 Open Access
Open Access
CASE REPORT
Emergency Hybrid Correction in a Newborn with Critical Aortic Valve Stenosis with Acute Pulmonary Edema in the First Hour after Birth
Saint Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
* Corresponding Author: Vitaliy Suvorov. Email:
(This article belongs to the Special Issue: Nightmare Case Reports in Congenital Heart Disease)
Congenital Heart Disease 2023, 18(1), 57-65. https://doi.org/10.32604/chd.2023.025522
Received 20 July 2022; Accepted 22 October 2022; Issue published 09 January 2023
Abstract
Critical aortic valve stenosis in newborns is the cause of a severe clinical condition with the onset of symptoms during first hours after birth. We present a clinical case of a successful surgical correction of a critical aortic stenosis using a hybrid method applied in a newborn during the first day of life. The infant was diagnosed with a hypoplastic left heart complex with an intact atrial septum (aortic and mitral valves stenosis variant), that led to the cardiogenic shock and acute pulmonary edema. The procedure included bilateral banding of the pulmonary artery branches and atrioseptostomy with stenting of the interatrial septum. The surgery was performed through a median sternotomy.Graphic Abstract
Keywords
Congenital aortic valve stenosis (AVS) accounts for about 5% of all congenital heart defects (CHD) in newborns [1]. The severity of the child’s clinical condition after birth depends on the degree of AVS [2]. In severe and critical aortic valve stenosis, hemodynamically significant disorders often develop [3]. Severe and critical AS lead to an acute increase of the left ventricular afterload worsened by a closure of the ductus arteriosus after birth. Impaired systemic perfusion develops as a result of obstruction of the aortic valve and reduced blood flow, which leads to acute left ventricular failure with acute pulmonary edema.
We present a case of the successful treatment of a newborn child with a hypoplastic left heart complex (HLHC), who immediately after birth developed acute pulmonary edema with acute left ventricular failure against the background of critical AVS. As a result circulatory arrest occurred, which required the implementation of cardiopulmonary resuscitation. The child was resuscitated, and after stabilization of this clinical condition, a hybrid surgical correction was performed on the first day of life. The procedure included: bilateral banding of the pulmonary artery branches, and atrioseptostomy with stenting of the interatrial septum. Patent ductus arteriosus (PDA) stenting was not performed. Due to the unstable clinical condition, continuous infusion of prostaglandin preparations was continued to prevent PDA closure. After a the successful hemodynamic correction, decompression of the left heart was achived with restoring the blood flow balance in the systemic and pulmonary circulation. This contributed to the regression of pulmonary edema and left ventricular failure, resulting in stabilization of the patient’s condition.
A newborn boy was born at the clinic of St. Petersburg State Medical University at a gestational age of 39 weeks. Congenital heart disease was detected prenatally at 27 weeks of gestation: Hypoplastic left heart syndrome, mitral valve hypoplasia, aortic valve atresia, aortic arch hypoplasia, restrictive open foramen ovale. The child weighted 3800 grams, with a body length of 51 cm. During the first minutes after birth, the child’s condition began to worsen. The clinical picture included: progression of hypoxemia (from 91% at the moment of birth to 64% within the first ten minutes after birth), tachypnea (respiratory rate was more than 80 per minute), episodes of hemodynamically significant bradycardia with a heart rate of 66–78 beats per minute, bilateral rales on the lungs auscultation, a coarse systolic murmur over the entire region of the heart. The baby was admitted to the intensive care unit in critical condition. Tracheal intubation was performed the background of parallel cardiopulmonary resuscitation. The infusion of vazaprostan, levosimendan, and adrenaline was established.
2.1 Diagnostic Methods, Surgery and Postoperative Period
In the preoperative period, an echocardiographic (ECHO) examination and chest X-ray were performed.
According to the transthoracic ECHO (Figs. 1–3): Situs solitus. The heart was formed and located correctly. Atrioventricular, ventriculoarterial connections were concordant. Signs of a critical CHD were determined: hypoplastic left heart complex. Hypoplasia of the left ventricle (LV) (border values, does not fulfill the apex of the heart). 2nd degree fibroelastosis of the LV. The LV ejection fraction was significantly reduced, (15% Simpson method). Enlarged of the LV (end-diastolic diameter (D) of the LV 27 mm). Enlargement of the left atrium. The atrial septum was intact. The mitral valve: fibrous ring D = 18 mm (Z = +2.8). Mild mitral valve stenosis (Pmean = 2.8 mm Hg) with severe insufficiency. The aortic valve: bicuspid, fibrous ring D = 6 mm (Z = −1.4), effective opening valve orifice D = 1.7 mm (Z = −14). Critical aortic valve stenosis (Pmean = 53 mm Hg). The tricuspid valve: fibrous ring D = 15 mm (Z = +0.7), Vmax 0.9 m/s, mild insufficiency. The pulmonary valve: fibrous ring D = 6.5 mm (Z = −1.2), Vmax = 1.1 m/s, function was not impaired. The patent ductus arteriosus (D = 4 mm, right-left shunt). Ductus-dependent systemic and coronary blood flow. The ascending aorta D = 7 mm (Z = −0.7). The aortic arch was left-sided, Vmax = 1.4 m/s at the isthmus level. The aortic arch segment “A” D = 4 mm (Z = −1.3), segment “B” D = 4.5 mm (Z = −1.5), segment “C” D = 5.5 mm (Z = −2.3). The descending aorta without features, Vmax = 1.3 m/s.
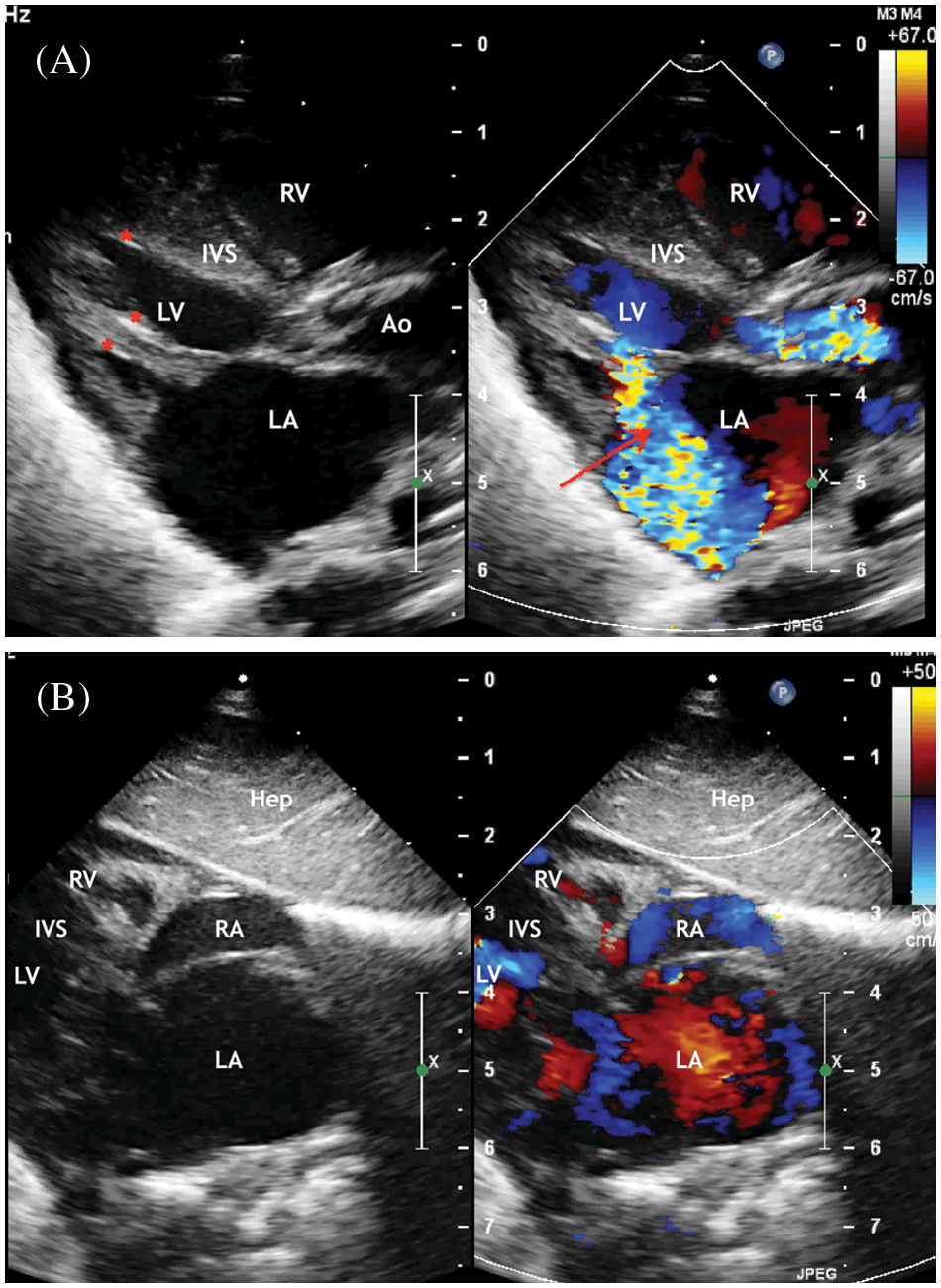
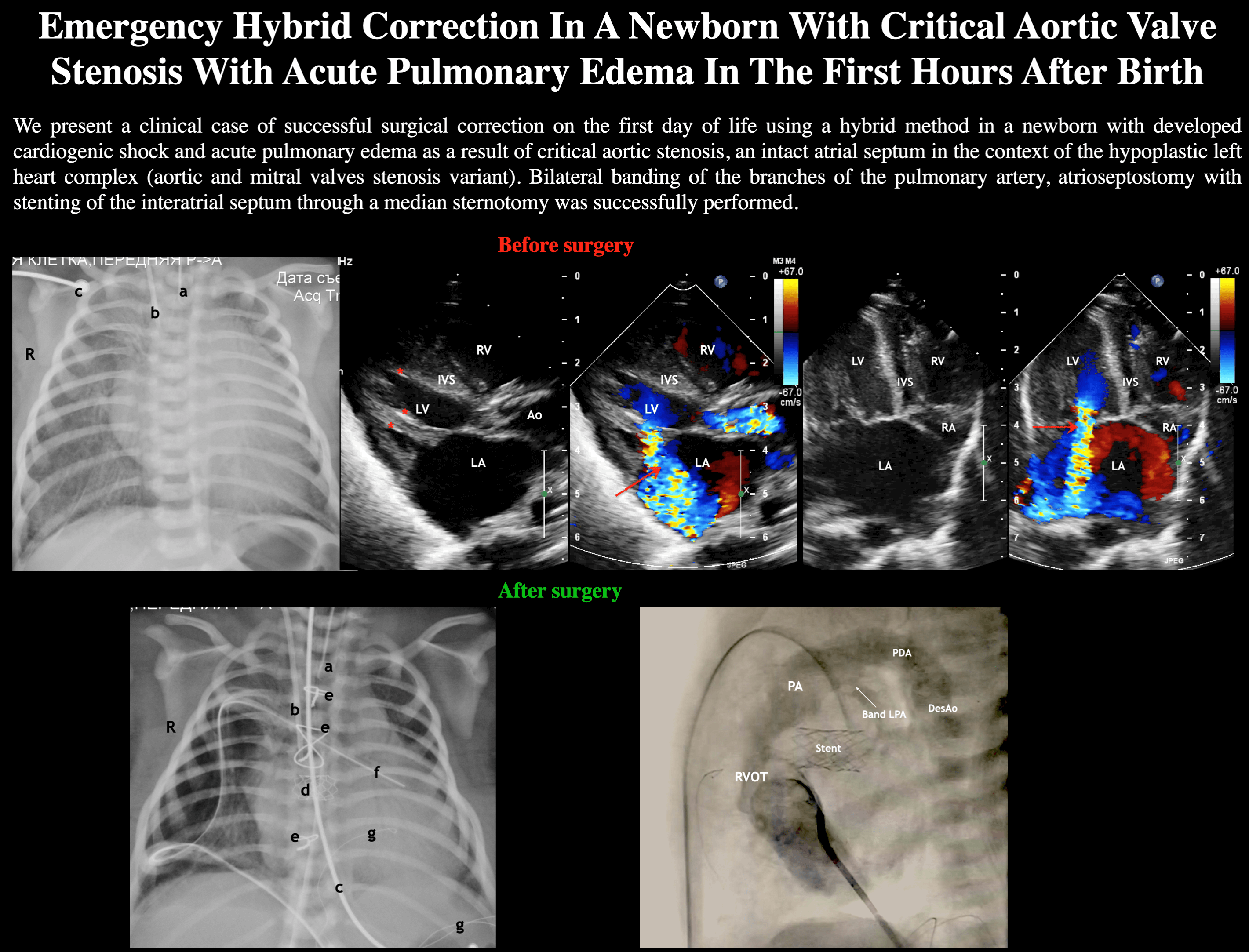
Figure 1: ECHO. (A) Parasternal long-axis view. Systole phase of the LV. LV myocardial hypertrophy, turbulent flow through the aortic valve is visualized. Aortic valve annulus diameter 6 mm but opening diameter 2.1 mm. Severe mitral regurgitation (marked with an arrow) with an enlarged of the left atrium. Areas of fibroelastosis of the LV (marked with an asterisk). (B) Subcostal view. Enlarged of the left atrium. Displacement of the interatrial septum to the right
Abbreviation: RV, the right ventricle; LV, the left ventricle; LA, the left atrium; RA, the right atrium; Ao, the ascending aorta. IVS, the interventricular septum; Hep, the liver.
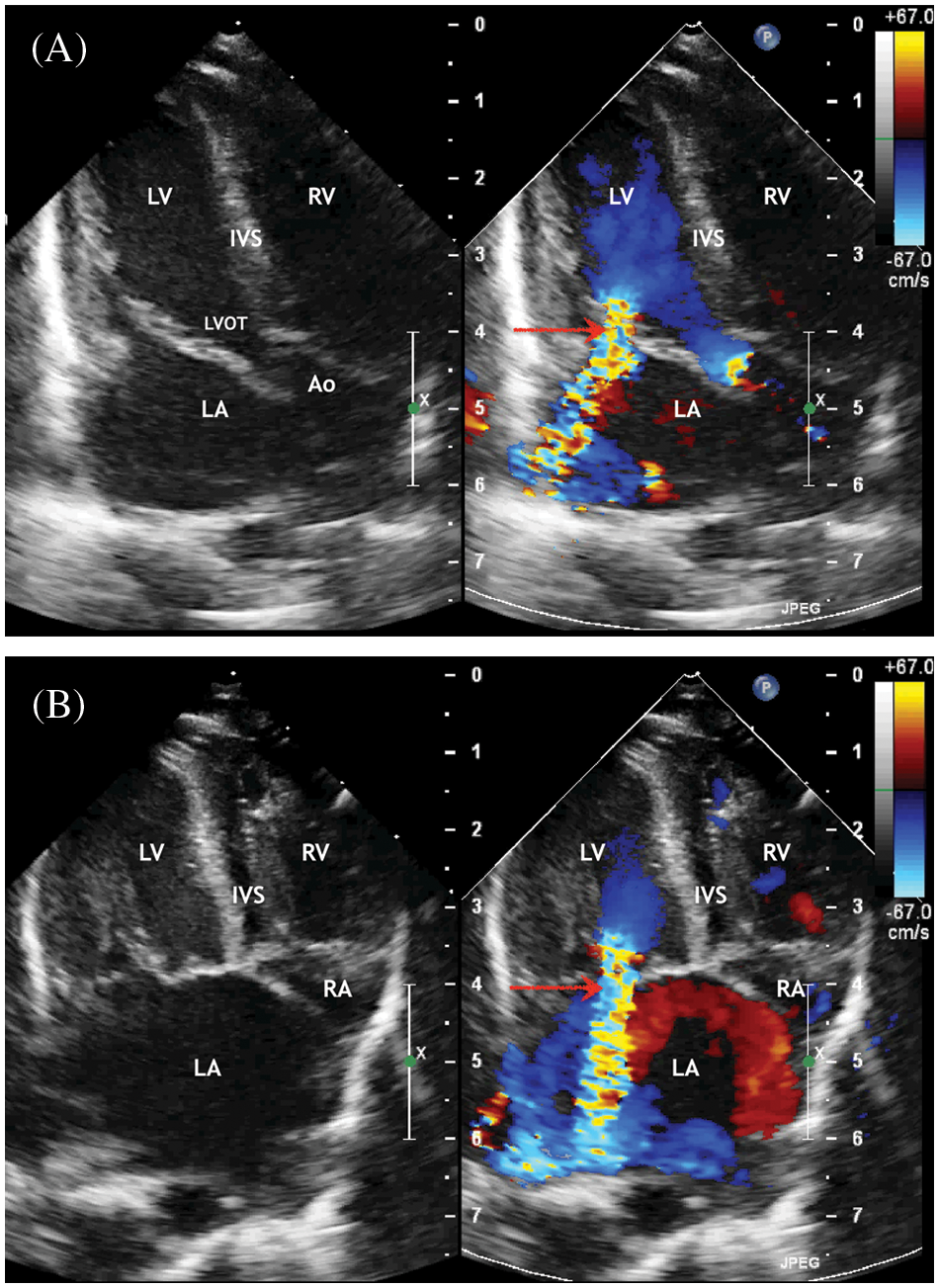
Figure 2: ECHO. (A) Five chambers view. Systole phase of the LV. Severe mitral regurgitation (marked with an arrow) with enlarged of the left atrium. (B) Four chambers view. Systole phase of the LV. Severe mitral regurgitation (marked with an arrow) with enlarged of the left atrium. Displacement of the interatrial septum to the right
Abbreviation: RV, the right ventricle; LV, the left ventricle; LA, the left atrium; RA, the right atrium; IVS, the interventricular septum; LVOT, the left ventricle output tract; LA, the left atrium; IVS, the interventricular septum; Ao, the ascending aorta.
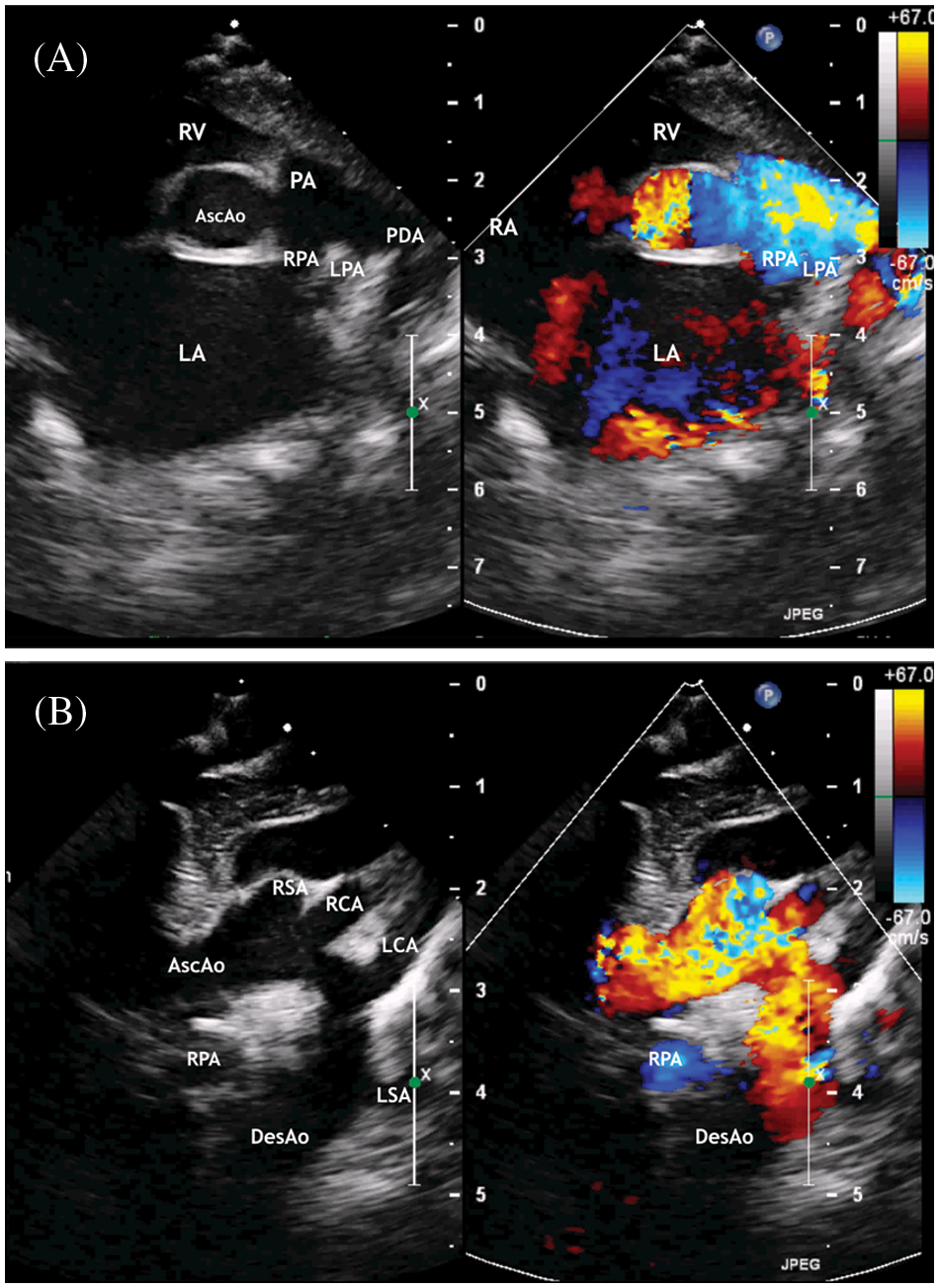
Figure 3: ECHO. (A) Parasternal short axis view. Enlargement of the left atrium. The patent ductus arteriosus with right to left shunt. (B) Suprasternal view. The ascending aorta is determined, mild aortic arch hypoplasia, and brachiocephalic vessels
Abbreviation: AscAo, the ascending aorta; DesAo, the descending aorta; LCA, RCA, the left and right common carotid artery; LSA, RSA, the left and right subclavian artery; RV, the right ventricle; RA, the right atrium; PA, the pulmonary artery; LPA, the left pulmonary artery; RPA, the right pulmonary artery; AscAo, the ascending aorta.
Mitral valve insufficiency was probably associated with severe volume overload of the left chambers and the absence of interatrial communication. As a result, the fibrous ring of the mitral valve was dilated. This is indirectly confirmed by the fact that after surgery and decompression of the left chambers, mitral valve insufficiency decreased to 1 degree.
Before surgery, the aortic valve and mitral valve had sufficient values and there were no signs of their hypoplasia, the subvalvular apparatus did not look abnormal (2 papillary muscles, good leaflets mobility), the aortic arch was slightly hypoplastic, possibly due to insufficient prenatal antegrade load. Therefore, we did not attribute this defect to the Shone’s complex, but to the hypoplastic left heart complex.
According to the X-ray of the chest: signs of acute pulmonary edema and pronounced zones of hypoventilation and atelectasis of the lungs on both sides were diagnosed (Fig. 4).
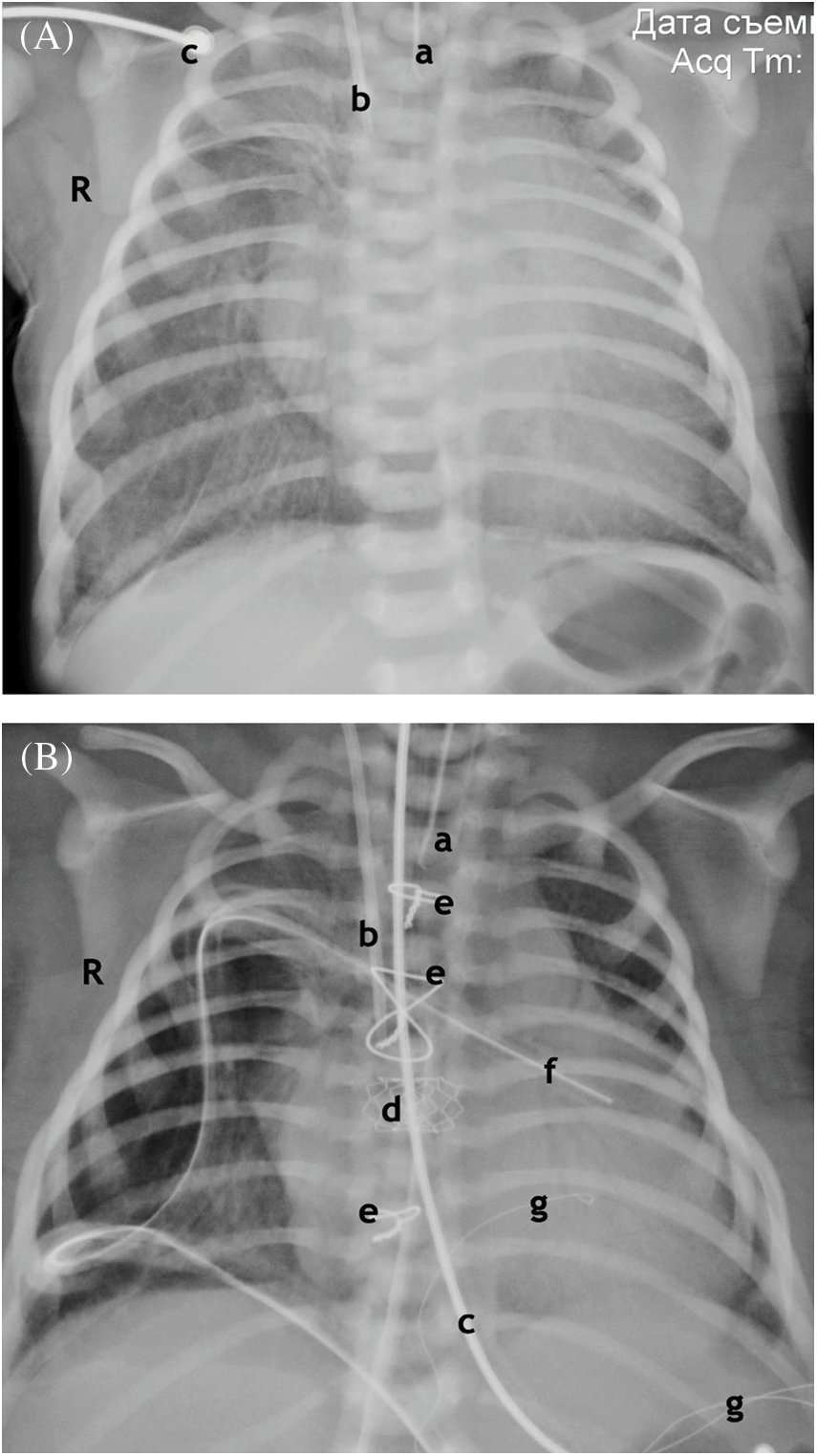
Figure 4: Chest X-ray. (A) Anterior projection. Before the operation. 1 h after birth. Enlargement of the shadow of the heart. Signs of pulmonary edema. Atelectasis of the upper lobe on the right, hypopneumatization on both sides. (B) Anterior projection. After operation. Age 7 days. Resolution of pulmonary edema. Atelectasis of the upper lobe on the right, Improvement of pneumatization. Shrinkage of the shadow of the heart
Notes: End of the endotracheal tube (a). End of the central venous catheter (b). ECG electrode (c). Stent at the atrial septum level (d). Wire ligatures of the sternum (e). The drainage of the right pleural cavity, pericardium (f). Temporary pacing electrodes (g).
After stabilization of the condition, considering the high risk of progression of the LV failure, acute pulmonary edema, a decision was made to perform a hybrid hemodynamic correction on the first day of life: percutaneous balloon atrioseptostomy, banding of the pulmonary artery branches. The purpose of the operation was to stop the causes of the LV failure and acute pulmonary edema, to ensure unhindered entry of oxygenated blood into the systemic circulation and to the blood flow balance in the pulmonary and systemic circulation.
Considering the critical clinical condition of the child, the presence of a critical CHD, which resulted in the development of acute LV heart failure, acute pulmonary edema, the futility of conservative therapy, indications for emergency surgical correction were determined.
The first stage was planned to perform percutaneous balloon atrioseptostomy. Performed angiography of the chambers of the heart, as a result of which the flow at the level of the interatrial septum was not determined. Many attempts to perform atrioseptostomy were ineffective. A decision was made to perform an open atrioseptostomy through a median sternotomy. After performing the access, a purse-string suture was formed on the auricle of the right atrium with polypropylene monofilament thread 5/0. After that, a puncture of the atrial septum was performed, a 6 Fr introducer was installed, a balloon artrioseptostomy was successfully performed, and stenting of the atrial communication with a stent with a diameter of 6 mm was performed (Figs. 4 and 5).
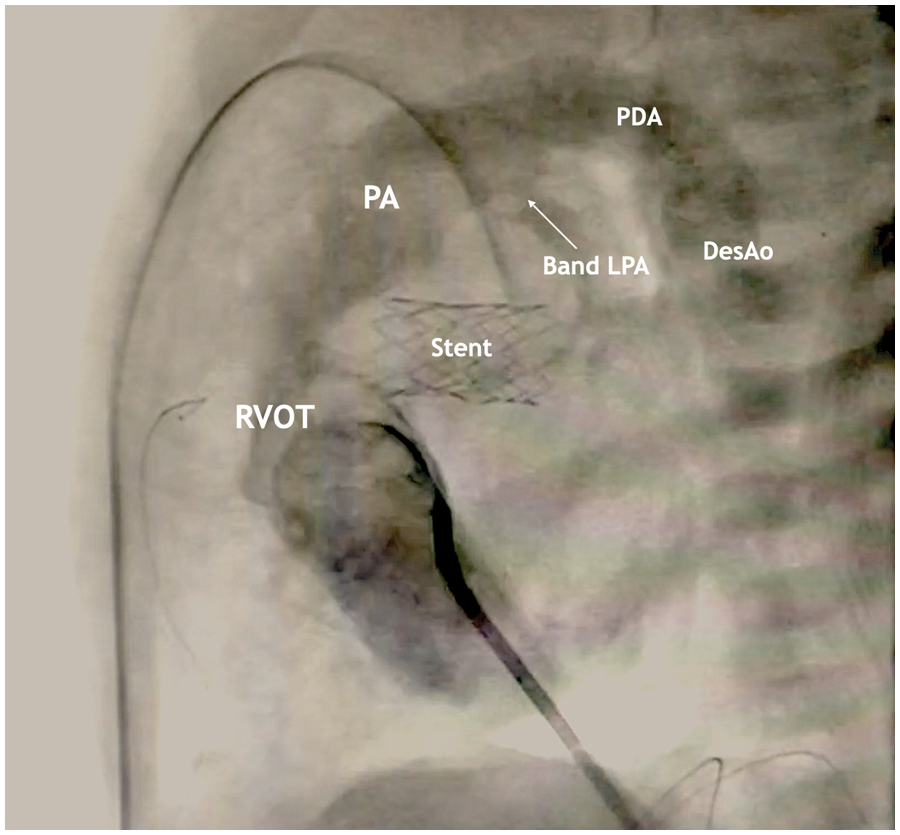
Figure 5: Lateral projection during cardiac catheterization with contrast
The stent is visualized in the projection of the atrial septal defect, the area of narrowing of the LPA, PDA with a diameter of 4 mm with a right-left shunt.
The next step was bilateral banding of the pulmonary artery branches [4]. During the operation, the target values of blood oxygen saturation (SaO2) were maintained at a level of about 80%. A cuff made of vascular PTFE (polytetrafluoroethylene) prosthesis was used to band of the PA branches. The prosthesis was cut in the longitudinal direction so that a strip 2 mm wide was obtained. The length of the strip required for the left pulmonary artery (LPA) was determined by the formula: 7 mm + patient weight, expressed as 1 mm for every 1 kg. For the right pulmonary artery (RPA) according to the formula: 7.5 mm + patient’s weight. During the banding of the pulmonary artery branches, the proportion of inhaled oxygen was maintained at the level of 21%. Intraoperatively ECHO was performed to detect a decrease in blood flow in the pulmonary veins and to assess blood flow at the site of banding of the left and right pulmonary arteries. PDA stenting was not performed. Due to the unstable clinical condition, continuous infusion of prostaglandin preparations was continued to prevent PDA closure. The sternotomy wound was sutured using the “open chest” technique.
The PDA was maintained by continuous infusion of prostaglandins, the average dose was 0.001–0.005 µg/kg/min. The dosage was changed if the diameter of the PDA changed. The target diameter values were considered to be no less than 5 mm. Echocardiography was performed daily to control the diameter of the PDA. PDA stenting was performed in a planned manner 26 days after the hybrid operation.
2.2 Сriteria for the Effectiveness of Balancing Systemic and Pulmonary Blood Flow
The success of the surgery in our study was achieved when the following criteria were met:
(1) Target values of various indicators of balanced pulmonary and systemic circulation (Qp/Qs = 1). Qp/Qs was assessed in accordance with recommendations of Klauwer et al. [3]:
– PaO2 ~ 40 (±5) mm Hg,
– SpO2 75%–85% with normal lung function (i.e., with SO2 in the pulmonary veins = 100%), normal ventilation (FiO 21%–30%), normal blood pressure and hemoglobin level.
(2) Intraoperative assessment of pulmonary arteries flow velocity in echocardiography: Vmax > 3 m/s, stenotic nature of the diastolic flow [4,5].
In the postoperative period, the child’s condition remained extremely serious, unstable for three days, due to the persistence of heart and respiratory failure, capillary leak syndrome. The consequences of impaired systemic perfusion resolved by day 6 after surgery: the level of lactate in the venous blood, the arteriovenous difference in oxygen (PaO2 and PvO2, SaO2 and SvO2), the level of oxygen delivery to tissues and its extraction normalized. The phenomena of heart failure resolved after 8 days, as evidenced by the improvement in contractile function according to ECHO, as well as signs of resolution of acute renal failure against the background of discontinuation of inotropic drugs. The child was extubated 17 days later with continued assisted ventilation using a non-invasive ventilation system. On the 35th day of life, the child was transferred to the neonatal pathology department. Discharged under the supervision of a pediatric cardiologist at the age of 1 month 10 days.
Critical CHD with systemic blood flow obstruction is the most common cause of acute heart failure in the neonatal period. Under such conditions, a syndrome of low systemic cardiac output develops, requiring surgical intervention. Clinical deterioration develops in most cases due to the rapid closure of fetal communications: PDA and patent foramen ovale (PFO). Early diagnosis and immediate treatment determine the prerequisites for a favorable outcome. These conditions are usually accompanied by progression of cyanosis without response to oxygen therapy, which can lead to the development of the cardiogenic shock, and difficult to distinguish from other causes of shock [1,2]. In addition to prostaglandin therapy to keep the ductus arteriosus open, a balanced pulmonary and systemic circulation should be one of the main goals [5,6]. In the case when a violation of blood shunting at the level of the atrial septum develops due to restrictive PFO and there is an obstruction of the systemic output, the child’s condition may progressively worse.
In order to be prepared for such situations, it is necessary to prenatally identify such critical defects. Considering that the prenatally open foramen ovale was present, albeit restrictive, and we did not detect it after birth (because it closed, possibly prenatally at a later date), the child quickly destabilized after birth as a result of the development of the cardiogenic shock and acute pulmonary edema.
We believe that it is difficult to be prepared for such situations since these are rare cases. To increase the chances of the successful treatment of such patients, childbirth should take place in a specialized hospital with a cardiac surgery service.
In the presented clinical case, the development of cardiogenic shock occurred within the first hour after birth, which required cardiopulmonary resuscitation and emergency surgery. Due to the fact that the child was born in a multidisciplinary hospital with the possibility of providing cardiac surgery and interventional care, it was possible to complete the necessary amount of treatment in the first hours after decompensation of the clinical condition. In addition, it should be noted that in the presented clinical case, the key to successful treatment was simultaneous decompression of the left heart and balancing the pulmonary and systemic blood flow. Given the borderline dimensions and pronounced fibroelastosis of the LV, the percutaneous balloon aortic valve plasty was accompanied by a dubious effect, especially with the development of complications in the form of total aortic insufficiency. Cardiopulmonary bypass surgery in children with acute pulmonary edema and in critical condition is not the procedure of choice for a number of reasons, the main of which is the need for cardioplegia and volemic load, which aggravates the function of the lungs and heart muscles in the postoperative period, including as a result of capillary leakage [3,7]. In our case, we resorted to restoring fetal circulation. A hybrid operation was successfully performed in the newborn on the first day of life through the median sternotomy approach: balloon artioseptostomy with stenting of the interatrial septum and banding of the pulmonary artery branches, as a result of which the child’s condition was stabilized.
After hemodynamic correction the child’s condition improved, but still remained unstable. Therefore, the implementation of stenting of the PDA was postponed. Given the borderline values of the left heart and the normal size of the aortic and mitral valves annulus, we thought that in the future it would be possible to perform a biventricular correction. But after 4 months at the follow-up examination, the left structures were not normal, and the child underwent the comprehensive stage 2 procedure (the Norwood operation and the bidirectional Glenn procedure).
The result of the presented clinical case of the successful stabilization of the critical condition of a newborn with critical CHD with systemic blood flow obstruction emphasizes the high efficiency of hybrid hemodynamic correction. Given the nature of the clinical course of critical CHD with systemic blood flow obstruction with the development of the cardiogenic shock and acute pulmonary edema in the first hours after birth, one cannot fail to note the importance of prenatal diagnosis of malformations in children and delivery of pregnant women in multidisciplinary clinics with the possibility of providing cardiac surgery and interventional care to newborns.
Acknowledgement: We thank Dietrich Klauwer, M.D for his help and support in the treatment of patients.
Authors Contribution: Vitaliy Suvorov has made substantial contributions to the intellectual content of an article in terms of the conception, drafting, and revising of the work and interpretation of the data. Vladimir Zaitcev has made substantial contributions to the general content of an article in terms of the conception and the acquisition of the data. Olga Tereshenko has made substantial contributions to the content of an article in terms of the drafting and revising of the work. Nikolay Pilyugov has made substantial contributions to the revising of the work and the drafting of the article. Michail Komissarov has made substantial contributions to the content, to the acquisition of the data, revising of the work.
Ethics Approval: The informed consent from the parents is obtained, and they granted permission to publish the images for this article.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Affolter, J. T., Ghanayem, N. S. (2014). Preoperative management of the neonate with critical aortic valvar stenosis. Cardiology in the Young, 24(6), 1111–1116. DOI 10.1017/S1047951114002029. [Google Scholar] [CrossRef]
2. Khalil, M., Jux, C., Rueblinger, L., Behrje, J., Esmaeili, A. et al. (2019). Acute therapy of newborns with critical congenital heart disease. Translational Pediatrics, 8(2), 114–126. DOI 10.21037/tp.2019.04.06. [Google Scholar] [CrossRef]
3. Klauwer, D., Neuhaeuser, C., Thul, J., Zimmermann, R. (2019). A practical handbook on pediatric cardiac intensive care therapy, vol. 560, pp. 421–424. Cham, Switzerland: Springer. DOI 10.1007/978-3-319-92441-0. [Google Scholar] [CrossRef]
4. Kitahori, K., Murakami, A., Takaoka, T., Takamoto, S., Ono, M. (2010). Precise evaluation of bilateral pulmonary artery banding for initial palliatio in high-risk hypoplastic left heart syndrome. The Journal of Thoracic and Cardiovascular Surgery, 140(5), 1084–1091. DOI 10.1016/j.jtcvs.2010.07.084. [Google Scholar] [CrossRef]
5. Suvorov, V., Zaitcev, V., Andrzejczyk, K. (2022). Effectiveness of bilateral pulmonary artery banding in patients with hypoplastic left heart syndrome and congenital heart defects with a functional single ventricle: A single-center retrospective study. Congenital Heart Disease, 17(3), 365–374. DOI 10.32604/chd.2022.019126. [Google Scholar] [CrossRef]
6. Galantowicz, M. (2009). The hybrid approach to hypoplastic left heart syndrome. Operative Techniques in Thoracic and Cardiovascular Surgery, 14(2), 74–85. DOI 10.1053/j.optechstcvs.2009.06.005. [Google Scholar] [CrossRef]
7. Khubulava, G. G., Marchenko, S. P., Dubova, E. V., Suvorov, V. V. (2016). Role of modified ultrafiltration in reduce of the systemic inflammatory response syndrome in cardiac surgery. Pediatrician, 7(1), 106–110. DOI 10.17816/PED71106-110. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools