 Open Access
Open Access
ARTICLE
Cardiac Surgery with Cardiopulmonary Bypass in Low-Weight or Preterm Neonates: A Retrospective Study Analyzing Early Outcome
1 Department of Cardiovascular and Thoracic Surgery, Université catholique de Louvain, Cliniques Universitaires Saint-Luc, Brussels, Belgium
2 Department of Pediatric Cardiology, Université catholique de Louvain, Cliniques Universitaires Saint-Luc, Brussels, Belgium
3 Department of Pediatric Intensive Care, Université catholique de Louvain, Cliniques Universitaires Saint-Luc, Brussels, Belgium
4 Department of Anesthesiology, Université catholique de Louvain, Cliniques Universitaires Saint-Luc, Brussels, Belgium
* Corresponding Author: Alain J. Poncelet. Email:
Congenital Heart Disease 2023, 18(2), 151-168. https://doi.org/10.32604/chd.2023.022636
Received 18 May 2022; Accepted 13 August 2022; Issue published 15 March 2023
Abstract
Background: Most outcome studies in congenital cardiac surgery for “low weight” neonates include patients undergoing surgery without cardiopulmonary bypass (CPB). The primary objective of our study was to identify risk factors for in-hospital mortality in neonates weighing less than 3 Kg and undergoing surgery with CPB. In addition, we compared the effect of early surgery with CPB (before 37W-gestational age (GA)) for congenital heart disease to delayed surgery until a corrected GA of 37 weeks in an attempt to promote weight gain. Methods: Retrospective single-center study including all patients operated between 1997 and 2017. Uni- and multivariable analysis were used to analyze outcome. Results: 143 patients were included. The median weight was 2.7 Kg and 49 (34.3%) weighted <2.5 Kg. 80% of the patients were Risk stratification STAT categories ≥3. 114 patients (80%) were operated without delay (usual timing, median age 9 days), whereas 29 patients (20%) entered a delayed strategy (median age 30 days). In-hospital mortality was 21.7%. By multivariate analysis, dysmaturity, preoperative positive ventilation, post-operative ECMO requirement or resuscitation, and any residual lesion were predictors of in-hospital death. In-hospital mortality in the usual timing group and the delayed group were 21.1% and 24.1%, respectively (p = 0.71). In-hospital mortality for neonates operated prior to 37W-GA (n = 10) was 27.3%. Conclusions: Predictors of in-hospital mortality in neonates less 3 Kg requiring CPB surgery did not differ from those unveiled in other contemporary studies. Our data demonstrates that a strategy of delaying surgery in selected patients resulted in similar clinical outcome.Keywords
Congenital heart surgery in low weight neonates (less 30 days) using cardiopulmonary bypass (CPB) conveys a significant risk of morbidity and mortality, and many of those procedures remain technically challenging [1–3].
Among patient-related risk factors for mortality, low weight at birth as well as prematurity are the most frequently studied [4,5]. Lung prematurity, other organ immaturity and dysfunction and body weight all contribute to the increased risk of morbidity associated with neonatal cardiac surgery [6,7]. Based on the recent literature, the term “low weight” (LW) relates to patients <2.5 Kg, while “very low weight” (VLW) relates to infants <2.0 Kg. However, the majority of studies include surgery without CPB inasmuch as half of their cohort [3–6,8]. Using such criteria of weight, Dollat et al. reported a 36% in-hospital mortality in a series of 28 preterm neonates operated under CPB at a mean weight of 2.3 Kg [9]. One could argue that using such weight definition of less than 2.5 Kg for LW in neonatal CPB surgery does not appropriately distinguish the threshold under which a marked decline in survival occurs.
For more than two decades, our centre has supported an algorithm based on delaying surgical procedure in LW neonates (prematurity or dysmaturity) requiring the use of CPB with the goal of achieving a corrected gestational age at surgery of 37 weeks, thereby allowing for weight gain and organ maturation. In theory, this philosophy of supportive care might decrease the cardiopulmonary bypass-related risk of intracranial haemorrhage, renal dysfunction, and coagulopathy [10,11].
However, contemporary studies from canters of expertise have shown that most of critical cardiac malformations can be surgically treated (repaired rather than palliated) within the first days of life with good results [4,5,12].
In addition to the debate about optimal timing, it has been shown that delay in intervention with non-physiological hemodynamic, persisting cyanosis, inadequate pulmonary blood flow or preoperative ventilation can be associated with increased complications, including ventilatory dependency, failure to thrive, absence of weight gain, sepsis, chronic pulmonary disease, necrotizing enterocolitis, and acute renal failure [13].
The primary objective of our study was to identify risk factors for in-hospital mortality in a selected cohort of neonates weighing less than 3 Kg and undergoing surgery with CPB. As a secondary objective, we compared the effect of early surgery with CPB (before 37W-GA and/or >37W with dysmaturity) for critical congenital heart disease (CHD) compared to delayed surgical strategy to achieve either a corrected gestational age of 37 weeks or in an attempt to promote weight gain and organ maturation for dysmature neonates. We also analysed the effect of low weight at CPB surgery (2.5 Kg or less) for critical congenital heart disease (CHD).
Our Institutional Ethical Board approved this retrospective analysis (Cliniques universitaires Saint-Luc, Brussels, Belgium, IRB 2021/0408/334). Informed consent was waived based on the retrospective design as well as the data anonymization.
For this study, we extracted from our institutional paediatric cardiac database all patients operated with the use of cardiopulmonary bypass and weighting less than 3 Kg from 1997 until 2017.
Since patients with a diagnosis of hypoplastic left heart syndrome (HLHS) and HLHS-like represented a subset of patients with known high procedural-related mortality, those were excluded from the present analysis.
2.1 Data Collection and Follow-Up
Information about preoperative, operative and postoperative variables were retrospectively collected by reviewing a computerized patient database, hospital charts, operative notes and autopsy reports if appropriate. Clinical follow-up was obtained from outpatient clinics records or from referring paediatric cardiologists.
In our cohort, the presence of heart failure was based on clinical symptoms such tachypnea, tachycardia, hepatic enlargement, feeding difficulties, inappropriate sweating, but also on imaging studies (cardiomegaly on chest XR). Besides, mechanical ventilation as well as the need for positive pressure ventilation was also used as criteria for heart failure. Finally, patients in whom the use of inotropes and/or diuretics was necessary to maintain a stable hemodynamic state were similarly included.
Outcomes of interest were observed for 3 months following the index procedure and included mortality, extra-corporeal life support (ECMO), unplanned reoperation or unplanned catheterization, atrio-ventricular block requiring pacemaker implantation, chylothorax, sepsis, stroke or intra-cranial bleeding and necrotizing enterocolitis. The hospital length of stay was calculated as date of intervention to date of discharge. Follow-up was 100% complete (143/143 patients). The median follow-up was 54 months (1.2–128).
Continuous data are presented as mean ± standard deviation or median (interquartile range) for respectively parametric and nonparametric data. Normality of the distribution was assessed with the Shapiro-Wilk test. Categorical data are presented as numbers and proportions and compared with the Chi-square test or the Fisher’s exact test, if appropriate. Differences between means or medians were compared using unpaired Student’s t-test or Mann–Whitney U test, according to the distribution. Survival curves were generated with the Kaplan–Meier estimator and were compared using the log-rank test.
To assess the factors associated with survival, a Cox proportional hazards model was generated by including pre- and intraoperative variables. To determine the independent predictors of each outcome of interest, variables with a p-value < 0.20 in the univariate analysis were entered into a multivariate conditional forward stepwise selection procedure. Results are displayed as hazard ratio or adjusted hazard ratio with 95% confidence interval. All tests were two-sided, with significance set at the 0.05 probability level (See Appendix A for a complete list of variables assessed in the logistic regression models). All statistical analyses were performed using the IBM SPSS Statistic version 26 (IBM Corp., Armonk, New York).
Between 1997 and 2017, 143 patients (78 boys and 65 girls) with a weight below ≤3 Kg were operated using cardio-pulmonary bypass in our center (median age at surgery: 13 days). This represents 4.5% of all children less 18 years old operated on in our center during the same period (n = 3123).
Patients’ characteristics are provided in Tables 1–2. The median weight was 2.7 Kg and 49 (34.3%) weighted ≤2.5 Kg at the time of surgery. Antenatal diagnosis was present in more than half of the patients. Prematurity, defined as a gestational age of <37 weeks was present in 31.5%. A neonate with a birth weight less than the 10th percentile for gestational age was considered “small for gestational age” or dysmature. Dysmaturity (LBW-GA) was present in 40.6% of the cohort.
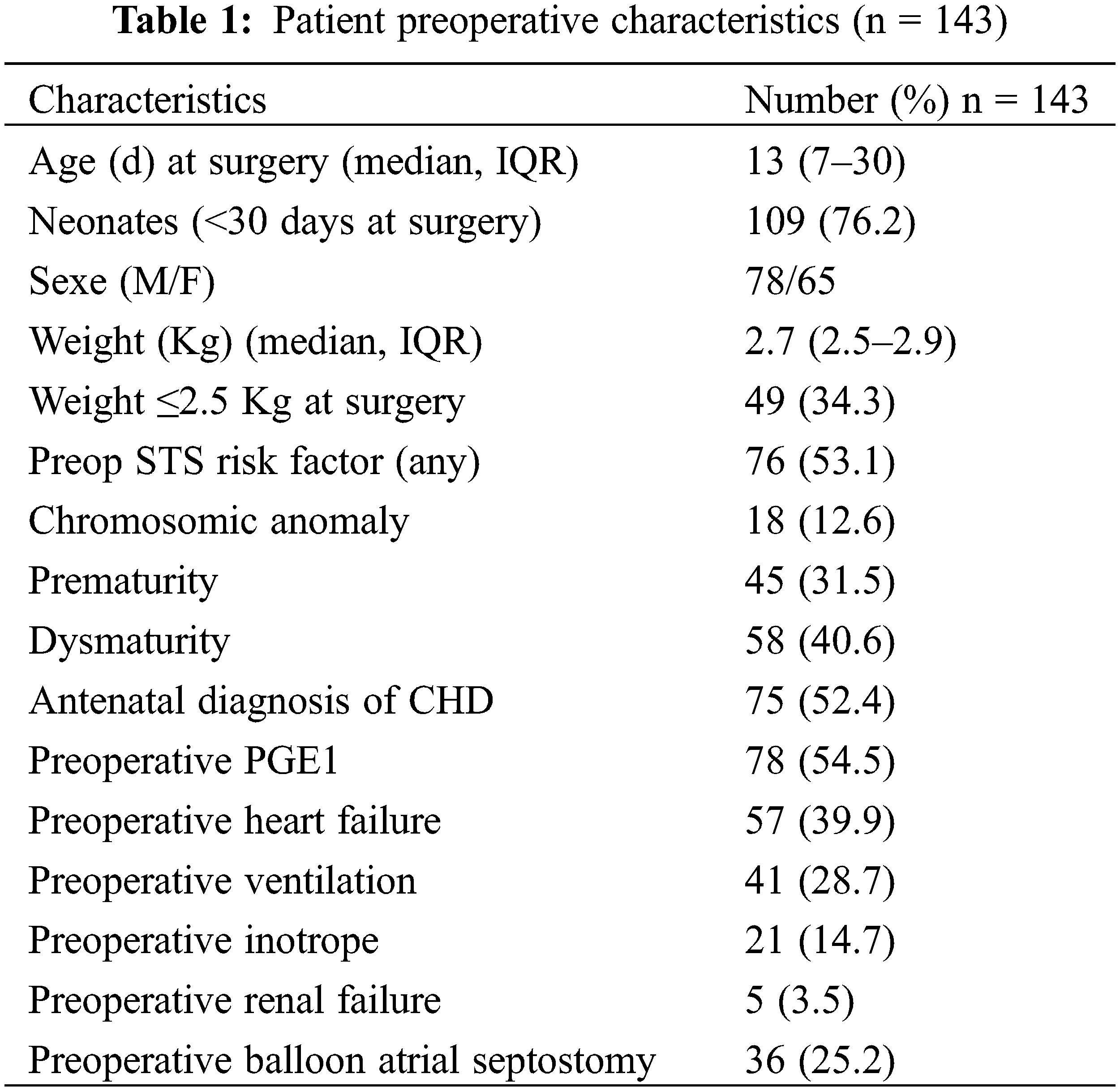
The majority of those neonates (54.5%) were intensive care unit (ICU)-bound and received prostaglandin infusion. With 40% of patients presenting preoperative heart failure, invasive ventilation and inotropic support were commonly used to stabilize patient’s condition prior to surgery.
Among the commonest diagnosis, transposition of the great arteries (TGA) (41%), ventricular septal defects (VSDs) (15%), truncus arteriosus (9%), pulmonary atresia (PA) (w/wo VSD) and extreme forms of Tetralogy of Fallot (TOF) (8%), interrupted aortic arch (5%), total abnormal pulmonary venous return (TAPVR) (5%) and aorto-pulmonary window (4%) altogether represented more than 90% of all diagnoses.
By risk stratification STAT(Society of thoracic surgeons/European Association for cardio-Thoracic surgery) scores, 26 patients (18.2%) were STAT 1or STAT 2 categories, 55 patients in STAT 3 (38.5%), 56 (39.2%) and 6 patients (4.2%) in STAT 4 and 5, respectively.
114 patients (79.7%) were operated without delay (median age at surgery nine days), whereas the surgical procedure was delayed in 29 patients (20.3%) in an attempt to promote weight gain and organ maturation (median age at surgery 30 days). Table 2 summarizes the diagnosis in each subset of patients.
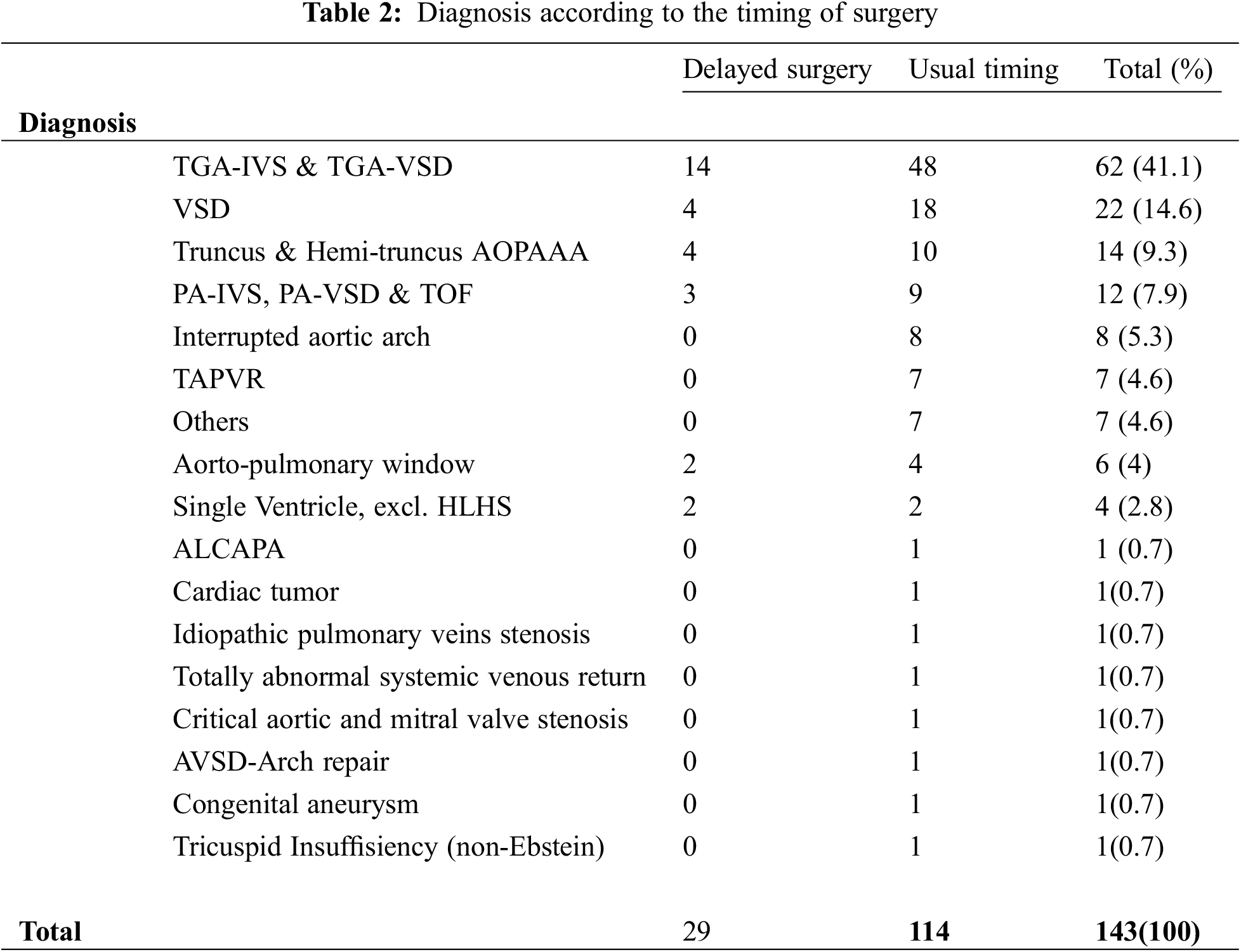
Despite our institutional policy, ten patients were operated prior to corrected 37 W. Four out of 10 were LBW-GA, and the mean GA was 34.4 weeks. Diagnosis were d-TGA-IVS (n = 4), d-TGA-restrictive VSD (n = 1), IAA-VSD (n = 2), A-P window (n = 1), total abnormal systemic venous return (n = 1) and cortriatum with native pulmonary veins stenosis (n = 1).
d-TGA patients underwent ASO prior to LV deconditioning. All other patients (except the total abnormal systemic venous patient) return were in overt heart failure, mechanically ventilated and with progressing secondary organ dysfunction despite maximal medical therapy.
Table 3 summarizes the operative characteristics of the entire cohort and the two subgroups.
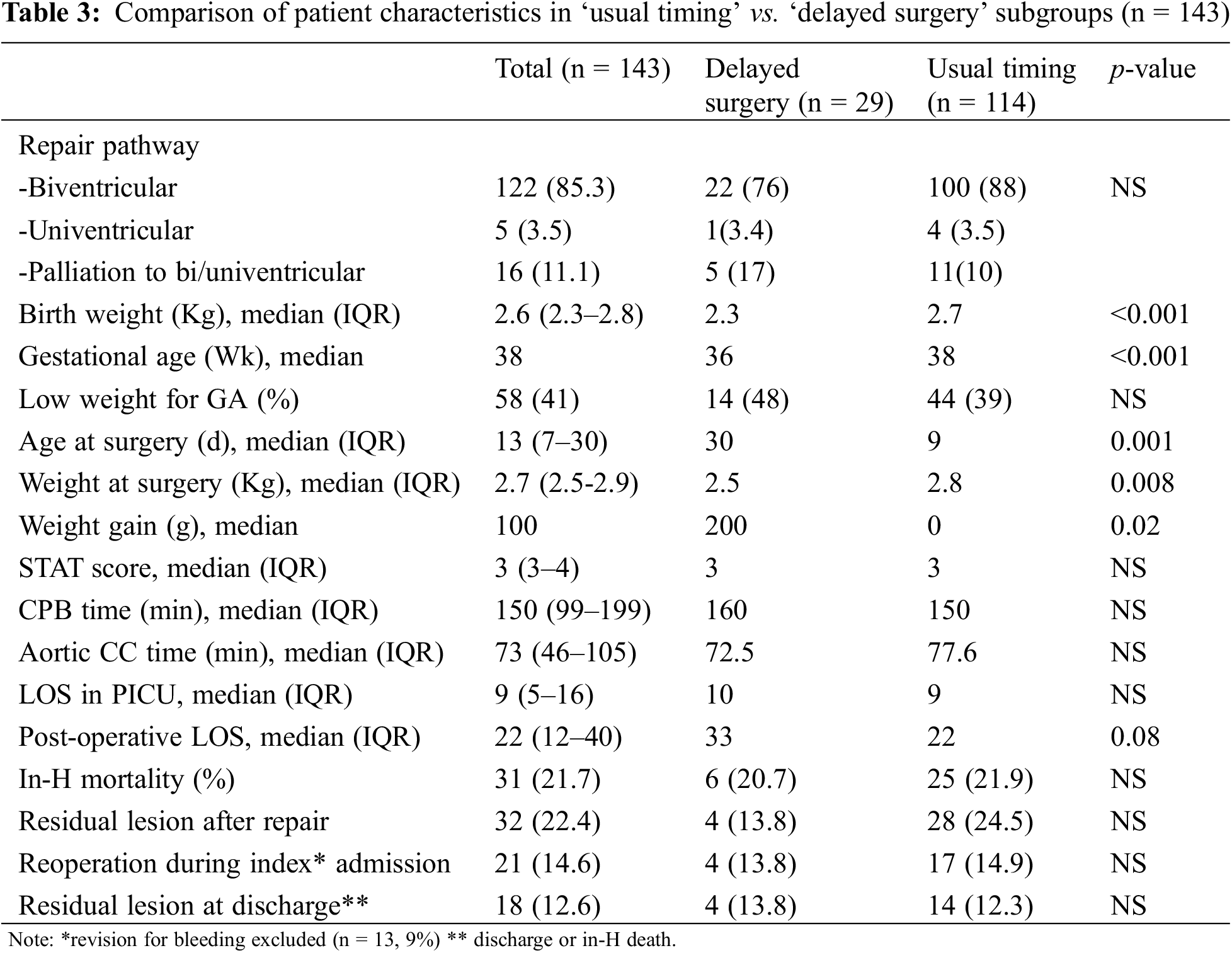
As illustrated, most patients underwent a bi-ventricular repair (n = 122, 85%) whereas palliation for uni/biventricular and for univentricular pathways were performed in 16 and five patients respectively. Risk categories (STAT), CPB and aortic cross clamp (ACC) time did not differ between groups.
Delayed sternal closure was performed in 52 patients of the cohort (36.4%) without difference between the delayed and the undelayed groups (37.9% vs. 36%, p = 0.8)
For the entire cohort, median intensive care and hospital stay were nine and 22 days, respectively. Despite nearly three weeks of conservative treatment, and a significant difference in weight gain (mean: 314 g vs. 134 g, p = 0.02), the «delaying strategy» resulted in similar post-operative intensive care length of stay (LOS) (13.5 d) and a longer post-operative hospital stay (mean: 37.5 vs. 27.5 days, p = 0.08).
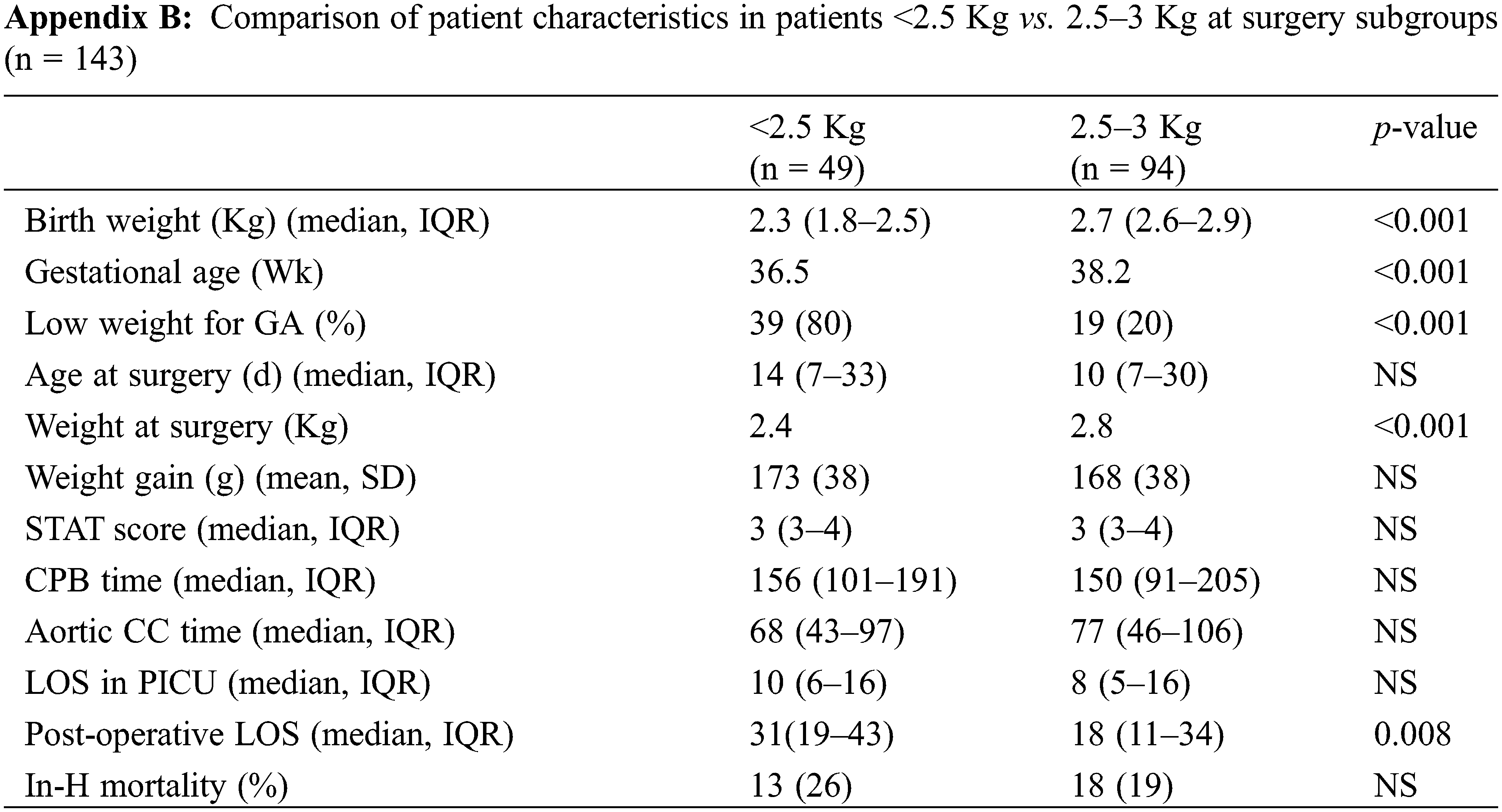
We repeated our analysis using a weight threshold of 2.5 Kg at surgery. As expected, the smallest, mostly immature neonates experienced a longer post-operative hospital stay (median: 31 vs. 18 days, p = 0.008) even though their intensive care LOS was not significantly different (median: 10 vs. 8 days, p = NS) (See Appendix B).
Excluding surgical revision for bleeding (n = 13, 9%), there were 21 reoperations (14.6%) and 16 patients (11.7%) required post-operative catheterization. The most common surgical reinterventions were ECMO procedures (n = 7) and pulmonary blood flow regulation procedures (n = 6). A detailed description of reintervention is provided in Table 4.
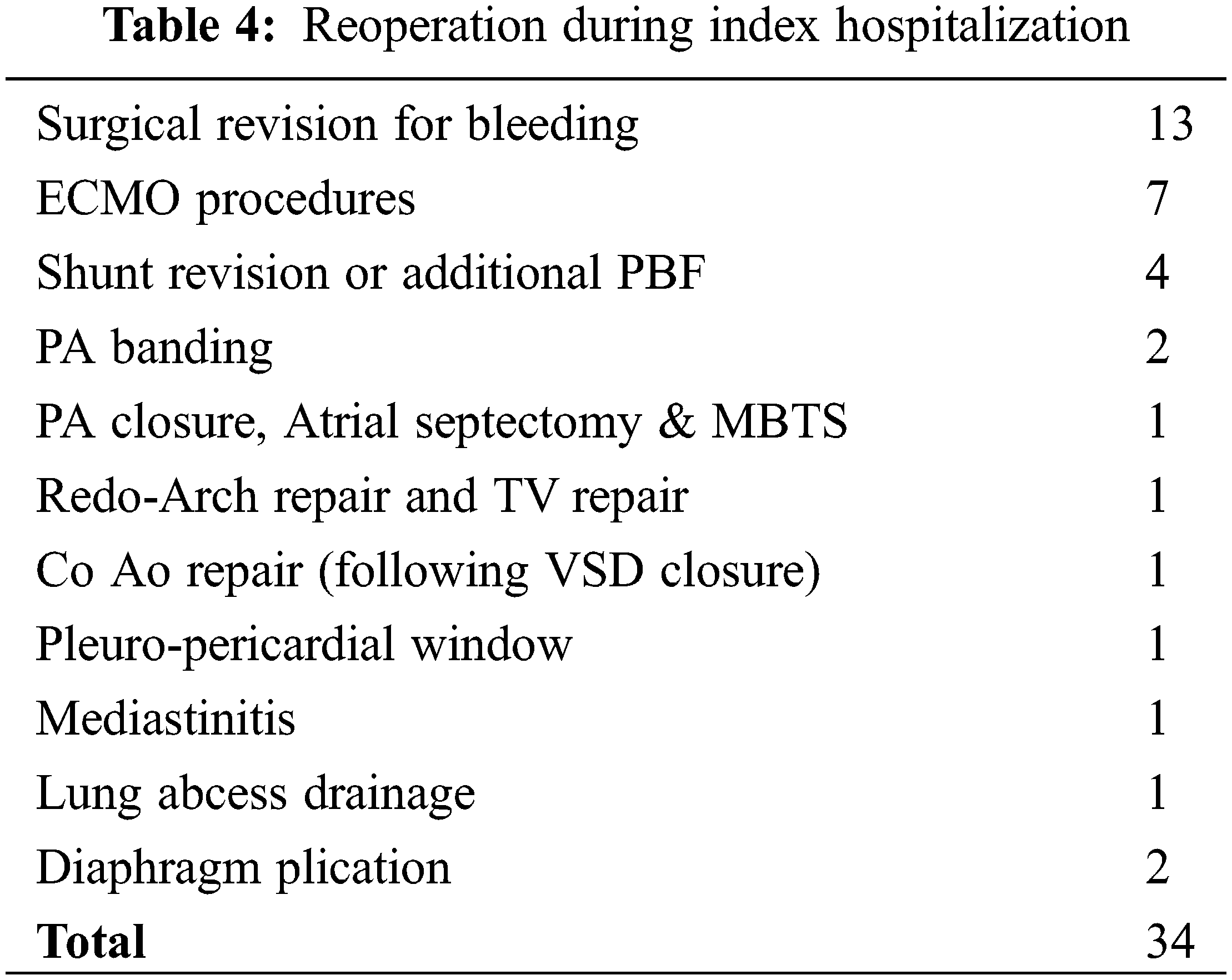
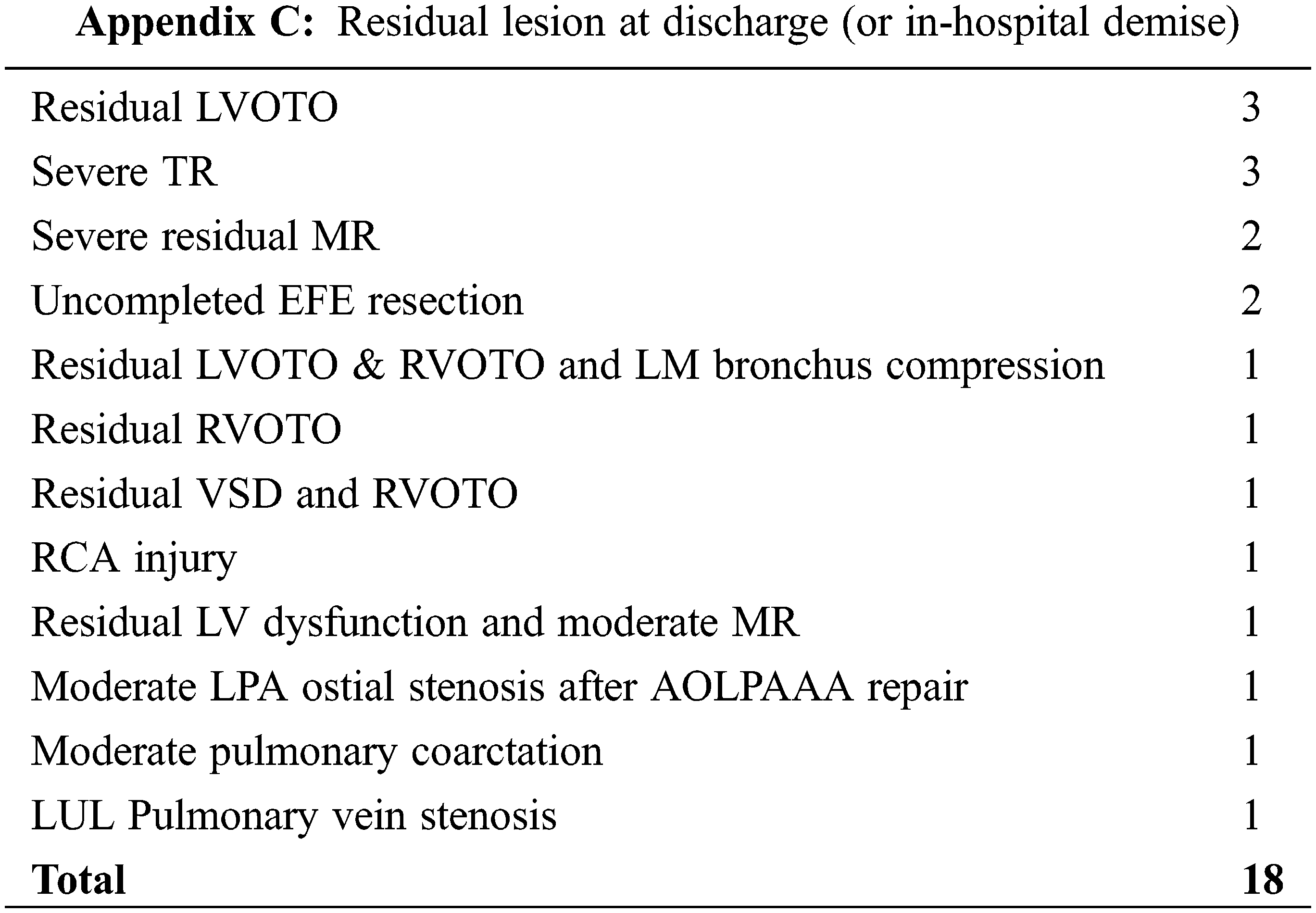
Finally, a residual lesion was present in 18 patients (12.6%) at hospital discharge or demise. Right and/or left ventricular outflow tract obstruction (n = 6), and severe AV valves regurgitation (n = 5) representing the commonest residual lesions. See Appendix C for the complete listing of residual lesions.
Out of 143 patients, 31 (21.7%) died during index hospitalisation, whereas overall survival at 1-yr and 5 yr for the entire cohort was 74.5% and 73.3%, respectively. The in-hospital mortality for the biventricular pathway group (n = 122) was 16.4%.
The in-hospital mortality for the usual timing group and the delayed surgery group were 21.1% and 24.1%, respectively (p = 0.71).
We analysed our subgroup of delayed surgery according to the presence or absence of weight gain during the optimization period (>10% weight gain). No significant difference in mortality was found (30.8% vs. 18.8%, p = 0.45).
For those neonates operated prior to 37W-GA, in-hospital mortality was 27.3% (vs. 21.2% if ≥37W-GA at surgery, p = 0.70).
For those neonates weighing 2.5 Kg or less at surgery, mortality was 28.6% (vs. 21.3% if weight 2.5–3 Kg, p = 0.08).
Kaplan-Meier survival curves according to the timing of surgery, weight at surgery and surgical era are illustrated in Figs. 1A–1C.
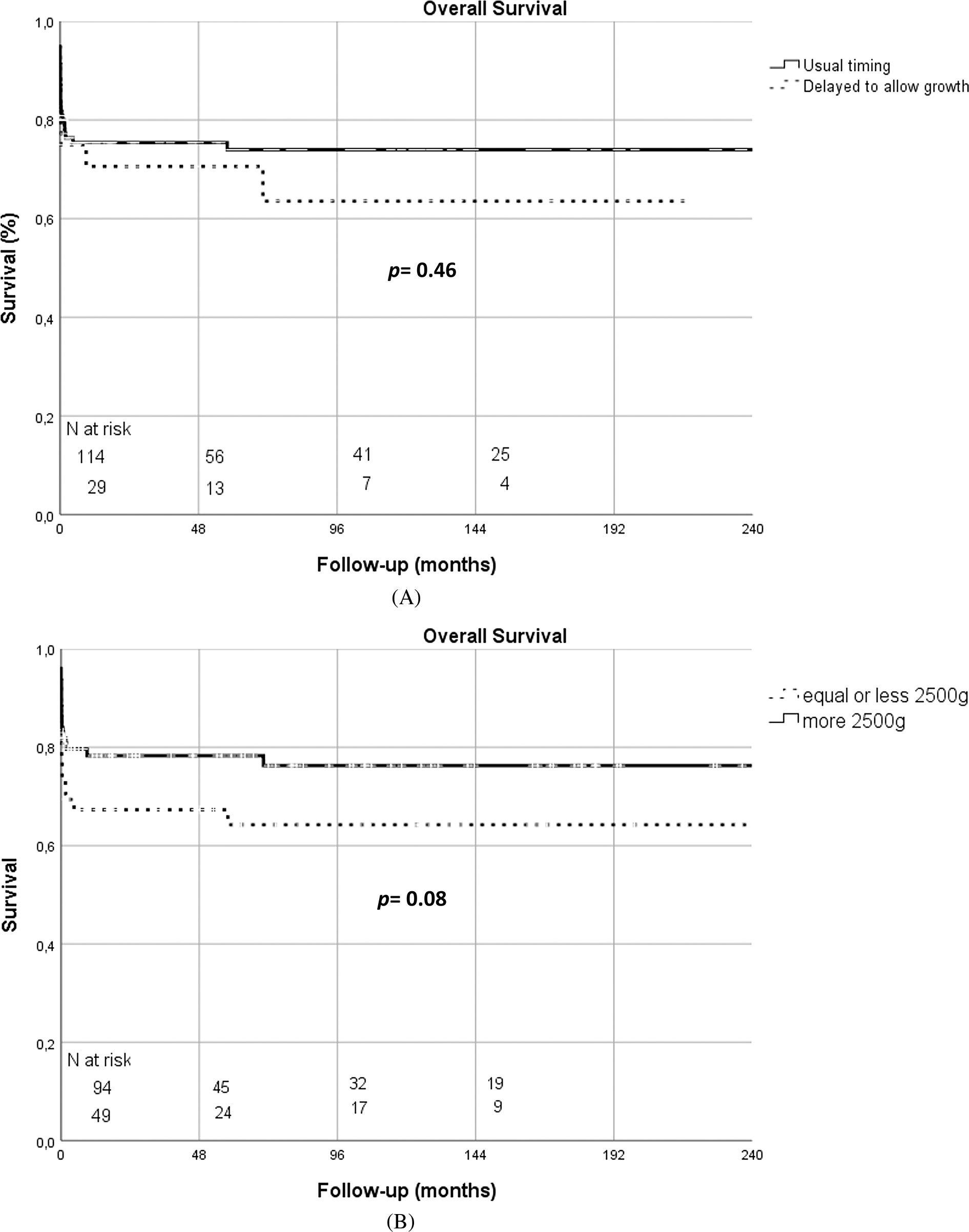
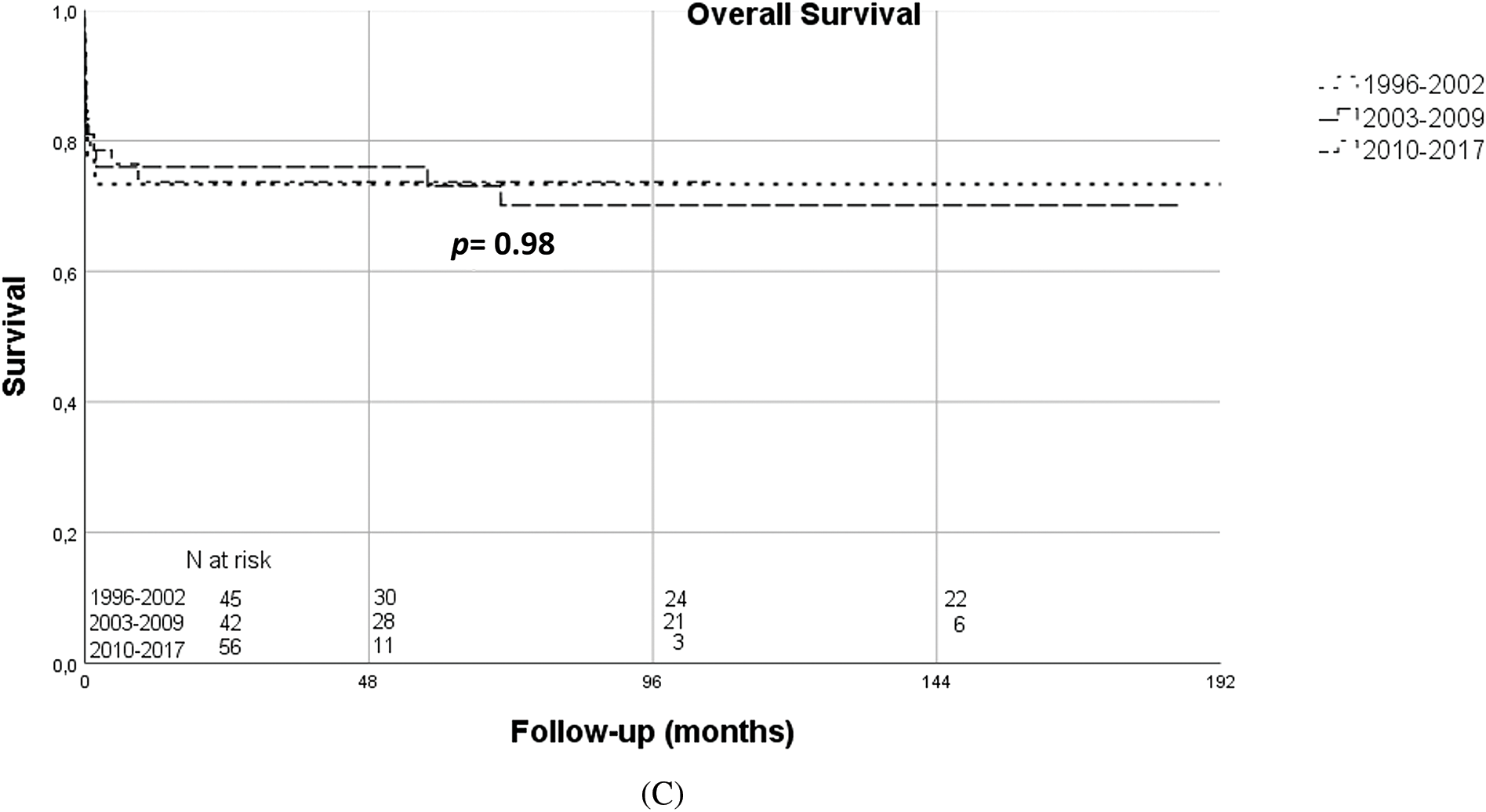
Figure 1: (A) Kaplan-Meier overall survival curves according to timing of surgery (usual timing vs. delayed surgery) (B) Kaplan-Meier overall survival curves according to weight at surgery (≤2500 g vs. >2500 g) (C) Kaplan-Meier overall survival curves according to surgical era (1996–2002 vs. 2003–2009 vs. 2010–2017)
As illustrated in Figs. 2A and 2B, we also analysed survival at 3 months as well as the rate of peri-operative complications by corrected gestational age and weight at time of surgery. The rate of survival increased with weight and no significant difference was noted between the 2–2.5 Kg and 2.5–3 Kg subgroups (p = 0.77). Survival increased with corrected-GA with a plateau in patients with 37 W GA or more. In-hospital morbidity decreased with corrected-GA and weight, with those >2.5 Kg having a 54% rate of complications (p = 0.04).
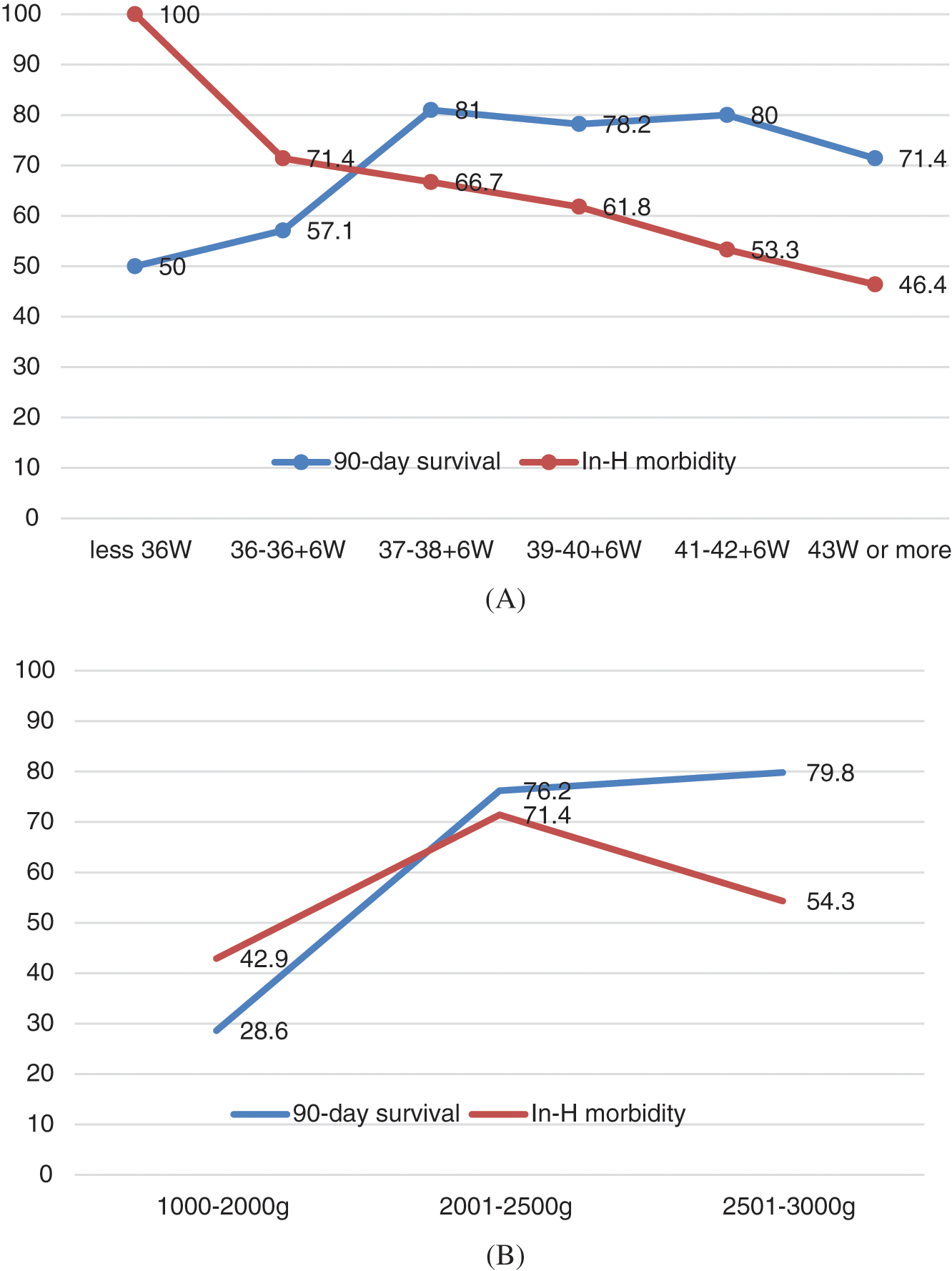
Figure 2: (A) Survival at 3 months and rate of complications of interest by corrected gestational age at the time of intervention (B) Survival at 3 months and rate of complications of interest by weight at time of intervention
For the entire cohort, by univariable analysis (Table 5), preoperative variables such as dysmaturity (p = 0.03) and risk categories (STAT, p = 0.007, Fig. 1) were strong predictors of in-hospital death. However, neither prematurity (<37W-GA), chromosomal abnormality nor a weight of less than 2.5 Kg were related to a poorer outcome (p = 0.87, p = 0.67, and p = 0.63, respectively). Dividing the cohort in terciles, and considering the most recent subgroup (2010–2017) as the reference, the Era effect was neutral (1996–2002: HR 0.9 (CI 0.4–2) (p = 0.83) and 2003–2009: HR 0.8 (CI 0.3–1.9) (p = 0.63).
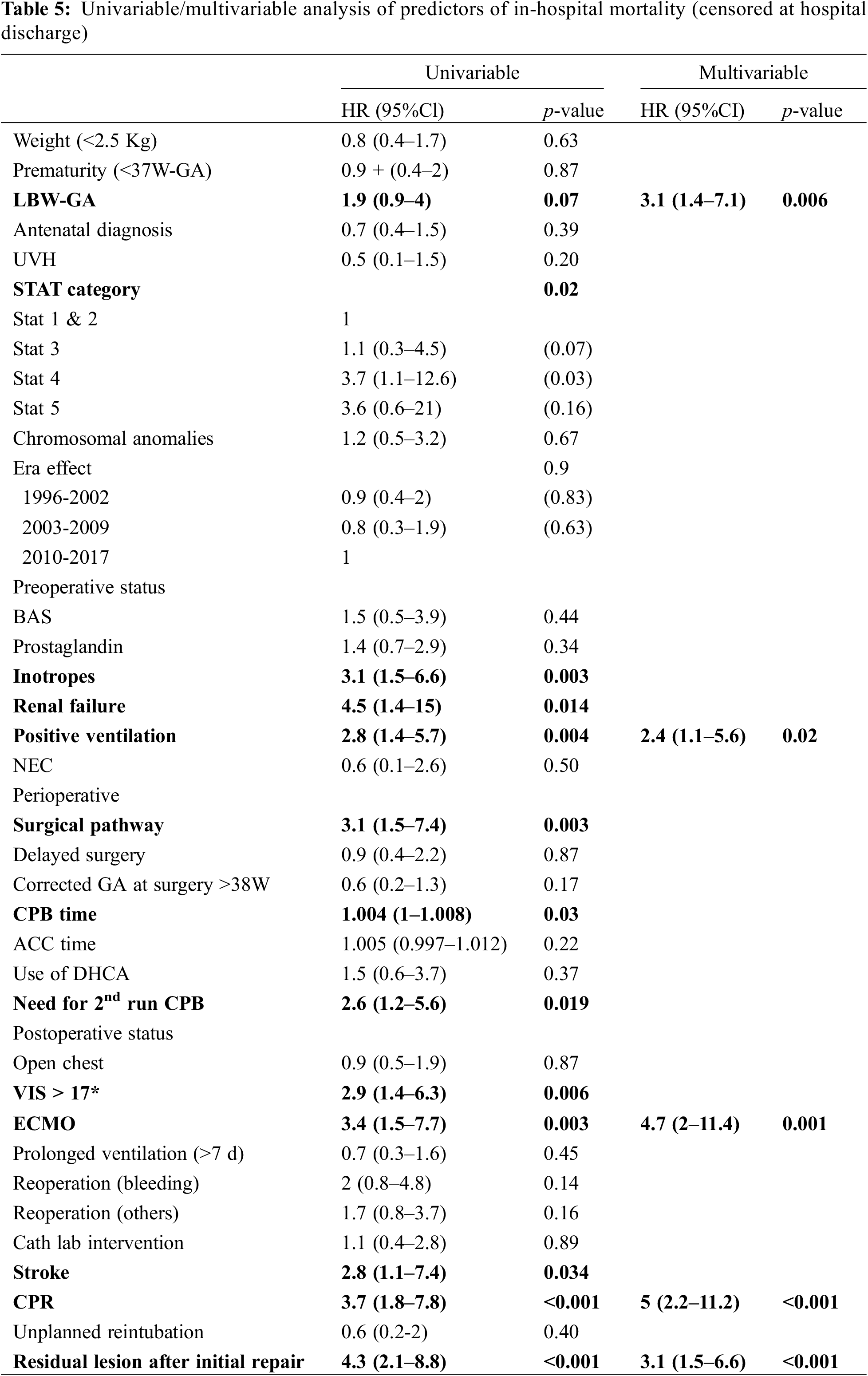
Relative to patient condition prior to surgery, inotropic support (Hazard Ratio (HR) 3.4, 95%CI [1.6–7.1]) and positive ventilation (HR 3, 95%CI [1.5–6]) were significant predictors of death.
Among the operative variables, surgical pathways other than biventricular repair (HR 3.9, 95%CI [1.9–8.2]), the need for additional run of CPB (HR 2.9, 95%CI [1.4–6.4]), the need for extra-corporeal membrane oxygenator (ECMO) (HR 4.3, 95%CI [1.9–9.7]), a higher postoperative vasoactive inotropic score (HR 3.2, 95%CI [1.5–6.9]), surgical revision for bleeding (HR 2.5, 95%CI [1–6.1]) and resuscitation (HR 4.3, 95%CI [2.1–8.9]) were all strong predictors of death. The presence of a residual lesion after initial repair was also a significant predictor of in-hospital mortality (HR 4.4, 95%CI [2.2–6.8]).
In multivariable analysis, dysmaturity (HR 3.1, 95%CI [1.4–7.1]), preoperative positive ventilation (HR 2.4, 95%CI [1.1–5.6]), post-operative ECMO requirement (HR 4.7, 95%CI [2–11.4]) or resuscitation (HR 5, 95%CI [2.2–11.2]), and any residual lesion after repair (HR 3.1, 95%CI [1.5–6.6]) were all predictors of in-hospital death (Table 5).
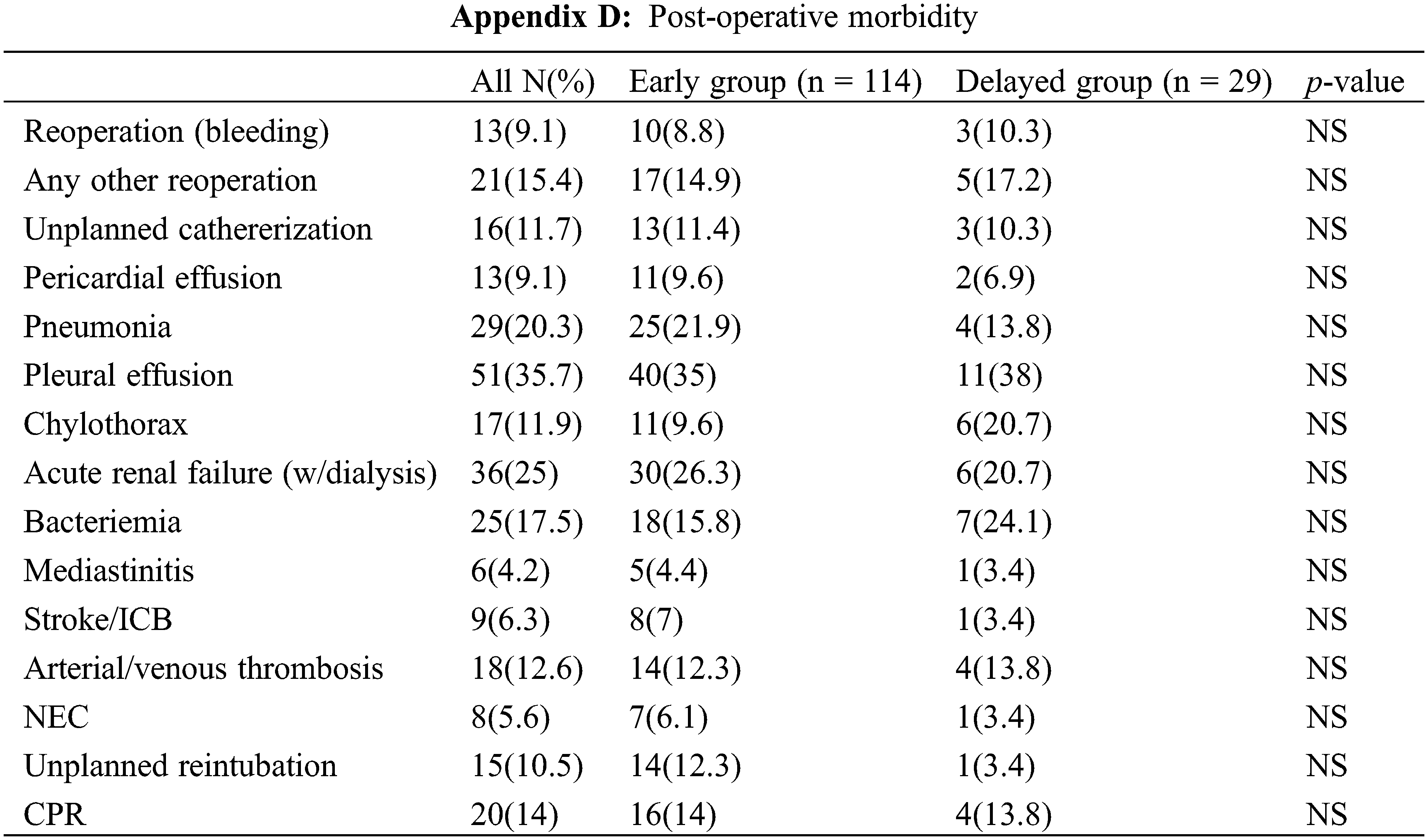
Importantly, 105 (73%) of these «high risk» patients had at least one of the listed post-operative complications, the most prevalent being pleural effusion (35.7%), renal dysfunction requiring peritoneal dialysis (25%), pneumonia (20.3%) and bacteraemia (17.5%). Appendix D provides an overview of the complications encountered in this subset of patients.
Comparison between the usual timing group and the delayed surgery group did not reveal any significant difference in the frequency of complications.
Over the past decades, prematurity and low birth weight have remained significant risk factors for mortality after neonatal cardiac surgery with or without the use of cardio-pulmonary bypass [6,14]. Improvement in surgical techniques and in neonatal medical care have resulted in a decreased operative mortality so that lower weight thresholds are set for operability.
Most studies on neonatal cardiac surgery have demonstrated that the relationship between birth weight and survival was nonlinear with 2.5 Kg being the weight under which a marked decline in survival occurs [1,4,8,15]. This threshold was determined based on studies with mixed cohort of non-CPB and CPB neonatal surgeries.
In 2015, Dollat et al. [9] reported on a subset of 28 premature babies (<37W-GA), undergoing early cardiopulmonary bypass surgery with a median weight of 2.3 Kg. The in-hospital mortality and overall mortality were 36% and 43%, respectively. These results question whether using such “weight” definition for neonatal CPB surgery appropriately distinguishes the threshold under which a marked decline in survival will occur. It also underscores the need for a multidisciplinary approach to investigate all potential strategies that could optimize outcome in those high-risk patients. However, Kim et al. were able to demonstrate encouraging results in term of survival in both 2.0–2.5 Kg and 2.5–3.0 Kg subgroups in their analysis of their most recent cohort of operated LBW neonates (including 15% patients without CPB). Overall, their in-hospital mortality was 11.8%, much lower than the same group previous report (24%) [15].
For the last two decades, our institutional policy has been to conservatively nurture those babies, to optimize their preoperative status as much as possible and to delay the surgical procedure in an attempt to promote weight gain and organ maturation [10]. However, several authors have challenged this policy [13] and CPB surgery in LW and VLW neonates is nowadays common practice in many centres of expertise [5,7,12].
In this retrospective study, we investigated our results in patients weighing less than 3 Kg with critical congenital heart disease requiring cardiopulmonary bypass, with a specific analysis of a subset of 29 neonates for whom we elected to use a primary conservative approach. These were deemed “high-risk” patients with a birth weight below 2.5 Kg (median 2.2 Kg), of whom 14 (48%) were both premature (<37W-GA) and dysmature (LBW-GA).
Comparison of our two subgroups (Table 3) showed that CHD diagnosis, risk category, risk stratification, CPB time and aortic clamping time were equivalent in the usual timing and delayed groups allowing for a meaningful comparison of post-operative mortality and morbidity.
Our results show that such approach allowed for a significant additional weight gain in the delayed group (mean 314 g, median 200 g), bringing those neonate’s weight up just over 2500 g. However, we did not find any survival benefit in the « delayed group » strategy (24.1% vs. 21.1% in the usual timing group).
While comparison with other studies is sometimes blurred by the inclusion of non-CPB neonatal cardiac surgery, our overall in-hospital mortality of 21.7% is in agreement with the existing literature [7,14,16] and reflects both the severity of their CHD diagnosis (82% ≥ STAT-3) and precarious preoperative state (heart failure (40%), ventilation (29%)).
In a recent study from Melbourne [7], reporting on 171 preterm and LBW infants, the overall six-month mortality was 23%, ranging from 9.2% (VSD repair) to 24% (TGA repair). Our analysis of the inflection point for both mortality and morbidity is in agreement with their results, since survival was similar for 2–2.5 Kg and 2.5–3.0 Kg subgroups, at the expenses of an increased in-hospital morbidity.
From the STS database studies, we know that STAT category will reflect on in-H mortality and that the presence of established preoperative risk factors will further worsen patients’ outcome. In neonates with preoperative shock (resolved or persistent at surgery), ventilation, or renal dysfunction, in-hospital mortality ranges from 15% to 36% [6].
In univariable analysis, unexpectedly, we could not confirm that the threshold of 2.5 Kg defined an increased risk of in-hospital mortality. We hypothesized that the comparison group which only included neonates with less 3 Kg at surgery (median weight 2.8 Kg) and more than 80% of STAT-3 category or higher patients represented an already challenging population, so that the excess mortality for the LW group could not be unmasked.
We also hypothesize that the absence of significant improvement in survival over time (the “era effect”) in our study could be related to fact that we did not yet alter our algorithm of treatment for those high-risk patients during the study period. In their last study, Kim et al. suggested that some of the incremental survival in the most recent cohort was likely related to a gradual change in practice pattern to preferentially offer catheter-based or hybrid intervention to LBW neonates when feasible especially in the univentricular subgroup. Our 16.4% mortality in the bi-ventricular group (n = 122, 85%) support this hypothesis.
Our multivariable analysis confirmed that common preoperative patient’s factors such as dysmaturity (LBW-GA) or preoperative ventilation to treat cardio-pulmonary failure were predictors of mortality.
In their study, Alsoufi et al. [4] suggested that this increased mortality hazard found in LW patients older than 31 days might indicate that significant delays in surgery might have a negative effect on survival, especially in those who fail to gain weight. Our data does not corroborate this hypothesis, as it showed that an increase in weight >10% during the optimization period resulted in a slightly worse outcome, though statistically not significant (30.8% vs. 18.8% in-H death). We speculate that part of this weight gain might reflect fluid overload or tissue swelling secondary to long-standing PGE1 infusion [17,18].
Post-operative organ dysfunction such as renal failure requiring dialysis, prolonged ventilation (>seven days), intracranial haemorrhage/stroke or bacteraemia have been shown to potentially be related to the use of CPB in premature/LBW neonates [19]. In our study, the prevalence of those potentially CPB-related complications are in agreement with recent studies [15].
Subgroup analysis demonstrated that they were as frequently encountered in each group.
Predictors of in-hospital mortality in neonates less 3 Kg requiring CPB surgery did not differ from those unveiled in other contemporary studies.
From the mortality and morbidity data provided in this study, we demonstrate that a strategy of delaying surgery in selected patients resulted in similar early clinical outcome. Importantly, this algorithm did affect our institutional resources since this small subset of neonates were hospitalized for an additional two-three weeks preoperatively, of whom 41.4% were ventilated. Based on the neutral effect observed in term of mortality as well as our excess mortality in the non-biventricular pathway groups, our current recommendation would be a) to delay surgical repair in patients <2 Kg and/or <37W corrected GA and a diagnosis suitable for temporisation and b) to explore alternative treatment modalities of palliation in patient with univentricular hearts as well as those with yet undetermined biventricular reparability (hybrid Norwood, ductal stenting,...).
This study suffers many limitations such as its retrospective design, the long period of inclusion and the small number of patients. On the other hand, the long-standing institutional policy of conservative optimization as well as the limited number of surgeons (JR and AP) have likely limited the variability in outcome in this heterogeneous cohort of patients.
This study also fails to address the effect of surgical timing on neurodevelopmental outcome, a very important aspect in those critically ill neonates, besides mortality and morbidity, although we know from some recent studies that the pathway of early surgical repair seems to limit long-term neurodevelopmental sequelae [20–22].
Acknowledgement: The authors thank Dr. D. Castanares Zapatero for his help in statistical reviewing and Mrs P. Segers for her expertise in editorial help.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Alain J Poncelet, Maureen Peers de Nieuwburgh, Mona Momeni; data collection: Alain J Poncelet, Maureen Peers de Nieuwburgh, Thierry Detaille; analysis and interpretation of results: Alain J Poncelet, Maurren Peers de Nieuwburgh, Stéphane Moniotte, Mona Momeni; draft manuscript preparation: Alain J Poncelet, Maureen Peers de Nieuwburgh, Stéphane Moniotte, Geoffroy de Beco, Karlien Carbonez, Jean E Rubay, Laurent Houtekie, Mona Momeni. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: The Institutional Ethical Board approved this retrospective analysis (Cliniques Universitaires Saint-Luc, Brussels, Belgium, IRB 2021/0408/334). Informed consent was waived based on the retrospective design as well as the data anonymization.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Pawade, A., Waterson, K., Laussen, P., Karl, T. R., Mee, R. B. (1993). Cardiopulmonary bypass in neonates weighing less than 2.5 kg: Analysis of the risk factors for early and late mortality. Journal of Cardiac Surgery, 8(1), 1–8. [Google Scholar] [PubMed]
2. Netz, B. C., Hoffmeier, A., Krasemann, T., Zahn, P., Scheld, H. H. (2005). Low weight in congenital heart surgery: Is it the right way? The Thoracic and Cardiovascular Surgeon, 53(6), 330–333. [Google Scholar] [PubMed]
3. Bové, T., Francois, K., de Groote, K., Suys, B., de Wolf, D. et al. (2004). Outcome analysis of major cardiac operations in low weight neonates. Annals of Thoracic Surgery, 78(1), 181–187. [Google Scholar]
4. Alsoufi, B., Manlhiot, C., Mahle, W. T., Kogon, B., Border, W. L. et al. (2014). Low-weight infants are at increased mortality risk after palliative or corrective cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 148(6), 2508–2514. [Google Scholar] [PubMed]
5. Kalfa, D., Krishnamurthy, G., Duchon, J., Najjar, M., Levasseur, S. et al. (2014). Outcomes of cardiac surgery in patients weighing <2.5 kg: Affect of patient-dependent and -independent variables. Journal of Thoracic and Cardiovascular Surgery, 148(6), 2499–2506. [Google Scholar] [PubMed]
6. Costello, J. M., Pasquali, S. K., Jacobs, J. P., He, X., Hill, K. D. et al. (2014). Gestational age at birth and outcomes after neonatal cardiac surgery: An analysis of the society of thoracic surgeons congenital heart surgery database. Circulation, 129(24), 2511–2517. https://doi.org/10.1161/CIRCULATIONAHA.113.005864 [Google Scholar] [PubMed] [CrossRef]
7. Alarcon Manchego, P., Cheung, M., Zannino, D., Nunn, R., d’Udekem, Y. et al. (2018). Audit of cardiac surgery outcomes for low birth weight and premature infants. Seminars in Thoracic and Cardiovascular Surgery, 30(1), 71–78. https://doi.org/10.1053/j.semtcvs.2018.02.013 [Google Scholar] [PubMed] [CrossRef]
8. Shepard, C. W., Kochilas, L. K., Rosengart, R. M., Brearley, A. M., Bryant III, R. et al. (2010). Repair of major congenital cardiac defects in low-birth-weight infants: Is delay warranted? Journal of Thoracic and Cardiovascular Surgery, 140(5), 1104–1109. https://doi.org/10.1016/j.jtcvs.2010.08.013 [Google Scholar] [PubMed] [CrossRef]
9. Dollat, C., Vergnat, M., Laux, D., Stos, B., Baruteau, A. et al. (2015). Critical congenital heart diseases in preterm neonates: Is early cardiac surgery quite reasonable? Pediatric Cardiology, 36(6), 1279–1286. https://doi.org/10.1007/s00246-015-1158-9 [Google Scholar] [PubMed] [CrossRef]
10. Jennings, E., Cuadrado, A., Maher, K. O., Kogon, B., Kirshbom, P. M. et al. (2012). Short-term outcomes in premature neonates adhering to the philosophy of supportive care allowing for weight gain and organ maturation prior to cardiac surgery. Journal of Intensive Care Medicine, 27(1), 32–36. https://doi.org/10.1177/0885066610393662 [Google Scholar] [PubMed] [CrossRef]
11. Hickey, E. J., Nosikova, Y., Zhang, H., Caldarone, C. A., Benson, L. et al. (2012). Very low-birth-weight infants with congenital cardiac lesions: Is there merit in delaying intervention to permit growth and maturation? Journal of Thoracic and Cardiovascular Surgery, 143(1), 126–136. https://doi.org/10.1016/j.jtcvs.2011.09.008 [Google Scholar] [PubMed] [CrossRef]
12. Kim, M., Okunowo, O., Ades, A. M., Fuller, S., Rintoul, N. E. et al. (2021). Single-center comparison of outcomes following cardiac surgery in low birth weight and standard birth weight neonates. Journal of Pediatrics, 238, 161–167. https://doi.org/10.1016/j.jpeds.2021.06.059 [Google Scholar] [PubMed] [CrossRef]
13. Chang, A. C., Hanley, F. L., Lock, J. E., Castaneda, A. R., Wessel, D. L. (1994). Management and outcome of low birth weight neonates with congenital heart disease. Journal of Pediatrics, 124(3), 461–466. https://doi.org/10.1016/S0022-3476(94)70376-0 [Google Scholar] [PubMed] [CrossRef]
14. Curzon, C. L., Milford-Beland, S., Li, J. S., O’Brien, S. M., Jacobs, J. P. et al. (2008). Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the society of thoracic surgeons congenital heart database. Journal of Thoracic and Cardiovascular Surgery, 135(3), 546–551. https://doi.org/10.1016/j.jtcvs.2007.09.068 [Google Scholar] [PubMed] [CrossRef]
15. Ades, A. M., Dominguez, T. E., Nicolson, S. C., Gaynor, J. W., Spray, T. L. et al. (2010). Morbidity and mortality after surgery for congenital cardiac disease in the infant born with low weight. Cardiology in the Young, 20(1), 8–17. https://doi.org/10.1017/S1047951109991909 [Google Scholar] [PubMed] [CrossRef]
16. Seo, D. M., Park, J. J., Yun, T. J., Kim, Y. H., Ko, J. K. et al. (2011). The outcome of open heart surgery for congenital heart disease in infants with low body weight less than 2500 g. Pediatric Cardiology, 32(5), 578–584. https://doi.org/10.1007/s00246-011-9910-2 [Google Scholar] [PubMed] [CrossRef]
17. Kaufman, M. B., El-Chaar, G. M. (1996). Bone and tissue changes following prostaglandin therapy in neonates. Annals of Pharmacotherapy, 30(3), 269–277. https://doi.org/10.1177/106002809603000311 [Google Scholar] [CrossRef]
18. Lewis, A. B., Freed, M. D., Heymann, M. A., Roehl, S. L., Kensey, R. C. (1981). Side effects of therapy with prostaglandin E1 in infants with critical congenital heart disease. Circulation, 64(5), 893–898. https://doi.org/10.1161/01.CIR.64.5.893 [Google Scholar] [PubMed] [CrossRef]
19. Hsia, T. Y., Gruber, P. J. (2006). Factors influencing neurologic outcome after neonatal cardiopulmonary bypass: What we can and cannot control. Annals of Thoracic Surgery, 81(6), S2381–S2388. https://doi.org/10.1016/j.athoracsur.2006.02.074 [Google Scholar] [PubMed] [CrossRef]
20. Lynch, J. M., Buckley, E. M., Schwab, P. J., McCarthy, A. L., Winters, M. E. et al. (2014). Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery, 148(5), 2181–2188. https://doi.org/10.1016/j.jtcvs.2014.05.081 [Google Scholar] [PubMed] [CrossRef]
21. Anderson, B. R., Ciarleglio, A. J., Hayes, D. A., Quaegebeur, J. M., Vincent, J. A. et al. (2014). Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. Journal of the American College of Cardiology, 63(5), 481–487. https://doi.org/10.1016/j.jacc.2013.08.1645 [Google Scholar] [PubMed] [CrossRef]
22. Lim, J. M., Porayette, P., Marini, D., Chau, V., Au-Young, S. H. et al. (2019). Associations between age at arterial switch operation, brain growth, and development in infants with transposition of the great arteries. Circulation, 139(24), 2728–2738. https://doi.org/10.1161/CIRCULATIONAHA.118.037495 [Google Scholar] [PubMed] [CrossRef]
Appendix A: Variables assessed in predictive models
Weight (<2500 g)
Prematurity (<37W-GA)
LBW-GA
Antenatal diagnosis
UVH
STAT category (Stat 1 & 2, Stat 3, Stat 4, Stat 5)
Chromosomal anomalies
Era effect (1996–2002; 2003–2009; 2010–2017)
Variables reflecting preoperative status
BAS
Prostaglandin
Inotropes
Positive ventilation
NEC
Perioperative variables
Surgical pathway (Biventricular vs. palliation for Uni/biV pathway)
Delayed surgery
Corrected GA at surgery >38 W
CPB time
ACC time
Use of DHCA
Need for 2nd run CPB
Postoperative status
Open chest
VIS > 17*
ECMO
Prolonged ventilation (>7 d)
Reoperation (bleeding)
Reoperation (others)
Cath lab intervention
Stroke
CPR
Unplanned reintubation
Residual lesion after initial repair
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools