 Open Access
Open Access
CASE REPORT
“Treat-Repair-Treat”: Management of Left Main Coronary Compression by a Pulmonary Artery Aneurysm in a Patient with Atrial Septal Defect and Significant Pulmonary Hypertension
1 Carol Davila University of Medicine and Pharmacy, Bucharest, 020021, Romania
2 Department of Cardiac Surgery, C. C. Iliescu Emergency Institute for Cardiovascular Diseases, Bucharest, 022322, Romania
3 Department of Cardiology, C. C. Iliescu Emergency Institute for Cardiovascular Diseases, Bucharest, 022322, Romania
4 Department of Cardiovascular Anesthesia and Intensive Care, C. C. Iliescu Emergency Institute for Cardiovascular Diseases, Bucharest, 022322, Romania
* Corresponding Author: Andrei George Iosifescu. Email:
(This article belongs to the Special Issue: Nightmare Case Reports in Congenital Heart Disease)
Congenital Heart Disease 2023, 18(1), 67-72. https://doi.org/10.32604/chd.2023.026598
Received 14 September 2022; Accepted 07 November 2022; Issue published 09 January 2023
Abstract
Left main coronary compression syndrome (LMCS) may complicate pulmonary artery aneurysms (PAA), usually developed in the context of pulmonary arterial hypertension (PAH). We report the case of a 51-year-old female patient with an atrial septal defect (unsuitable for device closure) complicated by a PAA generating a 90% left main stenosis. The significant PAH held us back from immediate surgery. After specific dual PAH-targeted therapy (sildenafil and bosentan), the atrial septal defect could be closed with a unidirectional valved patch; the PAA-induced LMCS was treated by reductive arterioplasty. The postoperative course was uneventful. Follow-up showed clinical improvement, but PAH treatment was still needed. After three months, coronary angiography showed only an insignificant residual left main stenosis, proving that reductive pulmonary arterioplasty was effective in treating LMCS. Any PAA requires further evaluation for LMCS, a dangerous but treatable complication. The “treat-repair-treat” strategy and shunt-closure with a unidirectional valved patch can both improve surgical prospects of LMCS with shunt-related PAH.Graphic Abstract
Keywords
Left main coronary compression syndrome (LMCS), a potential complication of pulmonary artery (PA) aneurysm (PAA), usually develops after long-standing pulmonary arterial hypertension (PAH). Although left main (LM) compression can lead to ischemic chest pain, myocardial infarction [1], and sudden death [2], it is still underdiagnosed, and its symptoms are erroneously attributed to PAH. Given the increased PAH-related surgical risk, LM stenting is usually the first therapeutical option for patients with LMCS. However, surgery is required in some cases of congenital heart disease without prohibitive PAH or in rare instances of PAA without PAH [3]. Here, we report a surgically treated case of PAA-related LMCS in an adult with atrial septal defect (ASD) and significant PAH.
A 51-year-old female patient was referred to the cardiology unit for increasing exertional dyspnea (class III heart failure) and atypical chest pain. The patient had a large ASD, unsuitable for device closure (due to the deficient aortic and superior rims); the patient had refused surgical repair in the past. Transthoracic echocardiography (TTE) showed a large ostium secundum ASD (38 mm), dilated right heart and a PAA (diameter = 58 mm), preserved right ventricular (RV) function, mild tricuspid regurgitation, and significant PAH. The cardiac catheterization (CC) found signs of pre-capillary pulmonary hypertension. The PA pressures were elevated: systolic (PAPs) = 72 mmHg and mean (PAPm) = 39 mmHg-at a peak systemic pressure = 126 mmHg; pulmonary artery wedge pressure (PAWP) = 7 mmHg; and increased pulmonary vascular resistance (PVR) = 4.3 Wood units (WU). The pulmonary and systemic vascular resistance ratio (PVR/PVS) was 0.31, and the pulmonary to systemic flow ratio (Qp/Qs) was 1.3. Nitric oxide testing showed an incomplete effect (9 mmHg decrease in PAPm). PAH-targeted therapy was prescribed before deciding if surgery was the most appropriate choice. The patient received sildenafil (3 × 20 mg/day) and diuretics. After three months, mild clinical improvement was obtained (from WHO functional class III to class II). The patient covered 400 m in the 6-minute walk test (without significant systemic desaturation at the end), and BNP was mildly increased (90 ng/L). However, CC reassessment was inconclusive (PAPs = 77 mmHg, PAPm = 41 mmHg, PVR = 4 WU, peak systemic pressure = 131 mmHg, PAWP = 8, Qp/Qs = 2). The clinical and biological parameters placed the patient in the intermediate-mortality PAH risk group; thus, treatment was supplemented with bosentan. Coronary angiography (CA) found a proximal, “pencil-like,” 90% LM stenosis (Fig. 1A); PAA aneurysm was confirmed on contrast-enhanced computed tomography (Figs. 2 and 3A), which also revealed PAAs close relationship with the downwards displaced LM origin. All findings suggested LMCS, and the patient underwent surgery.
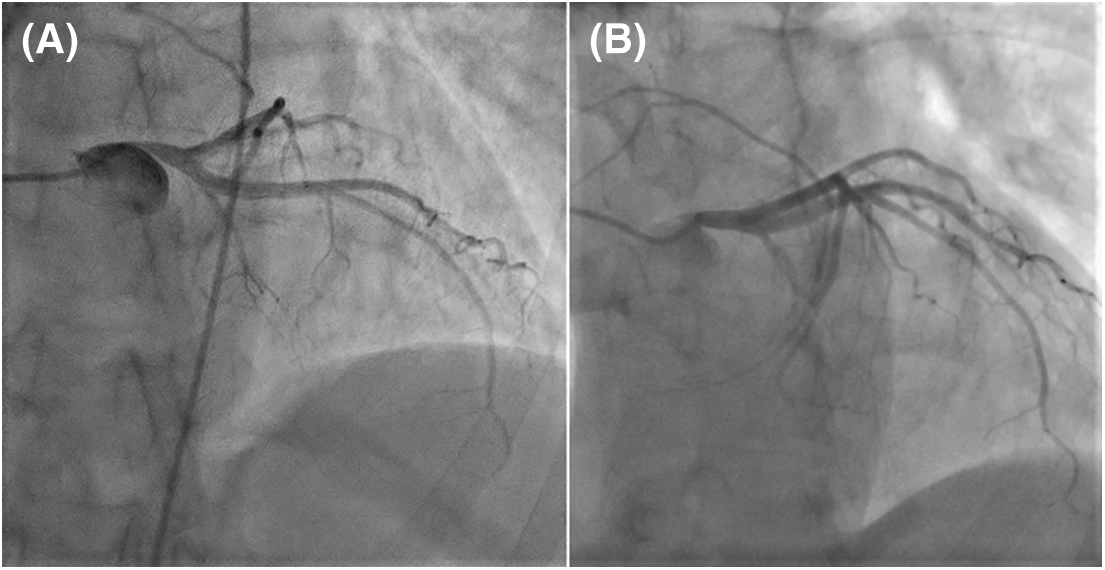
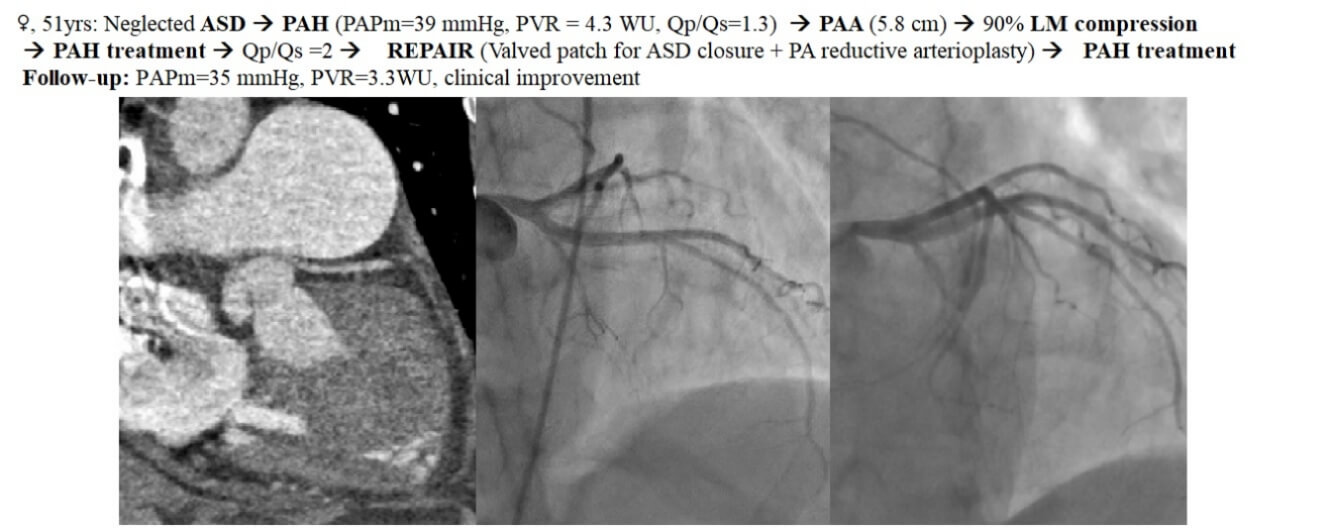
Figure 1: Coronary angiography (CA): (A). Preoperative left CA (LAO 15° CRA 30°): Severe LMCS with 90% stenosis; LM take-off angle <30°. (B). Postoperative CA (LAO 30° CRA 30°): Non-significant LM residual stenosis (30%); less steep LM take-off angle (∼45°) (LAO–left anterior oblique; CRA–cranial)
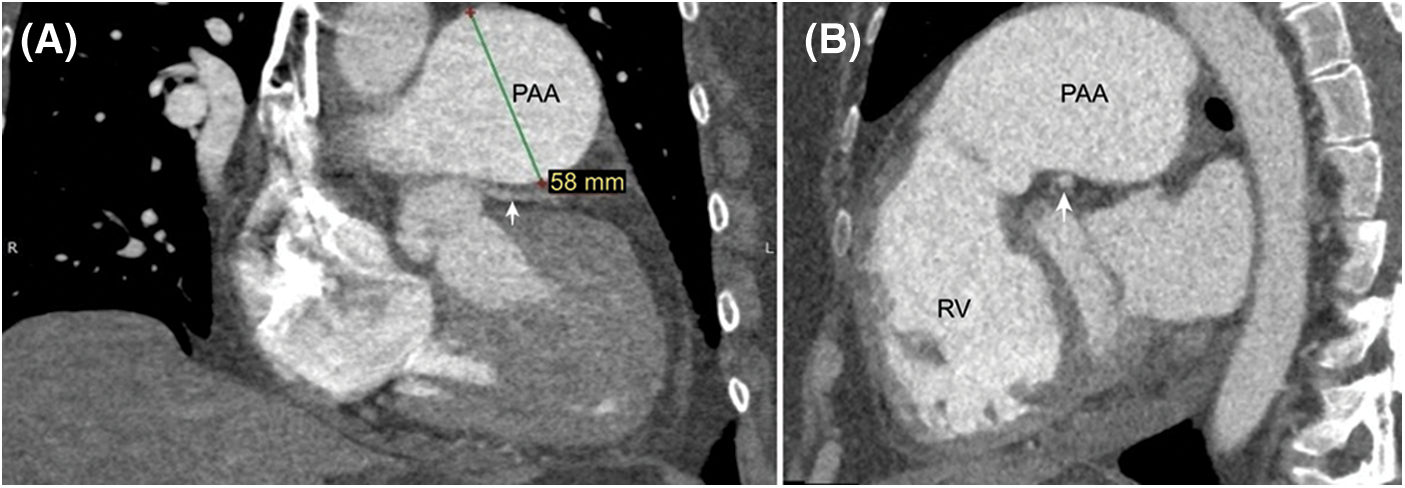
Figure 2: Thoracic contrast-enhanced computed tomography (A) Frontal image reveals the PAA closely related with a downward displaced LM (white arrow) having a sharp take-off angle from the aorta (≈30°). (B) LAO section shows the dilated RV and the PAA compressing the LM (white arrow)
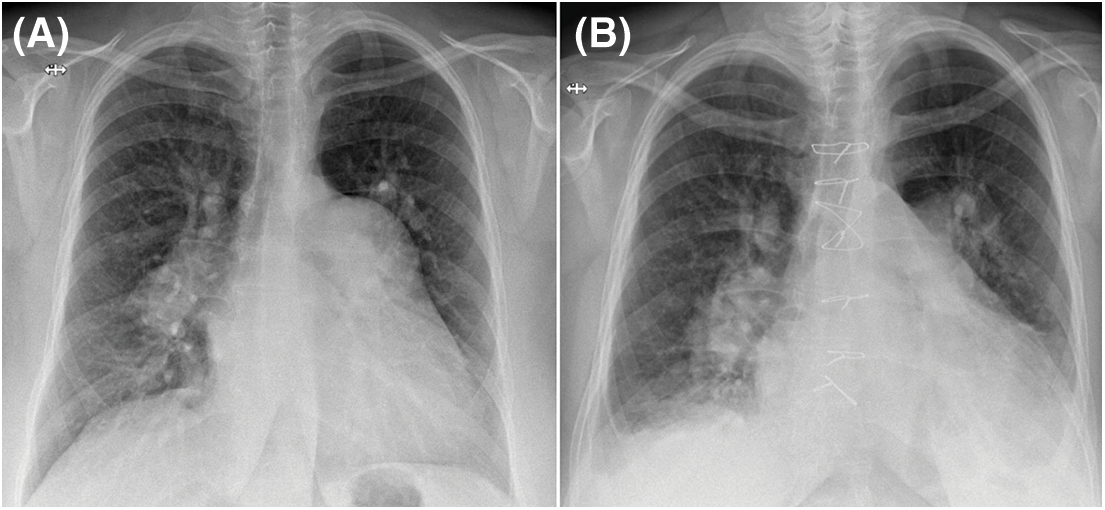
Figure 3: Chest X-ray PA view (A) PAA is conspicuous on the preoperative exam. (B) The PAA is not visible anymore postoperatively
The ASD was closed with a unidirectional valved patch; a reductive pulmonary arterioplasty treated the PAA and LMCS. The valved patch (with a 7 mm fenestration) was constructed before cardiopulmonary bypass (CPB) from a 5 × 8 cm rectangular piece of fresh pericardium using a previously described technique [4]. Two semi-circular segments were resected from either side of the PAA incision, aiming for a 30 mm reconstructed PA. CPB was weaned-off under mild doses of dobutamine and inhaled nitric oxide. CPB and aortic cross-clamping times were 103 and 89 min, respectively. The patient’s postoperative course was uneventful (no PAH crisis occurred). The patient was extubated six hours after the procedure and discharged on the eighth postoperative day (Fig. 3B).
PAH-specific dual therapy was continued postoperatively. After three months, exercise tolerance significantly improved, there was no residual shunt, reverse remodeling of the right ventricle had occurred (Fig. 4), PA diameter was 33 mm. An invasive reassessment found PAPs = 54 mmHg, PAPm = 35 mmHg, PVR = 3.3 WU, and a non-significant LM residual stenosis (30%) (Fig. 1B).
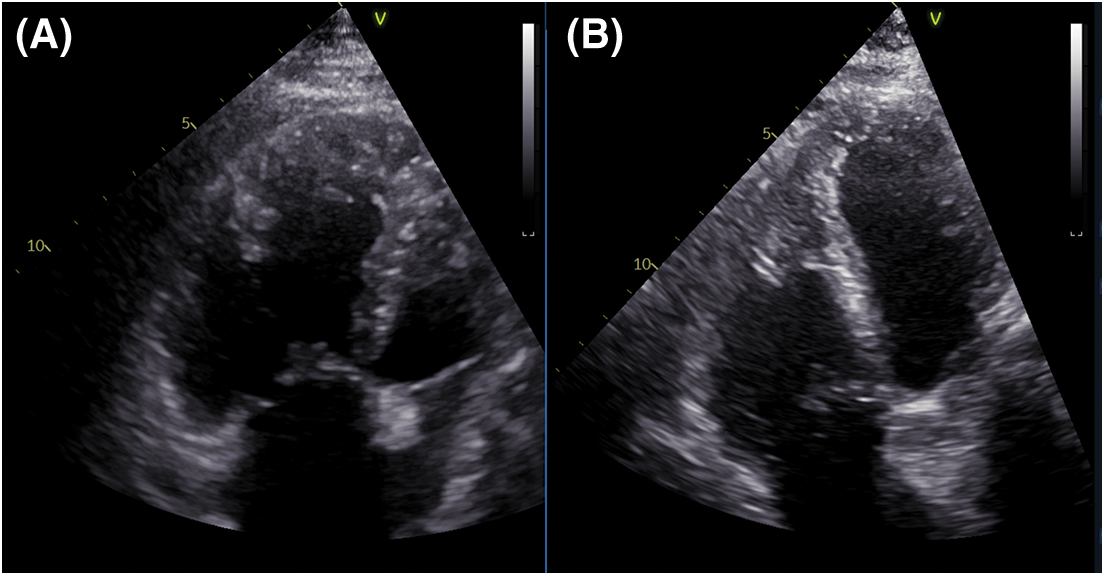
Figure 4: RV remodeling demonstrated by TTE (A) Preoperative exam shows a dilated RV. (B) Three months postoperatively, the TTE revealed significant revers-remodeling of the RV
In the most extensive study on PAH-associated LMCS, Galié et al. [5] found significant LM stenosis (⩾50%) in 6% of patients with PAH and 40% of those with PAH-associated chest pain; the best predictors of a severe LMCS were a PA diameter >40 mm and PA/aortic diameter ratio >1.5. In addition to “pencil-like” ostial stenosis, patients with LMCS exhibit inferior displacement of the LM by the PAA, generating a decreased LM take-off angle from the aorta [6] (<50°) and a curved LM and initial LAD path (Fig. 2A), indicating LMCS. This complication should be considered in any patient with significant PAH and either PA dilation or symptoms suggestive of myocardial ischemia.
Initially, it appeared that the patient had presented too late for surgery. However, recently either the “treat-and-repair” [7] or a “treat-repair-treat” [8] strategy has been recommended for ASD closure in patients with significant PAH; the latter approach was adopted here to maximize the patient’s chances. After pulmonary vasodilator treatment with specific PAH monotherapy, the hemodynamic measurements mildly improved; the Qp/Qs increased from 1.3 to 2, suggesting operability, while the PAPs of >50% of the peak systemic pressure ruled against surgery [9]. The clinical and hemodynamic condition placed the patient in a zone of intermediate risk for shunt closure (3 < PVR < 5 WU), in which current recommendations suggest considering surgery after the individual risk evaluation [9]. Even if stenting could have resolved the LMCS, the presence of an almost 6 cm PAH-related PAA had a poor prognosis [10], favoring the decision to repair. To prevent the risk of postoperative acute RV failure, the ASD was closed using a unidirectional valved patch [11]. The use of this technique in cases complicated by PAA and LMCS has not been reported yet. According to echographic measurements, the valved patch was prepared before CPB, thus reducing the aortic cross-clamping time.
The therapeutic choice in LMCS has not been clearly established. This depends on the patient’s underlying disease and the medical team’s experience. In cases of PAH with contraindications to surgery, interventional treatment with LM stenting has shown promising results [5]; however, it does not address the PAA. If surgical treatment is still possible, some may only treat the underlying disease and PAA (resection and graft interposition [12] or, more frequently, reductive arterioplasty [13]); others will perform coronary bypasses to the LM branches [14]. In cases with a significant shunt and no signs of myocardial ischemia, some may avoid treating 50% LMCS, counting on the beneficial effects of shunt suppression on the PA dimensions [15]. This attitude would not have been appropriate for the severe LMCS in the present case. Therefore, we opted to perform pulmonary arterioplasty, which significantly reduced the LM compression. A more aggressive PA reduction (PA target diameter of 25 mm) would have possibly been more effective.
Dilated PA (>40 mm) demands further monitoring and explorations to exclude LMCS. PAH-targeted therapy and surgery are complementary in the management of neglected left-to-right shunts in adult patients. The “treat-repair-treat” strategy and shunt-closure with a unidirectional valved patch can both improve surgical prospects of LMCS with shunt-related PAH. The benefits of pulmonary reductive arterioplasty on the PAA-induced LMCS were demonstrated by CA during follow-up. Late postoperative results of pulmonary arterioplasty for LMCS are yet to be studied, but our early experience was encouraging.
Authorship: The authors confirm their contribution to the paper as follows: study conception and design: A.G. Iosifescu, R. Enache; data collection: A.G. Iosifescu, R. Enache, I. Marinică, C. Radu; analysis and interpretation of results: A.G. Iosifescu, R. Enache, V.A. Iliescu; draft manuscript preparation: A.G. Iosifescu, R. Enache. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The informed consent is obtained, and the patient granted permission to publish the images for this article.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sharma, H., Doshi, S. N., Nadir, M. A. (2021). Acute myocardial infarction due to external compression of the left main coronary artery by a large pulmonary artery aneurysm. Case Reports in Cardiology, 2021, 8850044. DOI 10.1155/2021/8850044. [Google Scholar] [CrossRef]
2. Demerouti, E. A., Manginas, A. N., Athanassopoulos, G. D., Karatasakis, G. T. (2013). Complications leading to sudden cardiac death in pulmonary arterial hypertension. Respiratory Care, 58(7), 1246–1254. DOI 10.4187/respcare.02252. [Google Scholar] [CrossRef]
3. Yeh, D. D., Ghoshhajra, B., Inglessis-Azuaje, I., MacGillivray, T., Liberthson, R. et al. (2015). Massive pulmonary artery aneurysm causing left main coronary artery compression in the absence of pulmonary hypertension. Texas Heart Institute Journal, 42(5), 465–467. DOI 10.14503/THIJ-15-5028. [Google Scholar] [CrossRef]
4. Choudhary, S. K., Talwar, S., Airan, B. (2007). A simple technique of unidirectional valved patch for closure of septal defects. Journal of Thoracic and Cardiovascular Surgery, 134(5), 1357–1358. DOI 10.1016/j.jtcvs.2007.08.003. [Google Scholar] [CrossRef]
5. Galiè, N., Saia, F., Palazzini, M., Manes, A., Russo, V. et al. (2017). Left main coronary artery compression in patients with pulmonary arterial hypertension and angina. Journal of the American College of Cardiology, 69(23), 2808–2817. DOI 10.1016/j.jacc.2017.03.597. [Google Scholar] [CrossRef]
6. Kajita, L. J., Martinez, E. E., Ambrose, J. A., Lemos, P. A., Esteves, A. et al. (2001). Extrinsic compression of the left main coronary artery by a dilated pulmonary artery: Clinical, angiographic, and hemodynamic determinants. Catheterization and Cardiovascular Interventions, 52(1), 49–54. DOI 10.1002/(ISSN)1522-726X. [Google Scholar] [CrossRef]
7. Takaya, Y., Akagi, T., Sakamoto, I., Kanazawa, H., Nakazawa, G. et al. (2022). Efficacy of treat-and-repair strategy for atrial septal defect with pulmonary arterial hypertension. Heart, 108(5), 382–387. DOI 10.1136/heartjnl-2021-319096. [Google Scholar] [CrossRef]
8. He, Y., Li, Q., Zhang, C., Keller, B., Gu, H. (2021). Successful outcomes for atrial septal defect associated with pulmonary arterial hypertension using a “treat-repair-treat” strategy. International Journal of Cardiology Congenital Heart Disease, 2, 100075. DOI 10.1016/j.ijcchd.2020.100075. [Google Scholar] [CrossRef]
9. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2021). ESC scientific document group. 2020 ESC guidelines for the management of adult congenital heart disease. European Heart Journal, 42(6), 563–645. DOI 10.1093/eurheartj/ehaa554. [Google Scholar] [CrossRef]
10. Duijnhouwer, A. L., Navarese, E. P., Van Dijk, A. P., Loeys, B., Roos-Hesselink, J. W. et al. (2016). Aneurysm of the pulmonary artery, a systematic review and critical analysis of current literature. Congenital Heart Disease, 11(2), 102–109. DOI 10.1111/chd.12316. [Google Scholar] [CrossRef]
11. Rosic, M., Susak, S., Redzek, A., Velicki, L. (2016). Closure of an atrial septal defect with a one-way flap patch in a patient with severe pulmonary hypertension. Interactive Cardiovascular and Thoracic Surgery, 22(6), 856–858. DOI 10.1093/icvts/ivw032. [Google Scholar] [CrossRef]
12. Araújo, I., Escribano, P., Lopez-Gude, M. J., Lopez-Guarch, C. J., Sanchez, M. A. et al. (2011). Giant pulmonary artery aneurysm in a patient with vasoreactive pulmonary hypertension: A case report. BMC Cardiovascular Disorders, 11, 64. DOI 10.1186/1471-2261-11-64. [Google Scholar] [CrossRef]
13. Amaral, F. T., Alves Jr, L., Granzotti, J. A., Manso, P. H., Lima Filho, M. O. et al. (2007). Extrinsic compression of left main coronary artery from aneurysmal dilation of pulmonary trunk in an adolescent: Involution after surgery occlusion of sinus venosus atrial septal defect and pulmonary trunk plasty for reduction. Arquivos Brasileiros de Cardiologia, 88(2), e40–43. DOI 10.1590/s0066-782x2007000200022. [Google Scholar] [CrossRef]
14. Jodocy, D., Friedrich, G. J., Bonatti, J. O., Müller, S., Laufer, G. et al. (2009). Left main compression syndrome by idiopathic pulmonary artery aneurysm caused by medial necrosis erdheim-gsell combined with bicuspid pulmonary valve. Journal of Thoracic and Cardiovascular Surgery, 138(1), 234–236. DOI 10.1016/j.jtcvs.2008.02.076. [Google Scholar] [CrossRef]
15. Fujiwara, K., Naito, Y., Higashiue, S., Takagaki, Y., Goto, Y. et al. (1992). Left main coronary trunk compression by dilated main pulmonary artery in atrial septal defect: Report of three cases. Journal of Thoracic and Cardiovascular Surgery, 104(2), 449–452. DOI 10.1016/S0022-5223(19)34802-0. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools