 Open Access
Open Access
ARTICLE
Ventricular Septal Crypts: Remnants of Spontaneous Interventricular Defect Closure?
Cardiology Unit, Misericordia Hospital, Grosseto, Italy
* Corresponding Author: Andrea Picchi. Email:
Congenital Heart Disease 2023, 18(1), 1-6. https://doi.org/10.32604/chd.2023.025639
Received 23 July 2022; Accepted 25 October 2022; Issue published 09 January 2023
Abstract
Background: Ventricular crypts are quite a common finding during cardiac imaging, but their etiology is unclear. A possible final result of a spontaneous ventricular septal defect closure has been supposed but never investigated in earlier studies. Method: From January 1997 to December 2020, all newborns diagnosed to have a ventricular septal defect were prospectively entered in our database and those with an isolated defect were included in the study. Ventricular septal defects were classified into four types: perimembranous, trabecular muscular, inlet and outlet. A long-term follow up was performed in order to visualize the possible residual formation of a septal myocardial crypt. Results: A total of 376 isolated ventricular septal defects (314 muscular and 54 perimembranous, 4 inlet, 4 outlet) were detected. Follow up ranged from 1 to 23 years and showed that, among muscular type, a spontaneous closure occurred in 284 (91%), 26 did not close (8,28%), 2 required surgical intervention (0,63%), 3 were lost at follow up (0,95%). During this period, after spontaneous defect closure closure, 20 crypts were found (6,4%). Conclusion: This study shows that a muscular ventricular septal defect may evolve in the 6.4% of cases in a residual septal crypt. Although septal crypts occur more frequently in patients affected by hypertrophic and hypertensive cardiomyopathy, they may also represent the evolution of a spontaneous closure of a muscular interventricular defect.Graphic Abstract
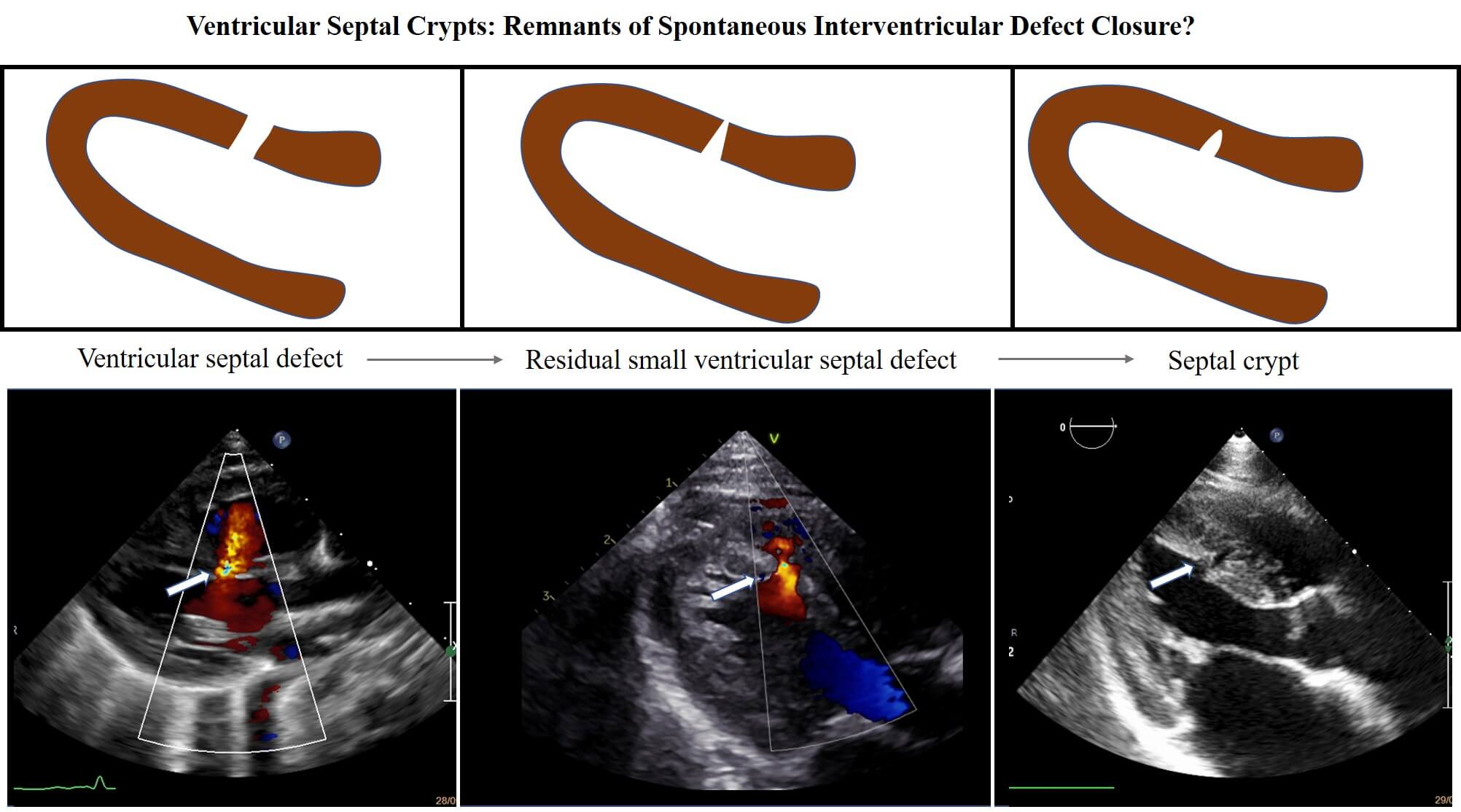
Keywords
Ventricular septal crypts are quite a common finding in the context of cardiac imaging. With the widespread use of Cardiac Computed Tomography (CT) and Magnetic Resonance (CMR), even small ventricular outpouchings (CVOs) are easily found. These CVOs include diverticula (CVD), aneurysms (CVA), clefts and crypts. Several definitions have been used to differentiate these outpouchings and our group has recently proposed a simple classification [1].
Focusing on ventricular septal crypts or diverticula, their appearance, in the adult patient, resembles that of a septal congenital muscular defect which has spontaneously closed. When a septal crypt is discovered in an adult patient, without clear anamnestic data of a previous interventricular defect (VSD), a definite etiology cannot be diagnosed. In clinical practice, these crypts are often hypothesized to be the final result of a closed VSD. However, a correlation between ventricular muscular septal defects and crypts has never been demonstrated. They may also appear in the phenotypic expression of a hypertrophic cardiomyopathy, a pathology which is necessary to rule out.
Objective of our study was to evaluate the presence and incidence of septal crypts in a large population of patients who underwent spontaneous VSD closure, documenting the evolution from VSD to a crypt in a long-follow-up period.
From January 1997 to December 2020, all newborns with a suspicion to have a congenital heart disease (CHD) were referred to the echocardiography laboratory of our Cardiology Unit. Newborns diagnosed to have a VSD were prospectively entered in our database and those with an isolated VSD were included in the study. Exclusion criteria were VSDs associated with other CHDs, such as atrioventricular septal defect, tetralogy of Fallot, transposition of the great arteries, tricuspid atresia, pulmonary stenosis, and coarctation of the aorta.
VSD were classified according to Lopez et al. [2]: perimembranous, trabecular muscular, inlet, outlet.
We focused on patients affected by a trabecular muscular VSD. We performed a follow up until a spontaneous closure was observed: these patients represent the study population and a long-term follow up was performed in order to visualize the possible residual formation of a septal myocardial crypt.
The defect size was measured by bi-dimensional imaging, unless borders were not clearly detectable; in that case the thickness of the color jet through the septum was used, following the classification proposed by Liu et al. [3]. Thus, VSDs were defined “small,” when less than 3 mm, “medium,” in case they were 3–5 mm, or “large” if more than 5 mm, respectively. Spontaneous closure was defined as the absence of color flow mapping shunt. Myocardial crypts were defined as narrow, deep invaginations within the myocardial wall penetrating more than 50% of the thickness of the adjoining compact myocardium; visible in diastole, they shrink or completely close in systole. The term “cleft” has been used by few Authors with the same significance as “crypt”: as this may be a source of confusion, we preferred to use “crypt”.
“Recesses” or “partial crypts” penetrating 25%–50% of the wall thickness which are difficult to be distinguished from trabeculations impinging the endocardial contour without any clinical significance, were decided to be excluded from our study [1].
Follow-up was scheduled at fixed time intervals: 1 month, 6 months, 1 year, 2 years, and then annually if the defect was still present. Follow up was considered in case of complete spontaneous defect closure.
All echocardiographic exams were performed at the echocardiography laboratory of our Cardiology Unit by two experienced pediatric cardiologists who were certified by the Italian Society of Echocardiography and CardioVascular Imaging (SIECVI) with a Level III competence. An Acuson Sequoia 512 ultrasound system model (Siemens Healthcare Ultrasound System, Erlangen, Germany) was used from 1998 to 2001 and a Vivid-7 ultrasound system model (GE-Healthcare, Milan, Italy) from 2002 to 2015. From 2015 to 2021 a Philips Epiq (Philips Healthcare, USA) and a GE e95 ultrasound Machine. Both Systems were equipped with dedicated neonatal and pediatric high frequency probes (bandwidth 10–4 MHz).
A final echocardiographic follow up was performed in all patients with a muscular isolated VSD in the data base during the years 2021 and 2022, while those in whom a septal crypt was found were prospectively registered.
Categorical variables were expressed as number of cases and percentages. Continuous variables were expressed as mean ± SD. Event(s) rates (spontaneous closure or surgical closure) were estimated with Kaplan-Meier curves and compared by the log-rank test. The association of selected variables with outcome were assessed with Cox’s proportional hazard model using stepwise multivariable procedures, a significance of 0.05 was required for a variable to be included into the multivariable model. Hazard ratios (HR), with the corresponding 95% confidence interval (CI), were estimated. The following co-variates were analyzed: age, sex, type, number of defects, severity, associated pathologies. Statistical significance was set at p < 0.05. Statistical Package for the Social Sciences (SPSS 17.0, SPSS Inc., Chicago, IL, USA) was used for the analysis.
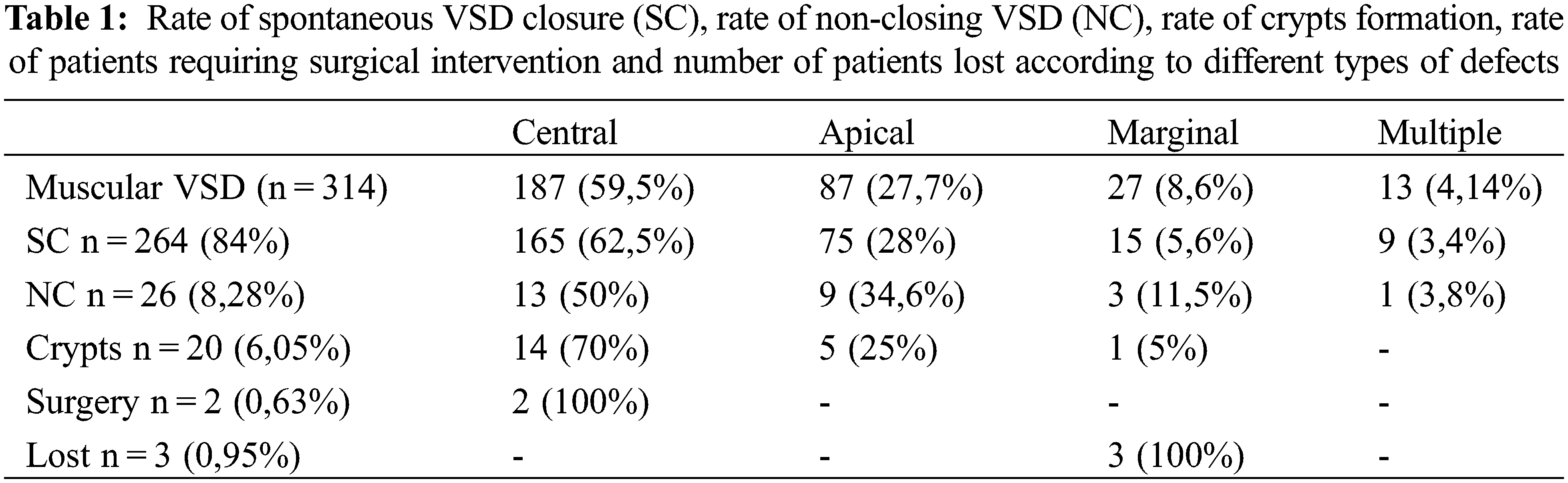
Total diagnoses of isolated VSD in the population were 376 (314 muscular and 54 perimembranous, 4 inlet, 4 outlet). Muscular VSD locations were as follows: 187 centrals (59,5%), 87 apical (27,7%), 27 marginals (8,6%), and 13 multiples (4,14%). Follow up lasted from 1 to 23 years. During follow up, among muscular type, a spontaneous closure occurred in 284 (91%), 26 did not close (8,28%), 2 required surgical intervention (0,63%), 3 were lost at follow up (0,95%). During this period, after spontaneous VSD closure, 20 crypts were found (6,4%). Among these, 14 derived from muscular central VSD, 5 from apical and 1 from a marginal defect (Table 1). During follow up, we found no echo-graphic signs of cardiomyopathies, no pathological changes in electrocardiograms and none of our patients presented symptoms or arrhythmias.
The main finding of this study is that residual crypts of the interventricular septum may represent the possible evolution of a spontaneously closing VSD (Fig. 1). This happened in 6.4% of our population of 314 trabecular VSD patients prospectively followed up for a long period (1 to 23 years, median 12 years). We have previously published a large case-series VSD documenting a very high incidence of spontaneous closure, especially among muscular ones, with different frequency, timing and pathophysiology [4].
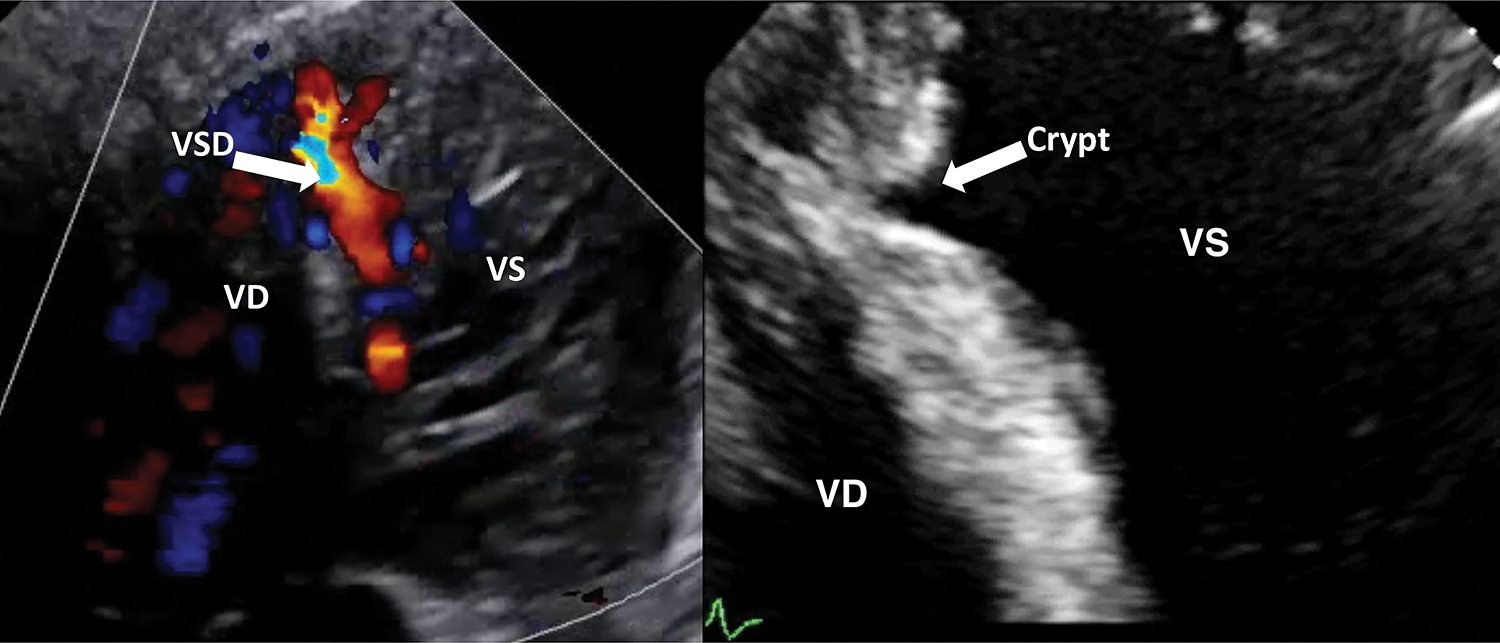
Figure 1: A case of spontaneous closure evolved in a septal crypt formation
Ventricular crypts are quite a common finding during cardiac imaging (echo, CT or MR), but their etiology is unclear. A possible final result of a spontaneous VSD closure had been supposed but never investigated in earlier studies. Crypts may be found in every segment of the left ventricle, more often, however, in the lateral wall and in patients affected by an HCM. The incidence of septal crypts in the general population is not known and only single centers case-series have been published. In a retrospective study, among 399 CMR cases, Johansson et al. described 27 basal inferior and 24 septal clefts: 13/120 (15.6%) were healthy volunteers and 5/19 (5.5%) were patients affected by HCM, whereas 11.4% were hypertensive subjects [5]. Germans et al. demonstrated crypts in 81% of HCM mutations carriers (13/16) and none in the control group (0/16) [6]. In a more recent larger study, Maron et al. disclosed a prevalence of 4% (10/261) in HCM patients, 61% (19/31) in mutation carriers, and similarly no clefts were visualized in the control group (0/98) [7]. Both studies are prospective, investigated a predefined cohort of abnormal sarcomeric protein carriers for HCM, and used a control group without LV hypertrophy or HCM family history. Interestingly, the relatively low prevalence of these clefts in frank HCM in the latter study was theorized to be attributed to the possibility of regression of these invaginations with subsequent LV wall thickening and remodeling. Therefore, they may identify family members who should be considered for further diagnostic imaging or genetic testing. In HCM patients, they are caused by myocardial fibers disarray or by focal areas of “non-compaction”. In CHD patients referred for a CMR, Petryka et al. found a prevalence of crypts in 8.3% [8]. Their presence has been described even in LV hypertrophy due to secondary causes with a frequency of 13.6% and 27% of hypertensive heart disease patients [8,9].
Our study population included only isolated VSD cases, and in patients without imaging features or anamnestic suspicion of a cardiomyopathy. During the follow up period, we found no echographic signs of cardiomyopathies and none of our patients presented symptoms or arrhythmias nor developed a Bundle-Branch-Block. We believe that the pathological development of crypts in the setting of an HCM is different from that of septal ones derived from VSD closure.
It is also important to differentiate our findings from pseudo-aneurysms derived from spontaneous closure of peri-membranous VSD. The natural history of peri-membranous VSD, the most represented (80%) in medical records, has been widely studied. Eroğlu et al. reviewed 685 VSD records and among 450 patients with peri-membranous defect, they described aneurysmal transformation in 56%, left ventricular-to-right atrial shunt in 8.4%, subaortic ridge in 5.8%, aortic valve prolapses in 11.7%, and aortic regurgitation in 7.3% [10]. On the contrary, among 211 subjects with trabecular muscular defects, 57% (120) closed spontaneously but no cases of crypt formation or other complications were described.
Cox et al. [11] in a retrospective study, observed a spontaneous closure in 59% of muscular VSD but no cases of residual crypts were described, but this was not the aim of their observations. Kantarci et al. [12] reviewed the records of 2,725 cardiac CT scans and found 18 cases of septal outpouches which they supposed to represent a possible consequence of spontaneous closure of muscular septal defects, but this opinion was not supported by any previous studies, as they admit in the discussion. Their suggestion was motivated by a theoretical hypothesis and by the imaging of spontaneous closure of ventricular septal defects that may resemble the diagnostic features of a septal crypt.
We did not include, in our evaluation, recesses, penetrating less than 50% of the wall, as “indentations” of the endocardial septal profile is a quite common finding at echocardiographic exams, especially when trabeculae or accessory muscular or tendinous fibers insert in the septal profile; the spatial resolution of ultrasound machines is not so high to permit a definite diagnosis of real recesses in many cases. Therefore, as ours is an echocardiographic study, in order not to overestimate the incidence of septal outpouchings, we chose to avoid those cases in which the invagination depth was lower than 50% of the septal thickness, thus defining only real crypts.
Unfortunately, no further studies have investigated this topic; consequently, ours can be regarded as the first study to demonstrate the possible evolution of a muscular VSD in a residual septal crypt. Therefore, a comparison to previous literature is not possible. Further long-term prospective researches are needed to confirm our conclusions and to evaluate the clinical significance of such septal crypts in the long-term follow up.
Despite a recent interest in the clinical significance of myocardial crypts, the multitude of the studies shows that they are a relatively common finding and are not pathognomonic of a specific disease. They do, however, occur more frequently in HCM and hypertensive cardiomyopathy although, because of the variability in the imaging protocols used and their relative frequency within the normal population, they should not be used to diagnose or clinically stratify these patients. Our study is the first to show the possible evolution of a muscular VSD in a residual septal crypt thanks to a longer echocardiographic follow up. We established that the etiology of a septal crypt may be the evolution of a spontaneous closure of a muscular interventricular defect.
Authorship: The authors confirm contribution to the paper as follows. Study conception and design: Alberto Cresti, Stefania Stefanelli. Data collection: Alberto Cresti, Stefania Sparla. Analysis and interpretation of results: Alberto Cresti, Andrea Picchi, Ugo Limbruno. Draft manuscript preparation: Alberto Cresti, Andrea Picchi, Ugo Limbruno. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Given the observational nature of the study, ethical committee approval was not required.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Cresti, A., Cannarile, P., Aldi, E., Solari, M., Sposato, B. et al. (2018). Multimodality imaging and clinical significance of congenital ventricular outpouchings: Recesses, diverticula, aneurysms, clefts, and crypts. Journal of Cardiovascular Echography, 28(1), 9–17. DOI 10.4103/jcecho.jcecho_72_17. [Google Scholar] [CrossRef]
2. Lopez, L., Houyel, L., Colan, S. D., Anderson, R. H., Béland, M. J. et al. (2018). Classification of ventricular septal defects for the eleventh iteration of the international classification of diseases-striving for consensus: A report from the international society for nomenclature of paediatric and congenital heart disease. The Annals of Thoracic Surgery, 106(5), 1578–1589. DOI 10.1016/j.athoracsur.2018.06.020. [Google Scholar] [CrossRef]
3. Liu, J. X., Wang, J. H., Yang, S. R., Liu, M., Xu, Y. et al. (2013). Clinical utility of the ventricular septal defect diameter to aorta root diameter ratio to predict early childhood developmental defects or lung infections in patients with perimembranous ventricular septal defect. Journal of Thoracic Disease, 5(5), 600–604. [Google Scholar]
4. Cresti, A., Giordano, R., Koestenberger, M., Spadoni, I., Scalese, M. et al. (2018). Incidence and natural history of neonatal isolated ventricular septal defects: Do we know everything? A 6-year single-center Italian experience follow-up. Congenital Heart Disease, 13(1), 105–112. DOI 10.1111/chd.12528. [Google Scholar] [CrossRef]
5. Johansson, B., Maceira, A. M., Babu-Narayan, S. V., Moon, J. C., Pennell, D. J. et al. (2007). Clefts can be seen in the basal inferior wall of the left ventricle and the interventricular septum in healthy volunteers as well as patients by cardiovascular magnetic resonance. Journal of the American College of Cardiology, 50(13), 1294–1295. DOI 10.1016/j.jacc.2007.06.026. [Google Scholar] [CrossRef]
6. Germans, T., Wilde, A. A., Dijkmans, P. A., Chai, W., Kamp, O. et al. (2006). Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. Journal of the American College of Cardiology, 48(12), 2518–2523. DOI 10.1016/j.jacc.2006.08.036. [Google Scholar] [CrossRef]
7. Maron, M. S., Rowin, E. J., Lin, D., Appelbaum, E., Chan, R. H. et al. (2012). Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circulation. Cardiovascular Imaging, 5(4), 441–447. DOI 10.1161/CIRCIMAGING.112.972760. [Google Scholar] [CrossRef]
8. Petryka, J., Baksi, A. J., Prasad, S. K., Pennell, D. J., Kilner, P. J. (2014). Prevalence of inferobasal myocardial crypts among patients referred for cardiovascular magnetic resonance. Circulation. Cardiovascular Imaging, 7(2), 259–264. DOI 10.1161/CIRCIMAGING.113.001241. [Google Scholar] [CrossRef]
9. Child, N., Muhr, T., Sammut, E., Dabir, D., Ucar, E. A. et al. (2014). Prevalence of myocardial crypts in a large retrospective cohort study by cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 16(1), 66. DOI 10.1186/s12968-014-0066-0. [Google Scholar] [CrossRef]
10. Eroğlu, A. G., Oztunç, F., Saltik, L., Bakari, S., Dedeoğlu, S. et al. (2003). Evolution of ventricular septal defect with special reference to spontaneous closure rate, subaortic ridge and aortic valve prolapse. Pediatric Cardiology, 24(1), 31–35. DOI 10.1007/s00246-002-1345-3. [Google Scholar] [CrossRef]
11. Cox, K., Algaze-Yojay, C., Punn, R., Silverman, N. (2020). The natural and unnatural history of ventricular septal defects presenting in infancy: An echocardiography-based review. Journal of the American Society of Echocardiography, 33(6), 763–770. DOI 10.1016/j.echo.2020.01.013. [Google Scholar] [CrossRef]
12. Kantarci, M., Duran, C., Bozkurt, M., Guven, F., Ceviz, N. et al. (2009). Cardiac multidetector computed tomography (MDCT) of spontaneously closed ventricular septal defect. La Radiologia Medica, 114(3), 370–375. DOI 10.1007/s11547-009-0381-y. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools