 Open Access
Open Access
REVIEW
The Prevalence of Congenital Heart Disease among School-Age Children in China: A Meta-Analysis and Systematic Review
1 Department of Public Health, Medical College, Qinghai University, Xining, China
2 School of Math and Statistics, Qinghai Minzu University, Xining, China
3 Department of Cardiothoracic Surgery, Qinghai University Affiliated Hospital, Xining, China
4 Department of Cardiothoracic Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, China
5 Clinical Medical College, Qinghai University, Xining, China
* Corresponding Authors: Jirong Qi. Email: ; Huilian Yang. Email:
# Shuqin Zhang and Bin Zhang contributed equally to this work
Congenital Heart Disease 2023, 18(2), 127-150. https://doi.org/10.32604/chd.2023.025616
Received 22 July 2022; Accepted 19 September 2022; Issue published 15 March 2023
Abstract
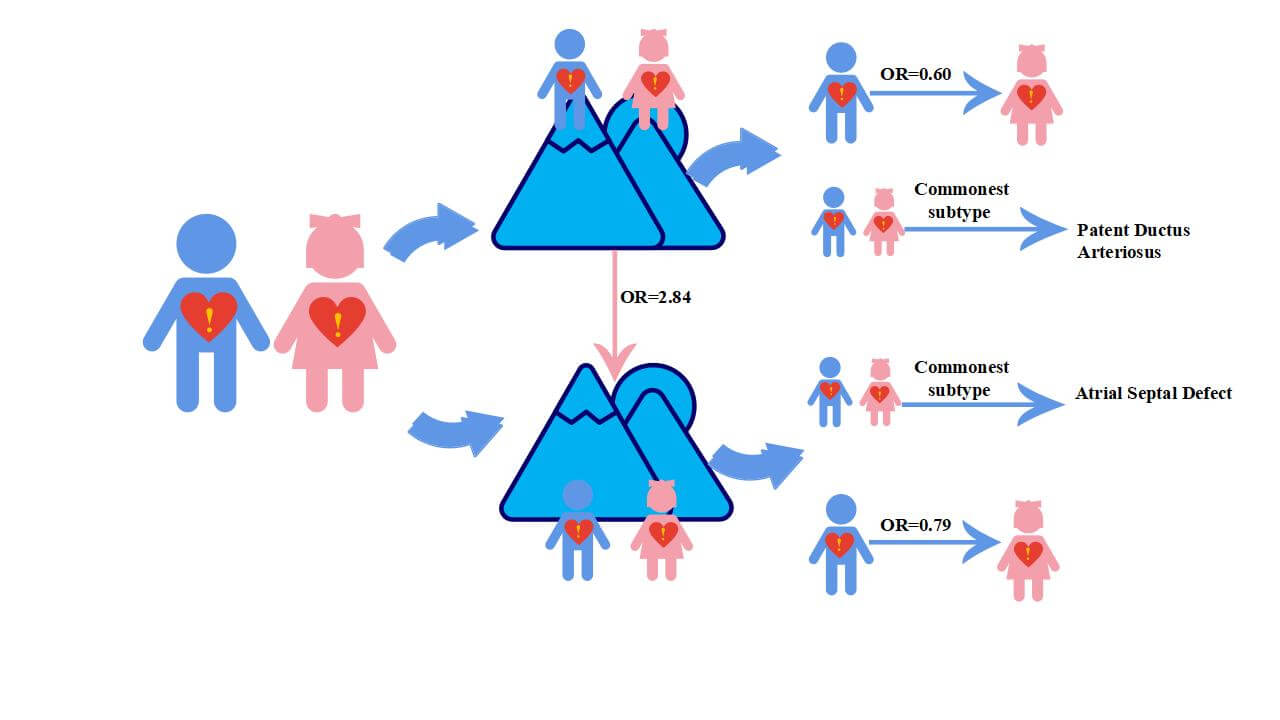
Objectives: To estimate the prevalence of Congenital Heart Disease (CHD) in school-age children, to identify the extent to which altitude affects the prevalence of the disease, and to examine trends in prevalence over time in China. Methods: Seven databases were systematically searched and last retrieved on September 10, 2021 for all studies reporting the prevalence of CHD in children after 1970 in China, which were then divided into high and low altitude regions based on 2500 meters above sea level. The random-effected model was used to combine prevalence data and subgroups analysis. The baseline data of all cases and individuals were used for comparison to calculate the odds ratio (OR) for overall and different altitude prevalence. Results: A total of 12,926,083 individuals (aged 3-18 years), with 31,835 cases from 86 studies, were included in the analysis. The pooled CHD prevalence of total children was 4.69 [95% confidence interval (CI): 4.10 to 5.29] per 1000 children. Overall, temporal trends analysis indicated that the prevalence of CHD in children continuously decreased with time, from 6.19 (95% CI: 4.50 to 7.88) per 1000 children in 1976–1985 to 3.30 (95% CI: 2.49; 4.38) per 1000 children in 2016–2021. The OR for the prevalence of CHD in children from high and low altitudes with baseline data was 2.84 (95% CI: 2.48 to 3.27) and 1.31 (95% CI: 1.13 to 1.53) (χ2 = 53.89, p < 0.01), respectively. The OR of the prevalence of CHD in male children compared to females was 0.60 (95% CI: 0.53 to 0.68) at high altitudes and 0.79 (95% CI: 0.71 to 0.89) at low altitudes. Among the seven most common subtypes, patent ductus arteriosus was the most common at high altitudes, while atrial septal defects were the most common at low altitudes. Conclusion: This study provides valuable insights for further disease prevention and etiological exploration. The overall decreasing trend in the prevalence of CHD in children over time may indicate a positive effect of perinatal management and treatment during infancy.Graphic Abstract
Keywords
Congenital Heart Disease (CHD) is defined as a functionally significant structural heart or intrathoracic great vessels disease present at birth [1]. This, in fact, is the most common congenital disability accounting for approximately one-third of all major congenital anomalies. The reported prevalence of CHD substantially varies worldwide, ranging from 0.6%–9.4% [2,3]. This discrepancy can be attributed to the age of diagnosis, the definition of the disease (e.g., whether or not patent foramen ovale was classified as CHD), and the diagnostic technique [4,5]. The prevalence of CHD seems to be constantly changing as diagnostic technology evolves and diagnostic criteria are being updated.
China is the country with the highest burden of CHD worldwide. It is also a country with diverse topography, vast area, and uneven economic development [6–8]. Early studies indicated that the prevalence of CHD differs in gender, geographical factors, economic status, and similar [7,9]. In their study, Zhao et al. extended the regime to study the prevalence of CHD at live birth in China [6]. In contrast to studies of CHD live births conducted with large birth registries, sample sizes for studies of school-age children with CHD are usually derived from cross-sectional surveys with relatively modest sizes. Moreover, individual studies of CHD prevalence in children reported widely varying rates, ranging from 0.70% to 13.35% [10,11]. These factors limit the generalizability at the level of the whole country. Previous studies have also shown substantial differences in the prevalence of CHD disease in relation to altitude [12–14]. Therefore, a comprehensive study of the prevalence of CHD in children and exploration of the subgroup heterogeneity is necessary. Accordingly, in the present study, we examined the discrepancy based on the medically significant altitude of 2500 meters above sea level [15]. The altitude level above 2500 m was defined as high altitude and the altitude below 2500 m as low altitude.
The prevalence of CHD among the global school-aged between 1970 and 2017 was reported. This review article pointed common subtypes of unrepaired CHD and conducted subgroup analysis for different economics levels and genders [16]. Nonetheless, CHD prevalence among school-aged children in China hasn’t been analyzed separately. A summarized data on the prevalence of CHD among Chinese school-aged is missing. Furthermore, altitude is a factor that has a significant influence on the occurrence of vascular disease, however, the altitude impact to CHD prevalence is unclear. To fill this gap, we perform this study to estimate the prevalence of CHD of school-age children in China, to identify the extent to what altitude affects the prevalence of the disease, and to examine trends of the prevalence over time in China between 1970 and 2021.
The systematic review and meta-analysis were conducted with the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17,18]. The protocol was preregistered on the International Prospective Registered of Systematic Reviews (PROSPERO: CRD42021277019).
We systematically reviewed publications that reported on the prevalence of total CHD among school-age children (3–18 years old). Relevant publications were retrieved by searching PubMed, Web of Science, Embase, China Biology Medicine disc database, Wanfang database, China National Knowledge Infrastructure, and Weipu on August 31, 2021. We used the following search terms (formatted for PubMed search): (“Heart Defect, congenital” [Mesh] OR “Heart Abnormality” [ti/ab] OR “Congenital Heart Disease” [ti/ab] OR “Congenital Heart Defect” [ti/ab] OR “Heart Abnormalities” [ti/ab]) AND (“Prevalence” [Mesh] OR” Period Prevalence” [ti/ab] OR “Point Prevalence” OR “Epidemiology” [Mesh] OR “Social Epidemiology” [ti/ab] OR “Epidemiology, social” [ti/ab] OR “Incidence” [Mesh] OR “Incidence proportions” [ti/ab] OR” Incidence Rate” [ti/ab]) AND (“China” [Mesh] OR “People’s Republic of China”[All Fields] OR “Mainland China”[ti/ab] OR “Chinese” [All Fields]). Studies published before 1970 were excluded from the analysis.
The articles that met the following criteria were included: (a) participants were Chinese school-age children (3–18 years old); (b) the publications were in Chinese or English language; (c) the relevant data on CHD prevalence or its subtypes among school-age children could be extracted; (d) articles were published after 1970; (e) quality assessment JBI-PCAT (Joanna Briggs Institute–Prevalence Critical Appraisal Tool) score ⩾ 4. Studies that reported on CHD prevalence in specific children, such as handicapped children, were excluded.
2.4 Data Extraction and Quality Assessment
Two authors (Shuqin Zhang and Jin Luo) who independently performed reviewing were responsible for data extraction and quality evaluation. Any disagreements were solved through discussion. The main information extracted from the literature contained title, publication year, investigation time, altitude, geographic region, common subtypes, total subjects, and CHD cases. We used the JBI-PCAT evaluation tool to appraise the methodological quality of the literature, which was specifically developed for systematic reviews of prevalence data [19,20]. The JBI-PCAT contains nine aspects of the problem, which are answered with Yes, No, Unclear, and Not applicable. According to the number of answers “Yes”, we classified the studies into three levels, i.e., high quality ⩾6; moderate quality 4–5; low quality ⩽3. Low-quality studies were discarded seen in Supplementary Table 1.
R (4.1.1) Meta package was used for data analysis. For heterogeneity analysis, we applied the Cochran Q test (p < 0.1 indicated significant difference) to make statistical inference and forest plot to perform statistical description; I2 was further used to quantify the size of heterogeneity. I2 > 50%, indicated significant heterogeneity, and the random-effect model was selected to combine the effect size with 95% CI. Egger’s regression test and the funnel plot were used to analyze publication bias. The odds ratio (OR) for CHD prevalence was calculated for each study using the pooled data from the included studies (containing all cases and individuals) as baseline data. Subtype analysis was conducted based on altitude (altitude > 2500 m or altitude ⩽ 2500 m), gender (male or female), income level (low, lower-middle, upper-middle, high), and geographic region (Central region, North region, Northeast region, Eastern region, South region, Southwest region, Northwest region). The Chi-square test was used to compare the prevalence of total CHD among subgroups in school-age children, using the Bonferroni method to adjust p values. p < 0.05 indicated statistically significant difference. Time trends were plotted using the Cubic spline smoothing technique. We divided income groups into low income (⩽$1045), lower-middle-income ($1046 to $4095), upper-middle income ($4096 to $12695), and high income (⩾$12,696) according to World Bank Income Groups [21].
3.1 Literature Screening and Characteristics
The initial search yielded 8,364 potential eligible publications from seven databases. After excluding duplicates, titles, abstracts, and full-text, 86 studies were finally included in the meta-analysis, involving 31,835 CHD cases and 12,926,083 children. Among these 86 studies, 59 were from low-altitude areas and 24 were from high-altitude areas, and 3 additional studies reported prevalence at both altitudes (Fig. 1). All the included studies were cross-sectional designs with a high JBI-PCAT score of 7.9 ± 1.4 (mean ± SD, rang 4–9). In most studies (93.1%), the main diagnostic tool was echocardiography; the remaining studies applied combinations of diagnostic tools, such as X-rays, physical examination, and electrocardiographs.
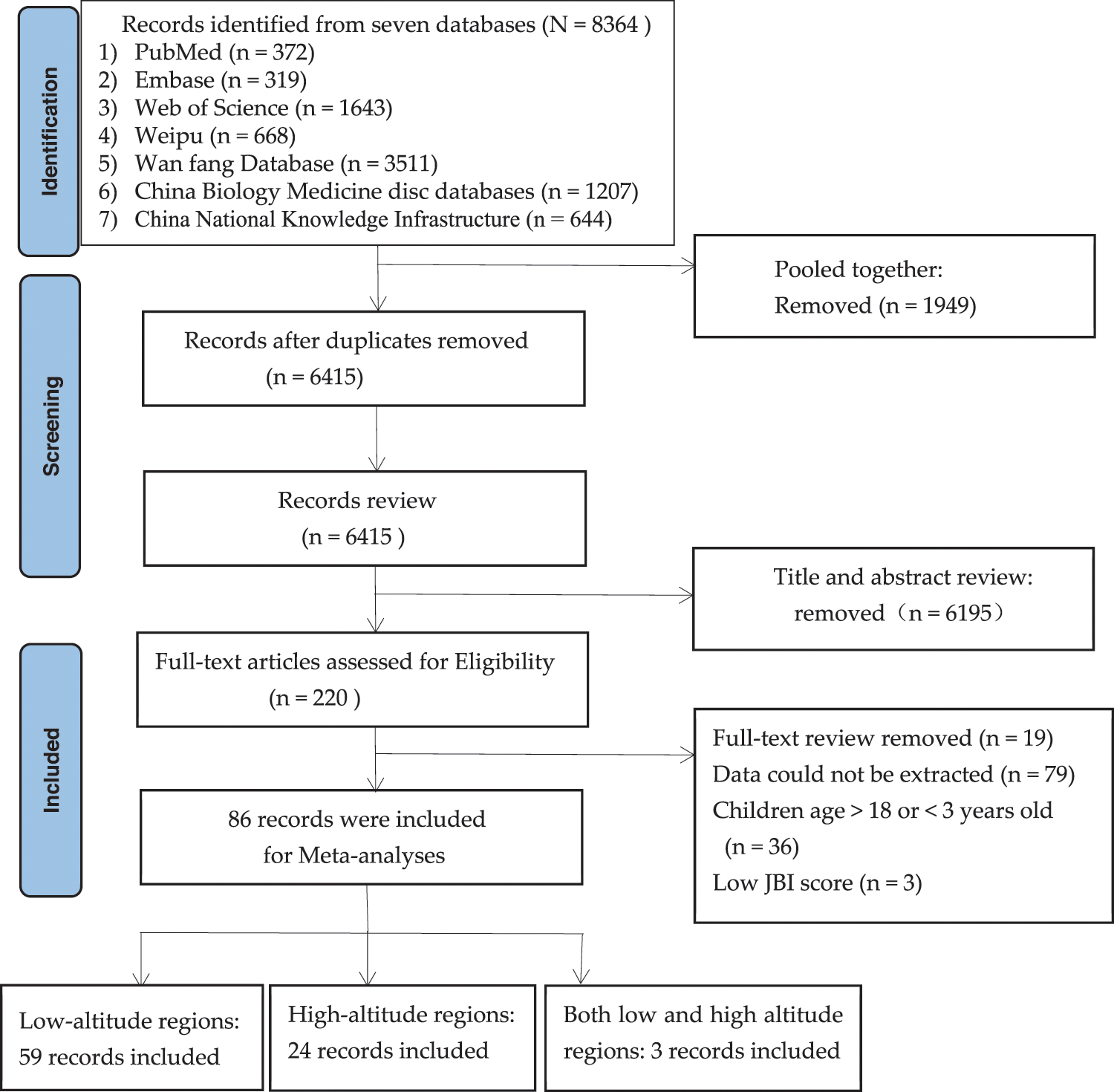
Figure 1: The PRISMA flow chart to identify and screen literature
3.2 Children’s CHD Prevalence Overall and Its Altitude Differences
A total of 86 papers were included in the study, of which 27 and 62 reported the prevalence of CHD at high and low altitudes, involving 1,434,399 children (CHD identified in 8,334 individuals) and 11,491,684 children (CHD identified in 23,501 individuals), respectively. The average overall prevalence of CHD in school-age children and that at high and low altitudes (per 1000 children) in China between 1976 and 2021 was 4.69 (95% CI: 4.10 to 5.29), 6.80 (95% CI: 5.65 to 8.05), and 3.20 (95% CI: 2.76 to 3.73), respectively.
Compared with the pooled OR [1.67 (95% CI: 1.45 to 1.91)] of 86 studies included, the OR for CHD prevalence in children at high altitude was 2.84 (95% CI: 2.48 to 3.27), and the OR for CHD prevalence in children at low altitude was 1.31 (95% CI: 1.13 to 1.53) (χ2 = 53.89, p < 0.01) (Fig. 2) (Supplementary Table 1).
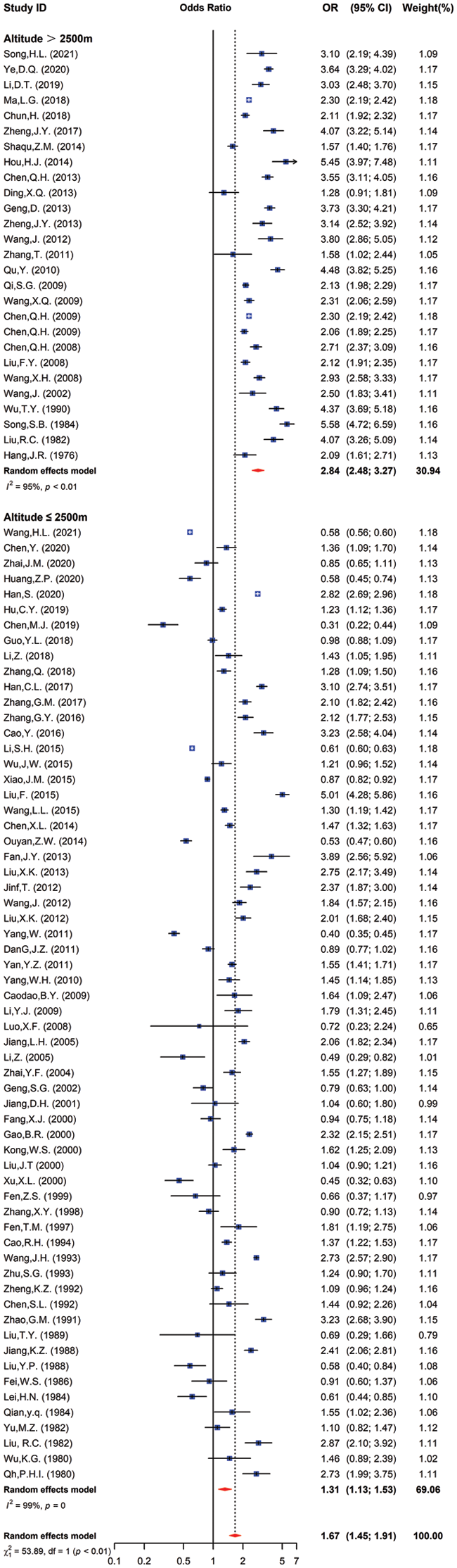
Figure 2: The odds ratio of CHD prevalence in children from different altitudes vs. all school children in China
3.3 Prevalence of CHD in Children over Time
As shown by time trend analysis, the overall trend in CHD prevalence in children decreased over time, except for a small rise between 2006 and 2015, from 6.19 per 1000 children in 1976–1985 to 3.30 per 1000 children in 2016–2021 (Fig. 3A). At the same time, a decreasing trend in CHD prevalence in children with was observed at different altitudes. At high altitudes, the prevalence of CHD children decreased from 10.01 per 1000 children in 1976–1985 to 6.25 per 1000 children in 2016–2021, and at low altitudes, the prevalence of CHD children decreased from 4.05 per 1000 children in 1976–1985 to 2.52 per 1000 children in 2016–2021. Of note, a steep decline was observed between 1986 and 1995 and a stable rise in later years in high-altitude regions (Fig. 3B).
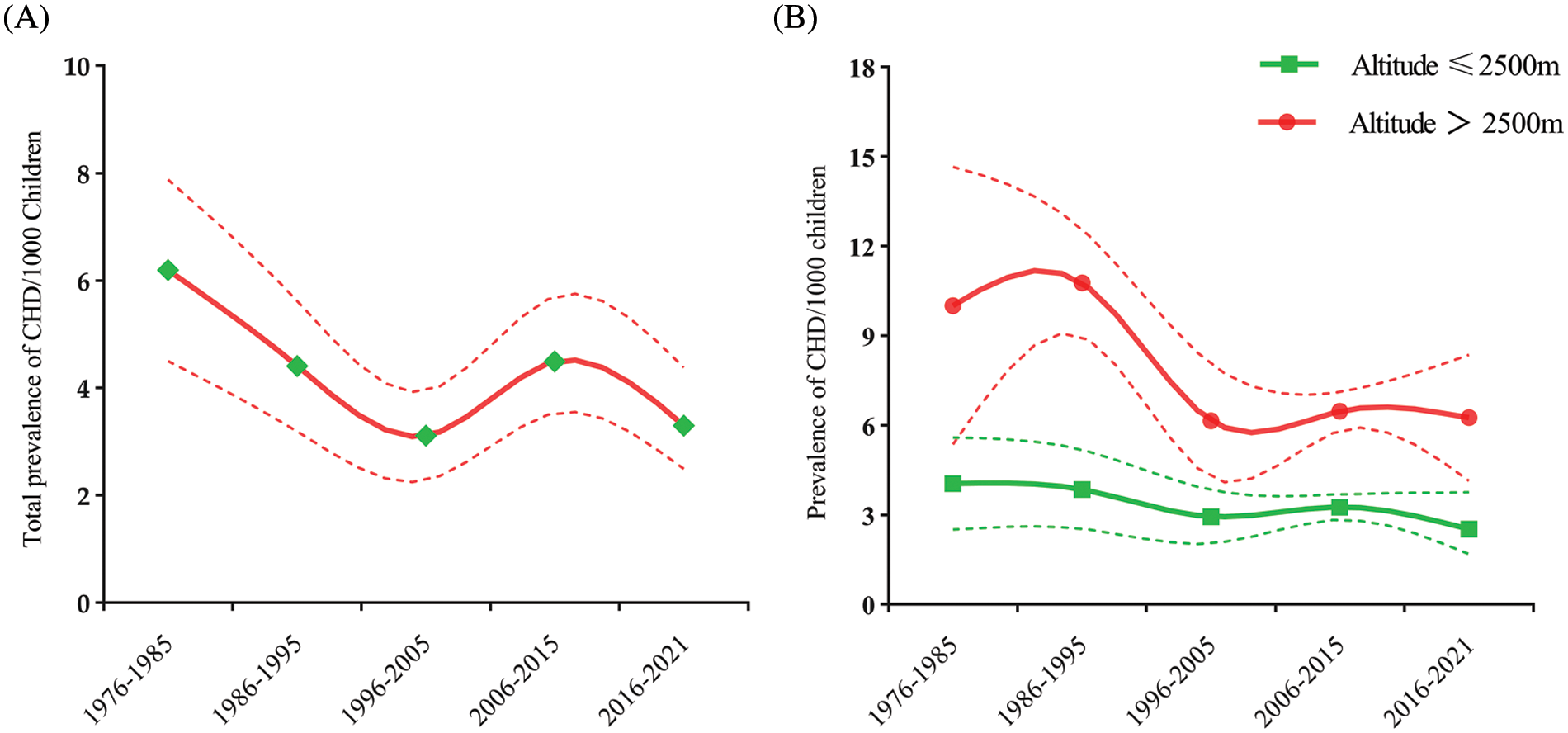
Figure 3: (A). Total CHD prevalence in children over time from 1976 to 2021 in China. The full line is the estimated overall prevalence of CHD; the dotted line represents the 95% CI. (B). The CHD prevalence in children over time at high and low altitude regions in China. The full line is the estimated prevalence of CHD; the dotted line represents the 95% CI
3.4 Total Prevalence of CHD in Children in Relation to Gender
A total of 49 studies reported on the total prevalence of CHD in school-age children in relation to gender, involving 1,755,808 males (CHD identified in 7,078 individuals) and 1,528,686 females (CHD identified in 8,267 individuals). Among these, 20 studies in high-altitude regions included 629,171 males (CHD identified in 2,989 individuals) and 555,253 females (CHD identified in 4,047 individuals), and 29 studies in low-altitude regions, included 1,126,637 males (CHD identified in 4,089 individuals) and 973,433 females (CHD identified in 4,220 individuals).
Reported total CHD prevalence in school-age children was 3.97 per 1000 children (95% CI: 3.47 to 4.53) among males, and 5.90 per 1000 children (95% CI: 5.08 to 6.87) among females (χ2 =141.32, p < 0.001). In both high and low-altitude areas, the prevalence of CHD was significantly lower in male children than in females (Fig. 4A). Additionally, the OR for male compared with female CHD children was 0.60 (95% CI: 0.53 to 0.68) at high altitudes (Fig. 4B) and 0.79 (95% CI: 0.71 to 0.89) at low altitudes (Fig. 4C) (Supplementary Table 1).
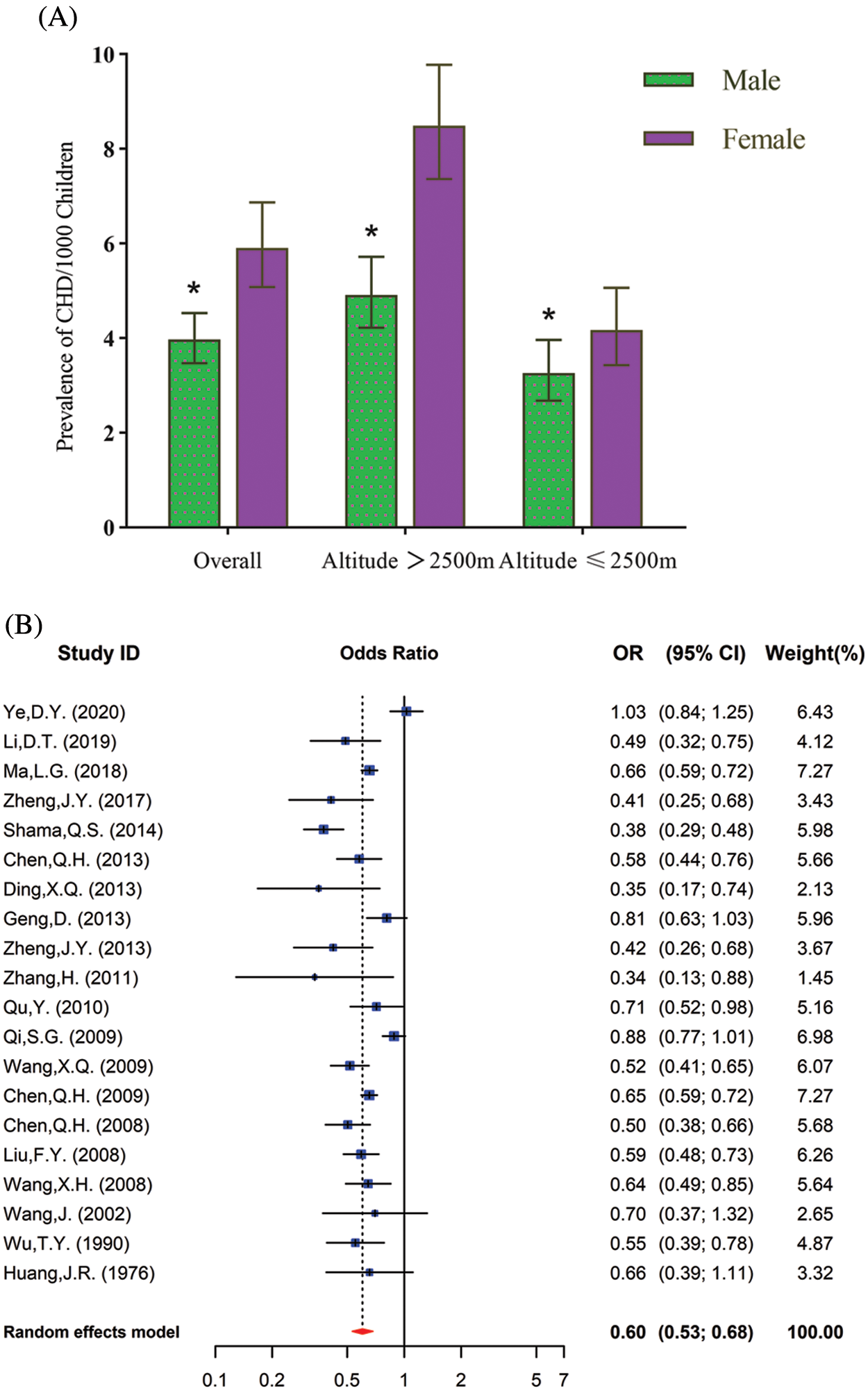
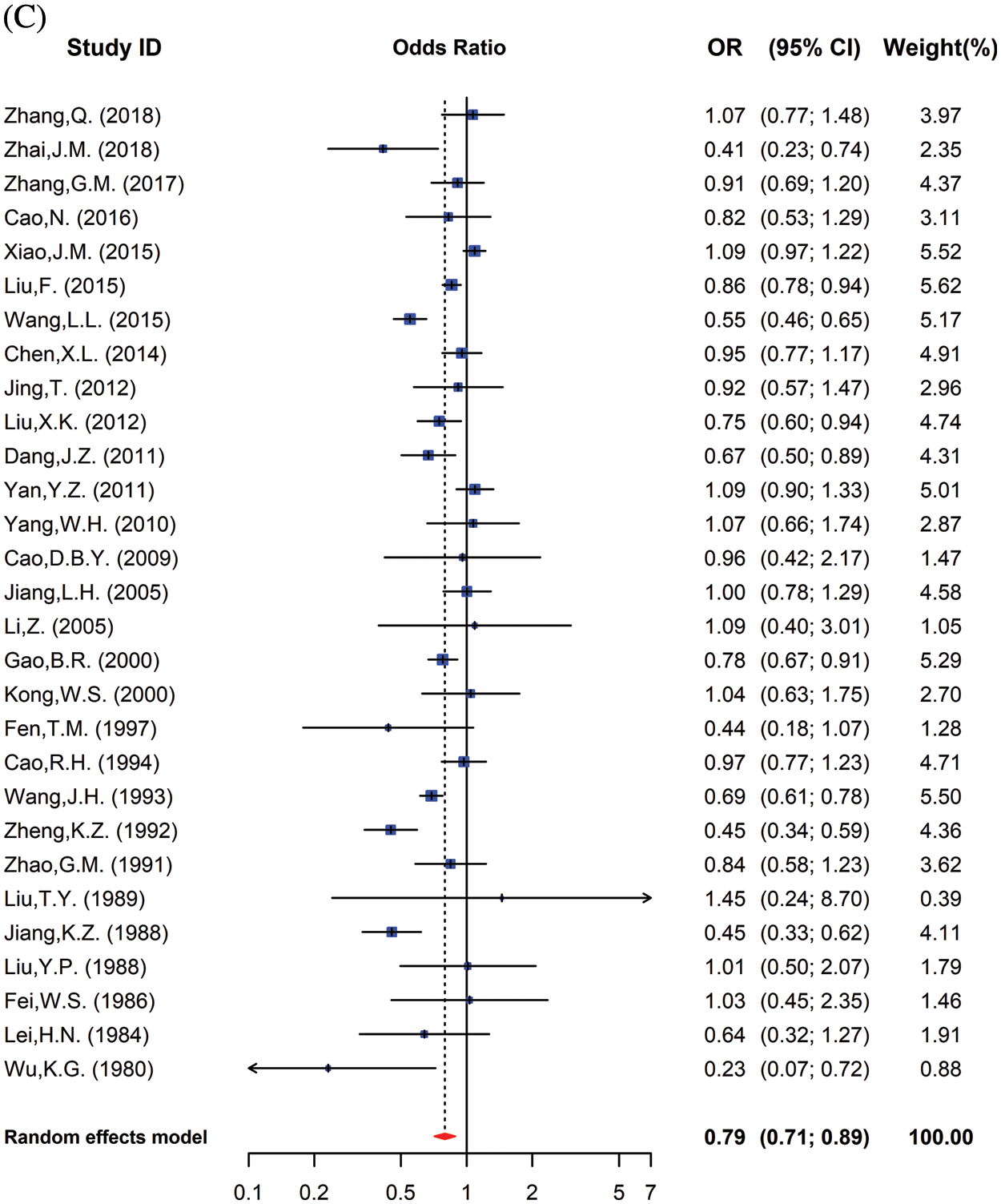
Figure 4: (A). The CHD prevalence in children from the overall high-altitude and low-altitude regions in China in relation to gender. Data are presented as Mean ± SE. *, p < 0.05, compared with different genders. (B). Meta-analysis of risk of CHD prevalence in male and female school children in high altitudes. Values <1 reflect the lower proportion of males. (C) Meta-analysis of risk of CHD prevalence among male and female school children in low altitudes. Values <1 reflect the lower proportion of males
3.5 Prevalence of Common Subtypes of CHD in Children
The CHD prevalence in children and percentage of the 7 most common subtypes are shown in Table 1, and the cumulative percentages of the 7 most common children CHD subtypes, overall and at high and low altitude are shown in Fig. 5A. Overall, the reported prevalence in CHD subtypes among children (per 1000 children) was as follows: VSD, 1.19 (95% CI: 1.02 to 1.38); ASD, 1.52 (95% CI: 1.24 to 1.83); PDA, 0.96 (95% CI: 0.77 to 1.17); PS, 0.11 (95% CI: 0.08 to 0.14); TOF, 0.10 (95% CI: 0.08 to 0.14); TGA, 0.03 (95% CI: 0.01 to 0.04); COA, 0.11 (95% CI: 0.04 to 0.21). The prevalence (per 1000 children) of 3 commonest CHD subtypes in children were PDA [2.7 (95% CI: 2.09 to 2.37)], ASD [2.32 (95% CI: 2.02 to 2.63)], and VSD [1.13 (95% CI: 0.88 to 1.41)] in high altitude areas, while in low altitude areas they were ASD [2.32 (95% CI: 2.02 to 2.63)], VSD 1.22 (95% CI: 1.01 to 1.45)], and PDA [0.49 (95% CI: 0.39 to 0.61)] (Fig. 5B), respectively.
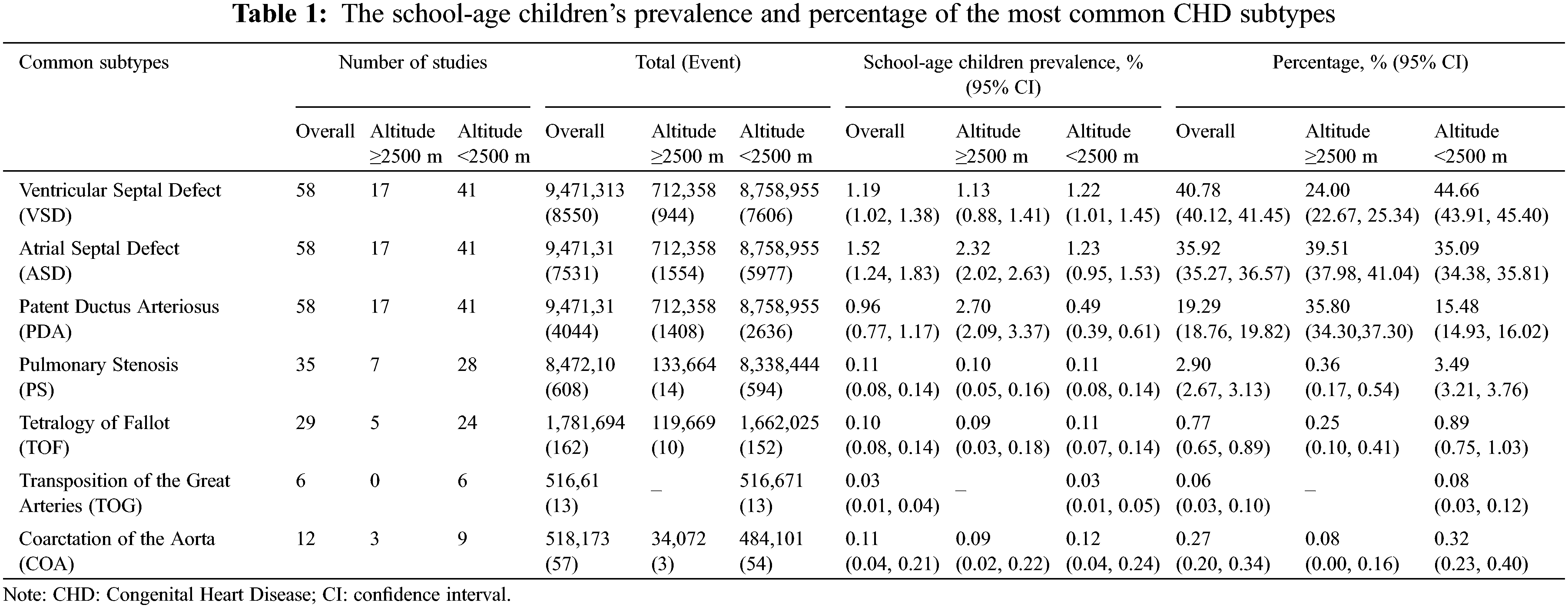
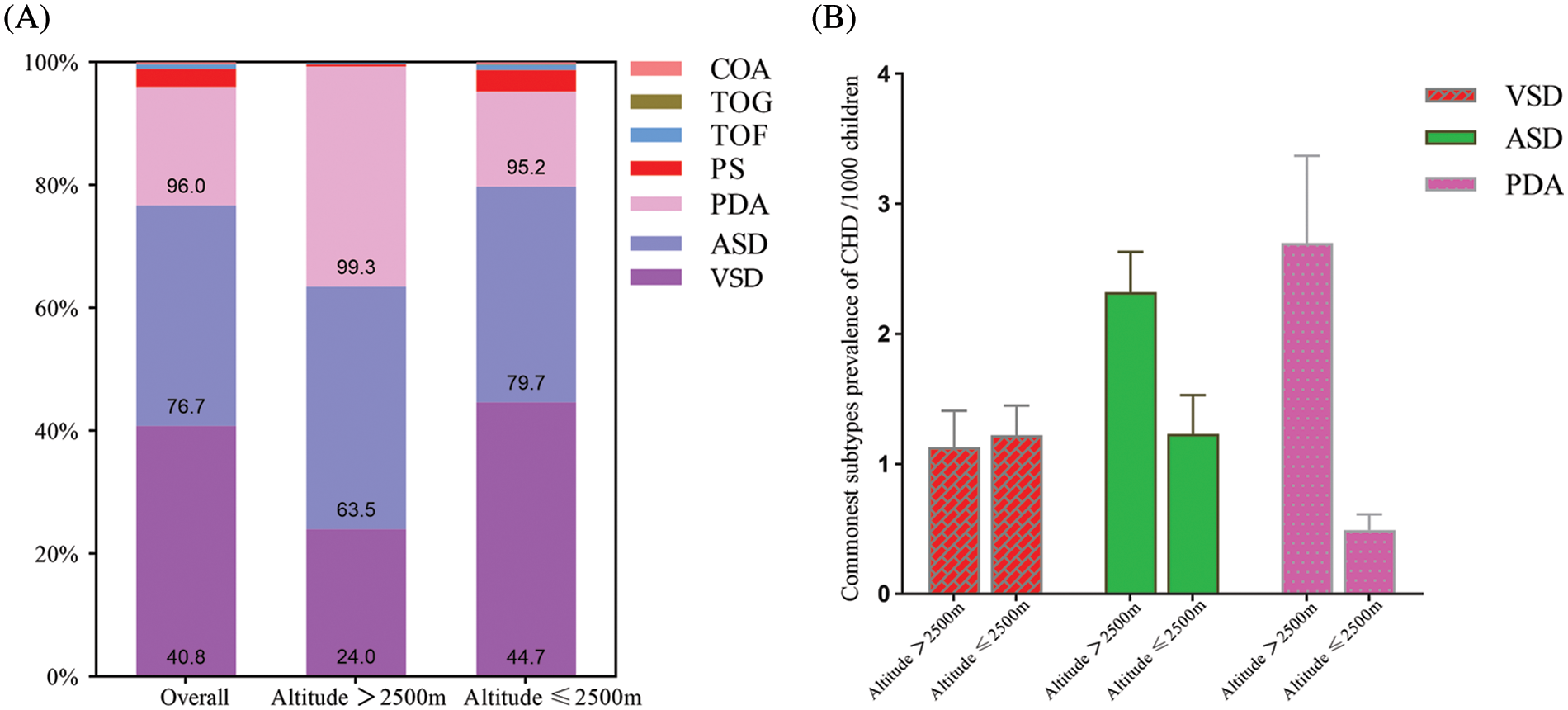
Figure 5: (A) Seven specific subtypes cumulative percentage of CHD children in China. (B) The prevalence of 3 most common subtypes of CHD in children in China
3.6 Prevalence of CHD in Children in Relation to Different Geographical Regions
Significant regional differences were detected. The highest reported prevalence of total CHD among school-age children was in the Northwest region [5.97 per 1000 children (95% CI: 4.93 to 7.11)], while the lowest was in the Central region [1.32 per 1000 children (95% CI: 1.15 to 1.50)]. The reported total prevalence of CHD among children in the Northwest region was significantly higher compared with all other regions (all, p < 0.05). The Southwestern region was the second highest reporting total CHD prevalence in children [4.33 per 1000 children (95% CI: 3.38 to 5.40)] (Fig. 6).
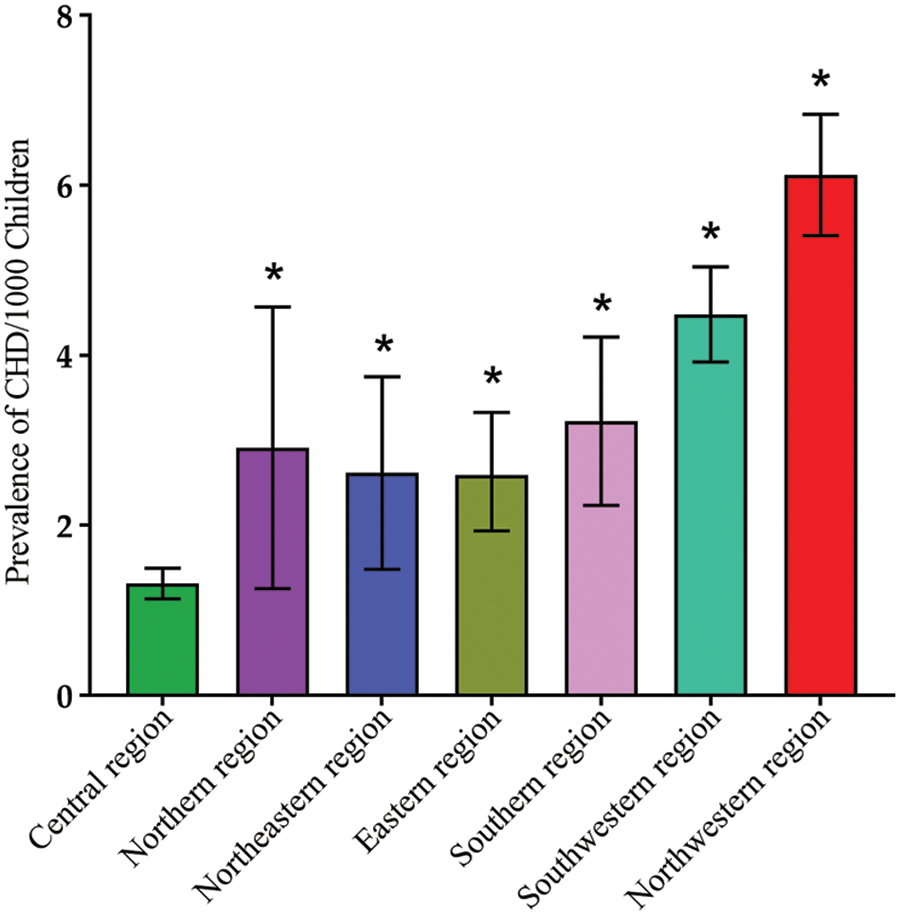
Figure 6: CHD prevalence in different geographic regions in China. Data are presented as Mean ± SE. *, p < 0.05, compared with central region
3.7 Prevalence of CHD in Children in Relation to Different Income Regions
Only lower-middle and upper-middle regions data were available. The significant difference between two income groups were found: the reported prevalence of total CHD among school-age children in lower-middle regions 4.61 (95% CI: 3.93 to 5.35) per 1000 and upper-middle regions 2.53 (95% CI: 1.97 to 3.26) per 1000 (χ2 = 9048.08, p < 0.001) (Fig. 7).
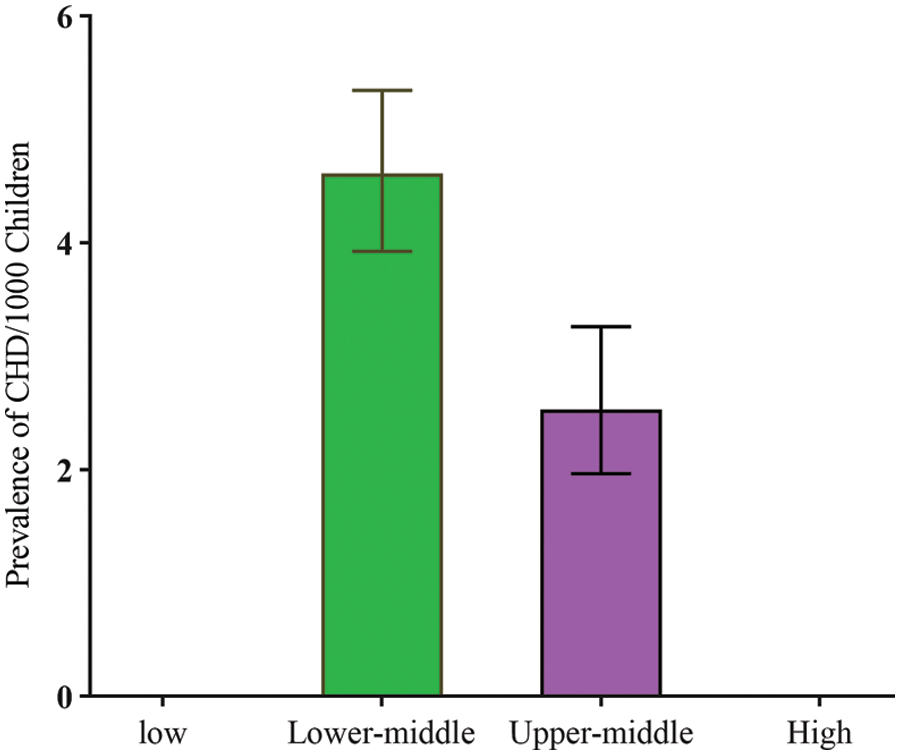
Figure 7: CHD prevalence and income level in China
3.8 Heterogeneity, Subgroup Analyses, and Publication Bias
Heterogeneity in subgroups by altitude, gender, and income levels was explored in pooled estimates, revealing statistical significance (I2 rang 84.0%–99.4%; Q statistic, all p < 0.001) (Table 2). Estimates of prevalence in school-age children did not significantly change after excluding any of the individual studies. However, egger’s regression test (t = 7.06, p < 0.001) and the funnel plot showed publication bias seen in Supplementary Fig. 1.
To the best of our knowledge, this is the first meta-analysis of the prevalence of CHD in school-age children in China. This review study included 31,835 CHD cases and 12,926,083 individuals from 86 published literature.
The prevalence of total CHD in school-age children was 4.69 per 1000 children, and overall prevalence decreased over time from 1976 to 2021, except for a rise during the period 2006–2015. The reported average CHD prevalence in school-age children in China was higher than birth prevalence (2.50 per 1000 birth) [9]. The birth prevalence increased from 0.21 per 1000 children to 4.91 per 1000 children from 1980–2015, revealing a 24-fold increase, while the prevalence rate decreased from 6.19 per 1000 children to 3.30 per 1000 children from 1976–2021, reaching a roughly two-fold decrease. Therefore, the total average prevalence of CHD at birth was higher than the prevalence of CHD in school-age children, which may not represent the true situation.
In the comparison of time trend analysis, the prevalence trend among school-age children was opposite to birth prevalence [2,3,9]. The following reasons might explain this phenomenon: (1) with the development of interventions, surgeries, and medications, many mild lesions of CHD may be cured in infancy [22–25]. Consequently, these children can normally go to school; (2) as socioeconomic level, sonographer skills, and diagnostic equipment evolve, prenatal screening has become increasingly important [26–29], especially for expectant mothers with offspring at high risk of CHD, such as habitual abortion and CHD family history. Once critical Congenital Heart Disease (CCHD) is detected by obstetric examinations, termination of pregnancy may be preferred [30]. (3) According to the research result of Boris Groisman et al., the perinatal mortality rate of CCHD is about 25% [31], which might explain why critical lesions only accounted for 9.7% among CHD school-age children. We also found the opposite time trend compared to the previous meta-analysis by Liu et al. [16]. This might be due to the limited sample size in the previous study, which involved 46 studies from around the world, thus failing to report the prevalence and time trend of CHD among school-age children in China. Therefore, it might not truly reflect the condition of CHD prevalence in school-age children in China.
Another notable finding was that the CHD prevalence in school-age children was 2-fold higher in higher altitude regions than in lower altitude regions. These findings confirm the results of previous studies, reporting a higher prevalence of CHD in high altitudes [13,32–34]. This huge difference could be attributed to the geographical environment, socio-economical level, and ethnic diversity. Atmospheric oxygen levels decrease as altitude increases, and low oxygen tension results in restricted vasoconstriction, which is thought to be the mechanism of PDA. Meanwhile, high pulmonary vascular resistance and right heart pressure persist at high altitudes, thus inhibiting early closure of the foramen ovale [35–38]. In addition, affected by altitude and cold, the incidence of various cardiovascular diseases also tends to increase [39]. High-altitude areas are economically underdeveloped regions, which could cause social problems, especially in women’s and children’s health care. Due to the special geographical environment, inconvenient transportation, and lack of medical and health professionals, it is difficult for pregnant women to receive regular obstetric examinations and screening for common neonatal diseases [40,41]. Consequently, many mild diseases in newborns are not timely treated or even discovered until school age. The high-altitude areas are dominated by ethnic minorities, especially Tibetans; however, it remains unknown whether CHD is more common in the Tibetan population, which future studies should address.
Meantime, CHD prevalence in males was significantly lower than in female school children, both in high altitude or low altitude areas. This was consistent with the result of Yoo et al. [42], who reported the total CHD prevalence over time was higher in females in adult and pediatric populations. Additionally, specific CHD prevalence varies according to gender, with males being more prone to severe and complex lesions and females to simple lesions, which corresponds to higher mortality rates in males than females [43,44]. So far, the mechanism underlying gender differences in prevalence has not yet been clarified and should be addressed by further studies.
The present study also revealed a geographical discrepancy in CHD prevalence in children. The Northwest district reported the highest total prevalence of CHD in children in China. However, in a previous meta-analysis of CHD prevalence at birth, the prevalence was relatively low in the Northwest. This result could be partly attributed to inadequate maternal antenatal and postnatal screening systems, which might make some diseases of perinatal infant diseases difficult to detect (e.g., Congenital Heart Disease) [34,40]. Furthermore, most of the Northwest Territories are located at high altitudes and are economically underdeveloped, which may be one of the important reasons for the high CHD prevalence.
Heterogeneity tests revealed high levels of heterogeneity in altitude, gender, geographic regions, and income level in our study. Some factors may contribute to heterogeneity, including the research design of the original papers, socioeconomic situation, study population selection, prenatal care service, ethnic background, and diagnostic tools used. However, the present study included very large sample sizes, which made point estimates very precise and SEs very small. Therefore, heterogeneity was expected and inevitable in cross-sectional studies.
This study has several limitations. First, the study design and diagnostic skills of each original paper varied, which could result in a bias in the reported prevalence and may be present in our estimates. Second, in addition to reporting the total number of cases and participants, not each study reported subgroups in detail, which may affect the stability of subgroup analysis outcome. Finally, whilst studying the CHD prevalence in children at different altitudes, due to the limitation of sample size, we simply defined 2500 m as the segmentation point between high and low altitudes, without further division of altitude, which makes it impossible to determine the prevalence of CHD with increasing altitude.
The overall CHD prevalence in school-age children decreased over time, and the prevalence in high altitude areas was more than twice as high as in low altitude areas, thus representing a serious disease burden at high altitudes. The prevalence of CHD was significantly lower in male than in female children, and the difference was more pronounced, especially at high altitudes. We also found that the prevalence of different subtypes of CHD was correlated with altitude. The prevalence of CHD in different regions remains uncertain, as it is not confirmed whether the differences are real or just methodological. Based on our results, we suggest that the Chinese government pay more attention to high-altitude and economically underdeveloped areas in allocating medical resources and the health care of women and children.
Funding Statement: This research was supported by Qinghai Provincial Science and Technology Department (Grant No. 2021-ZJ-751).
Author Contributions: Conceptualization, Shuqin Zhang and Jirong Qi; methodology, Jianying Wu and Jin Luo; software, Bin Zhang and Haomin Shi; validation, Shuqin Zhang and Huilian Yang; formal analysis, Shuqin Zhang, Jin Luo, and Bin Zhang; investigation, Jin Luo and Haomin Shi; resources, Shuqin Zhang and Jirong Qi; data curation, Jianying Wu and Bin Zhang; writing—original draft preparation, Shuqin Zhang and Bin Zhang; writing—review and editing, Shuqin Zhang, and Huilian Yang, Jirong Qi; visualization, Jin Luo; supervision, Jirong Qi and Huilian Yang; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Mitchell, S. C., Korones, S. B., Berendes, H. W. (1971). Congenital Heart Disease in 56,109 births. Incidence and natural history. Circulation, 43(3), 323–332. [Google Scholar] [PubMed]
2. van der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of Congenital Heart Disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. https://doi.org/10.1016/j.jacc.2011.08.025 [Google Scholar] [PubMed] [CrossRef]
3. Liu, Y., Chen, S., Zühlke, L., Black, G. C., Choy, M. K. et al. (2019). Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology, 48(2), 455–463. https://doi.org/10.1093/ije/dyz009 [Google Scholar] [PubMed] [CrossRef]
4. Plana, M. N., Zamora, J., Suresh, G., Fernandez-Pineda, L., Thangaratinam, S. et al. (2018). Pulse oximetry screening for critical congenital heart defects. The Cochrane Database of Systematic Reviews, 3(3), CD011912. https://doi.org/10.1002/14651858.CD011912.pub2 [Google Scholar] [PubMed] [CrossRef]
5. Karim, J. N., Bradburn, E., Roberts, N., Papageorghiou, A. T. (2022). First-trimester ultrasound detection of fetal heart anomalies: Systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology, 59(1), 11–25. https://doi.org/10.1002/uog.23740 [Google Scholar] [PubMed] [CrossRef]
6. Zhao, Q. M., Liu, F., Wu, L., Ma, X. J., Niu, C. et al. (2019). Prevalence of Congenital Heart Disease at live birth in China. The Journal of Pediatrics, 204, 53–58. https://doi.org/10.1016/j.jpeds.2018.08.040 [Google Scholar] [PubMed] [CrossRef]
7. GBD 2017 Congenital Heart Disease Collaborators (2020). Global, regional, and national burden of Congenital Heart Disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet Child & Adolescent Health, 4(3), 185–200, 2020. [Google Scholar]
8. He, Y., Xu, W., Su, Z., Liu, K., Zhang, H. (2020). Addressing the rising burden of Congenital Heart Disease in China. The Lancet Child & Adolescent Health, 4(4), e7. https://doi.org/10.1016/S2352-4642(20)30061-4 [Google Scholar] [PubMed] [CrossRef]
9. Zhao, L., Chen, L., Yang, T., Wang, T., Zhang, S. et al. (2020). Birth prevalence of Congenital Heart Disease in China, 1980–2019: A systematic review and meta-analysis of 617 studies. European Journal of Epidemiology, 35(7), 631–642. https://doi.org/10.1007/s10654-020-00653-0 [Google Scholar] [PubMed] [CrossRef]
10. Zheng, J. Y., Qiu, Y. G., Li, D. T., He, J. C., Chen, Y. et al. (2017). Prevalence and composition of CHD at different altitudes in Tibet: A cross-sectional study. Cardiology in the Young, 27(8), 1497–1503. https://doi.org/10.1017/S1047951117000567 [Google Scholar] [PubMed] [CrossRef]
11. Song, H. L., L., J., Zhou, R. F., Wang, L. (2021). Prevalence of Congenital Heart Disease in community screening of primary and secondary school students in five Chinese provinces in relation to altitude and ethnicity. Chinese Journal of Public Health, 37(10), 1514–1516. [Google Scholar]
12. Chen, Q. H., Wang, X. Q., Qi, S. G. (2008). Cross-sectional study of Congenital Heart Disease among Tibetan children aged from 4 to 18 years at different altitudes in Qinghai Province. Chinese Medical Journal, 121(24), 2469–2472. https://doi.org/10.1097/00029330-200812020-00001 [Google Scholar] [CrossRef]
13. Zheng, J. Y., Tian, H. T., Zhu, Z. M., Li, B., Han, L. et al. (2013). Prevalence of symptomatic Congenital Heart Disease in Tibetan school children. The American Journal of Cardiology, 112(9), 1468–1470. https://doi.org/10.1016/j.amjcard.2013.07.028 [Google Scholar] [PubMed] [CrossRef]
14. García, A., Moreno, K., Ronderos, M., Sandoval, N., Caicedo, M. et al. (2016). Differences by altitude in the frequency of congenital heart defects in Colombia. Pediatric Cardiology, 37(8), 1507–1515. https://doi.org/10.1007/s00246-016-1464-x [Google Scholar] [PubMed] [CrossRef]
15. González-Andrade, F. (2020). High altitude as a cause of congenital heart defects: A medical hypothesis rediscovered in Ecuador. High Altitude Medicine & Biology, 21(2), 126–134. https://doi.org/10.1089/ham.2019.0110 [Google Scholar] [PubMed] [CrossRef]
16. Liu, Y., Chen, S., Zühlke, L., Babu-Narayan, S. V., Black, G. C. et al. (2020). Global prevalence of Congenital Heart Disease in school-age children: A meta-analysis and systematic review. BMC Cardiovascular Disorders, 20(1), 488. https://doi.org/10.1186/s12872-020-01781-x [Google Scholar] [PubMed] [CrossRef]
17. Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C. et al. (2021). The PRISMA, 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal (Clinical Research Edition), 372, n71. [Google Scholar]
18. Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C. et al. (2021). PRISMA, 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. British Medical Journal (Clinical Research Edition), 372, n160. https://doi.org/10.1136/bmj.n160 [Google Scholar] [PubMed] [CrossRef]
19. Munn, Z., Moola, S., Riitano, D., Lisy, K. (2014). The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. International Journal of Health Policy and Management, 3(3), 123–128. https://doi.org/10.15171/ijhpm.2014.71 [Google Scholar] [PubMed] [CrossRef]
20. Ma, L. L., Wang, Y. Y., Yang, Z. H., Huang, D., Weng, H. et al. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Military Medical Research, 7(1), 7. https://doi.org/10.1186/s40779-020-00238-8 [Google Scholar] [PubMed] [CrossRef]
21. The World Bank. World Bank Country and Lending Groups (2021). https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Google Scholar]
22. Shiina, Y., Toyoda, T., Kawasoe, Y., Tateno, S., Shirai, T. et al. (2011). Prevalence of adult patients with Congenital Heart Disease in Japan. International Journal of Cardiology, 146(1), 13–16. https://doi.org/10.1016/j.ijcard.2009.05.032 [Google Scholar] [PubMed] [CrossRef]
23. Ávila, P., Mercier, L. A., Dore, A., Marcotte, F., Mongeon, F. P. et al. (2014). Adult Congenital Heart Disease: A growing epidemic. The Canadian Journal of Cardiology, 30(12), S410–419. https://doi.org/10.1016/j.cjca.2014.07.749 [Google Scholar] [PubMed] [CrossRef]
24. Marelli, A. J., Ionescu-Ittu, R., Mackie, A. S., Guo, L., Dendukuri, N. et al. (2014). Lifetime prevalence of Congenital Heart Disease in the general population from 2000 to 2010. Circulation, 130(9), 749–756. https://doi.org/10.1161/CIRCULATIONAHA.113.008396 [Google Scholar] [PubMed] [CrossRef]
25. Yeh, S. J., Chen, H. C., Lu, C. W., Wang, J. K., Huang, L. M. et al. (2015). National database study of survival of pediatric Congenital Heart Disease patients in Taiwan. Journal of the Formosan Medical Association, 114(2), 159–163. https://doi.org/10.1016/j.jfma.2012.10.006 [Google Scholar] [PubMed] [CrossRef]
26. Donofrio, M. T., Moon-Grady, A. J., Hornberger, L. K., Copel, J. A., Sklansky, M. S. et al. (2014). Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation, 129(21), 2183–2242. https://doi.org/10.1161/01.cir.0000437597.44550.5d [Google Scholar] [PubMed] [CrossRef]
27. Hunter, L. E., Simpson, J. M. (2014). Prenatal screening for structural Congenital Heart Disease. Nature Reviews: Cardiology, 11(6), 323–334. https://doi.org/10.1038/nrcardio.2014.34 [Google Scholar] [PubMed] [CrossRef]
28. Hopkins, M. K., Dugoff, L., Kuller, J. A. (2019). Congenital Heart Disease: Prenatal diagnosis and genetic associations. Obstetrical and Gynecological Survey, 74(8), 497–503. https://doi.org/10.1097/OGX.0000000000000702 [Google Scholar] [PubMed] [CrossRef]
29. Arnaout, R., Curran, L., Zhao, Y., Levine, J. C., Chinn, E. et al. (2021). An ensemble of neural networks provides expert-level prenatal detection of complex Congenital Heart Disease. Nature Medicine, 27(5), 882–891. https://doi.org/10.1038/s41591-021-01342-5 [Google Scholar] [PubMed] [CrossRef]
30. Lytzen, R., Vejlstrup, N., Bjerre, J., Petersen, O. B., Leenskjold, S. et al. (2019). Mortality and morbidity of major Congenital Heart Disease related to general prenatal screening for malformations. International Journal of Cardiology, 290(9), 93–99. https://doi.org/10.1016/j.ijcard.2019.05.017 [Google Scholar] [PubMed] [CrossRef]
31. Groisman, B., Barbero, P., Liascovich, R., Brun, P., Bidondo, M. P. (2022). Detection of critical Congenital Heart Disease among newborns in Argentina through the national surveillance system of Congenital Heart Disease (RENAC). Archivos Argentinos de Pediatria, 120(1), 6–13. [Google Scholar] [PubMed]
32. Ma, L. G., Chen, Q. H., Wang, Y. Y., Wang, J., Ren, Z. P. et al. (2018). Spatial pattern and variations in the prevalence of Congenital Heart Disease in children aged 4–18 years in the Qinghai-Tibetan Plateau. The Science of the Total Environment, 627(10), 158–165. https://doi.org/10.1016/j.scitotenv.2018.01.194 [Google Scholar] [PubMed] [CrossRef]
33. Chun, H., Yue, Y., Wang, Y., Dawa, Z., Zhen, P. et al. (2019). High prevalence of Congenital Heart Disease at high altitudes in Tibet. European Journal of Preventive Cardiology, 26(7), 756–759. https://doi.org/10.1177/2047487318812502 [Google Scholar] [PubMed] [CrossRef]
34. Li, J. J., Liu, Y., Xie, S. Y., Zhao, G. D., Dai, T. et al. (2019). Newborn screening for Congenital Heart Disease using echocardiography and follow-up at high altitude in China. International Journal of Cardiology, 274, 106–112. https://doi.org/10.1016/j.ijcard.2018.08.102 [Google Scholar] [PubMed] [CrossRef]
35. Alzamora-Castro, V., Battilana, G., Abugattas, R., Sialer, S. (1960). Patent ductus arteriosus and high altitude. American Journal of Cardiology, 5(6), 761–763. https://doi.org/10.1016/0002-9149(60)90052-7 [Google Scholar] [PubMed] [CrossRef]
36. Miao, C. Y., Li, W. X., Geng, D., Tao, L. A., Zuberbuhler, J. S. et al. (1988). Effect of high altitude on prevalence of Congenital Heart Disease. Chinese Medical Journal, 101(6), 415–418. [Google Scholar] [PubMed]
37. Miao, C. Y., Zuberbuhler, J. S., Zuberbuhler, J. R. (1988). Prevalence of congenital cardiac anomalies at high altitude. Journal of the American College of Cardiology, 12(1), 224–228. https://doi.org/10.1016/0735-1097(88)90378-6 [Google Scholar] [PubMed] [CrossRef]
38. Forsey, J. T., Elmasry, O. A., Martin, R. P. (2009). Patent arterial duct. Orphanet Journal of Rare Diseases, 4(1), 17. https://doi.org/10.1186/1750-1172-4-17 [Google Scholar] [PubMed] [CrossRef]
39. Whayne Jr, T. F. (2014). Altitude and cold weather: Are they vascular risks? Current Opinion in Cardiology, 29(4), 396–402. https://doi.org/10.1097/HCO.0000000000000064 [Google Scholar] [PubMed] [CrossRef]
40. Foggin, P. M., Torrance, M. E., Dorje, D., Xuri, W., Marc Foggin, J. et al. (2006). Assessment of the health status and risk factors of Kham Tibetan pastoralists in the alpine grasslands of the Tibetan plateau. Social Science & Medicine, 63(9), 2512–2532. https://doi.org/10.1016/j.socscimed.2006.06.018 [Google Scholar] [PubMed] [CrossRef]
41. Labasangzhu, Bjertness, E., McNeil, E. B., Deji, Guo, Y. et al. (2018). Progress and challenges in improving maternal health in the Tibet Autonomous Region. China Risk Management and Healthcare Policy, 11, 221–231. [Google Scholar] [PubMed]
42. Yoo, B. W. (2018). Epidemiology of Congenital Heart Disease with emphasis on sex-related aspects. Advances in Experimental Medicine and Biology, 1065, 49–59. https://doi.org/10.1007/978-3-319-77932-4 [Google Scholar] [CrossRef]
43. Aubry, P., Demian, H. (2016). Sex differences in Congenital Heart Disease. Annales de Cardiologie et D’angeiologie, 65(6), 440–445. https://doi.org/10.1016/j.ancard.2016.10.006 [Google Scholar] [PubMed] [CrossRef]
44. Shang, W. J., Ye, J. L., Pan, X. P. (2020). The analysis on the change and trend of the Congenital Heart Disease mortality rate in children aged 0–1 in China from 2004 to 2018. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine], 54(11), 1249–1254. [Google Scholar] [PubMed]
Supplementary File
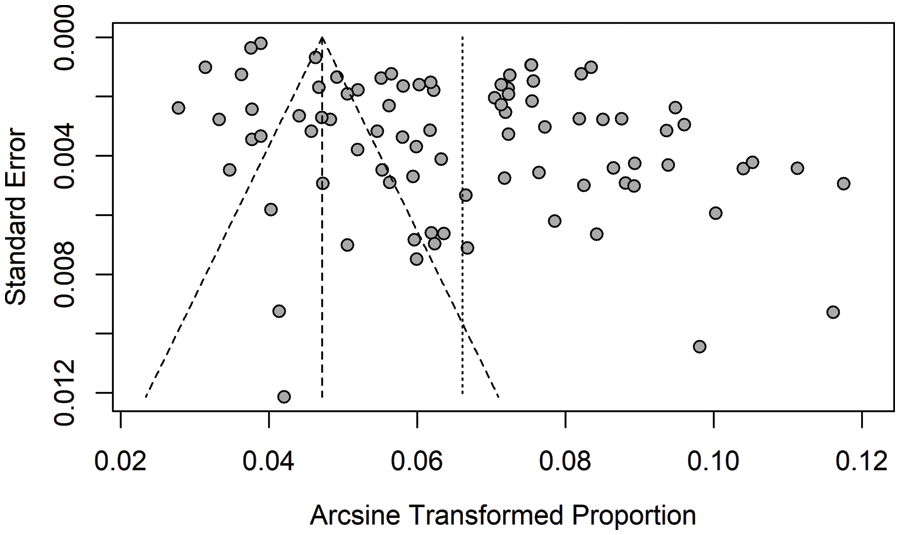
Supplementary Figure 1: Analysis of publication bias with the funnel plot
References of Studies Included in the Systematic Review and Meta-Analysis
1. Munn, Z., Moola, S., Lisy, K., Riitano, D., Tufanaru, C. (2015). Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. International Journal of Evidence-Based Healthcare, 13(3), 147–153. https://doi.org/10.1097/XEB.0000000000000054
2. Ma, L. L., Wang, Y. Y., Yang, Z. H., Huang, D., Weng, H. et al. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Military Medical Research, 7(1), 7. https://doi.org/10.1186/s40779-020-00238-8
3. Liberali, R., Kupek, E., Assis, M. A. A. (2020). Dietary patterns and childhood obesity risk: A systematic review. Childhood Obesity, 16(2), 70–85. https://doi.org/10.1089/chi.2019.0059
4. Wu, K. G., Lin, M. H., Huang, C. F., Wang, Y. H., Lin, X. Y. et al. (1980). A survey on the prevalence of Congenital Heart Disease in 4466 people in Shigu Commune, Yongchun County. Journal of Fujian Medical University, 1, 60–61.
5. Qh, P. H. I. (1980). Report on the screening of 5,658 school-aged children with Congenital Heart Disease in Xining area. Qinghai Medicine, (3), 2–3.
6. Huang, J. R., Wang, Q., Gong, C. J., Wang, L. S., Gesang, L. M. et al. (1981). Report of Congenital Heart Disease screening in children in Lhasa. Tibetan Medicine, (2), 32–39.
6. Yu, M. Z. (1982). A survey of cardiovascular disease in school-aged children in Shijingshan District, Beijing. Journal of Cardiopulmonary Vascular Disease, x(1), 10.
8. Liu, R. C., Wu, T. Y., Ge, R. L., Wang, X. Z., Xiao, S. J. et al. (1982). A survey of Congenital Heart Disease in children on the Qinghai plateau. Chinese Journal of Cardiovascular Diseases, 10(4), 241–242.
9. Qian, Y. Q., Zheng, D. Y., Zeng, F. Y., Li, M. J., Sun, Z. Q. (1984). Epidemiological survey of Congenital Heart Disease in children under six years of age in Chengdu. Chinese Journal of Cardiovascular Diseases, 1(12), 4.
10. Lei, H. N., Luo, Q. Z., Luo, S. X. (1984). A survey report on cardiovascular disease in 22,497 primary and secondary school students in Ganzhou City. Jiangxi Medicine, x(2), 23–26.
11. Song, S. B., Chen, X. Y. (1984). A survey of 10,262 school-age children with Congenital Heart Disease in Huanzhong area. Chinese Journal of Cardiovascular Diseases, (2), 85.
12. Fei, W. S. (1986). Preliminary analysis of Congenital Heart Disease survey and related prevalence factors among 10,211 adolescents in Xinyu City. Jiangxi Medicine, x(2), 521–522.
12. Liu, Y. P. (1988). Survey on Congenital Heart Disease in primary and secondary school students in Dandong city. School Health, 9(2), 52.
14. Jiang, K. Z., Zheng, K. Z. (1988). A survey of congenital heart disease in 27,419 children and adolescents in Li and Miao Autonomous Prefecture, Hainan Island. New Medicine, x(12), 632–633.
15. Liu, T. Y., Zhang, Y. H., Liu, L., Chen, S. L., Qiang, Y. et al. (1989). Investigation and analysis of Congenital Heart Disease and blood pressure reference values in 2925 primary and secondary school students in suburban Yinchuan City. Ningxia Medical Journal, 11(6), 327–329.
16. Wu, T. Y., Ge, E. L., Xiao, S. J., Wang, X. Z. (1990). Epidemiological study of congenital heart disease in children on the Qinghai-Tibetan plateau. Journal of Cardiopulmonary Angiology, 9(3), 131–135.
17. Zhao, G. M., Wang, F., Wang, D. M., Wang, G. R., Guo, G. Z. et al. (1991). Epidemiological survey of Congenital Heart Disease in 13,821 school-age children in the Bai region. Chinese Journal of Circulation, 6(4), 301–302+336.
18. Zheng, K. Z., Jiang, K. Z., Li, D. S., Wei, H. Z. (1992). Report on the epidemiological survey of Congenital Heart Disease in children and adolescents in five cities and counties of Hainan Island. Chinese Journal of Circulation, 114(2), 348–349+399.
19. Chen, S. L., Chen, C. Y., Lu, X. Y., Huang, C. Z., Wei, N. et al. (1992). Epidemiological survey analysis of Congenital Heart Disease in urban and rural areas of Yinchuan City. Ningxia Medical Journal, (6), 364–366.
20. Zhu, S. G., Ma, L. J., Yuan, T. H., Xiang, Y. Z., Wu, Y. et al. (1993). Investigation of factors associated with Congenital Heart Disease in 12,468 primary and secondary school students in the Yimeng Mountains. Chinese Journal of Epidemiology, 14(3), 163–165.
21. Wang, J. H., Hou, S. C., Meng, R. H., Guo, S. H. (1994). Epidemiological survey of 165,929 primary and secondary school students with Congenital Heart Disease in six counties and districts of Jincheng City, Shanxi Province. Shanxi Journal of Medicine, 23(3).
22. Cao, R. H., Ge, H. M., Chang, Y. L., Dong, G. X., Dong, Z. Q. (1994). A survey of 92,593 primary and secondary school students with Congenital Heart Disease in urban and rural areas of Changzhi. Chinese Journal of Circulation, 9(9).
22. Fen, T. M., Ni, G. M., Huang, J. K., Liao, Y. L., Wang, B. Y. et al. (1997). Current prevalence levels of Congenital Heart Disease and rheumatic heart disease in rural primary and secondary school students in Tanzhou Town, Panyu, Guangdong. Lingnan Journal of Cardiovascular Diseases, 3(4), 4–5+61.
22. Zhang, X. Y., Li, R., Wang, Z. Z., Huang, Z. W. (1998). Analysis of heart disease among students in Gansu Province. Journal of Gansu College of Traditional Chinese Medicine, 15(1).
25. Kuang, Z. S., Liu, Y. B., Zheng, P., Liu, S. B., Xiong, R. S. et al. (1999). Epidemiological survey of Congenital Heart Disease in children and adolescents in Guilin. Journal of Guangxi Medical University, 16(3).
26. Fang, X. J., Gao, A. J., Chu, D. G., Lu, R. X., Wang, G. Q. (2000). A survey on the prevalence of Congenital Heart Disease among some secondary school students in Dongcheng District. School Health in China, 21(6), 449.
26. Gao, B. R., Yue, F. Z. (2000). An epidemiological study of Congenital Heart Disease in six cities of Gansu Province. Chinese Journal of Circulation, 15(5).
26. Liu, J. T., Liu, Q. J., Li, S. L. (2000). Epidemiological survey of Congenital Heart Disease in children aged 0–17 years in Kunming. Chinese Journal of Eugenics and Genetics, 8(2), 5–7.
29. Kong, W. S., Guo, S. Y., Zheng, X. M., Zhang, J. R., Qu, F. et al. (2000). Report on the screening of 14,774 primary and secondary school students with precocious heart disease in Xiamen. Fujian Journal of Medicine, 22(3), 114.
30. Xu, X. L., Jin, H. Y., Wang, B. H. (2000). Discussion on the prevalence and prevention of cardiovascular disease in primary and secondary school students in Zhejiang Province. China Cardiovascular Journal, 5(1), 3–6.
31. Jiang, D. H. (2002). Investigation of congenital cardiovascular disease in school-age children in Keshan County. School Health in China, 23(1), 74.
32. Wang, J., Wang, Z. N., Li, S. Z., Wang, L. J., Chen, Z. D. et al. (2002). Survey of 6500 elementary school students with Congenital Heart Disease in Lhasa. Tibet Science and Technology, (1), 12–14.
32. Geng, S. G., Wang, G. F., Lv, B. L., Zhao, L., Ge, T. (2002). Investigation of congenital heart disease and physiological murmur in secondary school students in Jianhu County. School Health in China, (4), 346–347.
34. Zhai, Y. F., Ma, J., Chen, J. H., Xiao, Z. M., Wang, W. (2004). A survey on the detection rate of heart murmurs and the incidence of Congenital Heart Disease in 25,488 people aged 2–17 years in Jiuquan, Gansu Province. Chinese Journal of Cardiovascular Disease Research, 2(10), 785–787.
35. Jiang, L. H., Duan, C. Q., Ma, Z. Q., Zhu, L. J., Yin, W. J. et al. (2005). A survey on the prevalence of Congenital Heart Disease in people aged 3 to 18 years in some areas of Yunnan Province. Chinese Journal of Epidemiology, 26(3).
35. Li, Z., Wang, J. D., Li, A. M., Zhou, X. H., Zhao, Y. M. (2005). Epidemiological survey of Congenital Heart Disease in children and adolescents in Dali Prefecture. Journal of Dali College (Natural Science), 14(3), 53–54.
35. Chen, Q. H., Wang, X. Q., Tong, Y. F. (2008). Epidemiological survey of Congenital Heart Disease in a population aged 4–17 years in Huangnan Prefecture, Qinghai Province. Journal of Qinghai Medical College, 29(2), 73–76.
38. Luo, X. F. (2008). Survey on the prevalence of Congenital Heart Disease in children aged 0~5 years in urban areas of Nanyang City. Henan Journal of Preventive Medicine, 19(4), 281–282.
39. Liu, F. Y., Wang, X. Q., Wang, Q., Zhao, G. Q., Wang, Q. et al. (2008). Current distribution of Congenital Heart Disease in the (4–17)-year-old population in Haixi, Qinghai. Journal of Highland Medicine, 18(1), 54–57.
40. Wang, X. H., Chen, Q. H., Dong, Y. F., Zhu, C. K., Qu, Y. et al. (2008). Epidemiological survey of Congenital Heart Disease in Tibetan children aged 4 to 18 years at different altitudes in Qinghai Province. Chinese Journal of Epidemiology, 29(4), 317–320.
41. Chen, Q. H., Lu, L., Xu, X. L., Wang, Q., Zhao, G. Q. et al. (2009). Epidemiological survey of Congenital Heart Disease in a population aged 4 to 18 years in three counties of Haidong District, Qinghai Province. Chinese Journal of Preventive Medicine, 43(4), 319–321.
42. Caodao, B. Y., Li, W., Li, X. Y. (2009). Investigation of congenital heart disease in adolescents in Inner Mongolia. Modern Preventive Medicine, 36(21), 4014–4015.
43. Qi, S. G., Wang, X. Q., Chen, Q. H., Liu, F. Y., Hu, L. (2009). Comparison of the incidence of congenitalheart disease in children of different altitudes and ethnic groups. China Public Health, 25(12), 1493–1494.
44. Li, Y. J., Chen, J. H., Wei, Y. H., Li, W. H. (2009). Epidemiological survey of Congenital Heart Disease in children aged 0–14 years in the mountainous areas of southern Ningxia. Ningxia Medical Journal, 31(2), 113–114.
45. Chen, Q. H., Liu, F. Y., Wang, X. Q., Qi, G. R., Liu, P. F. et al. (2009). Epidemiological survey of Congenital Heart Disease in people aged 4 to 18 years at different altitudes in Qinghai Province. Chinese Journal of Epidemiology, 30(12), 1248–1251.
46. Wang, X. Q., Liu, F. Y., Guo, B. H., Wang, X. L., Qu, Y. et al. (2009). Survey on congenital heart disease in a population aged 4 to 17 years in Hainan Tibetan Autonomous Prefecture. China Public Health, 25(4), 474–475.
47. Yang, W. H., Luo, X. M., Han, X., Yan, J. H., Zhou, C. Q. et al. (2010). A survey on Congenital Heart Disease in children aged 0–5 years in Kunshan City. China Maternal and Child Health Research, 21(6), 727–729.
48. Qu, Y., Qi, G. R., Lu, L., Yan, Y. P., Chen, Q. H. (2010). Epidemiological survey of precocious heart disease in children aged 4–8 years at high altitude in Qinghai. Beijing Medicine, 32(10), 813–815.
49. Dang, J. Z., Tan, P., Xu, Y. X., Tuo, C. D., Yang, Y. S. et al. (2011). A survey report on 87,672 primary and secondary school students with Congenital Heart Disease in Jingyuan County, Gansu Province. Western Chinese Medicine, 24(12), 38–39.
50. Zhang, H., Yang, R., Wang, X. Y., Li, J. (2011). Epidemiological survey of Congenital Heart Disease in adolescents in Muli County, Liangshan Prefecture. Medical Information, 24(12), 191–192.
51. Yan, Y. Z., Wu, H. B., Zhang, J. W. (2011). A survey of 108,864 elementary school students with Congenital Heart Disease in 11 counties under the jurisdiction of Changzhi City. Shanxi Journal of Medicine, 3(40), 254–255.
52. Liu, X. K., Rao, L., Ma, F. Y., Wang, P., Zhao, G. et al. (2012). Epidemiological survey of Congenital Heart Disease in children aged 5 to 14 years in Liangshan Yi Autonomous Prefecture. Chinese Modern Physician, 50(29), 14–15.
53. Yang, W., Gao, C. H., Gong, T. B. (2012). Analysis of physical health examination results of primary and secondary school students in Qianjiang District, Chongqing from 2009 to 2011. Chinese and Foreign Health Digest, 9(31), 38–41.
54. Wang, J. (2012). A survey on the prevalence of Congenital Heart Disease in Uyghur and Kirgiz children aged 5–14 years in Xinjiang (Master Dissertation). Xinjiang Medical University, China.
55. Jing, T., Tong, S. F., Guo, Y. L., Liu, J. P., Ran, Q. L. et al. (2012). A census of the prevalence of Congenital Heart Disease in 12,014 children and adolescents in Shizhu County, Chongqing. Chongqing Medical, 41(5), 472–473+476.
56. Zheng, J. Y., Qiu, Y. G., Li, D. T., He, J. C., Chen, Y. et al. (2017). Prevalence and composition of CHD at different altitudes in Tibet: A cross-sectional study. Cardiology in the Young, 27(8), 1497–1503. https://doi.org/10.1017/S1047951117000567
57. Chen, Q. H., Wang, X. H., Xu, X. L., Wang, Q., Zhao, G. Q. et al. (2013). A survey on the current situation of Congenital Heart Disease in Tibetan children and adolescents in areas above, 4000 m altitude. Journal of Liberation Army Medicine, 38(8), 657–660.
58. Liu, X. K., Sang, H., Xiu, J. F., Li, X., Li, J. et al. (2013). Survey on the prevalence of Congenital Heart Disease in school students in Liangshan Prefecture, Sichuan Province. Chinese Journal of Internal Medicine, 52(6), 494–497.
59. Geng, D., Yang, Y. P., Zhao, Y. S., Wang, L. B., Yue, S. F. et al. (2013). A survey on the incidence of Congenital Heart Disease in Yushu area. Journal of Highland Medicine, 23(2), 60–61.
60. Pan, J. Y., Yuan, C. G., Gao, C., Chen, Z. F., Ma, J. (2013). Epidemiological survey of Congenital Heart Disease in infants and children in Feidong County. Chinese Journal of Disease Control, 17(5), 446–450.
61. Ding, X. Q., Gao, Q., Wang, K., Wang, H. (2013). Distribution characteristics of Congenital Heart Disease in a population aged 4 to 17 years in Yushu County, Yushu Tibetan Autonomous Prefecture, Qinghai Province. Journal of Highland Medicine, 23(1), 60–61.
62. Hou, H. J. (2014). Analysis of Congenital Heart Disease screening results among Tibetan elementary school students in Suo County, Nagqu Region, Tibet Autonomous Region. Chinese Society of Ultrasound Medical Engineering, 456–457.
63. Chen, X. L., Lu, H. H., Zhong, C. J., Xcu, M., Xia, C. Q. (2014). Epidemiological survey of Congenital Heart Disease in infants and children in Nantong area and analysis of related factors. Journal of Interventional Radiology, 23(12), 1095–1098.
64. Shaqu, Z. M., Wang, X. Y., Yin, L., Wang, L. J. (2014). Epidemiological survey of congenital cardiovascular disease in children and adolescents aged 3–18 years at different altitudes in Liangshan Prefecture. Journal of Chinese Medicine, B07(29), 364–365.
65. Ouyang, Z. W., Liu, P., He, L., Luo, C. X., Shi, X. J. et al. (2014). An epidemiological survey on precocious heart disease in children aged 3~14 years in Shaoyang area. Southwest Defense Medicine, 24(11), 1274–1276.
66. Liu, F., Yang, Y. N., Xie, X., Li, X. M., Ma, X. et al. (2015). Prevalence of Congenital Heart Disease in Xinjiang Multi-Ethnic Region of China. PLoS One, 10(8), e0133961. https://doi.org/10.1371/journal.pone.0133961
67. Li, S. H., Dong, Y. L., Tong, Y. F., Liu, L. B. (2015). Epidemiological survey of Congenital Heart Disease in children aged 3 to 14 years in Jiangxi Province. Chinese Contemporary Medicine, 22(28), 174–176+179.
68. Wu, J. W. (2015). A survey of 24,861 primary and secondary school students with Congenital Heart Disease in Hunnan District, Shenyang in 2014. Liaoning Medical Journal, 29(4), 211.
69. Xiao, J. M., Chen, J. H., Wang, J. Y., Jin, H., Li, W. et al. (2015). A survey on the prevalence and treatment status of Congenital Heart Disease among school-age children with different household registration in Dongguan. Chinese Journal of Child Health, 23(4), 395–398.
70. Wang, L. L. (2015). Differences in the prevalence of precocious heart disease between Han and Yi ethnic groups among school children in Liangshan Prefecture, Sichuan Province. Medical Aesthetics and Beauty, 6(1), 566.
71. Zhang, G. M., He, L. Y., Ma, S. F. (2016). Epidemiological survey of congenital heart disease in children in Ulanqab region, Inner Mongolia. Chinese Journal of Medicine, 18(8), 858–859.
72. Cao, Y. (2016). Epidemiological survey of precocious heart disease in Yangbi County, Yunnan Province, and analysis of susceptibility genes and mutations in disseminated atrial septal defects (Doctoral Dissertation). Kunming Medical University, China.
73. Zhang, G. M., He, L. Y., Ma, S. F. (2017). An epidemiological survey and analysis of Congenital Heart Disease in children of ethnic minorities in Xinjiang. China Maternal and Child Health Research, 28(5), 593–594.
74. Zheng, J. Y., Qiu, Y. G., Li, D. T., He, J. C., Chen, Y. et al. (2017). Prevalence and composition of CHD at different altitudes in Tibet: A cross-sectional study. Cardiology in the Young, 27(8), 1497–1503. https://doi.org/10.1017/S1047951117000567
75. Han, C. L., Jiang, L. H., Hou, Z. L., Wang, W. J., Zhang, Y. (2017). A survey on the epidemiological characteristics of Congenital Heart Disease in people aged 3 to 18 years in different regions of Yunnan Province. Journal of Kunming Medical University, 38(8), 30–34.
76. Chen, M. J. (2019). Analysis of children’s physical fitness in public and private childcare institutions in Zhangjiagang City in 2018. China Maternal and Child Health Care, 34(22), 5089–5091.
77. Chun, H., Yue, Y., Wang, Y., Dawa, Z., Zhen, P. et al. (2019). High prevalence of Congenital Heart Disease at high altitudes in Tibet. European Journal of Preventive Cardiology, 26(7), 756–759. https://doi.org/10.1177/2047487318812502
78. Ma, L. G., Chen, Q. H., Wang, Y. Y., Wang, J., Ren, Z. P. et al. (2018). Spatial pattern and variations in the prevalence of Congenital Heart Disease in children aged 4–18 years in the Qinghai-Tibetan Plateau. The Science of the Total Environment, 627(10), 158–165. https://doi.org/10.1016/j.scitotenv.2018.01.194
79. Zhang, Q., Zhang, Y. Q., Lu, F. X., Chen, G., Ren, D. Z. (2018). Analysis of epidemiological characteristics of Congenital Heart Disease in school-age children and its influencing factors in Qianxinan Prefecture. Journal of Clinical Rational Drug Use, 11(6), 98–100.
80. Li, Z., Wang, J. D., Li, A. M., Zhou, X. H., Zhao, Y. M. (2005). Epidemiological survey of Congenital Heart Disease in children and adolescents in Dali Prefecture. Journal of Dali College (Natural Science), 39(3), 53–54.
81. Guo, Y. L., Lu, J., Duan, Y. Y., Fan, J. H., Zhou, H. M. (2018). Epidemiological survey of Congenital Heart Disease in children aged 4–12 years in Dehong Prefecture, Yunnan Province. Chinese Journal of Eugenics and Genetics, 26(2), 79–80.
82. Li, D. T., Zhao, L., Cao, Y., Chen, Y., Qiu, Y. G. et al. (2019). A cross-sectional study on the prevalence of Congenital Heart Disease in children from different altitudes in Tibet. Hebei Medicine, 4(12), 1894–1898.
83. Hu, Z. Y., Liu, J. K., Jing, Q. Q., Zeng, X. W., You, K. (2019). Epidemiological survey of existing Congenital Heart Disease in children aged 3 to 14 years in Panzhihua City. Sichuan Medicine, 40(2), 173–175.
84. Ye, D. Q., Lu, L., Zhang, M. J. (2020). A cross-sectional study on the prevalence of Congenital Heart Disease in children from different altitudes in Huangnan Prefecture, Qinghai Province. Medical Aesthetics and Beauty, 29(21), 46.
85. Chen, Y., Chen, M. (2020). Survey on the prevalence of Congenital Heart Disease in preschool children in Nanjing area and analysis of the factors of its development. Practical Preventive Medicine, 29(11), 1384–1387.
86. Han, S., Wei, C. Y., Hou, Z. L., Li, Y. X., Ding, Y. C. et al. (2020). Prevalence of Congenital Heart Disease amongst Schoolchildren in Southwest China. Indian Pediatrics, 57(2), 138–141. https://doi.org/10.1007/s13312-020-1731-z
87. Zhai, J. M., Sun, H., Li, R. H., Sun, Y., Zhong, L. et al. (2020). A survey on the prevalence of Congenital Heart Disease in school children aged 4 to 14 years in Luchun County, Honghe Prefecture, Yunnan Province. Guangxi Medicine, 42(6), 725–728.
88. Huang, Z. P., Yang, W. (2020). Screening and intervention for Congenital Heart Disease in a county of Qiannan region in the age group of 3–18 years. Journal of Qiannan National Medical College, 33(2), 127–128.
89. Song, H. L., Lu, J., Zhou, R. F., Wang, L., Zhang, S. Y. et al. (2021). Prevalence of predilection for Community screening of primary and secondary school students in relation to altitude and ethnicity in five Chinese provinces. China Public Health, 37(10), 1514–1516.
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF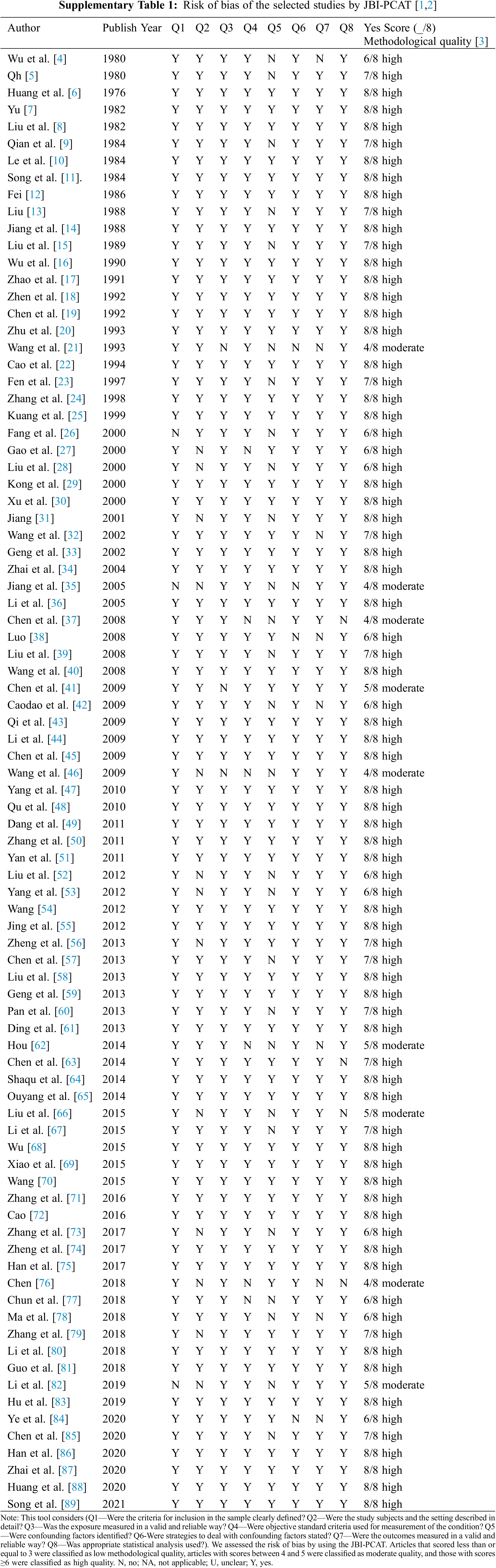
 Downloads
Downloads
 Citation Tools
Citation Tools