 Open Access
Open Access
ARTICLE
Transcatheter Ventricular Septal Defect Closure with Nit-Occlud Lê VSD Device—Five Years’ Experience and Literature Review
1 Cardiology Department, Mother and Child Health Institute of Serbia “Dr Vukan Čupić”, Belgrade, 11070, Serbia
2 Faculty of Medicine, University of Belgrade, Belgrade, 11070, Serbia
* Corresponding Author: Sergej M. Prijić. Email:
Congenital Heart Disease 2023, 18(3), 361-371. https://doi.org/10.32604/chd.2023.026533
Received 09 November 2022; Accepted 05 March 2023; Issue published 09 June 2023
Abstract
Introduction: Transcatheter closure is an alternative to ventricular septal defect (VSD) occlusion surgery. Nit-Occlud Lê VSD coil is a new device yet to be evaluated. The study aimed to evaluate immediate and midterm results after transcatheter closure with the Nit-Occlud Lê VSD device. Methods: The retrospective analysis included 30 patients with VSD referred for closure during the period from October 2015 to December 2020. Results: At the time of intervention, the patients’ mean age and body weights were 7.5 ± 5.6 years and 29.3 ± 19.1 kg. The majority of the defects had perimembranous location (24/30), four defects had muscular and two outlet subaortic position. The mean effective right-side diameter of the VSDs was 3.6 ± 1.3 mm. Single ventricular fibrillation, device embolization, and hemolysis developed in different patients and were successfully treated. None of the patients had a complete atrioventricular block. The coil was successfully placed in 25/30 (83.3%) patients. The majority of the devices were 10 mm × 6 mm (11/25) and 12 mm × 8 mm (8/25) in size. Two patients required the implantation of a second device. The follow-up period was 2.1 ± 1.4 years. Complete VSD closure was achieved in 48% of cases immediately after the intervention, 74% during 2.1 ± 1.6 months after the procedure, and 81% over follow-up. The remaining patients had a trivial residual defect. During the follow-up, approximately one-third of patients developed trivial aortic and mitral valve regurgitation, and half of the patients acquired trace/mild tricuspid regurgitation. Standardized (z-score) left ventricular end-diastolic diameter (0.15 ± 0.37 vs. 0.92 ± 0.82, p = 0.005) and left atrium dimension (0.47 ± 0.58 vs. 1.89 ± 1.11, p = 0.005), as well as the left atrium to aortic root ratio (1.2 ± 0.1 vs. 1.4 ± 0.2, p = 0.005) showed a significant decrease over follow-up related to the period before intervention. Conclusion: Intervention with Nit-Occlud® Lê VSD coil showed appropriate results regarding VSD closure rate, complications, and chamber remodeling. The introduction of this device into clinical practice is a significant step forward in transcatheter perimembranous VSD occlusion.Keywords
Ventricular septal defect (VSD) is the most common congenital heart disease, representing up to 25% of all congenital heart defects (CHD). Based on the location VSD can be classified as muscular, perimembranous, inlet and outlet. The most common are the perimembranous VSDs (pmVSDs), which account for up to 70% of all VSDs and up to 80% of all surgically treated ventricular septal defects. Although surgical pmVSD closure has been a standard treatment for more than 60 years, it carries minimal, but noticeable risks, such as complete post-surgical atrio-ventricular block (cAVB) in 1%–5% of the cases, significant residual VSD in 1%–10% of the subjects, the necessity for re-operation in 2% of the patients and even death in rare cases. Furthermore, infections, tachyarrhythmias, and neurological complications may occur after surgery [1,2]. Thus, transcatheter pmVSD closure appears to be a promising and attractive alternative to standard surgical treatment. This especially holds true for a subgroup of asymptomatic children who present with mild left side chambers volume overload and in whom potential risks of open-heart surgery may overweigh the potential benefits [3].
This report shows our experience in transcatheter closure of VSD with Nit-Occlud Lê VSD device.
All procedures were conducted in accordance with the ethical standards of Helsinki Declaration and with the informed consent obtained from the patients’ legal guardians.
This retrospective analysis included 30 patients with VSD referred for closure at Mother and Child Health Institute of Serbia during the period from October 2015 to December 2020. Primary indications for transcatheter VSD closure were left chambers volume overload (left ventricular end-diastolic diameter (LV-EDD) z-score > 1.5, left atrium (LA) z-score > 1.5 or LA to aorta (Ao) ratio > 1.5), pulmonary to systemic flow ratio > 1.5 and absence of pulmonary hypertension. Selection criteria included the distance between the VSD rim and aortic annulus of more than 2 mm with estimated final coil position without touching the aortic valve, body weight over 9 kg and age older than 12 months. Six patients were excluded from the study because angiography showed a small defect with no indication for transcatheter closure.
Nit-Occlud Lê VSD coil (PFM: Produkte für die Medizin AG, Cologne, Germany), for pmVSD transcatheter closure, was introduced in 2010 and has gained a CE mark in Europe [4–6]. The Nit-Occlud Lê VSD device is a coil made of nitinol wire. It has a cone-in-cone configuration, which means that the proximal cone is reversed. Polyester fibers are attached to the reinforced left side loops with the aim to enhance the closure. The device is mostly designed for closure of pmVSDs with aneurysmal septum and cone-shaped muscular VSD, but also for doubly committed VSDs. Nit-Occlud Lê VSD coil is produced in sizes 8/6, 10/6, 12/6, 12/8, 14/8, and 16/8 mm (left/distal and right/proximal end, respectively) and the implantation is performed via 6-F or 7-F sheaths. The size of the device was determined according to the recommendations: left ventricular diameter of the device was either twice as large as the minimal right side VSD opening or equal to (or 1–2 mm larger than) the left side VSD diameter [6]. In case of multiple VSD openings or septal aneurysms, the coil was chosen based on the left side diameter of the aneurysm.
All the patients were subjected to pre-interventional transthoracic echocardiography and routine laboratory analyses. Catheterisations were performed under general anesthesia, with pre- and post-procedural antibiotic prophylaxis and heparinisation during the procedure (heparin bolus of 100 IU/kg body weight). Almost all of the interventions (27/30) were carried out in cooperation with the international proctor who participated in designing the device (Dr. Trong Phi Le, Heart Center Bremen, Klinikum Links der Weser, Germany). Initially, left ventricular angiogram was made in order to define the size and location of the defect. The VSD diameter was measured at the diastolic phase and an occluder was selected based on the largest measurement. The device implantation technique (Fig. 1) was described in detail in previous publications [4,6].
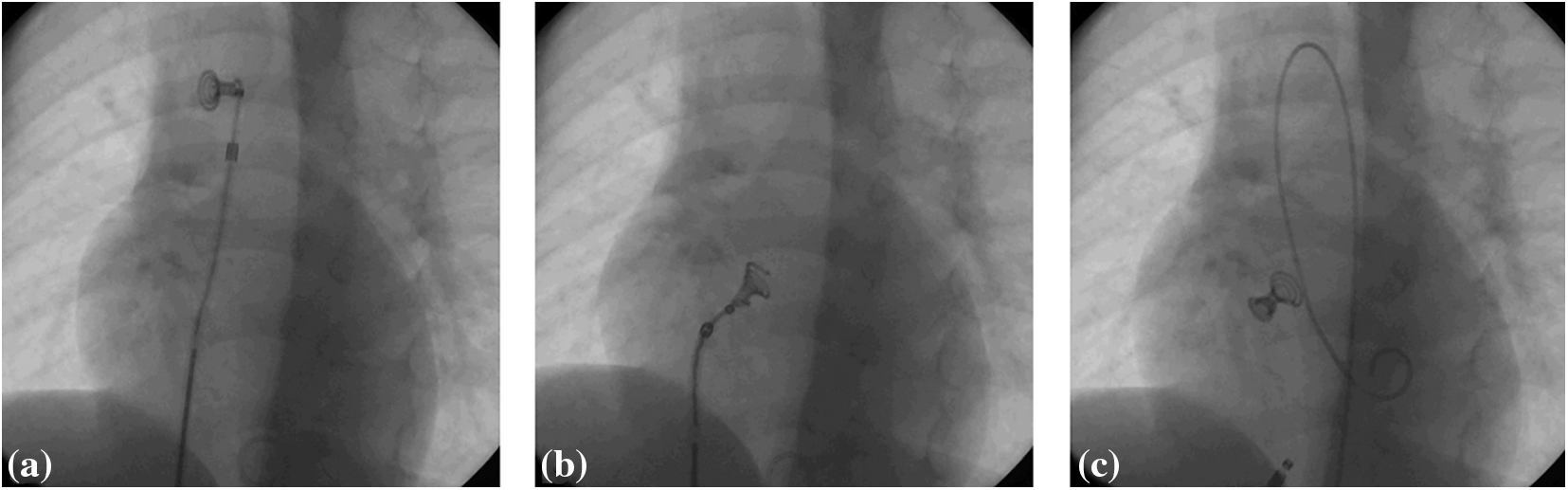
Figure 1: The final steps of Nit-Occlud Lê VSD coil implantation. (a) Complete left coil is deployed in ascending aorta; (b) the whole system is retracted through the aortic valve and left ventricle until the coil enters VSD and the final two loops are deployed at the right side of the defect; (c) if the position of the device is considered adequate, it is released
Follow-up period was 2.1 ± 1.4 years and included clinical and echocardiographic evaluation after the VSD closure. Left ventricular end-diastolic diameter, left atrium, and aortic dimensions were observed by M-mode, 2-D echocardiography, and z-scores estimations. Aortic, mitral and tricuspid regurgitation were scored as none (0), trace (0.5+), mild (1+), moderate (2+), and severe (3+) by combination of Doppler echocardiography methods (length and area of regurgitation jet, vena contracta, pressure half-time, retrograde aortic flow), left atrial and left ventricular end-diastolic diameter measurement.
Standard descriptive statistics included mean values with standard deviations, medians with interquartile ranges and percentages of monitored parameters. The analytic strategy included one-way ANOVA and Fisher test for comparisons between VSD sizes and closure rates, respectively. The comparison between the pre-interventional and follow-up findings (LV-EDD z-score, LA z-score, LA:Ao ratio and valvar regurgitation) was done using the Student paired T and Wilcoxon tests. Probability value of p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 18.0 (IBM Corp., Armonk, NY, USA) for Windows.
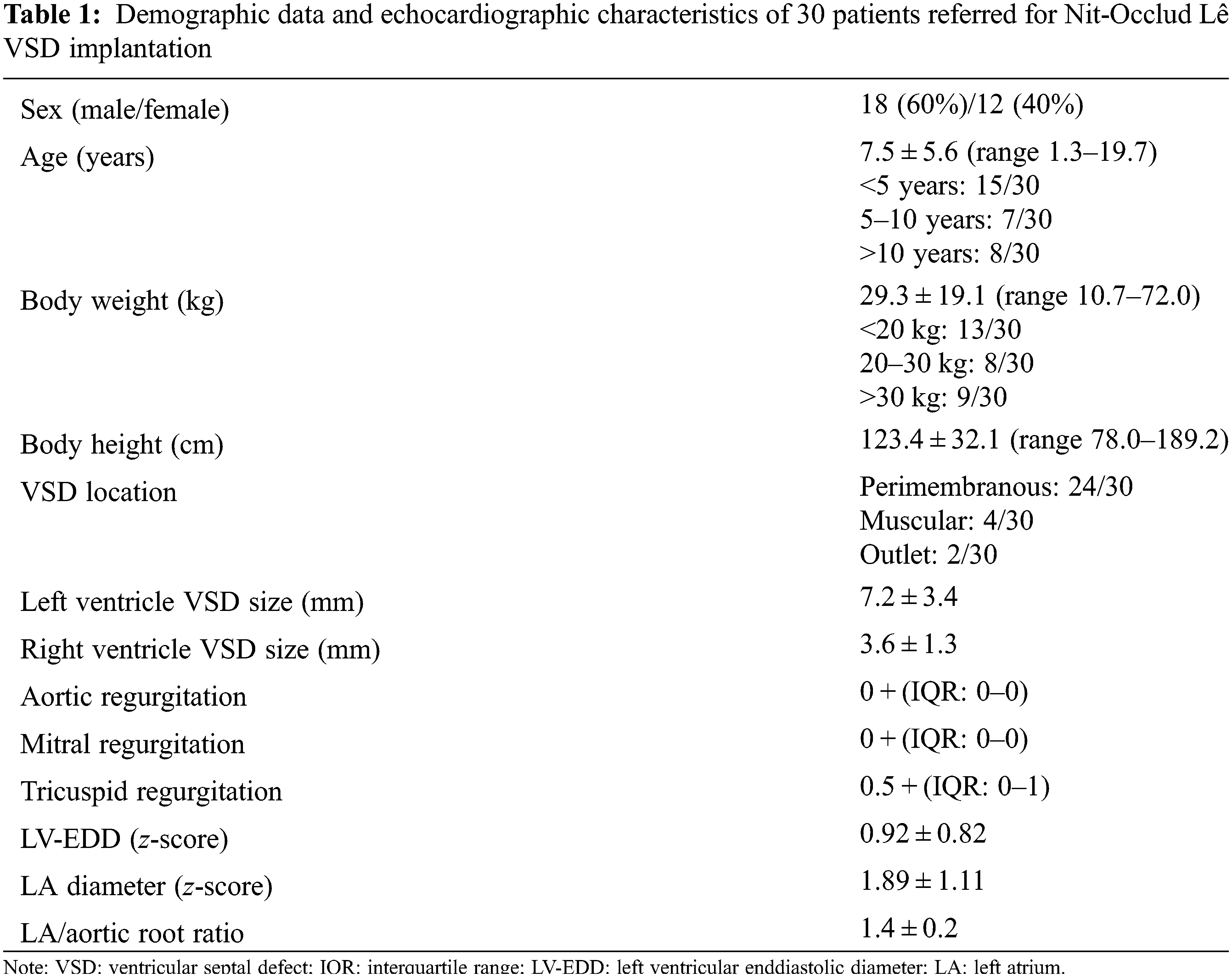
The intervention was performed in 30 children (Table 1). A large majority of our patients had a pmVSD (n = 24) with left side diameter of 7.8 ± 3.2 mm and right-side diameter of 3.5 ± 0.9 mm. The remaining 6 patients had either muscular or outlet VSD with an effective diameter of 3.2 ± 1.0 mm and 6.0 ± 2.8 mm, respectively. There were no patients with inlet or doubly committed VSD in the study. Patients with flexible device (proximal diameter of 6 mm) had smaller right-side diameter of the VSD, related to those with stiff device (proximal diameter of 8 mm) (3.2 ± 0.8 mm vs. 4.3 ± 1.7 mm; p = 0.032).
3.2 Immediate Results and Complications
In 25/30 (83.3%) interventions PFM coil was successfully placed. Most devices were 10 mm × 6 mm (11/25) and 12 mm × 8 mm (8/25) in size. Interventions with devices 8 mm × 6 mm, 12 mm × 6 mm and 16 mm × 8 mm in size were performed in three, two, and one patient, respectively. Two patients required the implantation of an additional device due to persistence of the important residual L-R shunt. The first patient had aneurysmal configuration of perimembranous septum, with multiple openings on the right side. After the implantation of the device (12 mm × 8 mm) into the largest defect, control angiography showed another significant shunt, which was occluded with another PFM device (12 mm × 8 mm). The second patient had a perimembranous defect with outlet extension, occluded with PFM device 16 mm × 8 mm. Two days after the procedure she had significant residual shunt with signs of haemolysis (macroscopic haematuria, haemoglobinuria and plasma haemoglobin concentration decline down to 7.2 g/dL), which was the indication for another device implantation (ADO II).
The procedure was not successfully completed in five patients. The first of these patients had a subaortic post-Rastelli procedure VSD on intracardiac tunnel, just beneath aortic valve. The catheterisation confirmed the lack of rim towards the aortic valve and the appropriate position of the device could not be achieved. During the manoeuvres with the catheters, he developed transitory ventricular fibrillation which was successfully terminated by a direct current (DC) shock. The second patient had an oblique mid-muscular VSD. Besides multiple repositions, the device could not fill-up the defect and protruded to the right ventricle. The third patient had multiple right-side openings of the muscular VSD, unsuitable for the guidewire placement. The fourth patient had occlusion with PDA occluder after failed attempt with PFM coil. The fifth patient’s device embolized into pulmonary artery with successful retrieval and it was concluded that there is a risk for stable placement of device due to very thin and mobile perimembranous septum.
Mild complications developed isolated in different patients. Contrast allergy resolved after one dose of methylprednisolone. Partial thrombosis of femoral artery was treated with streptokinase for 4 h and heparin for 24 h. Right bundle branch block was noted at first outpatient control after the catheterisation, confirmed on 24 h ECG Holter monitoring and persisted during one-year follow-up. Premature ventricular beats occurred the day after the procedure, in up to 8.4% of all beats, with one registered couplet. Propranolol was initially administered, and the therapy was omitted after three years, without deterioration of the finding on control 24 h ECG Holter monitoring. Severe complications, such as cAVB, need for urgent surgical intervention, or death, did not occur.
Mean follow-up (FU) period was 2.1 ± 1.4 years. Complete VSD closure was achieved in 48% of cases immediately after the intervention, 74% during 2.1 ± 1.6 months after the procedure, and 81% over FU. Remaining patients had trivial residual defects. Complete VSD closure rate depended on the type of device. Namely, patients with flexible device (proximal diameter of 6 mm) had complete occlusion rate of 92%, while patients with stiff device (proximal diameter of 8 mm) had closure rate of 63% during FU (p = 0.253).
During the follow up three patients had rhythm disturbances unnoticed before intervention, one had paroxysmal supraventricular tachycardia and two had premature ventricular beats. Two patients had episodes of headache. Over the FU, approximately one-third of the patients developed trace (0.5+) of aortic and mitral regurgitation, while the development of mild (1+) aortic and mitral regurgitation was noticed only once (p = 0.034 and p = 0.011, respectively) (Fig. 2). Half of the patients acquired trace/mild tricuspid regurgitation (p = 0.105) (Fig. 2). Nevertheless, the end-diastolic diameter of left ventricle showed statistically significant decrease over FU compared to period before intervention (p = 0.005), as well as the size of left atrium (p = 0.005) and left atrial/aortic root ratio (p = 0.005) (Fig. 3).
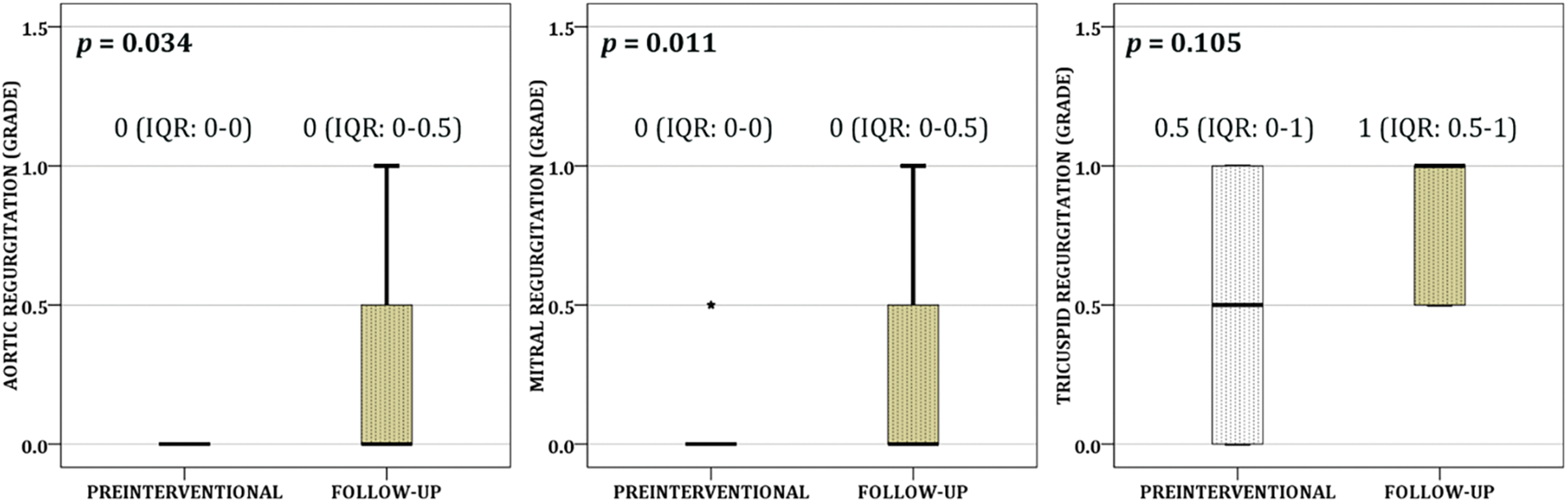
Figure 2: Development of the trace/mild aortic, mitral, and tricuspid regurgitation were demonstrated over the follow up (each box shows median and interquartile range (the box length), while asterisk represents value outside of the 1.5 box length)
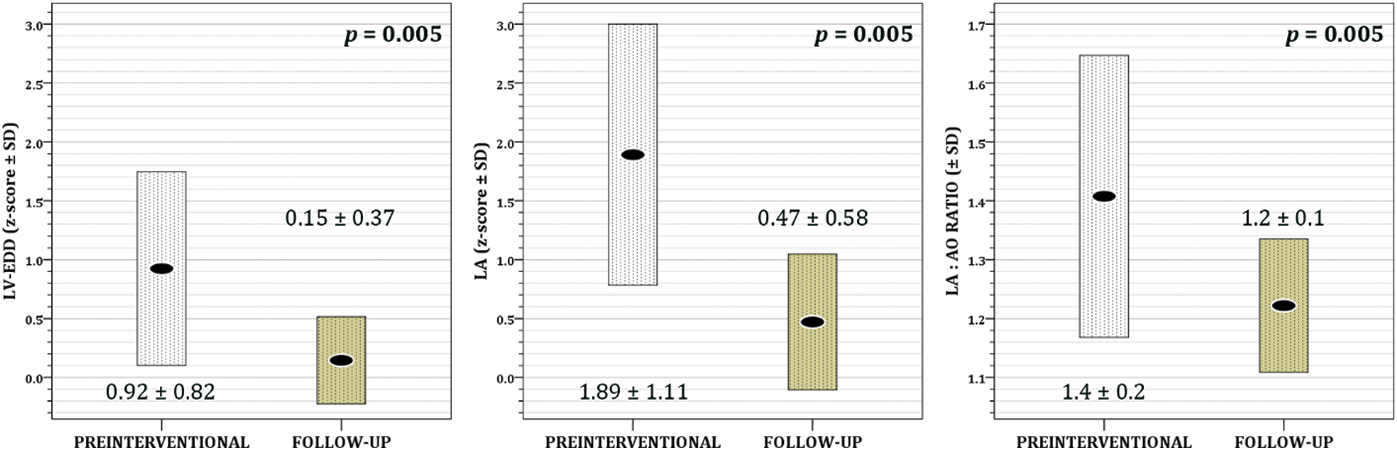
Figure 3: The effect of transcatheter VSD closure on left ventricular end-diastolic diameter (LV-EDD) z-score, left atrium diameter (LA) z-score and left atrium/aortic root (LA:Ao) ratio (each box represents mean value ± standard deviation)
Since pmVSD is the single most common CHD, its transcatheter closure was always a procedure of great interest to interventional cardiologists. This especially holds true for asymptomatic children with borderline VSD and signs of left chambers volume overload, in whom the open-heart surgery is questionable and in whom the transcatheter closure offers significant advantages, such as reduced psychological impact, no scar, shorter hospital stays, faster time to normal activities, etc. [1,3].
Although it has been nearly 35 years since Lock, in 1988, described the first successful transcatheter pmVSD closure, the ideal device is yet to be developed [7]. During the last three decades, many devices, such as Rashkind umbrella device, Starflex device, and Amplatzer duct occluders (ADO I and ADO II), have been used for pmVSD closure. Initially, large delivery sheaths were required, complex implantation techniques were needed, and interference with aortic and tricuspid valves and significant residual shunting were quite frequent [2]. Nonetheless, every new generation of devices was significantly better than the previous ones. The introduction of an Amplatzer membranous VSD device in the second half of the 1990s made the procedure significantly less risky to perform, increased the closure rate and reduced the valve complications, enabling the results to be comparable to the surgical ones [2,4]. Recently developed Nit-Occlud Lê VSD coil we used to close pmVSDs in our patients has many features which make it close to an ideal closing device. It is relatively easy to apply through a small venous sheath, suitable for different forms of VSD, retrievable, allows repositioning and the closure rate is high.
During the five-year period in our study, 25 interventions of VSD closure with a PFM device were performed. It is our policy not to close small VSD in patients who are asymptomatic and do not have unquestionable left chambers enlargement. On the other hand, in patients with left-side volume overload, closure is necessary in order to prevent pulmonary arterial hypertension, ventricular dysfunction, arrhythmias, aortic regurgitation and development of double-chambered right ventricle [2]. Immediate complete occlusion in our study (48%) is comparable to multicenter study on PFM device occlusion published by Haas (48%), European Registry study of Amplatzer-like discs (65%) and investigation by Yang et al., who mostly used the so-called Shanghai modification of original Amplatzer pmVSD disc (53%) [1,2,4]. Considering follow-up results after Nit-Occlud Lê VSD coil implantation, we showed complete closure in 74% and 81% of patients over two months and two years after the procedure, respectively. Hass et al. reported complete occlusion rates of 89%, 95%, and 99% three months, one, and two years after device implantation, while Odemis et al. showed occlusion rate of 85%–90% over 6–12 months FU (Table 2) [4,5,8]. We showed that the complete closure rate depends on the stiffness of the device (diameter of the proximal end). Although important, this finding in our group did not achieve statistical significance, presumably due to an insufficient number of patients. In our opinion, the main reason for different complete closure rates is based on the facts that VSDs occluded with “stiff” devices (proximal diameter of 8 mm) were initially larger and that “stiff” devices might be less adjusting, leaving the loops slightly less twisted and enabling a possibility for residual shunts, even beside the adequate size of the device. Therefore, the less favorable outcome in our group of patients, with respect to the persistence of a trivial residual shunt, might be explained by the fact that we used “stiff” device in 36% of patients, while it was used in 15% and only 5% of patients in the Haas and Odemis study, respectively. This is in agreement with a French study in which “stiff” device was used in 62% of patients, and residual shunt persisted in 15% of patients during 27 months FU [9]. However, Kozlik-Feldmann showed residual shunt in 46% and 31% of patients three months and two years after device implantation, respectively (Table 2) [10].
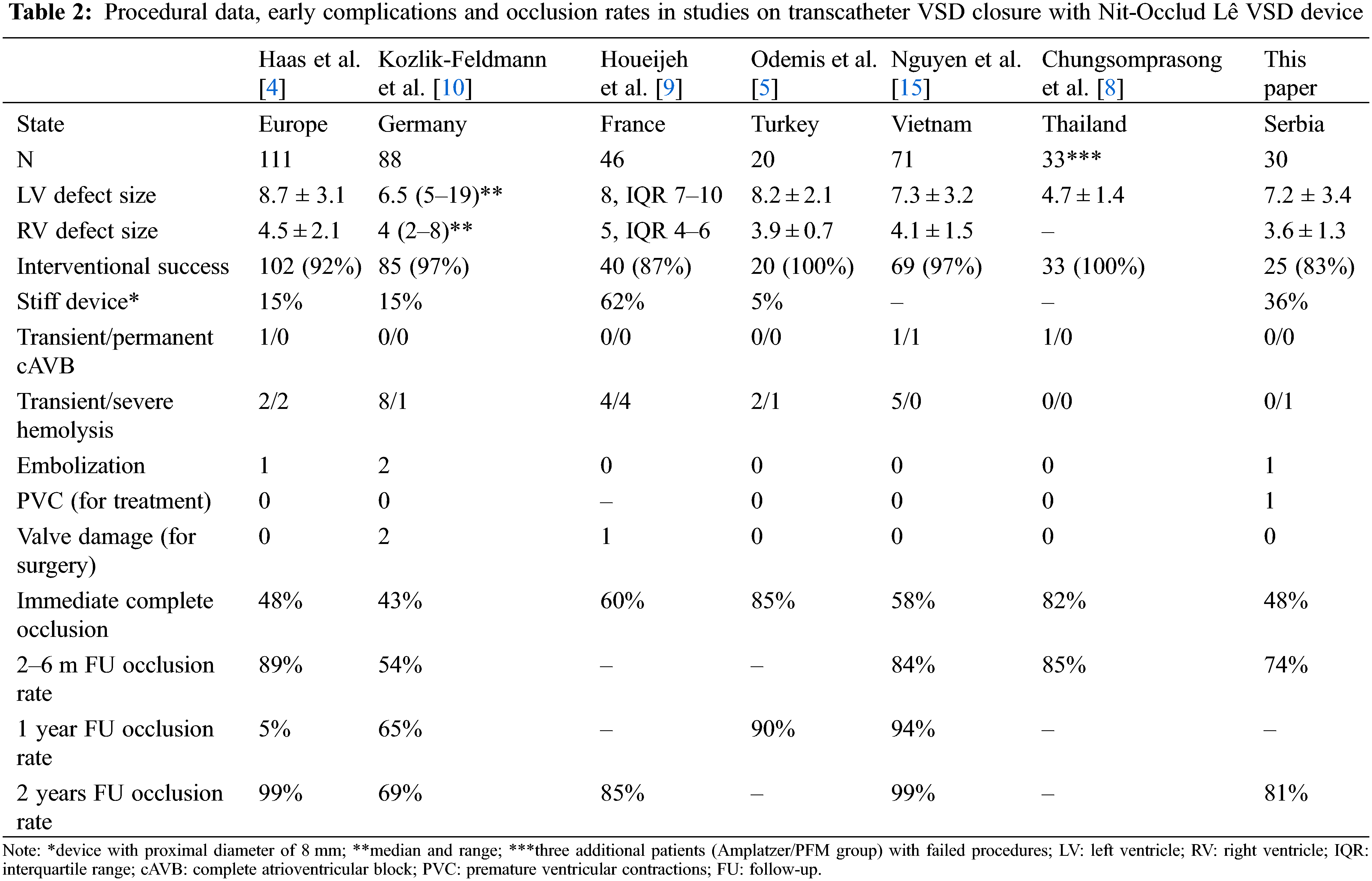
The percentage of post-interventional immediate and late permanent cAVB of nearly 4.8% after VSD closure with Amplatzer device was unacceptably high which, in most European centers, resulted in the abandonment of the procedure [2,4]. However, recent data from the multicentric study based on mostly Amplatzer device VSD occlusion showed a decrease in the occurrence of cAVB [11]. Chinese data suggest that rigidity of the discs and increasing clamping forces due to a very short distance between them might be the two most important causes of post-interventional cAVB [1]. Others believe that the concept of stenting and clamping the defect, per se, is responsible for the development of post-interventional cAVB, because of mechanical pressure exerted on surrounding tissue [2,4,5]. However, ADO II and Konar-MF occluders are promising devices for transcatheter pmVSD closure, with advantages in device softness, designed to provide high conformability to septal defects with lower risk of heart block [12–14]. Contrary to the “stent and clamp” occluding concept, flexible Nit-Occlud Lê VSD coil closes the hole by filling it, therefore it does not exert mechanical pressure on surrounding tissue. Closure with the coil is additionally achieved by its reinforced distal (left) loops covered with polyester fibers. Consequently, the risk of post-interventional cAVB is minimal and no permanent cAVB (with exception of one case from Vietnam) has been reported, after approximately 400 procedures having been described so far worldwide (Table 2) [4,5,8–10,15]. Similar to others, we found no cAVB after the procedure. Thus, Nit-Occlud Lê VSD offers the occlusion rate similar to Amplatzer-like devices, but at a higher safety level [4].
Hemolysis appears to be the single most important complication after PFM device VSD closure, with an incidence of 8% (Table 2) [4,5,8–10,15]. It is more frequent with PFM device than with Amplatzer disc, because the closure of a moderate/large VSD with PFM device is more likely to end with significant residual shunt, due to the design and softness of the device. Hemolysis is transitory in most cases (5.6%) and can be managed conservatively. Severe cases (2.4%) require placement of a second device or surgical explantation [16]. In the Haas study, 4/102 patients (3.9%) developed hemolysis. In 2 patients (1.9%) it resolved spontaneously, one needed surgical explantation, and one required a second device, same as the patient in our study.
Overall rate of embolization of the Nit-Occlud Lê VSD coil is 1% (Table 2) [4,5,8–10,15]. We had one patient with device embolization into pulmonary artery, which was resolved by retrieving the devices with a snare, as previously described [10].
With respect to conduction abnormalities and arrhythmias, we found one new-onset right bundle branch block (RBBB), which is in concordance with previously reported data. One patient with suboptimal coil position had a trivial residual shunt, a new-onset trace of aortic regurgitation (AR) and frequent premature ventricular contractions (PVCs) that needed anti-arrhythmic therapy. Of note is that frequent PVCs that would need pharmacological treatment after Nit-Occlud Lê VSD coil implantation were not reported so far. To avoid such complications, optimal coil positioning and configuration is mandatory. Finally, one patient had a paroxysmal supraventricular tachycardia (PSVT) attack one year after the PFM coil implantation. PSVT attacks did not recur after initiation of anti–arrhythmic therapy, but this also is an event that has not been previously reported regarding PFM device VSD closure follow-up.
Development of trivial aortic and mitral regurgitation was observed in approximately one third our patients, and half of the patients acquired trace/mild tricuspid regurgitation. In their multicenter study, Haas et al. reported also a trivial aortic and tricuspid post-interventional incompetence. Persistent trivial/mild AR post PFM device implantation was also observed by Chungsomprasong et al. [8]. The incidence of aortic and tricuspid valve damage that require surgery is less than 1% (Table 2) [4,5,8–10,15]. Absence of the important valvar incompetence was explained by the fact that PFM device is flexible and does not affect the valves significantly. Be that as it may, the frequency of new-onset AR, although it was only trivial/mild, is of concern. The real significance of AR requires longer follow-up and analysis of progression of the regurgitation, until then, it remains an obstacle in this technique and deserves attention. Explanation for the new-onset aortic regurgitation might be in affecting the valve by implantation technique (i.e., AV loop formation and crossing the aorta by the coil). Additionally, released device can “capture” the chords of tricuspid valve and change the anatomical relations in the heart [9]. However, ADO II and Konar-MF occluders can be introduced in flexible retrograde approach, without establishment of AV loop, which can avoid tricuspid and aortic valve injuries [14].
Introduction of Nit-Occlud Lê VSD device into clinical practice has made a significant step forward towards transcatheter small/moderate VSD occlusion as a first method of choice. Data presented in this study demonstrate that transcatheter VSD closure with PFM device is feasible in significant number of patients, with good initial success, acceptable follow-up occlusion rates and minimal risks and complications. The most important effect is normalization in size of both left ventricle and left atrium. Namely, acquired trivial valvar insufficiency is hemodynamically much less important than left-right shunt across the VSD. The findings of this study can be used to improve patient selection in order to obtain successful pmVSD closure with Nit-Occlud Lê VSD coil and to predict potential procedure-related immediate and medium-term complications. Moreover, one might consider transcatheter closure for achieving normality (including ability for sport activity) in patients with borderline dilatation of the left heart cavities and significant systolic murmur. More patients and longer follow-up in the years to come will define the real role of this device in transcatheter VSD closure.
Acknowledgement: Sincere gratitude is extended to the Proctor, Dr. Trong Phi Le, for cooperation and the feedback.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Prijić S, Vukomanović V, Košutić J, Cerović I, Stajević M, Ninić S; data collection: Cerović I, Popović S, Krasić S; analysis and interpretation of results: Prijić S, Košutić J, Vukomanović V, Dizdarević I; draft manuscript preparation: Cerović I, Košutić J, Prijić S. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and analyzed during the current study might be available if request is directed to corresponding author.
Ethics Approval: The study was performed in accordance with ethical standards of Helsinki Declaration and Ethical Committee of Mother and Child Health Institute of Serbia (Approval Number 08/20/23). The written informed consent for interventions was obtained from the patients’ legal guardians.
Conflicts of Interest: The authors declare that they have no conflicts of interest regarding the present study.
References
1. Yang, J., Yang, L., Wan, Y., Zuo, J., Zhang, J. et al. (2010). Transcatheter device closure of perimembranous ventricular septal defects: Mid-term outcomes. European Heart Journal, 31(18), 2238–2245. https://doi.org/10.1093/eurheartj/ehq240 [Google Scholar] [PubMed] [CrossRef]
2. Carminati, M., Butera, G., Chessa, M., de Giovanni, J., Fisher, G. et al. (2007). Investigators of the european VSD registry. Transcatheter closure of congenital ventricular septal defects: Results of the european registry. European Heart Journal, 28(19), 2361–2368. https://doi.org/10.1093/eurheartj/ehm314 [Google Scholar] [CrossRef]
3. Kosutic, J., Stajevic, M., Sehic, I. et al. (2010). Indikacije i optimalan uzrast za hiruršku korekciju izolovanog otvora međukomorske pregrade. In: Zdravkovic, D. (Ed.Issues in paediatrics, vol. 31, pp. 321–328. Belgrade: Zavod za udzbenike i nastavna sredstva [in Serbian]. [Google Scholar]
4. Haas, N. A., Kock, L., Bertram, H., Boekenkamp, R., de Wolf, D. et al. (2017). Interventional VSD-closure with the Nit-Occlud® Lê VSD-coil in 110 patients: Early and midterm results of the EUREVECO-registry. Pediatric Cardiology, 38(2), 215–227. https://doi.org/10.1007/s00246-016-1502-8 [Google Scholar] [PubMed] [CrossRef]
5. Odemis, E., Saygi, M., Guzeltas, A., Tanidir, I. C., Ergul, Y. et al. (2014). Transcatheter closure of perimembranous ventricular septal defects using Nit-Occlud® Lê VSD coil: Early and mid-term results. Pediatric Cardiology, 35(5), 817–823. https://doi.org/10.1007/s00246-013-0860-8 [Google Scholar] [PubMed] [CrossRef]
6. PFM medical (2014). Nit-Occlud® Lê VSD. https://www.pfmmedical.com/productcatalogue/occluder/nit_occludr_le_vsd/index.html [Google Scholar]
7. Lock, J. E., Block, P. C., McKay, R. G., Baim, D. S., Keane, J. F. (1988). Transcatheter closure of ventricular septal defects. Circulation, 78(2), 361–368. https://doi.org/10.1161/01.CIR.78.2.361 [Google Scholar] [PubMed] [CrossRef]
8. Chungsomprasong, P., Durongpisitkul, K., Vijarnsorn, C., Soongswang, J., Lê, T. P. (2011). The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil. Catheterization and Cardiovascular Interventions, 78(7), 1032–1040. https://doi.org/10.1002/ccd.23084 [Google Scholar] [PubMed] [CrossRef]
9. Houeijeh, A., Godart, F., Jalal, Z., Ovaert, C., Heitz, F. et al. (2020). Transcatheter closure of a perimembranous ventricular septal defect with Nit-Occlud Lê VSD coil: A French multicentre study. Archives of Cardiovascular Diseases, 113(2), 104–112. https://doi.org/10.1016/j.acvd.2019.11.004 [Google Scholar] [PubMed] [CrossRef]
10. Kozlik-Feldmann, R., Lorber, A., Sievert, H., Ewert, P., Jux, C. et al. (2021). Long-term outcome of perimembranous VSD closure using the Nit-Occlud® Lê VSD coil system. Clinical Research in Cardiology, 110, 382–390. https://doi.org/10.1007/s00392-020-01750-6 [Google Scholar] [PubMed] [CrossRef]
11. Yang, L., Tai, B. C., Khin, L. W., Quek, S. C. (2014). A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). Journal of Interventional Radiology, 27(3), 260–272. https://doi.org/10.1111/joic.12121 [Google Scholar] [PubMed] [CrossRef]
12. Tanidir, I. C., Baspinar, O., Saygi, M., Kervancioglu, M., Guzeltas, A. et al. (2020). Use of lifetech™ konar-MF, a device for both perimembranous and muscular ventricular septal defects: A multicentre study. International Journal of Cardiology, 310, 43–50. https://doi.org/10.1016/j.ijcard.2020.02.056 [Google Scholar] [PubMed] [CrossRef]
13. Haddad, R. N., Daou, L. S., Saliba, Z. S. (2020). Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: Midterm outcomes of the first middle-eastern experience. Catheterization and Cardiovascular Interventions, 96(3), E295–E302. https://doi.org/10.1002/ccd.28678 [Google Scholar] [PubMed] [CrossRef]
14. Sun, H., Luo, G., Du, Z., Ji, Z., Pan, S. (2021). Transcatheter closure of perimembranous ventricular septal defect using the amplatzer duct occluder II. Congenital Heart Disease, 16(2), 151–157. https://doi.org/10.32604/CHD.2021.014770 [Google Scholar] [CrossRef]
15. Nguyen, H. L., Phan, Q. T., Dinh, L. H., Tran, H. B., Won, H. et al. (2018). Nit-Occlud Lê VSD coil versus duct occluders for percutaneous perimembranous ventricular septal defect closure. Congenital Heart Disease, 13(4), 584–593. https://doi.org/10.1111/chd.12613 [Google Scholar] [PubMed] [CrossRef]
16. Bulut, M. O., Küçük, M., Ballı, Ş., Çelebi, A. (2016). Treatment of severe hemolysis following Nit-Occlud Lê VSD coil implantation with amplatzer duct occluder II. Archives of the Turkish Society of Cardiology, 44(7), 593–596. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools