 Open Access
Open Access
ARTICLE
Delivery Outcomes in Non-Tertiary Referral Centers for Women with Congenital Heart Disease
1 Medical College of Wisconsin, Milwaukee, WI, USA
2 Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA
3 Department of Internal Medicine, Medical College of Wisconsin, Milwaukee, WI, USA
* Corresponding Author: Matthew Buelow. Email:
Congenital Heart Disease 2023, 18(3), 315-323. https://doi.org/10.32604/chd.2023.027349
Received 26 November 2022; Accepted 22 February 2023; Issue published 09 June 2023
Abstract
Background: Women with congenital heart disease (CHD) have increased risk for adverse events during pregnancy and delivery. Prior studies have assessed pregnancy and delivery outcomes at tertiary referral centers (TRC). The aim of our study was to assess pregnancy outcomes in women with CHD who deliver in a non-tertiary referral center (non-TRC). Methods: Clinical demographics were collected, including anatomic complexity, physiologic state and pre-pregnancy risk assessment. Patients were stratified by delivery location, either TRC or non-TRC. Maternal and neonatal complications of pregnancy were reported. Results: Women with CHD who delivered in a TRC had a higher pre-pregnancy risk when assessed by the Zahar and CARPREG-II scores, and had more patients fall into a higher WHO classification. There was no difference in rates of maternal cardiac complications between delivery locations (11%) and neonatal complications (20%) between deliveries at TRC and non-TRC. Conclusions: There were not increased maternal cardiac or neonatal complications when delivery occurred at a non-TRC. Neonatal complications remained high regardless of delivery location. This study suggests that proper risk assessment may help identify women who are candidates for safe delivery at non-TRC in women with CHD, and that neonatal resources should be considered when planning delivery location.Graphic Abstract

Keywords
Congenital heart disease (CHD) is the most common birth defect in the United States and affects nearly 1% of births per year [1]. Due to advances in surgical and medical therapy, there are more adults with congenital heart disease (ACHD) than children with congenital heart disease in the United States [2]. With improved survival, there has been a substantial increase in pregnancies from women with CHD. In one study, the number of deliveries among women with CHD increased 34.9% from 1998–2007, relative to a 21.3% increase in births in the general population [3].
However, due to residual cardiac disease, women with CHD have increased risk for adverse events during pregnancy and delivery [4]. Prior studies have demonstrated women with CHD to have an 18-fold increased risk of death during admission for delivery, and sustained arrhythmia has been shown to be the most common adverse event [5,6]. While studies have primarily evaluated outcomes of mothers with CHD at tertiary referral centers (TRC), pregnancy and delivery outcomes at non-tertiary referral centers (non-TRC) have not yet been reported. The aim of our study was to assess delivery outcomes in women with CHD at non-TRC.
This was a retrospective chart review of patients followed in the Wisconsin Adult Congenital Heart Disease (WATCH) Program at the Medical College of Wisconsin. The WATCH program follows adult patients with CHD, with multiple outreach locations throughout the state outside of the tertiary referral center in Milwaukee, WI that is affiliated with our academic program. The study protocol was approved by the institutional IRB (Approval No. 1566396).
Pregnant patients (≥16 years of age) followed in the WATCH program from 2014–July 2021 were included. Demographic and clinical variables were collected, including medication use prior to pregnancy, history of cardiac arrhythmias, history of prior heart failure exacerbations, and pre-partum and pregnancy induced-hypertension. Exclusion criteria included patients with a history of acquired heart disease and cases where complete delivery data was not available.
Anatomic cardiac complexity and pre-pregnancy physiologic state was assessed in accordance with the 2018 ACC/AHA Care Guidelines for Adults with Congenital Heart Disease, along with pre-pregnancy NYHA functional class. Pregnancy associated cardiac risk assessment was performed utilizing the World Health Organization (WHO) classification of classes I, II, III or IV and Zahara and CARPREG II scores [6].
Composite adverse maternal cardiac events and composite adverse neonatal events were recorded. Adverse maternal events included decompensated heart failure (CHF), arrhythmias requiring treatment, aortic dissection, cardiac surgery that was required during pregnancy or after delivery, a diagnosis of pre-eclampsia and maternal mortality. Adverse neonatal events included intrauterine growth restriction (IUGR; estimated neonatal weight <10th percentile based on estimated gestational age, EGA), preterm delivery (<37 weeks EGA), intrauterine fetal demise, term birth weight <2500 g, neonatal CHD, and other congenital anomalies. Fetal CHD was diagnosed prenatally using fetal echocardiography and confirmed with post-natal echocardiography.
Delivery location was categorized as a non-tertiary referral center (non-TRC) if delivery occurred in a community hospital, or as a tertiary referral center (TRC) if the delivery occurred in the academic tertiary referral hospital affiliated with our program. Location of prenatal cardiac care was categorized as an outreach location, or a main campus clinic based on the primary location of cardiac care delivery. Delivery location was determined by shared decision making between the primary cardiologist, obstetric team, and the patient following an individualized risk assessment, including cardiovascular history, pre-partum cardiovascular risk assessment, and current physiologic state. Patients were followed carefully throughout pregnancy with close collaboration between the cardiac team and obstetrics providers.
Continuous data is presented as a mean or median with standard deviation, while categorical data are recorded as frequencies. Differences in these baseline characteristics and outcomes were examined using Student’s t-test for continuous variables and Fisher’s exact test or Chi-squared test for categorical data. Statistical analysis of the data was performed using IBM SPSS, version 27.0.0 Multilingual Multiplatform eAssembly (IBM, Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant.
There were 165 pregnancies in 136 women, including 87 births at TRC, and 78 births at non-TRC. There were no statistically significant differences in baseline demographics or clinical characteristics between patients delivering at TRC vs. non-TRC (Table 1). The most common maternal cardiac diagnoses were tetralogy of Fallot, bicuspid aortic valve, and coarctation of the aorta (Fig. 1). The vast majority (99%) were NYHA functional classes I–II, and most patients were classified in physiological stage A and anatomic complexity class II, without a difference between delivery location. As anticipated, patients delivering at a TRC had a higher WHO classification, had higher estimated pre-pregnancy risk as assessed by CARPREGII and Zahara indices, and had more patients at physiologic stage B or C (p = 0.05) than patients delivering at non-TRC.
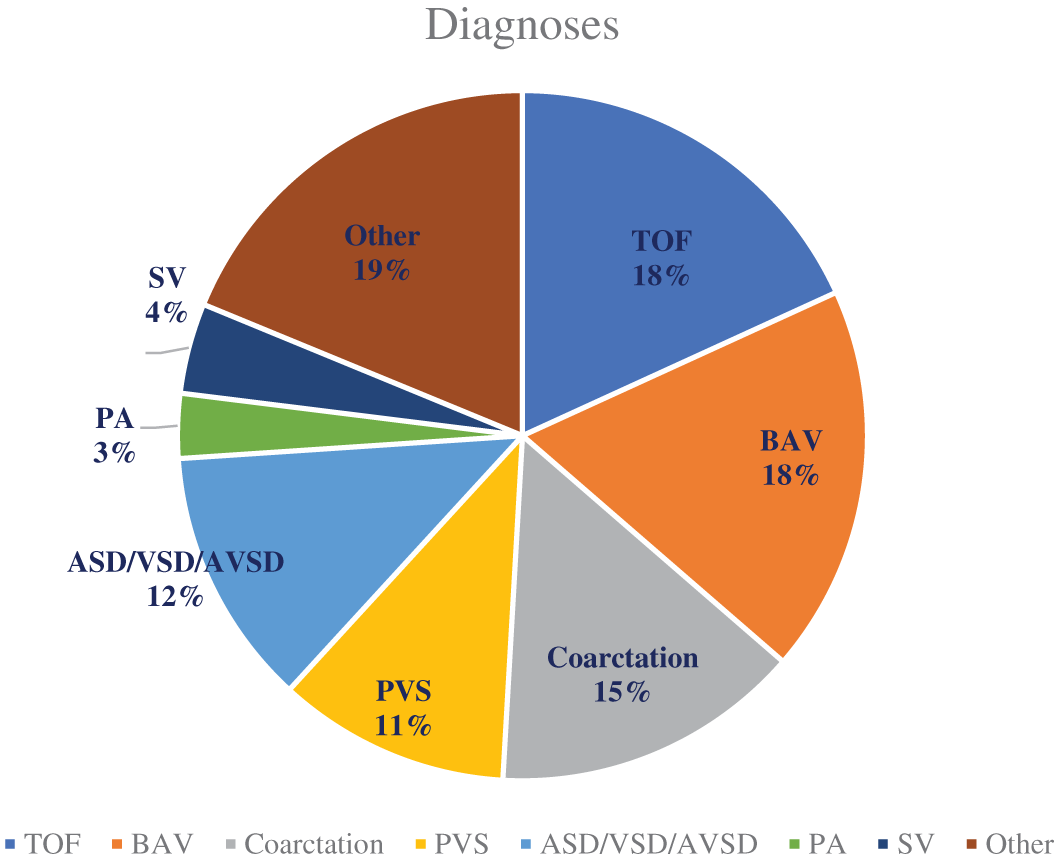
Figure 1: List of maternal diagnoses
TOF, or tetralogy of Fallot is the most common diagnosis with n = 30, followed by BAV (bicuspid aortic valve), and coarctation of the aorta. PVS (pulmonary vein stenosis), ASD/VSD/AVSD (atrial septal defect, ventricular septal defect, or atrioventricular septal defect), PA (pulmonary atresia), SV (single ventricle defect), and those under an “other” classification make up the remaining diagnoses.
Overall, there were 18 patients (10.9% of cohort) with a maternal cardiac complication, including 10 (11.5%) at TRC and 8 at non-TRC (10.3%; p = 0.79). Maternal mortality was observed in one patient (n = 1), while congestive heart failure (n = 6) and arrhythmias (n = 2) also occurred (Table 2). Additionally, there was a diagnosis of pre-eclampsia made in 9 patients (3 at a TRC and 6 at a non-TRC), though none progressed to eclampsia. No patients required surgical cardiac intervention or suffered an aortic dissection.
The single maternal mortality occurred in a 38-year-old G8P7007 with unrepaired congenital heart disease. The patient had a moderate sized anterior muscular ventricular septal defect, double chamber right ventricle, severe right ventricular hypertrophy with qualitatively normal right ventricular systolic function, with severe tricuspid valve regurgitation. The peak pulmonary outflow tract gradient was 70 mmHg. While her prior pregnancies were reportedly uncomplicated, she first presented to care at our institution with symptoms of CHF and atrial fibrillation in the second trimester. Following preterm premature rupture of membranes and delivery of a non-viable fetus at 21 weeks EGA, she developed a post-partum hemorrhage with subsequent arrhythmic cardiac arrest. Despite significant efforts, the patient was unable to be resuscitated.
In addition to the arrhythmic cardiac arrest noted above, a second patient developed atrial flutter at 22 weeks EGA. She was medically managed until 35 weeks EGA when she was admitted for delivery due to ongoing symptoms. Following successful delivery of a healthy infant, she was electrically cardioverted 3 days after delivery. Finally, a third patient who underwent a cesarean section at full term developed an episode of supraventricular tachycardia that required adenosine for conversion.
There were four patients at a TRC who experienced heart failure. One was functional class NYHA I prior to pregnancy, while the other three were all NYHA II. All patients had a WHO class of II or III, a CARPREGII average of 2.80 and a Zahara average of 1.95. All patients developed symptoms in the immediate post-partum period and received anti-congestive therapy, including oneafter delivering twins via cesarean section at 30 weeks EGA. Lastly, there were 3 women at a TRC who experienced issues with pre-eclampsia. All 3 were successfully treated with anti-hypertensive medication and aspirin and were able to deliver at full term. None of these patients progressed to eclampsia.
Maternal cardiac complications (n = 8) at a non-TRC were congestive heart failure (n = 2) and pre-eclampsia (n = 6). Of the patients with CHF, both had a WHO class of II and CAREPREGII and ZAHARA scores of 0. The first patient was a 17-year-old born with pulmonary vein stenosis, who unfortunately was found to have a non-viable fetus, secondary to intrauterine fetal demise. A second patient was a 25-year-old female with a history of ASVD and coarctation of the aorta, who had a vaginal delivery at 39 weeks. Following discharge, she returned to the emergency department with hypervolemia including extremity and pulmonary edema, and an elevated BNP level. Both patients responded well to decongestive therapy. Additionally, 6 patients developed pre-eclampsia prior to their delivery, 2 of which had a pre-partum diagnosis of hypertension. Two patients required emergent caesarean section at 32 weeks EGA, while the other 4 patients delivered at term.
In addition to the arrhythmic cardiac arrest noted above, a second patient developed atrial flutter at 22 weeks EGA. She was medically managed until 35 weeks EGA when she was admitted for delivery due to ongoing symptoms. Following successful delivery of a healthy infant, she was electrically cardioverted 3 days after delivery. Finally, a third patient who underwent a cesarean section at full term developed an episode of supraventricular tachycardia that required adenosine for conversion.
There were four patients at a TRC who experienced heart failure. One was functional class NYHA I prior to pregnancy, while the other three were all NYHA II. All patients had a WHO class of II or III, a CARPREGII average of 2.80 and a Zahara average of 1.95. All patients developed symptoms in the immediate post-partum period and received anti-congestive therapy, including oneafter delivering twins via cesarean section at 30 weeks EGA. Lastly, there were 3 women at a TRC who experienced issues with pre-eclampsia. All 3 were successfully treated with anti-hypertensive medication and aspirin and were able to deliver at full term. None of these patients had progressed to eclampsia.
Maternal cardiac complications (n = 8) at a non-TRC were congestive heart failure (n = 2) and pre-eclampsia (n = 6). Of the patients with CHF, both had a WHO class of II and CAREPREGII and ZAHARA scores of 0. The first patient was a 17-year-old born with pulmonary vein stenosis, who unfortunately was found to have a non-viable fetus, secondary to intrauterine fetal demise. A second patient was a 25-year-old female with a history of ASVD and coarctation of the aorta, who had a vaginal delivery at 39 weeks. Following discharge, she returned to the emergency department with hypervolemia including extremity and pulmonary edema, and an elevated BNP level. Both patients responded well to decongestive therapy. Additionally, 6 patients developed pre-eclampsia prior to their delivery, 2 of which had a pre-partum diagnosis of hypertension. Two patients required emergent caesarean section at 32 weeks EGA, while the other 4 patients delivered at term.
There were neonatal complications in 33 (21%) pregnancies, with 20 occurring in TRC and 13 in non-TRC (p = 0.31). Pre-term birth was seen in 6 patients in TRC and 6 patients at non-TRC. The average EGA for all infants was 37.8 weeks at a TRC and 37.5 weeks at a non-TRC, without a difference (p = 0.52) between locations. Of the infants born pre-term, there was no difference in EGA, or average weight (1.8 kg) between delivery locations, including 30.2 weeks EGA at TRC, compared to 32.7 weeks EGA at non-TRC. IUGR was seen in 6 neonates (2 at a TRC and 4 at a non-TRC), including an infant also born prematurely. Of these, 1 neonate was born at term (>37 weeks EGA) had a BW less than 2500 g and was delivered at a non-TRC (Table 2). There were 4 pregnancies that resulted in fetal demise (2 at a TRC, 2 at non-TRC); all pregnancies with intrauterine fetal demise between 20 and 31 weeks EGA.
Nine pregnancies resulted in neonatal congenital heart disease, including ventricular septal defect (n = 5), hypoplastic left heart syndrome (n = 2), transposition of the great arteries (n = 1), and tetralogy of Fallot (n = 1). One patient developed respiratory distress syndrome (RDS) and a second neonate had a congenital pulmonary airway malformation (CPAM), both delivering at TRC.
Of the 165 pregnancies, there were 27 (16%) pregnancies managed prenatally at an outreach clinical location. Ultimately, 6 (20%) were referred for delivery at TRC due to maternal cardiac risk (Zahara 1.57 and CARPREGII 1.71 and elevated WHO class of II–III), though none had a cardiac complication. Conversely, there was 138 (84%) pregnancies managed at main campus prenatally, of which 56 (40%) ultimately delivered at a non-TRC. Of these 56 patients, there were 2 maternal cardiac complications, and 7 neonatal complications (5 pre-term delivery, 1 IUGR, and 1 RDS). Patients were from 27 distinct counties, with highest frequency in southeastern Wisconsin where our academic medical center is located, as well as northeastern Wisconsin, where our program has additional outreach locations (Fig. 2).
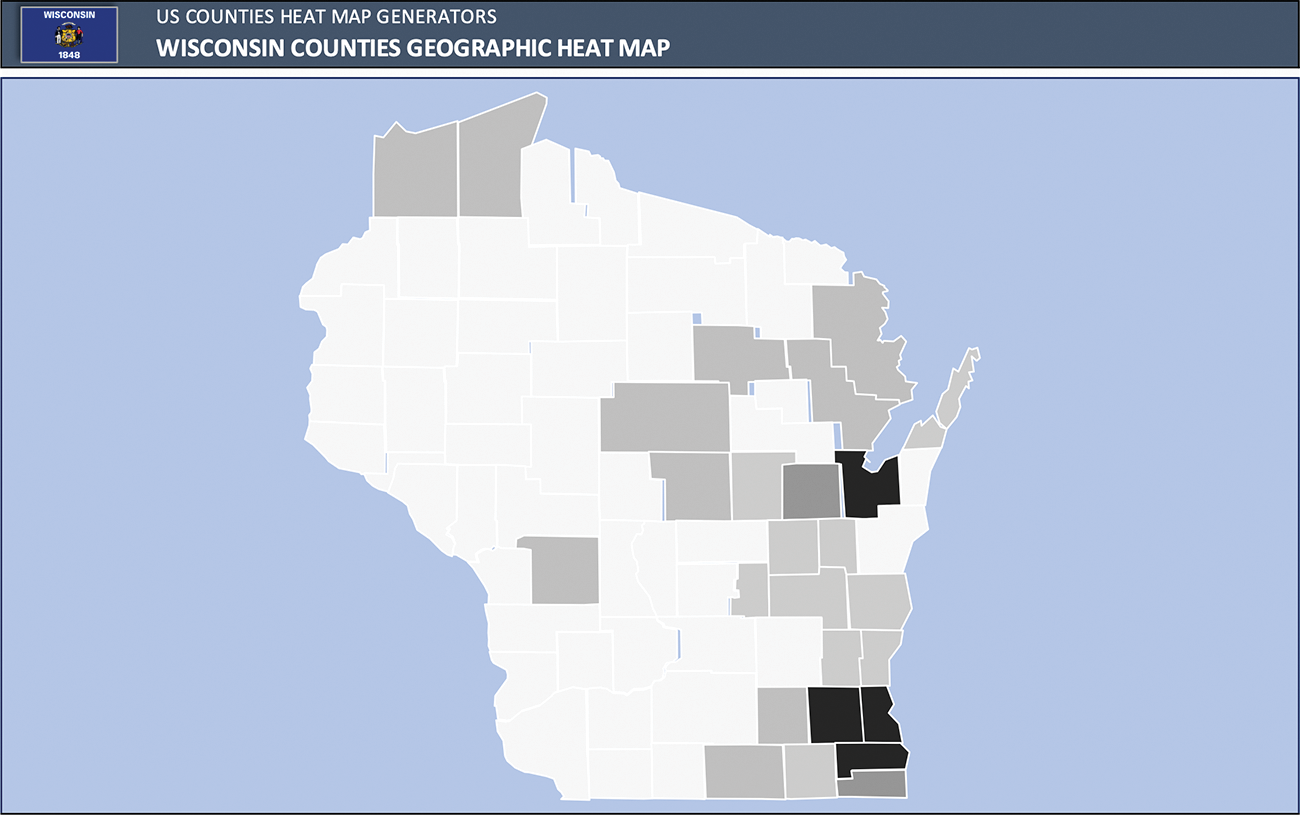
Figure 2: Heat map of patient’s home counties
While pregnancy in women with CHD has become an important area of research, most studies have focused on outcomes at tertiary referral centers. Our study, the first to assess maternal and neonatal outcomes in deliveries at non-TRC, suggests that delivery at non-TRC can be safe inappropriately selected patients. This is important for patients who wish to deliver at a community hospital closer to their home and family. Many ACHD programs serve patients throughout a large geography, with many patients living a significant distance from an academic medical center. This study shows through proper risk stratification, and care collaboration, many patients may be able to safely deliver closer to their home and families.
Previous studies have demonstrated that maternal cardiac risk assessment predicts adverse cardiac events [7,8]. In our study, as expected, patients who delivered at a TRC had higher WHO classifications, and higher CAREPREG II and Zahara scores. In addition to maternal cardiac risk scoring, we also assessed anatomic and physiologic classification. Steiner et al. reported that physiologic classification was the strongest predictor of maternal cardiac complication, likely as physiologic class can be dynamic due to the hemodynamic changes that occur in pregnancy. This contrasts with anatomic complexity which is fixed throughout pregnancy [9]. In our study, patients delivering at a TRC had higher degree of both anatomic and physiologic complexity. Furthermore, despite a favorable risk stratification, all maternal events at non-TRC were the result of increase in physiologic state throughout the pregnancy, ultimately resulting in symptoms of heart failure or the development of pre-eclampsia, despite lower assessed pre-pregnancy risk. This further highlights the importance of physiologic state, given the anticipated pregnancy induced hemodynamic changes.
As maternal CHD is a major determinant of risk to the fetus and can result in higher frequency of fetal and neonatal complications, we also assessed neonatal outcomes. In our study, while there was no difference in neonatal complications stratified by delivery location, there remained a high frequency of neonatal complications within the cohort at 20%. Most commonly, neonates were preterm, though IUGR and term infants with birth weight < 2500 g were also seen. Our study is consistent with prior reports, where women with existing cardiac disease are more likely to give birth to newborns with lower percentile growth and are more likely to have a preterm delivery [10,11]. However, in a subset analysis of premature infants, the EGA was younger in patients at a non-TRC, at approximately 30 weeks EGA, vs. 32.7 weeks EGA at a TRC, and additionally, there were 3 term neonates at a non-TRC with a BW < 2500 g. This suggests that fetal growth remains a concern even in patients with lower assessed cardiac risk.
As a result, given the high frequency of neonatal complications, including pre-term delivery and reduced birth weight, there should be careful consideration of neonatal resources for any delivery at a non-TRC. Even if a non-TRC has the resources to properly treat a low-risk maternal cardiac complication, they may not have the resources necessary for higher acuity neonatal patients including a neonatal intensive care unit if necessary.
As anticipated, patients followed in our outreach locations, referred for delivery at TRC, had high maternal cardiac risk. Conversely, when patients had acceptable cardiac risk, they still may deliver at non-TRC, even when primarily followed at the main campus location. This individualize risk assessment is best guided with awareness of local cardiac and neonatal resources, and transparent counseling regarding maternal and neonatal risk. The best-case scenario is pre-conception counseling, with comprehensive risk assessment in the pre-partum period, along with regular follow-up during pregnancy, by a team, that has experience in managing congenital heart disease and heart disease within pregnancy. This has been our approach, and while accepted as ideal state, has also been demonstrated to improved outcomes in a case series of high-risk patients [12].
It is important to note that regardless of maternal cardiac risk, delivery location may be determined by antenatally diagnosed fetal disease. This was notable given the observed neonatal CHD, 4 of whom had ductal dependent lesions. This further highlights the importance of multidisciplinary antenatal care, including fetal echocardiography, and delivery planning with the cardio-obstetrical teams to optimize the outcome for the both the patient and the newborn.
Our study was limited by retrospective design and tertiary referral bias, as only patients seen within our program were included. As many patients delivered at outside institutions, comprehensive neonatal records may not have been available, beyond gestational age and birth weight. As a result, composite outcomes of fetal events were not inclusive of all neonatal morbidity, such as transient tachypnea of the newborn or respiratory failure requiring mechanical ventilation. However, this limitation would only underestimate the extent of adverse neonatal events, and further emphasizes that neonatal resources need to be considered, regardless of assessed maternal risk, when planning for a delivery at a non-TRC. Finally, our analysis was limited to patients followed in our academic adult congenital heart disease program and did not include patients with cardiomyopathy, connective tissue disease, and women with acquired heart disease.
Overall, there was not increased maternal cardiac or neonatal complications when delivery occurred at a non-TRC. This study suggests that pregnancy risk assessment helps identify women who may be candidates for safe delivery at a non-TRC in women with CHD. Pregnancy and delivery risk assessment should be done by a health care team with cardiologists trained in the management of adults with congenital heart disease, in collaboration with obstetricians and high risk maternal fetal medicine physicians. Finally, due to the frequency of neonatal complications, neonatal resources should be considered at non-TRC before a choice is made to deliver.
Acknowledgement: The authors would like to thank Herma Heart Institute and the Medical College of Wisconsin for their support.
Funding Statement: This work was supported through Herma Heart Institute and the Medical College of Wisconsin.
Author Contributions: The authors confirm contribution to the studies as follows: study conception, design, data collection, analysis, interpretation of results, draft manuscript preparation: D. Sweeney and M. Buelow. Draft Manuscript preparation: S. Cohen, J. Gerardin, P. Bartz, S. Ginde.
Availability of Data and Materials: The datasets are not publicly available due to ethical consideration, though can be made available upon reasonable request.
Ethics Approval: Given the observational and retrospective nature of this study, ethical approval was not required. The study aligned with IRB requirements at our institution and patient identifiers were used to protect any patient information.
Conflicts of Interest: The authors declare they have no conflicts of interest to report regarding the present study.
References
1. van der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease world-wide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58, 2241–2247. https://doi.org/10.1016/j.jacc.2011.08.025 [Google Scholar] [PubMed] [CrossRef]
2. Marelli, A., Gilboa, S., Devine, O., Kucik, J., Ionescu-Ittu, R. et al. (2012). Estimating the congenital heart disease population in the United States in 2010–what are the numbers? Journal of the American College of Cardiology, 59. https://doi.org/10.1016/S0735-1097(12)60788-8 [Google Scholar] [CrossRef]
3. Opotowsky, A. R., Siddiqi, O. K., D’Souza, B., Webb, G. D., Fernandes, S. M. et al. (2012). Maternal cardiovascular events during childbirth among women with congenital heart disease. Heart, 98, 145–151. https://doi.org/10.1136/heartjnl-2011-300828 [Google Scholar] [PubMed] [CrossRef]
4. Mcilvaine, S., Feinberg, L., Spiel, M. (2021). Cardiovascular disease in pregnancy. Neoreviews, 747–759. https://doi.org/10.1542/neo.22-11-e747 [Google Scholar] [PubMed] [CrossRef]
5. Whittemore, R., Hobbins, J. C., Engle, M. A. (1982). Pregnancy and its outcome in women with and without surgical treatment of congenital heart disease. Journal of the American College of Cardiology, 50, 641–651. https://doi.org/10.1016/0002-9149(82)90334-4 [Google Scholar] [PubMed] [CrossRef]
6. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology, 73(12), 1494–1563. https://doi.org/10.1016/j.jacc.2018.08.1028 [Google Scholar] [PubMed] [CrossRef]
7. Lu, C. W., Shih, J. C., Chen, S. Y., Chiu, H. H., Wang, J. K. et al. (2015). Comparison of 3 risk estimation methods for predicting cardiac outcomes in pregnant women with congenital heart disease. Circulation Journal, 79(7), 1609–1617. https://doi.org/10.1253/circj.CJ-14-1368 [Google Scholar] [PubMed] [CrossRef]
8. Silversides, C. K., Grewal, J., Mason, J., Sermer, M., Kiess, M. et al. (2018). Pregnancy outcomes in women with heart disease: The CARPREG II study. Journal of the American College of Cardiology, 71(21), 2419–2430. https://doi.org/10.1016/j.jacc.2018.02.076 [Google Scholar] [PubMed] [CrossRef]
9. Steiner, J. M., Lokken, E., Bayley, E., Pechan, J., Curtin, A. et al. (2021). Cardiac and pregnancy outcomes of pregnant patients with congenital heart disease according to risk classification system. The American Journal of Cardiology, 161, 95–101. https://doi.org/10.1016/j.amjcard.2021.08.037 [Google Scholar] [PubMed] [CrossRef]
10. Gelson, E., Curry, R., Gatzoulis, M. A., Swan, L., Lupton, M. et al. (2015). Maternal cardiac and obstetric performance in consecutive pregnancies in women with heart disease. BJOG, 122(11), 1552–1559. https://doi.org/10.1111/1471-0528.13489 [Google Scholar] [PubMed] [CrossRef]
11. Hardee, I., Wright, L., McCracken, C., Lawson, E., Oster, M. E. (2021). Maternal and neonatal outcomes of pregnancies in women with congenital heart disease: A meta-analysis. Journal of the American Heart Association, 10(8), e017834. https://doi.org/10.1161/JAHA.120.017834 [Google Scholar] [PubMed] [CrossRef]
12. Gardner, R., Durbak, E., Baird, R., Singh, K., Chapa, J. et al. (2022). Pregnancy in patients with shone complex: A single-center case series. Congenital Heart Disease, 17(2), 147–160. https://doi.org/10.32604/chd.2022.017366 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools