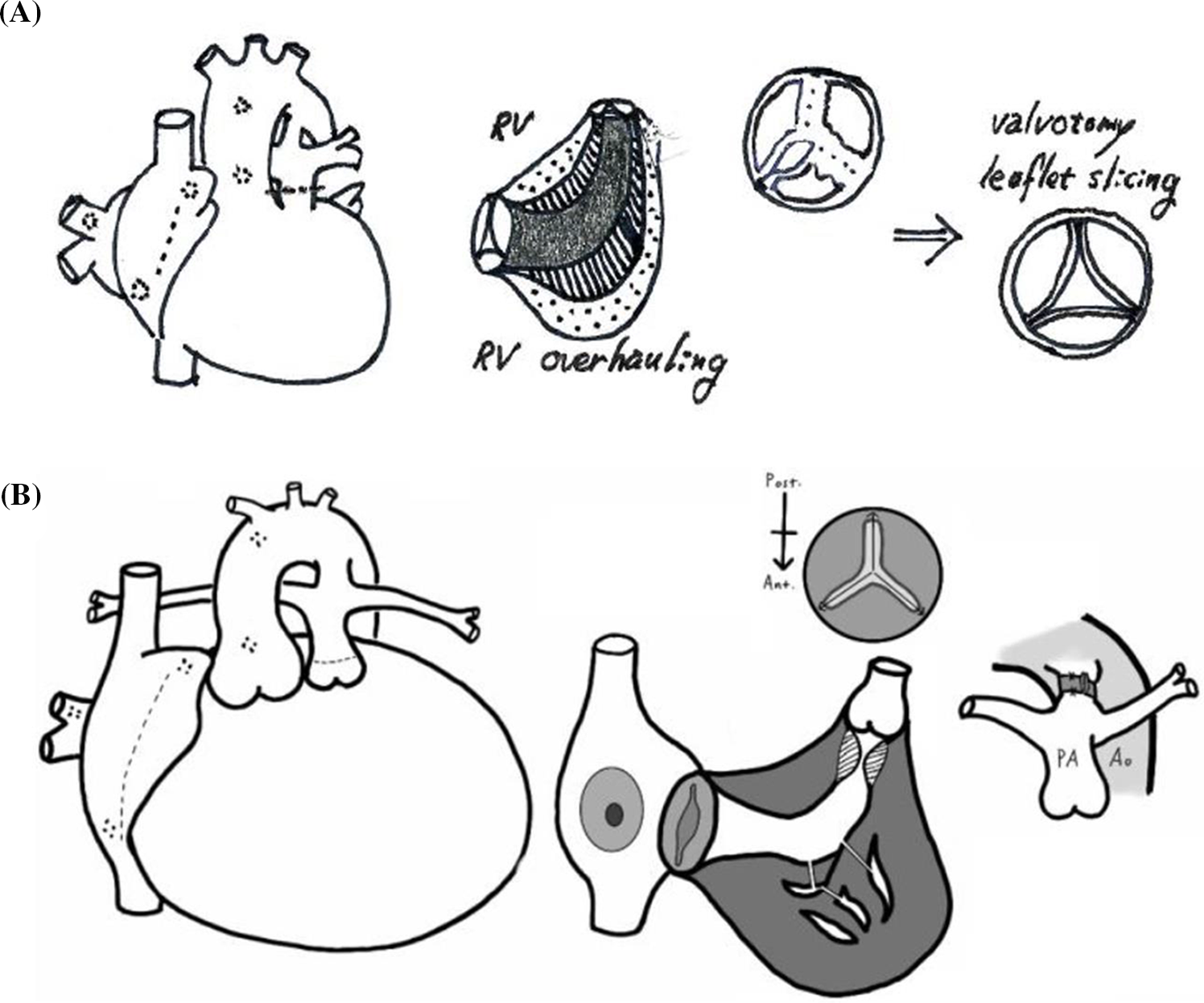
Figure 2: A schematic drawing of the modified right ventricular overhauling which is consisted of (1) resecting hypertrophied right ventricular trabecular muscle and peeling off the endocardial fibrotic tissue (A, B), (2) surgical pulmonary valvotomy (A, B) and modified Blalock-Taussig shunt or patent ductus arteriosus banding (B) under cardiopulmonary bypass support
Regarding the pulmonary blood source in neonates or young infants, we recently performed PDA banding in five patients, including four neonatal and one 32-day-old patient, who did not undergo any previous surgical intervention, including three balloon failure cases, instead of a BT shunt (Fig. 2B). For PDA banding, we used a 5-mm-diameter polytetrafluoroethylene (PTFE) strip to wrap the PDA with a 3–4 mm width for neonates. Then, we adjusted the band’s tightness by clipping the PTFE strip to achieve an internal diameter equal to the patient’s body weight in millimeters, as determined by echocardiography. To prevent the band’s migration, we made a couple of fixation sutures at the adventitia of the pulmonary artery. We continued to use prostaglandin E1 (PGE1) to maintain the PDA’s patency after banding for about 2 weeks while observing whether the enlarged RV could generate adequate forward flow to the pulmonary artery through the PV. If the RV could make adequate antegrade pulmonary blood flow during this period, we stopped PGE1 to allow the PDA to spontaneously close. Otherwise, we planned to place a stent in the PDA to supply pulmonary blood flow [11]. In this study, no patient required additional PDA stenting after PDA banding due to the lack of antegrade flow from the RV cavity following mRVoh.
All operations were performed under cardiac arrest with mild hypothermia (30°C–34°C). Hypertrophied trabecular muscle or fibrotic endocardium in the RV cavity was divided and resected using a dual approach through the TV and the main pulmonary artery. In patient with fibrotic endocardium, we peeled off the fibrotic tissue until the myocardial tissue was exposed. We tried not to make an additional separate external ventricular incision at the RV infundibular area to preserve the infundibular function. We believed that right ventriculotomy might be detrimental to future RV function and that the external RVOT patch following an external incision does not function well during RV contraction. Pulmonary valvotomy was cautiously performed along the vestigial commissure, with attempts to minimize valvular regurgitation and preserve the pulmonary annulus. Once pulmonary valvotomy was performed, hypertrophic muscle or thickened fibrotic tissue in the infundibular area was resected through a widened PV opening. We divided secondary or tertiary chordae for restrictive motion TV’s for better mobility. In cases of leaflet prolapse, we applied artificial chordae. Endothelial fibrotic tissue and hypertrophied muscle in the inlet and trabecular portion of the RV cavity were extensively removed through the TV (Figs. 1A and 1C). We considered concomitant reresection of RV fibrotic tissue or hypertrophic muscle if it regrew after the prior operation, when other surgical procedures for TV or PV problems after initial surgical management were also required. RV myectomy through the increased TV tended to be much easier in grown patients. Even after extensive resection in prior operations, it was not uncommon for the RV endocardium to show fibrotic changes again. Our surgical procedures are more precisely depicted in our recently published paper, which includes a video clip [10].
Continuous variables were presented as means and standard deviations if the data showed a normal distribution; otherwise, medians with interquartile ranges (IQRs) were used. We used Wilcoxon’s signed rank test to compare the changes in paired variables over time.
3.1 Perioperative Patient Characteristics and Follow-Up
In our study, we included 22 cases from 21 patients. Among these cases, we present seven cases of neonates (<30 days old) and two cases of young infants (32 and 60 days old) who underwent mRVoh. Thirteen patients, including four neonates, underwent primary RV myectomy without “prior surgical treatment”. Additionally, one patient from abroad underwent mRVoh as primary operation at the age of 1.3 years.
In our cohort, 18 patients had undergone previous surgical or percutaneous interventions before mRVoh, including balloon pulmonary valvuloplasty alone or concurrent or sequential modified BT shunt or PDA stenting. Of these 18 patients, three had undergone a 1.5-V repair before mRVoh; two in other hospitals and one in our institution. Except for one patient who had undergone a 1.5-V repair in our institution (pulmonary valvectomy and RVOT muscle resection at the time of 1.5-V repair after balloon from another hospital), there were no “surgical” RVOT widening procedures performed before mRVoh in our cohort.
mRVoh was performed at a median age of 163.5 days (IQR: 21.0–560.0 days). The median body weight and BSA at the time of the operation were 7.1 kg (IQR: 3.7–10.5 kg) and 0.34 (IQR: 0.23–0.43), respectively.
The mean cardiopulmonary bypass (CPB) time was 130.8 ± 65.9 min, and the mean aortic cross clamping time was 76.5 ± 41.3 min. Most patients underwent one or more repair techniques concurrently for the TV and PV. Additionally, concomitant procedures included pulmonary artery angioplasty, mitral valve repair, atrial septal defect closure with or without fenestration, or bidirectional cavo-pulmonary connection.
Fibrotic tissue was observed on the endocardium of all patients, including neonates, with varying depth and extents. It tended to be thicker in patients who had been exposed to pulmonary stenosis for longer periods. The fibrotic tissue was most commonly detected in the infundibular region but also in the trabecular region.
Three patients had already undergone a 1.5-V repair in other institutions, and two of them were being considered for a Fontan procedure due to their hypoplastic RV and right-sided valvular structures. However, we believed that the patients’ RV and valvular problems could resolved with mRVoh, and we completed the 2-V repair for one of them. For another patient, we performed mRVoh (valvuloplasty + RV endocardial fibrosis and hypertrophied muscle resection) and are waiting for RV to grow. The third patient, who had been in a 1.5-V state for 10 years, showed pulmonary valvular problems requiring surgical repair. We deemed the size and function of the patient’s RV to be tolerable as a whole pulmonary pump and therefore performed mRVoh, converting the patient’s 1.5-V to 2-V concomitantly with pulmonary valvuloplasty.
Two patients who had planned to undergo single ventricular palliation in other institutions with a BT shunt received 1.5-V repair and 2-V repair concomitantly with mRVoh, respectively.
At the end of the study, 19 patients were in 2-V status (86.4%) and three patients still had a 1.5-V status. Recent cardiac MRIs of these patients showed tolerable RV volume and function for a bi-ventricular repair, and we are considering a bi-ventricular conversion soon. However, despite undergoing mRVoh in the neonatal period, the RV of the third patient did not grow sufficiently for bi-ventricular status, and this patient remained in a 1.5-V status until the end of the study.
There were no mortalities during the median follow-up duration of 3.0 years (IQR: 1.3~4.7 years).
3.3 Changes in the Size and Function of TV in the Imaging Study
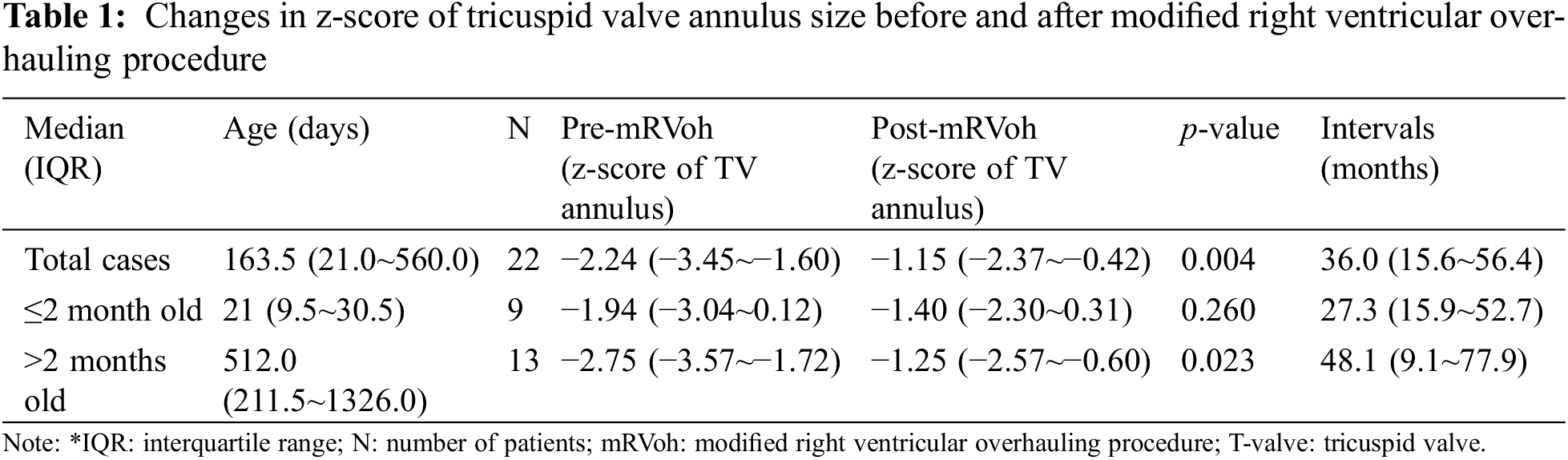
The most recent echocardiographic evaluations were performed at a median of 3.0 years (IQR: 1.34~4.73 years) after mRVoh. During the follow-up period, the median z-score of the TV annulus significantly increased from −2.24 (IQR: −3.4 to −1.60, before mRVoh) to −1.15 (IQR: −2.37 to −0.42, after mRVoh, p-value = 0.004). The change in the z-score of the TV annulus for individual patients before and after mRVoh is shown in Fig. 3.
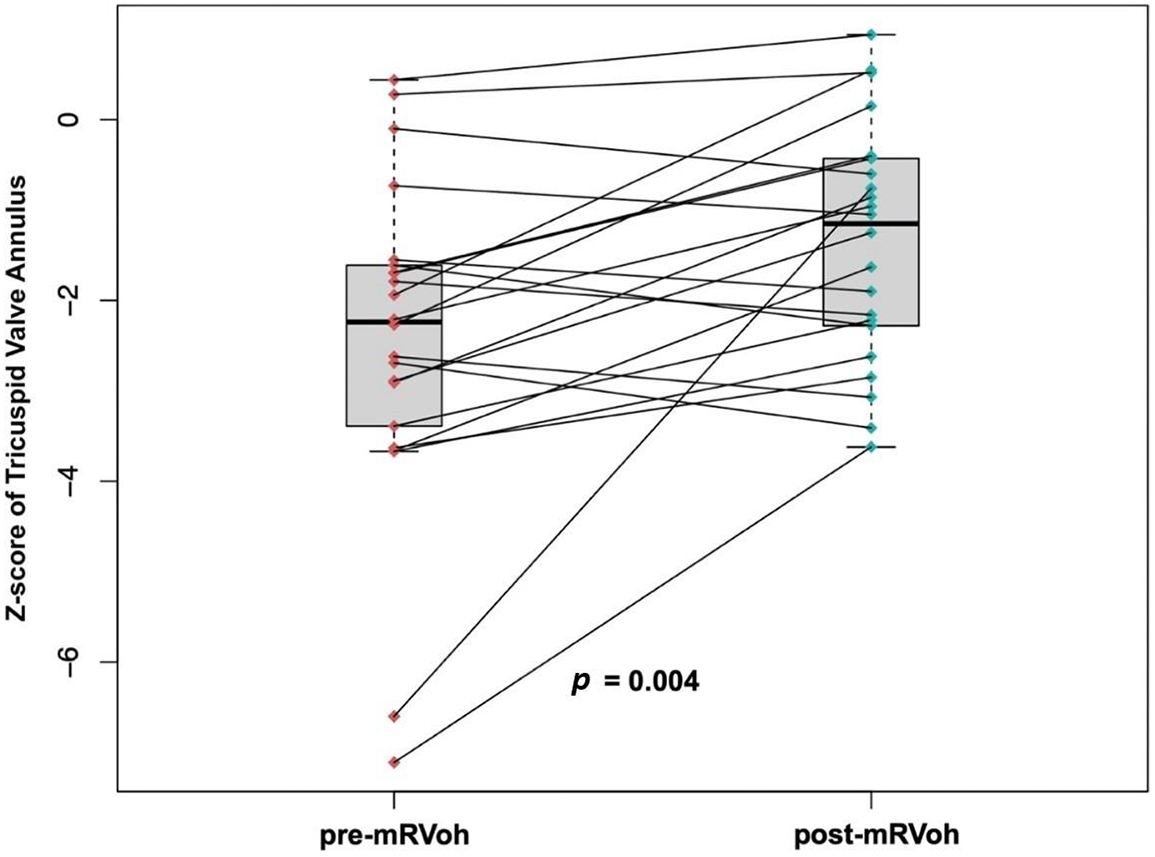
Figure 3: Changes of the z-score of the tricuspid valve annulus of individual patients before and after the modified right ventricular overhauling procedure
We divided the patients into two groups based on their age: (1) less than 2 months or (2) more than 2 months old. The younger group comprised neonates (n = 7) and infants (n = 2), and the median z-score of the TV annulus before mRVoh was −1.94 (IQR: −3.17–0.09) at a median of 21 days old. The z-score changed to −1.40 (IQR: −2.30–0.31) at 27.3 months (IQR: 15.9–52.7 years) after mRVoh, but this change was not statistically significant (p = 0.260, Table 1). In patients older than 2 months (median age: 17.1 months, IQR: 7.1–44.2 months), the z-score of the TV annulus before mRVoh (−2.75, IQR: −3.57 to −1.72) showed significant growth on the most recent echocardiography after mRVoh (−1.25, IQR: −2.57 to −0.60, p = 0.023, Table 1) for 48.1 months (IQR: 9.1–77.9 months).
Regarding TR, three patients showed an increase in TR degree (from trivial to mild in one patient and from mild to moderate in two patients), eight patients showed an improvement in TR degree (from mild to trivial in two patients, from moderate to mild in two patients, and from severe to moderate in four patients), and 11 patients showed no significant change in TR degree (trivial in four patients, mild in six patients, and moderate in one patient).
3.4 Effect of mRVoh: Comparison with Balloon Pulmonary Valvuloplasty Only
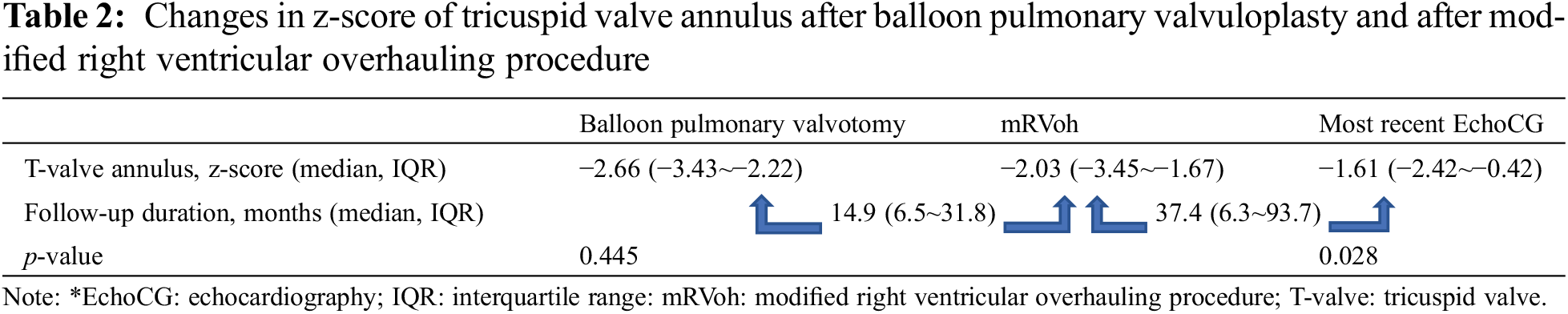
Seventeen patients underwent balloon pulmonary valvuloplasty once or more than once before the mRVoh procedure with or without concomitant or consecutive BT shunt formation or PDA stenting. Among these 17 patients, changes in the TV annulus were observed in ten patients who underwent mRVoh at least 6 months after the pre-mRVoh interventions (balloon pulmonary valvotomy only) to evaluate the effect of mRVoh on the growth of the TV annulus. We compared and analyzed the changes in z-scores of the TV annulus between two intervals: (1) just after interventions to pre-mRVoh (period without mRVoh) and (2) immediately after mRVoh to the most recent follow-up (period with mRVoh). The remaining seven patients were excluded from this comparison because their follow-up duration was insufficient to observe changes in the RV and TV.
The z-score of the TV annulus during the period after pulmonary valvuloplasty without mRVoh changed from −2.66 (IQR: −3.43 to −2.22) to −2.03 (IQR: −3.45 to −1.67) without statistical significance (p = 0.445), over a period of 14.9 months (IQR: 6.5–31.8 months). Meanwhile, the z-score of the TV annulus after mRVoh significantly changed (p = 0.028) from −2.03 (IQR: −3.45 to −1.67) to −1.61 (IQR: −2.42 to −0.42) for 37.4 months (IQR: 6.3–93.7 months) (Table 2).
3.5 PV Function on the Most Recent Echocardiography
When we define the degree of pulmonary stenosis (PS) into three grades based on the peak pressure, mild (peak pressure < 36 mmHg), moderate (36–64 mmHg) and severe (≥64 mmHg) [12], we had 19 patients with mild PS, two patients with moderate PS, and no patients with severe PS on the most recent echocardiography. Regarding pulmonary regurgitation (PR), six patients showed a moderate degree of PR, and three patients showed severe PR. Twelve patients had PR ≤ mild. The z-score of the PV annulus on the most recent echocardiography was −1.73 (IQR: −2.69 to −0.52).
4.1 Endocardial Fibrotic Tissue and Hypertrophied Muscle in the RV in PAIVS
Fibrotic tissue on the endocardium has been reported in various types of cardiac diseases, such as cardiomyopathies, diseases associated with infection or immunological problems, and congenital heart diseases [9,13–16]. Endocardial fibrotic tissue tends to restrict the growth of the left ventricle (LV) in some patients with a borderline size of small LV; therefore, the resection of this fibrotic tissue is believed to help increase the size of the small LV [17,18]. However, there are only a few reports about fibrotic tissue on the endocardium or similar fibrotic changes in the RV cavity [19,20]. To our knowledge, few studies have been found about the correlation between the resection of RV endocardial fibrotic tissue and RV and TV annular growth.
However, we have observed ineffective growth of the RV cavity and TV annulus even after RVOT widening or PV opening in some PAIVS patients. In almost all of these patients, we also observed endocardial fibrotic tissue and hypertrophied trabecular muscle in their RV cavities. This clinical observation led us to consider the effect of surgical resection of trabecular muscle and endocardial fibrotic tissue on the growth of the tricuspid annulus in these patients. Therefore, we hypothesized that leaving the endocardial fibrotic tissue and hypertrophied trabeculation tissue in the RV cavity without removal might not result in adequate RV cavity and TV annulus to support sufficient pulmonary circulation, although we could expect some degree of RV growth after RVOT alone.
4.2 Why Do We Call Our Surgical Approach “Modified” RV Verhauling?
Mee et al. reported their surgical strategy for patients with PAIVS based on the state of infundibular development [6]. Their step-by-step approach consisted of pulmonary valvotomy and BT shunt without CPB first, followed by resection of the hypertrophied RV muscle to achieve ultimate bi-ventricular status in the next stage of operation. They reported a high success rate in terms of achieving bi-ventricular circulation in these patients with almost 60% of their patients achieving this outcome.
Our current strategy differs from Dr. Mee’s original RV overhauling in two primary ways. First, we used CPB during pulmonary valvotomy. We believed this surgical approach would allow for to more precise and effective pulmonary valvotomy under direct vision, ultimately leading to reduced PR and improved PV function. Most patients in our study demonstrated PS⫹ of mild or lesser severity except for two patients with moderate PS. Furthermore, 57% of patients exhibited mild or lesser PR in the most recent echocardiography. We recognize that a longer follow-up is necessary to access the ultimate PV function, and future interventions for valve issue may be unavoidable for some patients in the future. However, fortunately only one patient required PV replacement during the study period. Some authors have reported PV incompetence after RV overhauling procedures or palliative RVOT widening procedures in most patients [6], or they have not even specifically commented on PV function [21]. Given that the function and morphology of the TV in these patients are typically unfavorable, we argue that the importance of PV function should be strongly considered.
Second, we concomitantly attempt to resect RV hypertrophied muscle and remove fibrotic endocardial tissue as an initial surgical procedure alongside pulmonary valvotomy. This is another critical reason for using CPB during the initial operation. Our resection range includes not only the inlet and trabecular portions but also the infundibular area. Thus, our modification seems advantageous in maintaining relatively steady antegrade flow from the RV to the PA with much less dynamic obstruction caused by hypertrophied infundibular muscle than the original RV overhauling procedure with pulmonary valvotomy alone.
We cannot say that we purely applied our surgical strategy to all patients in our study because some patients had previously undergone surgical or interventional pulmonary valvotomy with BT shunts at outside hospitals (36.4%, eight out of 22 cases) prior to RV cavity muscle or fibrotic tissue resection at our institution. However, at the time of their first concomitant surgery with the RVOT widening procedure and pulmonary blood flow source management, 13 patients underwent RV cavity muscle or fibrotic tissue excision, which was compatible with our current surgical strategy. In terms of RV and TV growth, as well as PV function, they nevertheless had acceptable surgical outcomes.
4.3 Effect of the mRVoh Procedure
Our surgical strategy covered a wide range of operative ages from neonates to almost adolescents, because a significant portion of the patients were referred from other institutions with various hemodynamic statuses such as BT shunt or PDA stenting and were either planning to undergo further single ventricle palliative procedures or were already in a 1.5-ventricle status. We believe that our mRVoh procedure can transform a small RV into a tolerable pulmonary pump. Regarding neonates who were diagnosed with PAIVS in our institutions, we have recently applied our mRVoh as a primary treatment option in cases of muscular pulmonary atretic area or severely hypertrophied infundibulum, where percutaneous intervention is not anticipated to be effective for RVOT widening.
In this study, the z-score of the TV annulus significantly increased after the mRVoh procedure compared to that after percutaneous pulmonary valvotomy alone before RV endocardial fibrotic tissue or muscle resection. The observation period after mRVoh was longer than the duration after the prior intervention. However, considering that the z-score includes the concept of somatic growth (height and weight) of a patient at a specific time point, we believe that mRVoh had a meaningful positive effect on RV growth in patients with PAIVS.
TR or PR of a significant degree might lead to dilatation of RV; however, we do not consider it as an ideal healthy “RV growth” process. Although we do not have exact measured data of RV volume for comparison between the groups according to the degree of right-sided valvular regurgitation, we expect that increased forward flow with preserved valvular function might promote healthy ventricular growth.
We expected younger patients who underwent mRVoh would have better RV growth. However, the younger patients (<2 months-old) who underwent mRVoh did not show better results compared with RV growth after mRVoh of the older (>2 months-old) patients. Although the effects of age at mRVoh on RV growth were unexpected, we still have the impression that older patients have a chance of RV growth after undergoing mRVoh (Table 1).
4.4 Study Limitations and Future Plans
This study was retrospectively performed based on a small and heterogeneous patient population who underwent our mRVoh procedure with or without prior surgical or interventional treatment.
In this study, we did not compare the outcomes of our surgical treatment with those of percutaneous interventional treatment (n = 14) that had been performed during the same study period. Comparing these two patient groups was difficult because their basic characteristics, especially in the pre-procedure TV annulus z-score, were significantly different. The pre-percutaneous interventional TV z-score of these 14 intervention-only patients was −1.11 (median, IQR: −1.65 to −0.41), which was significantly larger than that of our surgical treatment patients (pre-operative TV z-score of our patients: −2.24, IQR: −3.45 to −1.60, p = 0.006).
A longer follow-up duration is required to examine the effect of mRVoh in neonates, as it could reveal other concerns, such as arrhythmia, and the development of right-sided valvular problems [22]. The RV function and volume changes after mRVoh should also be evaluated using cMRI during follow-up. Currently, we are evaluating the mid- and long-term RV status of the patients who underwent mRVoh using cMRI, and we plan to analyze these data as soon as all the patients undergo a cMRI exam.
Regarding PDA banding as a pulmonary blood source, we need more data to verify the rationale of this technique. However, in our study, the banded PDA of all five patients worked very well as a temporary source for pulmonary flow until the RV allowed adequate antegrade flow through the opened RVOT after mRVoh.
In conclusion, despite the limitations of our study, we observed more effective growth of the tricuspid annulus and RV after mRVoh, which ultimately resulted in two-ventricle repair. Our mRVoh appeared to be much more effective regarding tricuspid annulus growth than the percutaneous intervention for RVOT widening alone.
Acknowledgement: Acknowledgement and reference heading should be left justified, bold, with the first letter capitalized but have no numbers. Text below continues as normal.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: JG Kwak, WH Kim, CH Lee; data collection: JG Kwak, T Yun, ER Kim, S Cho; analysis and interpretation of results: JG Kwak, WH Kim; draft manuscript preparation: JG Kwak. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data and materials belong to our institutions’ databases, and access will be granted only after permission from members of the data-security and ethics center and committee discussion.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Yeh, S. J., Chen, H. C., Lu, C. W., Wang, J. K., Huang, L. M. et al. (2015). National database study of survival of pediatric congenital heart disease patients in Taiwan. Journal of the Formosan Medical Association, 114(2), 159–163. [Google Scholar] [PubMed]
2. Toh, N., Kotani, Y., Akagi, T., Kuroko, Y., Baba, K. et al. (2020). Outcomes of patients with pulmonary atresia with intact ventricular septum reaching adulthood. Congenital Heart Disease, 15(1), 1–12. https://doi.org/10.32604/CHD.2020.011579 [Google Scholar] [CrossRef]
3. Hanseus, K., Bjorkhem, G., Lundstrom, N. R., Laurin, S. (1991). Cross-sectional echocardiographic measurements of right ventricular size and growth in patients with pulmonary atresia and intact ventricular septum. Pediatric Cardiology, 12(3), 135–142. [Google Scholar] [PubMed]
4. Humpl, T., Soderberg, B., McCrindle, B. W., Nykanen, D. G., Freedom, R. M. et al. (2003). Percutaneous balloon valvotomy in pulmonary atresia with intact ventricular septum: Impact on patient care. Circulation, 108(7), 826–832. [Google Scholar] [PubMed]
5. Lewis, A. B., Wells, W., Lindesmith, G. G. (1986). Right ventricular growth potential in neonates with pulmonary atresia and intact ventricular septum. The Journal of Thoracic and Cardiovascular Surgery, 91(6), 835–840. [Google Scholar] [PubMed]
6. Pawade, A., Capuani, A., Penny, D. J., Karl, T. R., Mee, R. B. (1993). Pulmonary atresia with intact ventricular septum: Surgical management based on right ventricular infundibulum. Journal of Cardiac Surgery, 8(3), 371–383. [Google Scholar] [PubMed]
7. Shimpo, H., Hayakawa, H., Miyake, Y., Takabayashi, S., Yada, I. (2000). Strategy for pulmonary atresia and intact ventricular septum. The Annals of Thoracic Surgery, 70(1), 287–289. [Google Scholar] [PubMed]
8. Neill, C. A., Ursell, P. (1984). Endocardial fibroelastosis and left heart hypoplasia revisited. Interniation Journal of Cardiology, 5(4), 547–550. [Google Scholar]
9. Ursell, P. C., Neill, C. A., Anderson, R. H., Ho, S. Y., Becker, A. E. et al. (1984). Endocardial fibroelastosis and hypoplasia of the left ventricle in neonates without significant aortic stenosis. British Heart Journal, 51(5), 492–497. [Google Scholar] [PubMed]
10. Pettersen, M. D., Du, W., Skeens, M. E., Humes, R. A. (2008). Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. Journal of the American Society of Echocardiography, 21(8), 922–934. [Google Scholar] [PubMed]
11. Cho, S., Kwak, J. G., Kim, W. H. (2022). Right ventricular sinus myectomy to facilitate right ventricle growth. Operative Techniques in Thoracic and Cardiovascular Surgery, 27(2), 206–217. [Google Scholar]
12. Nishimura, R. A., Otto, C. M., Bonow, R. O., Carabello, B. A., Erwin3rd, J. P. et al. (2014). 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. The Journal of Thoracic and Cardiovascular Surgery, 148(1), e1–e132. [Google Scholar] [PubMed]
13. Arya, S. O., Karpawich, P. P., Gupta, P., Buddhe, S., Singh, H. R. et al. (2012). Primary endocardial fibroelastosis presenting in a young child as incessant ventricular tachycardia and dilated cardiomyopathy. Texas Heart Institute Journal, 39(5), 714–718. [Google Scholar] [PubMed]
14. Ni, J., Bowles, N. E., Kim, Y. H., Demmler, G., Kearney, D. et al. (1997). Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation, 95(1), 133–139. [Google Scholar] [PubMed]
15. Nield, L. E., Silverman, E. D., Smallhorn, J. F., Taylor, G. P., Mullen, J. B. et al. (2002). Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. Journal of the American College of Cardiology, 40(4), 796–802. [Google Scholar] [PubMed]
16. Xu, X., Friehs, I., Zhong Hu, T., Melnychenko, I., Tampe, B. et al. (2015). Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circulation Research, 116(5), 857–866. [Google Scholar] [PubMed]
17. Emani, S. M., McElhinney, D. B., Tworetzky, W., Myers, P. O., Schroeder, B. et al. (2012). Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. Journal of the American College of Cardiology, 60(19), 1966–1974. [Google Scholar] [PubMed]
18. Emani, S. M., Bacha, E. A., McElhinney, D. B., Marx, G. R., Tworetzky, W. et al. (2009). Primary left ventricular rehabilitation is effective in maintaining two-ventricle physiology in the borderline left heart. The Journal of Thoracic and Cardiovascular Surgery, 138(6), 1276–1282. [Google Scholar] [PubMed]
19. Morgan, A. D., McLoughlin, T. G., Bartley, T. D., Shanklin, D. R. (1966). Endocardial fibroelastosis of the right ventricle in the newborn. Presenting the clinical picture of the hypoplastic right heart syndrome. American Journal of Cardiology, 18(6), 933–937. [Google Scholar] [PubMed]
20. Ucak, A., Onan, B., Inan, K., Ugur, M., Kucukodaci, Z. et al. (2010). Endocardial fibroelastosis of the right ventricle and tricuspid valve in a young adult with Behcet’s disease. Journal of Cardiac Surgery, 25(3), 347–349. [Google Scholar] [PubMed]
21. Kotani, Y., Kasahara, S., Fujii, Y., Eitoku, T., Baba, K. et al. (2016). A staged decompression of right ventricle allows growth of right ventricle and subsequent biventricular repair in patients with pulmonary atresia and intact ventricular septum. European Journal of Cardio-Thoracic Surgery, 50(2), 298–303. [Google Scholar] [PubMed]
22. Tamborrino, P. P., di Mambro, C., Marcolin, C., Vignaroli, W., Cafiero, G. et al. (2021). Arrhythmic risk in paediatric patients undergoing surgical repair for pulmonary atresia with intact ventricular septum. Congenital Heart Disease, 16(1), 85–94. https://doi.org/10.32604/CHD.2021.013038 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools