 Open Access
Open Access
ARTICLE
Quality of Life in Congenital Heart Disease Patients According to Their Anatomical and Physiological Classification
1
Cardiology Service, Complejo Hospitalario Universitario Insular-Materno Infantil, Las Palmas de Gran Canaria, 35016, Spain
2
Department of Medical and Surgical Sciences, Faculty of Health Sciences, Universidad de Las Palmas de Gran Canaria, Las Palmas
de Gran Canaria, 35016, Spain
3
Ophthalmology Service, Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria, 35012, Spain
* Corresponding Author: Efrén Martínez-Quintana. Email:
Congenital Heart Disease 2023, 18(2), 197-206. https://doi.org/10.32604/CHD.2021.013308
Received 01 August 2020; Accepted 13 November 2020; Issue published 15 March 2023
Abstract
Background: Living well is as important as living longer. The objective of this study is to assess quality of life (QoL) in congenital heart disease (CHD) according to current AHA/ACC anatomical and physiological classifi- cation. Methods: Cross-sectional study examining the World Health Organization QoL Bref questionnaire (WHOQoL-Bref) in consecutive outpatient CHD patients from a single unit. Results: 191 CHD patients were studied. Median age was 28 ± 13 years and 59% were male. 44 (23%), 115 (60%) and 33 (17%) CHD patients showed mild, moderate and great anatomical defects respectively while 69 (36%) patients were in physiological Stage A, 27 (14%) in Stage B, 84 (44%) in Stage C and 11 (6%) in Stage D. No significant differences were seen in relation the anatomical classification and the different sections of the WHOQoL-Bref questionnaire. CHD patients in Stages C and D had significant lower physical domain scores than patients in the Stage A (p < 0.05). However, no significant differences were seen in the psychological, social relationships and environmental domains. The binary logistic regression analysis showed that having a higher educational level was a protective factor [OR 0.32 (95% CI, 0.12–0.87), p = 0.026] while being married or cohabit was a risk factor [OR 3.46 (95% CI, 1.13–10.63), p = 0.030] for having a worse rated QoL. Meanwhile, having a worse functional class (NYHA ≥2) [OR 3.44 (95% CI, 1.20–9.81), p = 0.021] was associated with dissatisfaction with health. Conclusion: Patients with advanced physiological stages scored lower on the physiological domain. No statistical significance was seen, according to the anatomical and physiological classification, in the psychological, social relationship and environmental domains.Keywords
Living well is as important to most people as living longer and in recent years it has gained interest in knowing how to increase the quality of life (QoL) in congenital heart disease (CHD) patients. In fact, as more children and adults with CHD live longer it has become a topic of interest to know how they manage in daily life and how associated morbidities may impact on them. In this regard, previous systematic reviews and meta-analyses on QoL in CHD patients [1–4] illustrate that despite the increasing evidence it is still inconsistent. Factors such as the instruments used to measure QoL, research methodology or the CHD complexity have hampered the ability to draw firm conclusions. In fact, when QoL has been measured from a functional approach CHD patient normally do worse than healthy controls. Nonetheless when QoL is measured from a more holistic perspective no significant differences were seen [5].
Recently the American Heart Association/American College of Cardiology (AHA/ACC) [6] have released a major update to the first US guidelines on the management of adult CHD published a decade ago identifying a lot of physiological factors that are independent of their anatomy to some degree suggesting therefore the importance of these new classification in the prognosis, management and QoL of CHD patients. However, to date there are no data regarding QoL in CHD patients related to this new classification.
The objective of this study is (a) to assess the QoL in adolescents and young adult CHD patients (b) to establish how QoL changes in CHD patients attending to their underlying CHD complexity, anatomical and physiological and (c) to determine which physiological variables may affect QoL in CHD patients.
Individuals were selected among consecutive CHD patients older than 16 years with a structural CHD, verified by cardiac imaging, recruited from a single hospital outpatient CHD unit between October 2018 and April 2019. All patients or their parents/tutors gave their informed consent for participation. The protocol of the study was approved by the Complejo Hospitalario Universitario Insular-Materno Infantil’s Ethics Committee (CEIc/CEIm 890) and in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Date of birth, sex, weight, height and cardiovascular risk factors (systemic arterial hypertension, diabetes mellitus, dyslipidemia and smoking habit) were obtained from the clinical interview and/or the medical history. Patients were also asked their education level (none, primary, middle or university) and their marital status (single, divorced, separated, cohabit, married or widower). Body mass index (BMI) was calculated by the formula [weight (kg)/[height (m) × height (m)]. CHD patients were classified into diagnostic groups according to the newly elaborated AHA/ACC guidelines that intend to capture the complexity of CHD anatomy and physiology [6]. Cardiac defects were categorized as simple, moderate or great complexity from an anatomical point of view and the physiological Stage was graded as A, B, C and D from smaller to greater severity. The physiological classification system, unlike anatomical classification, considers the patient’s functional capacity (New York Heart Association (NYHA) classification) as well as other factors such as presence of valve disease, pulmonary hypertension, arrhythmias, aortic dilatation, end-organ function or hypoxemia [6].
QoL was defined as an individual´s satisfaction with his or her life dimensions comparing with his or her ideal life. The WHOQoL-Bref instrument was chosen for our study as it has been widely used in other sick populations, as a general measure and not disease related, and in CHD patients [7]. Also, the validation study of the WHOQoL-Bref in Spain, including data from primary care centres, has yielded positive evidence for acceptability, internal consistency, and convergent and discriminant validity [8].
The WHOQoL-Bref survey has 26 questions and produces a QoL profile. The first two questions of the survey are two stand-alone questions, one pertaining to the respondents’ rated QoL, and one related to their Satisfaction with Health. The rest of questions (24 items) establish the four domains. The Physical Health domain (7 items) includes questions pertaining to activities of daily living, dependence on medicinal substances and medical aids, energy and fatigue, mobility, pain and discomfort, sleep and rest and work capacity. The Psychological domain (6 items) focuses on bodily image and appearance, negative feelings, positive feelings, self-esteem, spirituality/religion/personal beliefs, thinking, learning, memory and concentration. The Social Relationships domain (3 items) includes questions pertaining to personal relationships, social support and sexual activity. The fourth domain, the Environment (8 items), includes questions related to safety and security, home and physical environment satisfaction, finance, i.e., does the respondent have enough money to meet their needs, health/social care availability, information and leisure activity accessibility and transportation satisfaction [9]. The investigators facilitated the QoL questionnaire to CHD patients, to fill it out during the physician consultation, after ensuring their ability to read and understand it.
Descriptive statistics (means and standard deviations, median and quartiles (25–75) and percentages) were used to characterize the sample. The differences in the distribution of the characteristics between groups were examined using chi-squared for categorical variables and t test for continuous variables. The Fisher exact test was employed instead of Pearson’s chi-square test when sample sizes were small. The two stand-alone questions included five-point response categories that were dichotomized for statistical purposes (0: very poor or poor overall QoL and 1:neither poor nor good, good and very good overall QoL; 0: very dissatisfied or dissatisfied health and 1:neither satisfied nor dissatisfied, satisfied or very satisfied health). Domain scores were scaled in a positive direction from 1 to 5 (i.e., higher scores denote higher QoL). The mean score of items within each domain was used to calculate the domain score. Mean scores were then multiplied by 4 in order to make domain scores comparable with the scores used in the WHOQOL-100. The second transformation method converted domain scores to a 0–100 scale [9]. One-Way Analysis of Variance (ANOVA) was used to test the equality of three or more means at one time by using variances. The Bonferroni post hoc test was done for multiple comparisons when the ANOVA test had a p value under 0.05. Logistic regression analysis was used to predict the dichotomous variable (rated QoL) and odds ratio (OR) was obtained after considering the effect of variables significant in the univariate analysis. The 95% confidence interval (CI) was used to estimate the precision of the odds ratio. A p values less than 0.05 was considered statically significant. Data analysis was carried out using SPSS 24.0 (SPSS, Chicago, IL).
One hundred and ninety one out of 200 CHD patients, mean age of 31 ± 13 years and 59% male patients, were included in the study. 7 CHD patients unable to understand the survey due to cognitive deficit (4 patients with Down syndrome and 2 patients with postoperative hypoxia) and 2 patients who did not completely fill out the questionnaire were excluded from de analysis. No one was excluded due to co-morbidity problems and no patient refused to participate in the study. According to CHD anatomy 44 (23%) patients were classified as having simple defects, 115 (60%) patients have moderate defects and 32 (17%) have great defects and according to the physiological CHD complexity classification 69 (36%) patients were in Stage A, 27 (14%) patients in Stage B, 84 (44%) patients in Stage C and 11 (6%) patients in Stage D. In relation to the NYHA classification 155 (81%) patients were in Class 1, 32 (17%) patients were in Classes 2 and 4 (2%) patients in Class 3. No patient was in Class 4.
Table 1 shows the results of the WHOQoL-Bref survey related to the rated QoL and the Satisfaction with Health questions and the four domain scores according to the anatomical and the physiological CHD classification. No significant differences were seen in relation the anatomical classification and the different sections of the WHOQoL-Bref questionnaire. However, from a physiological classification CHD patient in Stages C and D had significant lower physical domain scores than patients in the Stage A (p < 0.05). However, no significant differences were seen in the psychological, social relationships and environmental domains according to the physiological stages although there was a tendency to have lower psychological scores among CHD patients with more advanced physiological stages (p = 0.060). Similarly, although no significant difference was seen in the stand-alone question related to the rated QOL according to the anatomical and physiological classification, CHD patients with a Stage C and D physiological classification had a significant poorer satisfaction with their health.
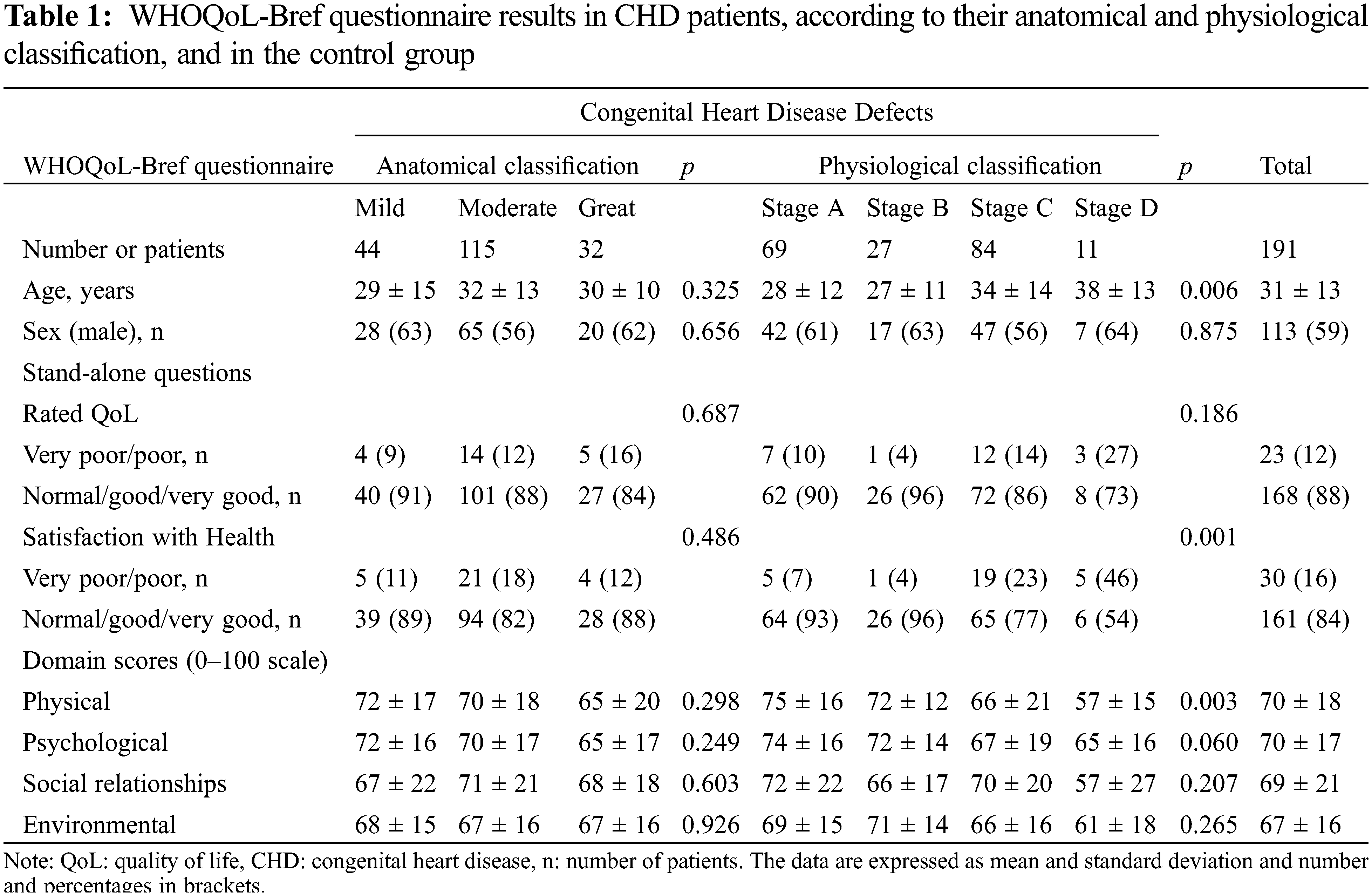
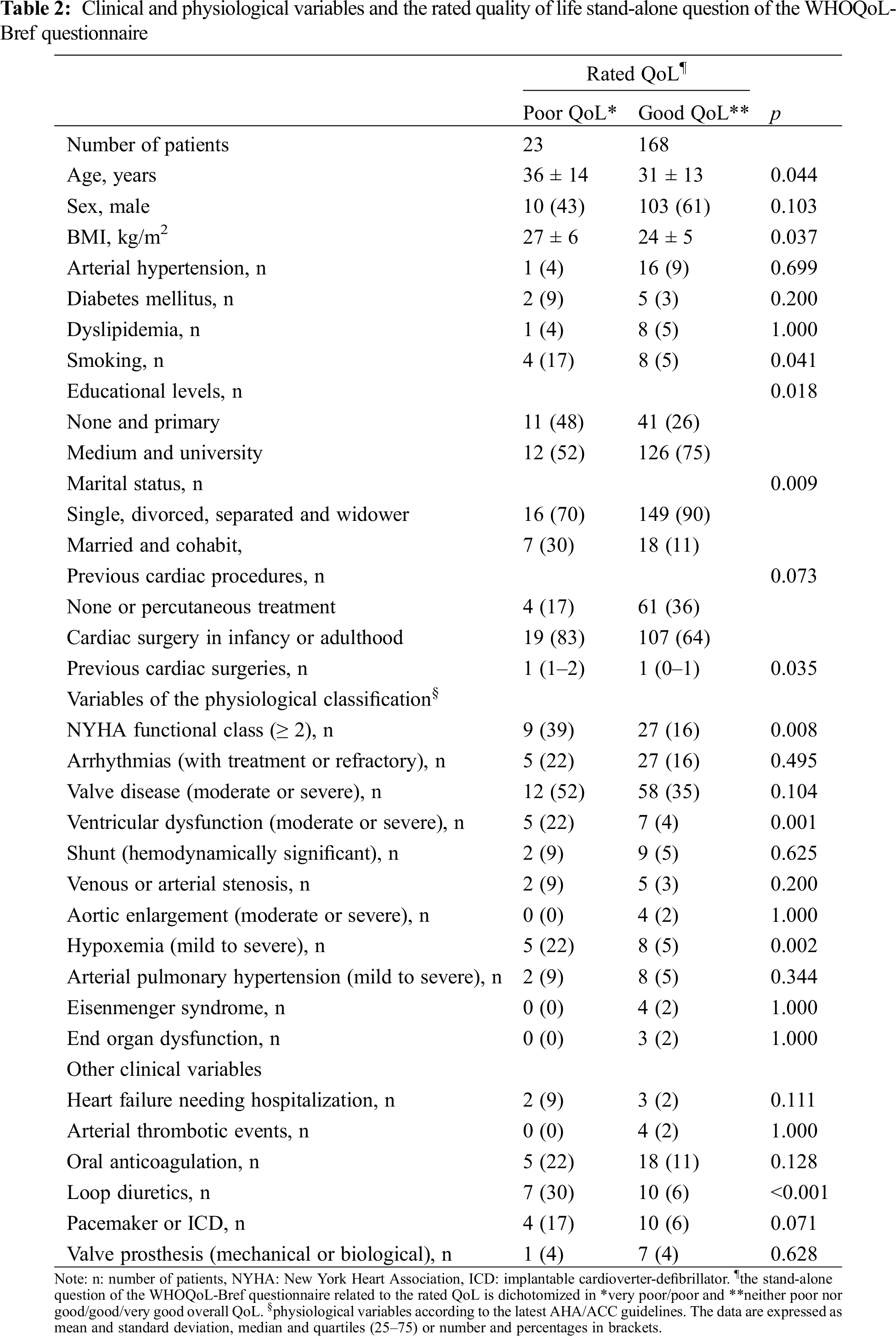
Table 2 shows the relation between the demographic, clinical and different physiological variables and the dichotomized rated QoL question. Also, heart failure needing hospitalization, arterial thrombotic events (stroke and myocardial infarction), being under oral anticoagulation or loop diuretic treatment, living with a pacemaker or an implantable cardioverter defibrillator (ICD) or having a cardiac valve prosthesis (metallic or biological) were included in the analysis. In it being older, with overweight, smoker, having a lower educational level, being married/cohabit, having a worse NYHA functional class, having ventricular dysfunction, being under diuretic treatment or hypoxemic were associated with a poorer overall QoL.
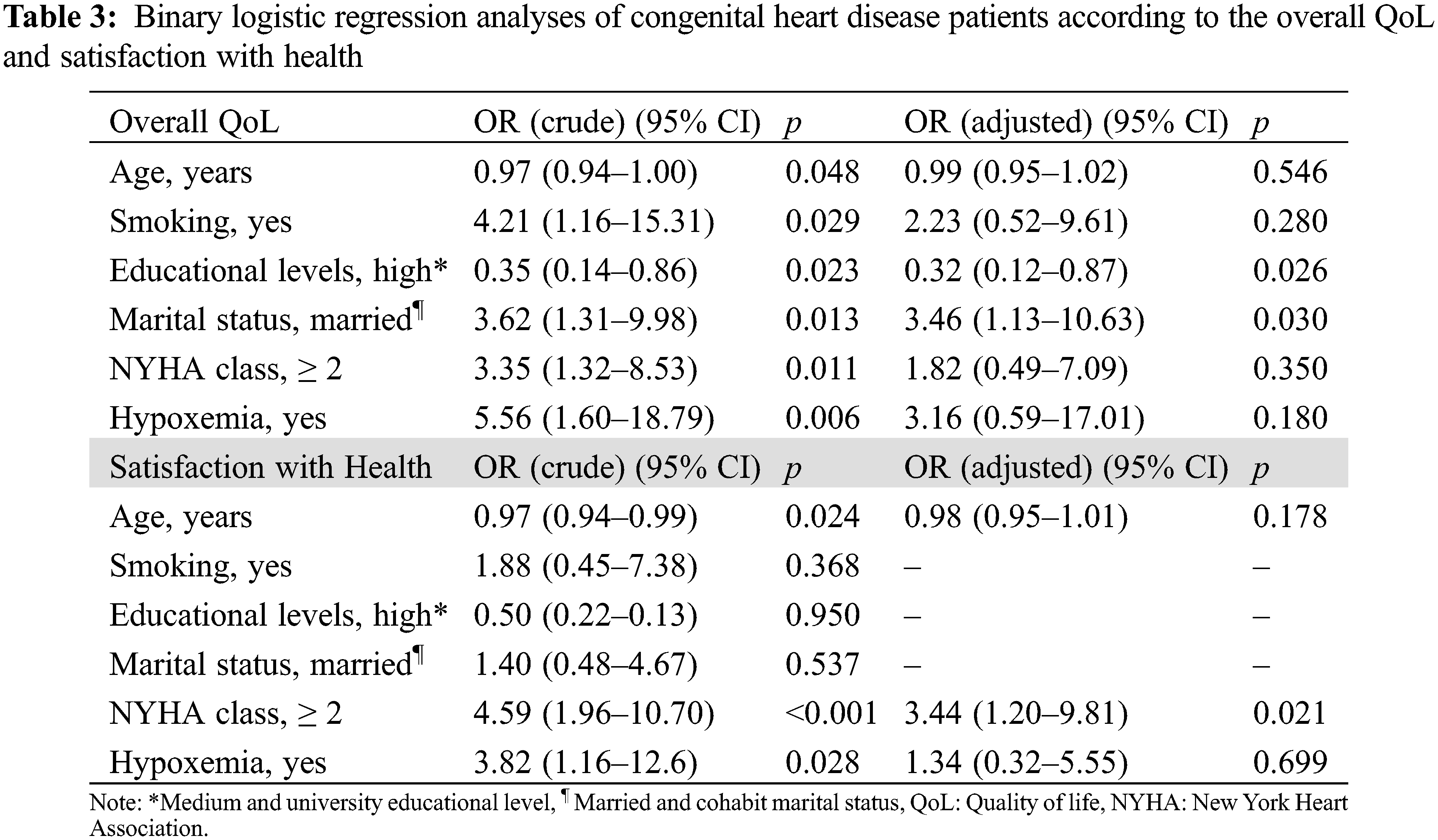
Meanwhile, the binary logistic regression analysis (Table 3) shows that having a higher educational level was a protective factor while being married or cohabit was a risk factor for having a worse rated QoL. The ventricular dysfunction and being under diuretic treatment variables were not included within the model due to the association between them and a worse NYHA functional class. On the other hand, the binary logistic regression analysis, showed having a worse functional class (NYHA ≥ 2) was a risk factor for having a greater dissatisfaction with health.
Like other patients with chronic illnesses, CHD with repaired or unrepaired defects continue to face physical, psychosocial, social and environmental challenges as they grow up [10]. In fact, numerous factors may affect them such as impaired peer relationships, family overprotection, sports activity restriction, delayed progression into independent adulthood, discrimination and bullying or the feeling of being different from healthy people [11–14].
In the last years, a large number of QoL studies have been published and the overall conclusion is that the QoL of adults with CHD is good. People with CHD can even have a better QoL than their healthy peers if QoL is measured holistically in terms of satisfaction with life. However, when QoL is measured in terms of physical functioning, patients with complex CHD defects tend to do worse than patients with moderate or mild defects and healthy individuals [5].
Recently, the AHA/ACC have released guidelines that classify CHD patients depending on anatomical defects and physiological situation since the latter seem to give extra information related to morbidity, prognosis or even QoL. Nonetheless, in our series we did not find significant differences between CHD patients and QoL according to their anatomical or physiological classification with the exception of the physical domain for the latter. This may be because some of the physiological variables such as having valvular disease, aortic enlargement or venous or arterial stenosis that classify patients as Stage C or D do not necessarily correlate with their clinical condition and therefore neither with their QoL. Moreover, inconsistent findings have been observed regarding the relationship between QoL and disease severity [15–17], severity of residual lesions [15,17] or cyanosis [17] indicating that symptoms experienced by patients may be more important contributory factors to QoL than defect complexity itself [18,19]. In this context, several years ago, Ganiats et al. [20] indicated that the NYHA classification was not a sensitive measure of QoL. In fact, although the NYHA provides insights in patients’ functional status, it may result in misleading findings in QoL. Actually, in our series it was shown that NYHA functional class was a predictive factor of health satisfaction but not of the rated QoL. These same conclusions reached Lane et al. [21] who found no statistically significant differences in the QoL parameters between surgically palliated and medically treated CHD patients, except for a poorer physical functioning among the former. They concluded that perhaps patients who have undergone palliative surgery have adjusted to and learnt to cope with their health problems and limitations and thus do not experience a poorer QoL compared to other CHD patients. In fact, having lived their whole life with a severe chronic disease, which has remained stable for most of the time in most cases, may have changed CHD patient’s expectations [22]. Similarly, Antonovsky [23] showed how a “sense of coherence” or way of making sense of the world, is a major factor in determining how well a person manages stress and stays healthy.
In regards to the marital status, previous research on mental health by age group has shown that the mental health of single people was better than that of married people in individuals younger than 30 years of age. Moreover, in the analysis by age group, the QoL of married men under the age of 30 years was lower than that of single men [24]. In fact, whereas QoL remains stable in patients with simple and moderate disease, patients with complex CHD are vulnerable for QoL deterioration, particularly beyond their third decade of life [25]. On the other hand, although there are still numerous questions and gaps remaining, the case for the positive effects of educational attainment on quality of life is in the balance very convincing [26].
Although we think that our series reflects the reality that exists in most CHD units there are limitations in our study that may impact our findings. Firstly, we did not look for other factors which may affect QoL such as morbidity problems or psychological factors. In fact, there has been a set of new factors that have been reported to have predictive value for QoL in CHD, such as dispositional orientation [27–29], self-efficacy [30], empowerment [31], illness perception [32], personality [33,34] and loneliness [35]. Secondly, we did not determine the economic level reached in the study subjects or the influence of parental hardships (employment, etc) on the family [36]. Thirdly, it should be noted that while there is an array of CHD diagnosis, even the moderate diagnoses tended to be on the simple side (e.g., coarctation of the aorta or valvular pulmonary stenosis). Finally, the small number of CHD patients in the physiological Stage D may have led to a lack of significance in three of the four domains of the WHOQoL-Bref survey, especially when CHD patients with worse physiological stages had lower domain scores.
We think that some of the variables chosen in the latest American guidelines for adult patients with CHD, such as dilatation of the aorta, the existence of residual shunts, venous stenosis or moderate valve lesions, do not have to lead to a worse physiological classification and therefore to a worse QoL. On the contrary, variables such as a worse NYHA functional class, ventricular dysfunction or being under diuretic treatment usually correlates with a more advanced CHD, a worse prognosis, and a greater dissatisfaction with health. Nonetheless, larger studies, with patients in a worse functional class, will be necessary to resolve the usefulness of this new classification in determining QoL in CHD patients.
In conclusion, in our series no significant differences were seen in the WHOQoL-Bref questionnaire among CHD patients depending on their anatomical and physiological complexity according to the latest AHA/ACC guidelines for the management of adults with CHD with the exception of the physical domain and satisfaction with health in patients with more advanced physiological stages.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Apers, S., Luyckx, K., Moons, P. (2013). Quality of life in adult congenital heart disease: What do we already know and what do we still need to know? Current Cardiology Reports, 15(10), 407. https://doi.org/10.1007/s11886-013-0407-x [Google Scholar] [PubMed] [CrossRef]
2. Schroder, M., Boisen, K. A., Reimers, J., Teilmann, G., Brok, J. (2016). Quality of life in adolescents and young adults with CHD is not reduced, a systematic review and meta-analysis. Cardiology in the Young, 26(3), 415–425. https://doi.org/10.1017/S104795111500181X [Google Scholar] [PubMed] [CrossRef]
3. Kahr, P. C., Radke, R. M., Orwat., S., Baumgartner, H., Diller, G. P. (2015). Analysis of associations between congenital heart defect complexity and health-related quality of life using a metaanalytic strategy. International Journal of Cardiology, 199, 197–203. https://doi.org/10.1016/j.ijcard.2015.07.045 [Google Scholar] [PubMed] [CrossRef]
4. Ladak, L. A., Hasan, B. S., Gullick, J., Gallagher, R. (2019). Health-related quality of life in congenital heart disease surgery in children and young adults, a systematic review and meta-analysis. Archives of Disease in Childhood, 104(4), 340–347. https://doi.org/10.1136/archdischild-2017-313653 [Google Scholar] [PubMed] [CrossRef]
5. Moons, P., Van Deyk, K., De Bleser, L., Marquet, K., Raes, E. et al. (2006). Quality of life and health status in adults with congenital heart disease, a direct comparison with healthy counterparts. European Journal Cardiovascular Prevention Rehabilitation, 13(3), 407–413. https://doi.org/10.1097/00149831-200606000-00017 [Google Scholar] [CrossRef]
6. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 139(14), e698–e800. [Google Scholar] [PubMed]
7. Khoshhal, S., Al-Harbi, K., Al-Mozainy, I., Al-Ghamdi, S., Aselan, A. et al. (2019). Assessment of quality of life among parents of children with congenital heart disease using WHOQOL-BREF: A cross-sectional study from Northwest Saudi Arabia. Health Quality Life Outcomes, 17(1), 183. https://doi.org/10.1186/s12955-019-1249-z [Google Scholar] [PubMed] [CrossRef]
8. Lucas-Carrasco, R. (2012). The WHO quality of life (WHOQOL) questionnaire, Spanish development and validation studies. Quality Life Research, 21(1), 161–165. https://doi.org/10.1007/s11136-011-9926-3 [Google Scholar] [PubMed] [CrossRef]
9. WHOQOL Group (1993). Study protocol for the World Health Organization Project to develop a quality of life assessment instrument (WHOQOL). Quality Life Research, 2(2), 153–159. https://doi.org/10.1007/BF00435734 [Google Scholar] [CrossRef]
10. Kim, G. B. (2014). Psychosocial adjustment and quality of life of adolescents and adults with congenital heart disease. Korean Journal of Pediatrics, 57(6), 257–263. https://doi.org/10.3345/kjp.2014.57.6.257 [Google Scholar] [PubMed] [CrossRef]
11. Dalieno, L., Mapelli, D., Volpe, B. (2006). Measurement of cognitive outcome and quality of life in congenital heart disease. Heart, 92(4), 569–574. https://doi.org/10.1136/hrt.2004.057273 [Google Scholar] [PubMed] [CrossRef]
12. Nakou, S. (2001). Measurement of quality of life in the health care field. Applications in child birth. Archives of Hellenic Medicine, 18(3), 254–266. [Google Scholar]
13. McMurray, R. L., Parsons, J. M., Quirk, J., Veldtman, G. R., Lewin, R. J. et al. (2001). A life less ordinary, growing up and coping with congenital heart disease. Coron Health Care, 5(1), 51–57. https://doi.org/10.1054/chec.2001.0112 [Google Scholar] [CrossRef]
14. Lee, S., Kim, S. S. (2010). The life of adolescent patients with complex congenital heart disease. Journal of Korean Academy of Nursing, 40(3), 411–422. https://doi.org/10.4040/jkan.2010.40.3.411 [Google Scholar] [PubMed] [CrossRef]
15. Teixeira, F. M., Coelho, R. M., Proença, C., Silva, A. M., Vieira, D. et al. (2011). Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatric Cardiology, 32(8), 1132–1138. https://doi.org/10.1007/s00246-011-0039-0 [Google Scholar] [PubMed] [CrossRef]
16. Vigl, M., Niggemeyer, E., Hager, A., Schwedler, G., Kropf, S. et al. (2011). The importance of socio-demographic factors for the quality of life of adults with congenital heart disease. Quality of Life Research, 20(2), 169–177. https://doi.org/10.1007/s11136-010-9741-2 [Google Scholar] [PubMed] [CrossRef]
17. Silva, A. M., Vaz, C., Areias, M. E., Vieira, D., Proença, C. et al. (2011). Quality of life of patients with congenital heart diseases. Cardiology in the Young, 21(6), 670–676. https://doi.org/10.1017/S1047951111000576 [Google Scholar] [PubMed] [CrossRef]
18. Bratt, E. L., Moons, P. (2015). Forty years of quality-of-life research in congenital heart disease: Temporal trends in conceptual and methodological rigor. International Journal of Cardiology, 195, 1–6. https://doi.org/10.1016/j.ijcard.2015.05.070 [Google Scholar] [PubMed] [CrossRef]
19. Apers, S., Kovacs, A. H., Luyckx, K., Thomet, C., Budts, W. et al. (2016). Quality of life of adults with congenital heart disease in 15 countries, evaluating country-specific characteristics. Journal American College of Cardiology, 67(19), 2237–2245. https://doi.org/10.1016/j.jacc.2016.03.477 [Google Scholar] [PubMed] [CrossRef]
20. Ganiats, T. G., Browner, D. K., Dittrich, H. C. (1998). Comparison of quality of well-being scale and NYHA functional status classification in patients with atrial fibrillation. American Heart Journal, 135(5), 819–824. [Google Scholar] [PubMed]
21. Lane, D. A., Lip, G. Y. H., Millane, T. A. (2002). Quality of life in adults with congenital heart disease. Heart, 88(1), 71–75. https://doi.org/10.1136/heart.88.1.71 [Google Scholar] [PubMed] [CrossRef]
22. Moons, P., Luyckx, K. (2019). Quality-of-life research in adult patients with congenital heart disease, current status and the way forward. Acta Paediatrica, 108(10), 1765–1772. https://doi.org/10.1111/apa.14876 [Google Scholar] [PubMed] [CrossRef]
23. Antonovsky, A. (1987). Unravelling the mystery of health: How people manage stress and stay well. San Francisco: Jossey-Bass. [Google Scholar]
24. Han, K. T., Park, E. C., Kim, J. H., Kim, S. J., Park, S. (2014). Is marital status associated with quality of life? Health and Quality of Life Outcomes, 12(1), 109. https://doi.org/10.1186/s12955-014-0109-0 [Google Scholar] [PubMed] [CrossRef]
25. Moons, P., Apers, S., Luyckx, K., Kovacs, A. H., Thomet, C. et al. (2018). Age and quality of life in adults with congenital heart disease worldwide: Evidence for a non-linear relationship. Circulation, 138, A14623. [Google Scholar]
26. Edgerton, J. D., Roberts, L. W., von Below, S. (2012). Education and quality of life. In: Land, K., Michalos, A., Sirgy, M. (eds.Handbook of social indicators and quality of life research. Dordrecht: Springer. [Google Scholar]
27. Apers, S., Moons, P., Goossens, E., Luyckx, K., Gewillig, M. et al. (2013). Sense of coherence and perceived physical health explain the better quality of life in adolescents with congenital heart disease. European Journal of Cardiovascular Nursing, 12, 475–483. [Google Scholar] [PubMed]
28. Neuner, B., Busch, M. A., Singer, S., Moons, P., Wellmann, J. et al. (2011). Sense of coherence as a predictor of quality of life in adolescents with congenital heart defects: A register-based 1-year follow-up study. Journal of Developmental and Behavioral Pediatrics, 32(4), 316–327. https://doi.org/10.1097/DBP.0b013e31821102ee [Google Scholar] [PubMed] [CrossRef]
29. Muller, J., Hess, J., Hager, A. (2014). Sense of coherence, rather than exercise capacity, is the stronger predictor to obtain health related quality of life in adults with congenital heart disease. European Journal Preventive Cardiology, 21(8), 949–955. https://doi.org/10.1177/2047487313481753 [Google Scholar] [PubMed] [CrossRef]
30. Thomet, C., Moons, P., Schwerzmann, M., Apers, S., Luyckx, K. et al. (2018). Self-efficacy as a predictor of patient-reported outcomes in adults with congenital heart disease. European Journal of Cardiovascular Nursing, 17(7), 619–626. https://doi.org/10.1177/1474515118771017 [Google Scholar] [PubMed] [CrossRef]
31. Acuña Mora, M., Sparud-Lundin, C., Burström, Å., Hanseus, K., Rydberg, A. et al. (2019). Patient empowerment and its correlates in young persons with congenital heart disease. European Journal of Cardiovascular Nursing, 18(5), 389–398. https://doi.org/10.1177/1474515119835434 [Google Scholar] [PubMed] [CrossRef]
32. Schoormans, D., Mulder, B. J., van Melle, J. P.,Pieper, P. G., van Dijk, A. P. et al. (2014). Illness perceptions of adults with congenital heart disease and their predictive value for quality of life two years later. European Journal of Cardiovascular Nursing, 13(1), 86–94. https://doi.org/10.1177/1474515113481908 [Google Scholar] [PubMed] [CrossRef]
33. Rassart, J., Luyckx, K., Goossens, E., Apers, S., Klimstra, T. A. et al. (2013). Personality traits, quality of life and perceived health in adolescents with congenital heart disease. Psychology Health, 28(3), 319–335. https://doi.org/10.1080/08870446.2012.729836 [Google Scholar] [PubMed] [CrossRef]
34. Rassart, J., Luyckx, K., Goossens, E., Oris, L., Apers, S. et al. (2016). A big five personality typology in adolescents with congenital heart disease: Prospective associations with psychosocial functioning and perceived health. International Journal of Behavioral Medicine, 23(3), 310–318. https://doi.org/10.1007/s12529-016-9547-x [Google Scholar] [PubMed] [CrossRef]
35. Luyckx, K., Goossens, E., Rassart, J., Apers, S., Vanhalst, J. et al. (2014). Parental support, internalizing symptoms, perceived health status, and quality of life in adolescents with congenital heart disease, influences and reciprocal effects. Journal of Behavioral Medicine, 37(1), 145–155. https://doi.org/10.1007/s10865-012-9474-5 [Google Scholar] [PubMed] [CrossRef]
36. Gleason, L. P., Deng, L. X., Khan, A. M., Drajpuch, D., Fulleret, S. et al. (2019). Psychological distress in adults with congenital heart disease, focus beyond depression. Cardiology in the Young, 29(2), 185–189. https://doi.org/10.1017/S1047951118002068 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools